Abstract
Objectives
The aims of the study were to compare the consumption of blood products before and after the implementation of a bleeding management algorithm in patients undergoing liver transplantation and to determine the feasibility of a multicentre, randomized study.
Background
Liver transplantation remains the only curative therapy for patients with end-stage liver disease, but it carries a high risk of surgical bleeding.
Materials and Methods
Retrospective study of patients treated before (group 1) and after (group 2) implementation of a haemostatic algorithm guided by viscoelastic testing, including use of lyophilized coagulation factor concentrates (prothrombin complex and fibrinogen concentrates). Primary outcome was the number of units of blood products transfused in 24 h after surgery. Secondary outcomes included hospital stay, mortality, and cost.
Results
Data from 30 consecutive patients was analysed; 14 in group 1 and 16 in group 2. Baseline data were similar between groups. Median total blood product consumption 24 h after surgery was 33 U (IQR: 11–57) in group 1 and 1.5 (0–23.5) in group 2 (p = 0.028). Significantly fewer units of red blood cells, fresh frozen plasma, and cryoprecipitate were transfused in group 2 versus group 1. There was no significant difference in complications, hospital stay, or in-hospital mortality between groups. The cost of haemostatic therapy was non-significantly lower in group 2 versus group 1 (7,400 vs. 15,500 USD; p = 0.454).
Conclusion
The haemostatic management algorithm was associated with a significant reduction in blood product use during 24 h after liver transplantation. This study demonstrated the feasibility and provided a sample size calculation for a larger, randomized study.
Keywords: Liver transplant, Viscoelastic testing, Haemostasis, Bleeding
Introduction
Liver transplantation remains the only curative therapy for patients with end-stage chronic liver disease. For these patients, the pathophysiological changes associated with worsening cirrhosis, together with the characteristics of the surgical procedure itself, confer a high risk of surgical bleeding [1–3]. In studies carried out at the beginning of the 21st century, up to around 30% of patients undergoing liver transplant lost more than 5,000 mL of blood [4], and 40% received six or more units of red blood cells (RBCs) [5]. In more recent experiences, even in centres where modern haemostatic management strategies were implemented, median RBC transfusion still remained around 3–4 intraoperative units [6–8]. In liver transplantation, the need for transfusion therapy is associated with the occurrence of postoperative complications such as infection [9], reintervention [10], increased hospital stay [11], and mortality [12–14]. In addition, the excessive use of blood products imposes additional stress on the health system as their sustainability is in crisis, especially after the COVID-19 pandemic [15, 16]. Interestingly, there is no specific current recommendation regarding the use of any particular surgical technique to reduce the amount of bleeding. Therefore, standardization of transfusion strategies plays a leading role in reducing the use of transfused components [17].
Evidence from a randomized, controlled trial has demonstrated that using viscoelastic testing (VET) during liver transplantation resulted in a significant reduction in the use of blood products [7]. Additionally, observational studies have demonstrated improvements in clinical outcomes associated with the intraoperative use of this technology [18, 19]. Likewise, the use of clotting factor concentrates during this surgery appears to be safe and effective [20, 21], and the use of both strategies together could facilitate the reduction of patient exposure to blood products, although at the moment, there is no high-quality evidence to support this hypothesis. Here, we report the results of a retrospective pilot study conducted to investigate the effect of introducing a haemostatic management algorithm in liver transplantation based on (1) haemostatic diagnoses obtained from VET and (2) priority treatment with lyophilized clotting factor concentrates, on the perioperative consumption of blood products, morbidity, and postoperative mortality.
Materials and Methods
This was a retrospective cohort study that included all adult patients with end-stage liver damage who underwent deceased donor liver transplantation consecutively between June 2016 and May 2022 at the Clínica Santa María tertiary hospital in Chile. Approval to collect data from medical records for this study was obtained from the local scientific ethical committee, Comité Ético Científico Clínica Santa María, with resolution number 162609-22.
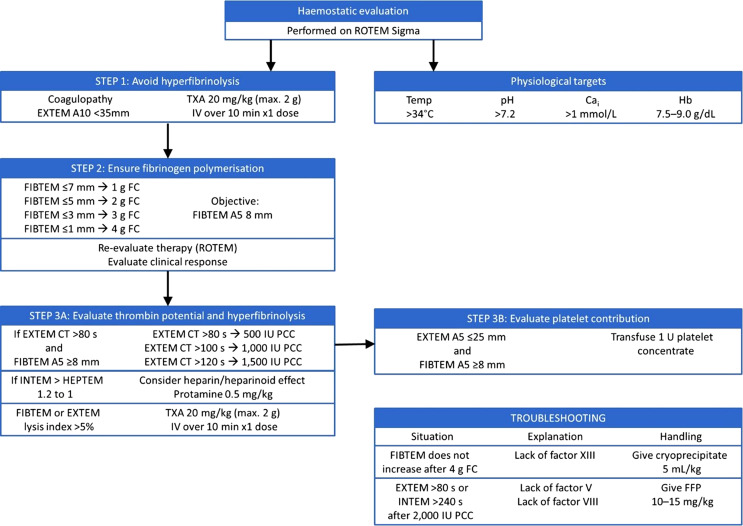
Haemostatic management of the first set of patients was performed at the discretion of the treating physician and without using any established protocol (group 1, n = 14), while the second set of patients were treated following the introduction of an intraoperative algorithm that included (1) diagnosis of coagulopathy by VET and (2) primarily treating these disorders with lyophilized concentrates of coagulation factors according to VET analysis (group 2, n = 16) (shown in Fig. 1). Additionally, the haemostatic management protocol included maintenance of physiological parameters within appropriate ranges [22], including haemoglobin levels between 7 and 9 g/dL, and the standardized use of tranexamic acid to prevent fibrinolysis.
Fig. 1.
Intraoperative bleeding management algorithm for liver transplantation. FC, fibrinogen concentrate; FFP, fresh frozen plasma; Hb, haemoglobin; IV, intravenous; PCC, prothrombin complex concentrate; TXA, tranexamic acid.
The object of the study was to compare the use of blood products between both groups before and after the implementation of this algorithm. The study was also conducted to determine the sample size necessary to design a future multicentre, randomized controlled trial to prospectively evaluate the implementation of this approach.
The primary outcome was the number of units of blood products transfused in the first 24 h after the start of surgery. Secondary outcomes included the total usage of blood products within the first 7 postoperative days; the first 24 h and 7 days of RBCs, fresh frozen plasma (FFP), cryoprecipitate, and platelet aphaeresis concentrates; and usage of lyophilized fibrinogen concentrate (Fibryga®, Octapharma AG, Switzerland); four factor prothrombin complex concentrate (PCC; Octaplex®, Octapharma AG); and use of antifibrinolytics (tranexamic acid, Espercil®, Taiyo Pharma Tech Co., Japan). Other secondary outcomes were estimated intraoperative bleeding, the occurrence of postoperative complications, hospital stay, and 28-day mortality. The total cost of surgery and the specific cost of haemostatic therapy were also analysed.
Immediate preoperative optimization of the patients consisted of RBC transfusions to maintain a haemoglobin concentration ≥7 g/dL. Prophylactic transfusions of other blood components were not performed. In case of preoperative bleeding, FFP was used to maintain a normalized international ratio (INR) of <2.0, transfusion of platelet aphaeresis concentrates to maintain a platelet count >50,000/μL, and transfusion of cryoprecipitates to maintain a plasma fibrinogen concentration of at least 1.5 g/L. Anaesthetic management followed a standardized protocol in all cases, consisting of induction with propofol, maintenance with sevoflurane, and use of remifentanil and rocuronium. Immunosuppression and antibiotic use were also standardized. Fluid administration was based on estimates of dynamic variables according to arterial line pulse contour analysis. All patients were ventilated protectively [23]. The surgical technique utilized for all patients was standard caval replacement [24]. The donor hepatectomy was completed using the Ligasure® device (Medtronic, USA). Portal reperfusion was performed first, followed by arterial reconstruction and subsequent reperfusion. All transplants were whole organ transplants, and as per national regulations, only donation in the case of diagnosed brain death was allowed. The use of blood salvage was used in all cases, except in cases of known malignancy. All blood products, as well as PCC, were available for the entire cohort. As the national health regulatory body had not given prior authorization, lyophilized human fibrinogen concentrate was only available for patients in group 2, who were treated as per the haemostatic management algorithm described in Figure 1.
VET was available for all patients. TEG®5000 (Haemonetics, USA) was used until case No. 8 (i.e., for the first 8 patients in group 1), and ROTEM® Sigma (Werfen, USA) was used from case No. 9 onwards (i.e., for 6 patients in group 1 and all patients in group 2). Sample collection was performed via the non-heparinized arterial line. Prior to the introduction of the haemostatic algorithm, the interpretation of the VET results and subsequent treatment were at the discretion of the treating physician, alongside haemostatic monitoring by means of classic laboratory tests if necessary. Postoperative transfusion management was not protocolized.
The model for end-stage liver disease-sodium (MELD-Na) score was calculated for both groups to evaluate the severity of cirrhosis before surgery, and the McCluskey index was calculated as a tool for predicting massive haemorrhage during liver transplantation. The cost of haemostatic therapy (units of blood products transfused plus use of clotting factor concentrates) was calculated using the institution’s internal financial records. As this is a private hospital, the details of the final cost of therapy are well defined at the time the monetary burden is passed onto the patient. To standardize the cost of therapy avoiding the effect of inflation, current prices at the time of the analysis were used to calculate the costs retrospectively. Other elements included in the cost of haemostatic therapy included the cost of the reagents and cartridges used for VET.
Statistical Analysis
In the present study, descriptive statistical data are presented with qualitative values expressed as frequencies and percentages, while quantitative variables are presented as the median and interquartile range (IQR). Analysis of quantitative variables was carried out using the Wilcoxon-Mann-Whitney test or Fisher’s exact test for categorical variables, as appropriate. Comparisons of results from the two groups were considered statistically significant where the p value <0.05.
Data analysis was performed using Stata 13.0 software (StataCorp, USA). Stata 13.0 software was also used to calculate the sample size of the prospective, randomized study to be carried out based on the findings of this study.
Results
Patient Population
Data from 30 consecutive patients were included in the analysis, which included all patients undergoing liver transplantation at the institution during the study period. The 30 patients were distributed in two groups: group 1 included 14 patients who were treated before the implementation of the haemostatic algorithm, and group 2 included 16 patients who were treated according to the implemented haemostatic algorithm. No patients were excluded due to lack of data.
The baseline data and clinical characteristics of the patients in the two groups are shown in Table 1. The median age of the patients in group 1 was 53 years (IQR: 37–58) and in group 2 was 57.5 years (44.5–60.5), with no statistically significant differences between the two groups. Regarding the patient severity assessment scales, group 1 had a median MELD-Na score of 22 points (IQR: 14–34), and group 2 had a median score of 21 points (10.8–30.5). The median McCluskey index was 2 for both groups. There were also no significant statistical differences in the characteristics of the donors, assessed according to validated standardized indices [25]. There were no statistically significant differences in laboratory parameters, including serum albumin and creatinine, nor any differences in haemoglobin, platelet, or fibrinogen levels between the two groups.
Table 1.
Baseline characteristics
| Group 1: before introduction of the algorithm (N = 14) | Group 2: following introduction of the algorithm (N = 16) | p value | |
|---|---|---|---|
| Age, years | 53 (37–58) | 57.5 (44.5–60.5) | 0.405 |
| Male, % | 64.3% | 68.8% | 0.550 |
| Preoperative MELD-Na | 22 (14–34) | 21 (10.8–30.5) | 0.677 |
| Transplant indication | |||
| Hepatocellular carcinoma, n | 3 | 3 | 0.287 |
| Viral disease, n | 1 | 1 | |
| Autoimmune hepatitis, n | 3 | 6 | |
| Alcoholic cirrhosis, n | 2 | 1 | |
| Non-alcoholic cirrhosis, n | 1 | 4 | |
| Biliary disease, n | 2 | 0 | |
| Other, n | 2 | 1 | |
| Patient history and comorbidities | |||
| McKluskey index | 2 (1–2.75) | 2 (1–3) | 0.491 |
| Anaemia, n | 10 | 11 | 0.569 |
| Preoperative thrombocytopaenia, n | 11 | 12 | 0.999 |
| Preoperative AKI, n | 5 | 3 | 0.263 |
| Diabetes mellitus, n | 5 | 9 | 0.225 |
| History of malignancy, n | 7 | 5 | 0.251 |
| Hospitalization within the last month, n | 8 | 11 | 0.706 |
| Previous abdominal surgery, n | 8 | 11 | 0.706 |
| Preoperative laboratory tests and others | |||
| Albumin, g/dL | 3.4 (2.8–4.0) | 3.5 (2.8–3.6) | 0.480 |
| Creatinine, mg/dL | 1.0 (0.8–1.2) | 0.7 (0.6–0.9) | 0.061 |
| Haemoglobin, g/dL | 10.3 (8.5–12.8) | 12.2 (8.9–13.3) | 0.454 |
| Platelet count, ×1,000/μL | 68 (48–96) | 46.5 (39.5–97.5) | 0.533 |
| Plasma fibrinogen, mg/dL | 139 (75–202) | 167 (129–195) | 0.358 |
| INR | 1.9 (1.2–2.6) | 1.8 (1.5–2.2) | 0.950 |
| Total ischaemia time, min | 367 (267–456) | 320 (265.5–407.5) | 0.637 |
| Donor risk index | 1.96 (1.60–2.09) | 1.88 (1.45–2.31) | 0.992 |
| Patients receiving any blood product preoperativelya, n | 4 | 5 | 0.999 |
Data are shown as median (inter-quartile range), unless indicated otherwise.
INR, international normalized ratio; AKI, acute kidney injury.
aFrom last hospital admission to completion of liver transplantation.
Effect of Implementation the Haemostatic Algorithm
The median total usage of blood products in the first 24 h following the start of surgery was 33 U (IQR: 11–57) in group 1 and 1.5 (0–23.5) in group 2 (p = 0.028). At 7 days following surgery, fewer units of blood products were transfused in group 1 compared with group 2 (36 vs. 4.5 U, respectively), although this difference did not reach significance (p = 0.062).
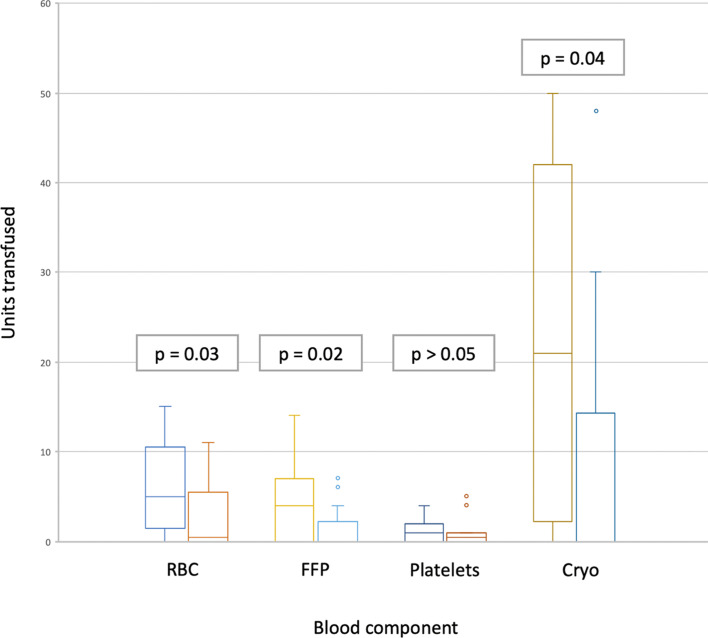
When analysing the usage of individual blood products, during the first 24 h following the start of surgery, significantly fewer units of RBCs, FFP, and cryoprecipitate were transfused in group 2, in which the haemostatic algorithm was used, as compared to group 1 (as shown in Fig. 2; Table 2). It is also noteworthy that the number of patients that received at least one unit of RBCs was statistically lower in group 2.
Fig. 2.
Blood component usage during the first 24-h peri-operative period. For each blood component, the left column corresponds to group 1, and the right column to group 2. Cryo, cryoprecipitate; FFP, fresh frozen plasma; Platelets, platelet concentrate; RBC, red blood cells; p, p value.
Table 2.
Haemostatic agents transfused in the first 24 h and 7 days following the start of surgery, in patients treated before and after introduction of the haemostatic algorithm
| Group 1: before introduction of the algorithm (N = 14) | Group 2: following introduction of the algorithm (N = 16) | p value | |
|---|---|---|---|
| 24 h following the start of surgery | |||
| Total blood products transfused, U | 33 (11–57) | 1.5 (0–23.5) | 0.028 |
| Patients receiving any blood product, n (%) | 12 (85.7) | 8 (50) | 0.057 |
| RBCs, U | 5 (2–10) | 0.5 (0–5) | 0.035 |
| Patients receiving RBC, n (%) | 11 (68.7) | 6 (37.5) | 0.032 |
| FFP, U | 4 (0–6) | 0 (0–1.5) | 0.024 |
| Patient receiving FFP, n (%) | 9 (64.2) | 4 (25) | 0.063 |
| Aphaeresis platelets, U | 1 (0–2) | 0.5 (0–1) | 0.560 |
| Patients receiving PC, n (%) | 8 (57.1) | 8 (50) | 0.730 |
| Cryoprecipitate, U | 21 (3–42) | 0 (0–13.5) | 0.043 |
| Patients receiving CP, n (%) | 11 (78.5) | 7 (43.7) | 0.071 |
| Fibrinogen concentrate, g | 0 (0–0) | 2.5 (1–5) | <0.001 |
| Patients receiving FC, n (%) | 0 (0) | 12 (75) | <0.001 |
| PPC, IU | 0 (0–0) | 1 (0–2) | 0.073 |
| Patients receiving PPC, n (%) | 3 (21.4) | 9 (56.2) | 0.071 |
| Tranexamic acid, g | 0 (0–0) | 2 (1–2) | <0.001 |
| Patients receiving TXA, n (%) | 1 (7.1) | 14 (87.5) | <0.001 |
| 7 days following the start of surgery | |||
| Total blood products transfused, U | 36 (11–63) | 4.5 (0–34) | 0.062 |
| Patients receiving any blood product, n (%) | 12 (85.7) | 8 (50) | 0.057 |
| RBCs, U | 6 (2–12) | 1.5 (0–8.5) | 0.198 |
| Patients receiving RBC, n (%) | 11 (68.7) | 8 (50) | 0.419 |
| FFP, U | 4.5 (0–8) | 0 (0–1.5) | 0.026 |
| Patient receiving FFP, n (%) | 9 (64.2) | 4 (25) | 0.063 |
| Aphaeresis platelets, U | 1 (0–3) | 1 (0–1) | 0.692 |
| Patients receiving PC, n (%) | 8 (57.1) | 9 (56.2) | 0.999 |
| Cryoprecipitate, U | 21 (3–42) | 0 (0–13.5) | 0.043 |
| Patients receiving Cryo, n (%) | 11 (78.5) | 7 (43.7) | 0.071 |
| Fibrinogen concentrate, g | 0 (0–0) | 2.5 (1–5) | <0.001 |
| Patients receiving FC, n (%) | 0 (0) | 12 (75) | <0.001 |
| PPC, IU | 0 (0–0) | 1 (0–2) | 0.073 |
| Patients receiving PPC, n (%) | 3 (21.4) | 9 (56.2) | 0.071 |
| Tranexamic acid, g | 0 (0–0) | 2 (1–2) | <0.001 |
| Patients receiving TXA, n (%) | 1 (7.1) | 14 (87.5) | <0.001 |
The amounts of transfusions are detailed only for transfused patients. Data are shown as median (inter-quartile range) or as numbers and percentages. Values in bold are where p < 0.05. Cryo, cryoprecipitate; FC, fibrinogen concentrate; FFP, fresh frozen plasma; PC, platelet aphaeresis concentrate; PPC, prothrombin complex concentrate; RBC, red blood cells; TXA, tranexamic acid.
At 7 days following surgery, statistically significant differences were seen for the number of units of FFP and cryoprecipitate transfused (Table 2). Additionally, significantly more tranexamic acid was administered to patients who were treated according to the algorithm, with a median of 0 g (IQR: 0–0) for group 1 and 2 g (1–2) for group 2 (p < 0.001) (Table 2).
Fibrinogen concentrates were only available for group 2. Accordingly, significantly more fibrinogen concentrate was administered to patients who were treated according to the algorithm (0 g [IQR: 0–0] for group 1 vs. 2.5 g [1–5] for group 2; p < 0.001). There was no significant difference in the use of PCCs between the two groups. There was only one use of protamine to counteract heparinoid effects in group 2 according to the algorithm, and there was no such use in group 1 treated prior to the implementation of the algorithm.
The volumes of intraoperative fluid administered are shown in Table 3. There were no statistically significant differences between the two groups in terms of crystalloids or albumin usage on the first postoperative day; furthermore, the use of the cell savage or the total volume of fluids infused intraoperatively was also the same.
Table 3.
Fluid therapy administered in the first 24 h following the start of surgery
| Group 1: before introduction of the algorithm (N = 14) | Group 2: following introduction of the algorithm (N = 16) | p value | |
|---|---|---|---|
| Volume of cell saver re-infused, mL | 250 (0–1,122) | 0 (0–202) | 0.127 |
| Crystalloids, L | 4.5 (40–5.4) | 4.6 (3.5–6.0) | 0.861 |
| Albumin, g | 60 (52.5–75) | 80 (60–82.5) | 0.131 |
| Total volume administered excluding blood products, L | 4.7 (4.2–6.1) | 5.0 (3.7–6.6) | 0.894 |
| Total volume administered including blood products, L | 7.0 (6.1–11.5) | 5.6 (4.1–9.9) | 0.092 |
Data are shown as median (inter-quartile range).
There were no statistically significant differences between the groups in intraoperative bleeding volume during the first postoperative day, in the occurrence of postoperative complications, the length of stay in the intensive care unit, or total hospital stay. Although there were no statistically significant differences between the groups concerning the total cost of haemostatic therapy, it is noteworthy that the cost was half in the group treated with the algorithm versus the group treated before its introduction (median, 13,800 USD [IQR: 4.6–25.1] vs. 7,200 USD [3.7–20.5], respectively; Table 4).
Table 4.
Bleeding, postoperative complications, hospital stay, and mortality during the first 24 h after the start of surgery, and the cost of haemostatic intervention
| Variable | Group 1: before introduction of the algorithm (N = 14) | Group 2: following introduction of the algorithm (N = 16) | p value |
|---|---|---|---|
| Estimated bleeding, mL | 1,450 (700–7,116) | 1,482 (475–2,496) | 0.547 |
| Postoperative mechanical ventilation duration, h | 10 (7–12) | 3.5 (0–31) | 0.397 |
| Postoperative complications | |||
| AKI KDIGO ≥2 | 8 (57.1) | 9 (56.3) | 0.626 |
| Major postoperative bleedinga, n (%) | 0 (0.0) | 4 (25.0) | 0.066 |
| Mechanical ventilation >24 h, n (%) | 3 (21.4) | 6 (37.5) | 0.299 |
| Reintubation, n (%) | 0 (0.0) | 1 (6.3) | 0.533 |
| Any infection, n (%) | 3 (21.4) | 4 (25.0) | 0.581 |
| Reoperation, n (%) | 2 (14.3) | 6 (37.5) | 0.154 |
| Reoperation for bleeding, n (%) | 0 (0) | 4 (25.0) | 0.066 |
| Thromboembolic events (DVP/PE), n (%) | 2 (14.2) | 3 (18.7) | 0.999 |
| Total complications, n | 1 (0–2) | 2 (0.5–4.5) | 0.243 |
| Hospital stay | |||
| ICU stay, days | 3 (2–6) | 4.5 (2.5–8.5) | 0.410 |
| Total hospital stay, days | 17 (13–24) | 17.5 (12–35.5) | 0.560 |
| Mortality | |||
| Mortality at 28 days, n (%) | 0 (0.0) | 1 (6.3) | 0.533 |
| Cost | |||
| Approximate cost of haemostatic intervention in the first 24 h following the start of surgery, 1,000 USD | 13.8 (4.6–25.1) | 7.2 (3.7–20.5) | 0.561 |
| Approximate cost of haemostatic intervention in the first 7 days following surgery, 1,000 USD | 15.5 (4.5–27.6) | 7.4 (3.7–20.5) | 0.454 |
Quantitative variables: median (inter-quartile range). Categorical variables: n (%).
AKI, acute kidney injury; DVP, deep vein thrombosis; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; PE, pulmonary embolism.
aMajor postoperative bleeding defined according to ISTH criteria [26].
Sample Size Calculation for Future Study
This pilot study has been proven feasible in the current setting in Chile, demonstrating that a larger, multicentre study to assess the benefits of such bleeding management could be conducted. To demonstrate a statistically significant reduction of 20% in total blood product consumption within the first postoperative day, and with a detection power of 80% allowing a drop-out rate of 15% lost to follow-up, 102 patients per group (204 in total) are required for the design of a randomized trial, taking into consideration the characteristics of the cohort described.
Discussion
This study evaluated the impact of the implementation of a haemostatic management algorithm based on VET, mainly focussing on the replenishment of haemostatic elements by means of clotting factor concentrates, on blood product consumption during liver transplant surgery. We found that the consumption of blood products in the first 24 h after the start of liver transplantation was significantly reduced after the implementation of the described algorithm.
These findings are in line with what has been previously described in the literature, where the use of viscoelastic methods during liver transplantation has reduced the use of blood products [2, 18, 27]. In a randomized study, Bonnet et al. [7] demonstrated that the use of VET-guided algorithms reduced the total intraoperative consumption of blood products and also the number of patients transfused with FFP. This could be explained by the ability to better select the type and correct amount of haemostatic products to be administered with VET. There is international consensus in recommending the use of VETs to guide haemostatic therapy during liver transplantation [1, 28, 29].
We believe that the priority use of lyophilized concentrates of coagulation factors over blood products has also allowed us to obtain significant reductions in the consumption of FFPs and cryoprecipitates. Unlike in other regions of the world, in Chile, both cryoprecipitate and fibrinogen concentrates are available for the treatment of hypofibrinogenemia. Given that there is evidence that fibrinogen concentrate is a safe and equally effective alternative for fibrinogen replacement [30, 31], it was decided to incorporate it as a priority in the haemostatic algorithm, seeking to reduce the time to delivery of therapy compared with that for cryoprecipitate [29]. Likewise, there is abundant evidence supporting the use of fibrinogen concentrate as a method of fibrinogen replacement both in a range of settings [30–34] and in liver transplantation specifically [35]. Of the 16 patients in group 2, only 7 patients required cryoprecipitate for the management of hypofibrinogenemia. The criterion for the use of cryoprecipitation in this group was the lack of expected increase in viscoelastic assay values after the use of 4 g of fibrinogen concentrates, as described above in the methods [36]. Priority use of lyophilized fibrinogen concentrates allowed 7 patients in group 2 (43.7%) to be exempted from the use of cryoprecipitate for the treatment of hypofibrinogenemia.
The use of PCC has not been prospectively evaluated in liver transplantation, but it has been used safely, guided by viscoelastic methods, as described in a number of observational studies [20, 21, 36, 37]. Furthermore, observational evidence from a single-centre study in liver transplantation, where one-third of patients required VET-guided coagulation factor replacement using an algorithm similar to ours, showed that prioritizing the use of PCC instead of FFP was not associated with an increase in thromboembolic complications [38]. Thus, further studies are warranted in this setting to evaluate whether such use of PCC would have a similar therapeutic value to FFP. This hypothesis has so far been prospectively evaluated only in cardiac surgery [39], where the use of PCC instead of FFP reduced RBC consumption and bleeding.
In this study, the application of the VET-guided algorithm for the management of bleeding resulted in 81.2% of patients receiving fibrinogen concentrate for hypofibrinogenaemia correction, while 56.2% of patients received coagulation factor replacement with PCC for thrombin optimization. These findings are similar to those reported in the literature [7, 21].
Significantly more patients in the group treated according to the algorithm had significantly higher use of tranexamic acid than those who were treated before its introduction. Although the use of antifibrinolytics is not systematically recommended in liver transplantation [40], current evidence suggests that their use in general surgery could reduce major bleeding events [41]. Our algorithm proposes the use of antifibrinolytics in cases of confirmed coagulopathy, in agreement with what has been suggested by experts [26, 42].
In our cohort, there was also a non-significant increase in the intraoperative use of albumin in the group treated according to the algorithm. This could be explained by the need to use colloids to replace the fluid intake provided by FFP in group 1, which was significantly reduced in group 2 (Tables 2, 3). Table 3 includes the use of FFP in the total fluid intake since, prior to the application of the algorithm, FFP could have been used to provide volume and coagulation factors. However, there were no significant differences in the total fluid therapy contribution.
Regarding the baseline risk of transfusion requirements, group 2 tended to have higher preoperative haemoglobin values compared with group 1 (10.3 (8.5–12.8) versus 12.2 (8.9–13.3) g/dL), but also lower platelet counts (68 (48–96) versus 46.5 (39.5–97.5) × 1,000/μL). The risk of massive transfusion was similar between the two groups. Evaluation through a randomized controlled trial would allow isolation of the impact of the transfusion strategy presented, allowing better assessment of the potential clinical benefit.
Consistent with previous studies [7], the incorporation of a VET-guided algorithm did not reduce the number of platelets transfused. However, there was a significant reduction in the use of RBCs. This was not associated with a decrease in intraoperative bleeding. Considering that the median preoperative haemoglobin values were statistically similar between the two groups, as was total fluid intake, this difference in usage could be due to an inadequate assessment of bleeding estimation or a reduction in haemodilution of the patients due to improvements in the clinical evaluation of blood volume, which did not achieve statistical significance due to the small size of the cohort. Interestingly, the significant reduction in the usage of blood products in group 2 was only maintained during the first 24 h. At 7 days following surgery, a large numerical difference was seen in the total use of blood products between the two groups, although this did not reach significance, most likely due to the small sample size. In this study, there was no standardized postoperative protocol for the use of blood products. It should be prospectively evaluated whether the maintenance of intraoperative strategies for maintaining haemostasis during the intensive care stay would allow further savings on transfusion therapy over a longer time period.
Regarding the safety and effectiveness profile of our protocol for the management of bleeding in liver transplantation, there was no evidence of a change in postoperative complications or hospital stay when using this VET-based haemostatic management algorithm, and our findings are in line with those described in the literature [7, 20, 21]. The lack of differences in these outcomes of the clinical approach may be due to the fact that adequate transfusion management is one aspect of an integrated strategy of accelerated recovery for this particular surgery [17]. The benefits associated with this specific form of protocolization of the perioperative management of patients undergoing liver transplantation are still part of the current research agenda.
It is noteworthy that the group treated according to the algorithm experienced four re-interventions due to bleeding. However, none of these occurred before 72 h postoperatively. These were a wall haematoma after a subcutaneous puncture that required drainage, two upper gastrointestinal bleeds that required therapeutic endoscopy, and ligation of bleeding haemorrhoids. Surprisingly, in group 2, there was a tendency to major postoperative bleeding; however, whether this tendency is connected to differences in the coagulation regime remains open for discussion. Four patients met the criteria for major postoperative bleeding according to the International Society on Thrombosis and Haemostasis (ISTH) [43], based on the need for transfusion of at least 2 units of RBCs in the first 48 postoperative hours. There was no bleeding at critical sites or fall in postoperative haemoglobin greater than 2 g/dL. It is possible that the restrictive intraoperative transfusion behaviours determined the need to transfuse postoperatively to maintain haemoglobin values above the safety limit of 7 g/dL [22]. However, as described here, there was no significant difference in transfusion requirements for RBCs in the 7 days following surgery, but there was a reduction in the consumption of RBCs and in the number of patients receiving RBC transfusions in the first 24 h. The influence of the algorithm on the presence of major postoperative bleeding should therefore be evaluated prospectively.
Although this is a retrospective study, it provides some guidance regarding the costs of haemostatic interventions in this specific population. Due to the characteristics of our institution as a private hospital, financial data associated with costs in patient care are easily extractable. As mentioned above, the median cost of treatment for patients treated according to the algorithm was around half that of patients treated before its introduction, although this difference most likely did not reach significance due to the small sample size. The use of patient blood management strategies in liver transplantation has been shown elsewhere to have a positive impact on the cost-effectiveness of haemostatic therapy [18]. At least, this study suggests that the implementation of alternative strategies to transfusion is feasible in liver transplantation and does not increase the cost of haemostatic intervention in these patients. On the contrary, there is a non-significant trend toward reduced costs of haemostatic therapy in patients exposed to the algorithm. We, therefore, believe it is worth setting up a larger randomized control trial following this pilot to further explore and study this association.
To determine if the proposed algorithm can achieve a 20% reduction in total blood product consumption, our pilot study has indicated that it will be necessary to include 204 patients in a proposed randomized clinical trial. In Chile, over the last 5 years, the average has been 109 cadaver donor liver transplants per year [44]. To achieve the level of recruitment necessary to carry out the study, multicentre recruitment pooled at the country level for at least 2 years would therefore be needed.
Limitations
This is a small, retrospective study in which it was arbitrarily decided to evaluate 30 consecutive patients from the beginning of the local liver transplantation program. Drawing conclusions from these findings is difficult, let alone establishing causality. This study has therefore been formulated from the beginning as a pilot study in order to obtain the required sample size to carry out a larger randomized controlled study regarding the impact of the application of this haemostatic algorithm.
The algorithm itself is a set of different haemostatic strategies. Therefore, conferring any of these a preponderance over another with respect to an eventual capacity to reduce the use of blood products is beyond the scope of this analysis due to the small sample size.
There was no variability in the surgical team; however, the cohort was treated over a period of almost 6 years, during which time the surgical skill and experience of the team will have evolved. In addition, the variability in management by anaesthesiologists should be taken into account since group 2 was treated by the same single anaesthesiologist, while, previously, there were four anaesthesiologists during the treatment of group 1. During these 6 years, there have been improvements in haemodynamic monitoring capacity with the incorporation of more modern pulse contour analysers, as well as a more user-friendly viscoelastic diagnostic method, which facilitates more frequent use during surgery. These factors could contribute to the way haemostatic therapy was utilized during liver transplantation over the course of the study.
Another limitation was the calculation of the estimated bleeding, which was not standardized and was based on the blood recovered by the blood salvage system, the collections of the aspiration canisters, and the visual assessment of the contents in gauze and compresses. The prospective study to be performed should have a standardized way of assessing this variable.
Conclusion
The use of a haemostatic management algorithm based on viscoelastic diagnostic tools and clotting factor concentrates was associated with a significant reduction in the consumption of blood products in the first 24 h after liver transplantation in this cohort. The analysis of the data obtained from this pilot study provides valuable information for the design of an adequately powered experimental study to explore the impact of this haemostatic algorithm on our local population.
Acknowledgments
The authors would like to thank the surgical, anaesthetic, critical care, and nursing teams of the Clínica Santa María, who contributed to the application of the haemostatic management algorithm. The authors would also like to thank Dagoberto Ojeda, MD (Universidad de los Andes), and Andrea Canals, MSc (Clínica Santa María), for their help with statistical analysis. The study was conducted independently by the investigators without external funding. We are grateful for the services of Portland Medical Communications Ltd., funded by Octapharma A.G., for translation into English.
Statement of Ethics
Approval to collect data from medical records for this study was obtained from the Local Scientific Ethical Committee, Comité Ético Científico Clínica Santa María, with resolution number 162609-22. Written informed consent was not required from patients due to the retrospective nature of the study.
Conflict of Interest Statement
I.A.S. reports personal fees and non-financial support from Octaphama and Werfen, outside the submitted work. M.F.G., J.C., and J.M. have no conflicts of interest to declare.
Funding Sources
No funding was provided for carrying out this study. For preparation of the manuscript, translation and editing services were provided by Portland Medical Communications Ltd., funded by Octapharma A.G.
Author Contributions
Ignacio Alonso Sarmiento contributed to the design of the study and manuscript drafting and approved the final version of the manuscript. María Fernanda Guzmán contributed to data collection and design of the work and approved the final version of the manuscript. Javier Chapochnick contributed to the design of the work and the critical revision of the article and approved the final version of the manuscript. Jens Meier contributed to the critical revision of the article and approved the final version of the manuscript.
Funding Statement
No funding was provided for carrying out this study. For preparation of the manuscript, translation and editing services were provided by Portland Medical Communications Ltd., funded by Octapharma A.G.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, I.A.S., upon reasonable request.
References
- 1. Bezinover D, Dirkmann D, Findlay J, Guta C, Hartmann M, Nicolau-Raducu R, et al. Perioperative coagulation management in liver transplant recipients. Transplantation. 2018 Apr;102(4):578–92. 10.1097/TP.0000000000002092. [DOI] [PubMed] [Google Scholar]
- 2. Blake BS, Aniskevich S, Thomas CS, Ladlie BL. Preoperative clinical characteristics that identify potential low-volume transfusion candidates among orthotopic liver transplant patients. Exp Clin Transpl. 2016 Aug;14(4):405–11. [PubMed] [Google Scholar]
- 3. Clevenger B, Mallett SV. Transfusion and coagulation management in liver transplantation. World J Gastroenterol. 2014 May 28;20(20):6146–58. 10.3748/wjg.v20.i20.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irita K. Risk and crisis management in intraoperative hemorrhage: human factors in hemorrhagic critical events. Korean J Anesthesiol. 2011 Mar;60(3):151–60. 10.4097/kjae.2011.60.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCluskey SA, Karkouti K, Wijeysundera DN, Kakizawa K, Ghannam M, Hamdy A, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl. 2006 Nov;12(11):1584–93. 10.1002/lt.20868. [DOI] [PubMed] [Google Scholar]
- 6. De Pietri L, Ragusa F, Deleuterio A, Begliomini B, Serra V. Reduced transfusion during OLT by POC coagulation management and TEG functional fibrinogen: a retrospective observational study. Transpl Direct. 2016 Jan;2(1):e49. 10.1097/TXD.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonnet A, Gilquin N, Steer N, Gazon M, Quattrone D, Pradat P, et al. The use of a thromboelastometry-based algorithm reduces the need for blood product transfusion during orthotopic liver transplantation: a randomised controlled study. Eur J Anaesthesiol. 2019 Nov;36(11):825–33. 10.1097/EJA.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 8. Roullet S, Freyburger G, Cruc M, Quinart A, Stecken L, Audy M, et al. Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry-based algorithm. Liver Transpl. 2015 Feb;21(2):169–79. 10.1002/lt.24030. [DOI] [PubMed] [Google Scholar]
- 9. Benson AB, Burton JR Jr, Austin GL, Biggins SW, Zimmerman MA, Kam I, et al. Differential effects of plasma and red blood cell transfusions on acute lung injury and infection risk following liver transplantation. Liver Transpl. 2011 Feb;17(2):149–58. 10.1002/lt.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hendriks HG, van der Meer J, de Wolf JT, Peeters PM, Porte RJ, de Jong K, et al. Intraoperative blood transfusion requirement is the main determinant of early surgical re-intervention after orthotopic liver transplantation. Transpl Int. 2005 Jan;17(11):673–9. 10.1007/s00147-004-0793-5. [DOI] [PubMed] [Google Scholar]
- 11. Fayed NA, Abdallah AR, Khalil MK, Marwan IK. Therapeutic rather than prophylactic platelet transfusion policy for severe thrombocytopenia during liver transplantation. Platelets. 2014;25(8):576–86. 10.3109/09537104.2013.849335. [DOI] [PubMed] [Google Scholar]
- 12. Ramos E, Dalmau A, Sabate A, Lama C, Llado L, Figueras J, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003 Dec;9(12):1320–7. 10.1016/jlts.2003.50204. [DOI] [PubMed] [Google Scholar]
- 13. Rana A, Petrowsky H, Hong JC, Agopian VG, Kaldas FM, Farmer D, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013 May;216(5):902–7. 10.1016/j.jamcollsurg.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 14. Real C, Sobreira Fernandes D, Sa Couto P, Correia de Barros F, Esteves S, Aragao I, et al. Survival predictors in liver transplantation: time-varying effect of red blood cell transfusion. Transpl Proc. 2016 Dec;48(10):3303–6. 10.1016/j.transproceed.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 15. Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. Blood system. N Engl J Med. 2017 Oct 12;377(15):1485–8. 10.1056/NEJMsb1706496. [DOI] [PubMed] [Google Scholar]
- 16. Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, et al. Essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020 Jul;131(1):74–85. 10.1213/ANE.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollok JM, Tinguely P, Berenguer M, Niemann CU, Raptis DA, Spiro M, et al. Enhanced recovery for liver transplantation: recommendations from the 2022 International Liver Transplantation Society consensus conference. Lancet Gastroenterol Hepatol. 2023 Jan;8(1):81–94. 10.1016/S2468-1253(22)00268-0. [DOI] [PubMed] [Google Scholar]
- 18. Leon-Justel A, Alvarez-Rios AI, Noval-Padillo JA, Gomez-Bravo MA, Porras M, Gomez-Sosa L, et al. Point-of-care haemostasis monitoring during liver transplantation is cost effective. Clin Chem Lab Med. 2019 May 27;57(6):883–90. 10.1515/cclm-2018-0889. [DOI] [PubMed] [Google Scholar]
- 19. Schumacher C, Eismann H, Sieg L, Friedrich L, Scheinichen D, Vondran FWR, et al. Use of rotational thromboelastometry in liver transplantation is associated with reduced transfusion requirements. Exp Clin Transplant. 2019 Apr;17(2):222–30. 10.6002/ect.2017.0236. [DOI] [PubMed] [Google Scholar]
- 20. Hartmann M, Walde C, Dirkmann D, Saner FH. Safety of coagulation factor concentrates guided by ROTEM-analyses in liver transplantation: results from 372 procedures. BMC Anesthesiol. 2019 Jun 11;19(1):97. 10.1186/s12871-019-0767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srivastava P, Agarwal A, Jha A, Rodricks S, Malik T, Makki K, et al. Utility of prothrombin complex concentrate as first-line treatment modality of coagulopathy in patients undergoing liver transplantation: a propensity score-matched study. Clin Transplant. 2018 Dec;32(12):e13435. 10.1111/ctr.13435. [DOI] [PubMed] [Google Scholar]
- 22. Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017 Jun;34(6):332–95. 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 23. Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013 Aug 1;369(5):428–37. 10.1056/nejmoa1301082. [DOI] [PubMed] [Google Scholar]
- 24. Makowka L, Stieber AC, Sher L, Kahn D, Mieles L, Bowman J, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am. 1988 Mar;17(1):33–51. 10.1016/s0889-8553(21)00342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, et al. The Eurotransplant donor risk index in liver transplantation: et-dri. Am J Transplant. 2012 Oct;12(10):2789–96. 10.1111/j.1600-6143.2012.04195.x. [DOI] [PubMed] [Google Scholar]
- 26. Gorlinger K, Perez-Ferrer A, Dirkmann D, Saner F, Maegele M, Calatayud AAP, et al. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol. 2019 Aug;72(4):297–322. 10.4097/kja.19169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. 2017 November-December;16(6):916–23. 10.5604/01.3001.0010.5283. [DOI] [PubMed] [Google Scholar]
- 28. Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary and renal considerations: executive summary. Crit Care Med. 2020 Mar;48(3):415–9. 10.1097/CCM.0000000000004193. [DOI] [PubMed] [Google Scholar]
- 29. Roberts LN, Lisman T, Stanworth S, Hernandez-Gea V, Magnusson M, Tripodi A, et al. Periprocedural management of abnormal coagulation parameters and thrombocytopenia in patients with cirrhosis: guidance from the SSC of the ISTH. J Thromb Haemost. 2022 Jan;20(1):39–47. 10.1111/jth.15562. [DOI] [PubMed] [Google Scholar]
- 30. Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the FIBRES randomized clinical trial. JAMA. 2019 Nov 26;322(20):1966–76. 10.1001/jama.2019.17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roy A, Stanford S, Nunn S, Alves S, Sargant N, Rangarajan S, et al. Efficacy of fibrinogen concentrate in major abdominal surgery: a prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei. J Thromb Haemost. 2020 Feb;18(2):352–63. 10.1111/jth.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cushing MM, Haas T. Fibrinogen concentrate for perioperative bleeding: what can we learn from the clinical trials? Transfusion. 2019 Nov;59(11):3295–7. 10.1111/trf.15437. [DOI] [PubMed] [Google Scholar]
- 33. Fominskiy E, Nepomniashchikh VA, Lomivorotov VV, Monaco F, Vitiello C, Zangrillo A, et al. Efficacy and safety of fibrinogen concentrate in surgical patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2016 Oct;30(5):1196–204. 10.1053/j.jvca.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 34. Ross C, Rangarajan S, Karimi M, Toogeh G, Apte S, Lissitchkov T, et al. Pharmacokinetics, clot strength and safety of a new fibrinogen concentrate: randomized comparison with active control in congenital fibrinogen deficiency. J Thromb Haemost. 2018 Feb;16(2):253–61. 10.1111/jth.13923. [DOI] [PubMed] [Google Scholar]
- 35. Sabate A, Dalmau A. Fibrinogen: a clinical update on liver transplantation. Transplant Proc. 2015 Dec;47(10):2925–8. 10.1016/j.transproceed.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 36. Colavecchia AC, Cohen DA, Harris JE, Thomas JM, Lindberg S, Leveque C, et al. Impact of intraoperative factor concentrates on blood product transfusions during orthotopic liver transplantation. Transfusion. 2017 Dec;57(12):3026–34. 10.1111/trf.14328. [DOI] [PubMed] [Google Scholar]
- 37. Nascimento JCR, Neto EBL, da Silva EL, Nunes RR, Marinho DS, Muniz FN, et al. Analysis of the hemostatic therapy in liver transplantation guided by rotational thromboelastometry or conventional laboratory tests. Eur J Gastroenterol Hepatol. 2020 Nov;32(11):1452–7. 10.1097/MEG.0000000000001660. [DOI] [PubMed] [Google Scholar]
- 38. Kirchner C, Dirkmann D, Treckmann JW, Paul A, Hartmann M, Saner FH, et al. Coagulation management with factor concentrates in liver transplantation: a single-center experience. Transfusion. 2014 Oct;54(10 Pt 2):2760–8. 10.1111/trf.12707. [DOI] [PubMed] [Google Scholar]
- 39. Karkouti K, Bartoszko J, Grewal D, Bingley C, Armali C, Carroll J, et al. Comparison of 4-factor prothrombin complex concentrate with frozen plasma for management of hemorrhage during and after cardiac surgery: a randomized pilot trial. JAMA Netw Open. 2021 Apr 1;4(4):e213936. 10.1001/jamanetworkopen.2021.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Devereaux PJ, Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, et al. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med. 2022 May 26;386(21):1986–97. 10.1056/nejmoa2201171. [DOI] [PubMed] [Google Scholar]
- 42. Sabate A, Blasi A, Costa M, Reyes R, Beltran J, Torres F. Assessment of rotational thromboelastometry for the prediction of red blood cell requirements in orthotopic liver transplantation. Minerva Anestesiol. 2018 Apr;84(4):447–54. 10.23736/S0375-9393.17.12023-7. [DOI] [PubMed] [Google Scholar]
- 43. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010 Jan;8(1):202–4. 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 44.Instituto de Salud Pública de Chile [Internet]. Trasplante de Órganos y Listas de Espera por Mes. [cited 2022 Nov 24]. Available from: https://www.ispch.cl/biomedico/estadisticas-de-trasplante/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, I.A.S., upon reasonable request.