Abstract
Janus kinases (JAKs) are a family of cytosolic tyrosine kinases that regulate cytokine signal transduction, including cytokines involved in a range of inflammatory diseases, such as RA, psoriasis, atopic dermatitis and IBD. Several small-molecule JAK inhibitors (JAKis) are now approved for the treatment of various immune-mediated inflammatory diseases. There are, however, key differences between these agents that could potentially translate into unique clinical profiles. Each JAKi has a unique chemical structure, resulting in a distinctive mode of binding within the catalytic cleft of the target JAK, and giving rise to distinct pharmacological characteristics. In addition, the available agents have differing selectivity for JAK isoforms, as well as off-target effects against non-JAKs. Other differences include effects on haematological parameters, DNA damage repair, reproductive toxicity and metabolism/elimination. Here we review the pharmacological profiles of the JAKis abrocitinib, baricitinib, filgotinib, peficitinib, tofacitinib and upadacitinib.
Keywords: abrocitinib, baricitinib, filgotinib, mode of action, peficitinib, tofacitinib, upadacitinib
Rheumatology key messages.
Janus kinase inhibitors (JAKis) have differential selectivity for JAK isoforms and different off-target binding profiles.
Effects on haematological parameters, DNA damage repair and reproductive toxicity differ across JAKis.
Differences between JAKis may translate to distinct pharmacological characteristics in patients.
Introduction
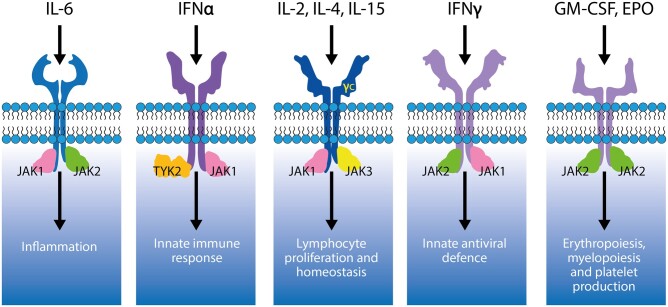
The Janus kinase (JAK) family of cytosolic tyrosine kinases includes four members in mammals: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) [1]. JAKs regulate cytokine signalling transduction by binding to membrane-proximal, intracellular domains of cytokine receptors [1–3]. Cytokine receptors with heterodimeric subunits recruit multiple JAKs, while homodimeric receptors exclusively bind JAK2 [2]. JAK1/JAK2/TYK2 signalling mediates the effects of several key pro-inflammatory cytokines, including IFN-γ and IL-6 [2, 3], that are implicated in inflammatory disorders, including rheumatological, dermatological and gastrointestinal diseases [4, 5]. JAK1/JAK3 heterodimers mediate common γ chain cytokine (IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21) signalling, regulating aspects of lymphocyte development, growth, differentiation and survival, as well as B cell class differentiation and immunoglobulin switching [6]. JAK2/TYK2 heterodimers regulate the signalling of IL-12 and IL-23, key immunoregulatory cytokines implicated in CD4+ Th1 and Th17 cell differentiation, respectively [7, 8]. JAK2 homodimers are crucial for the regulation of erythropoiesis, thrombopoiesis and myelopoiesis [5, 9–12] (Fig. 1).
Figure 1.
Cytokine receptors are associated with distinct JAK pairs. γc: common γ chain; EPO: erythropoietin; JAK: Janus kinase; TYK: tyrosine kinase
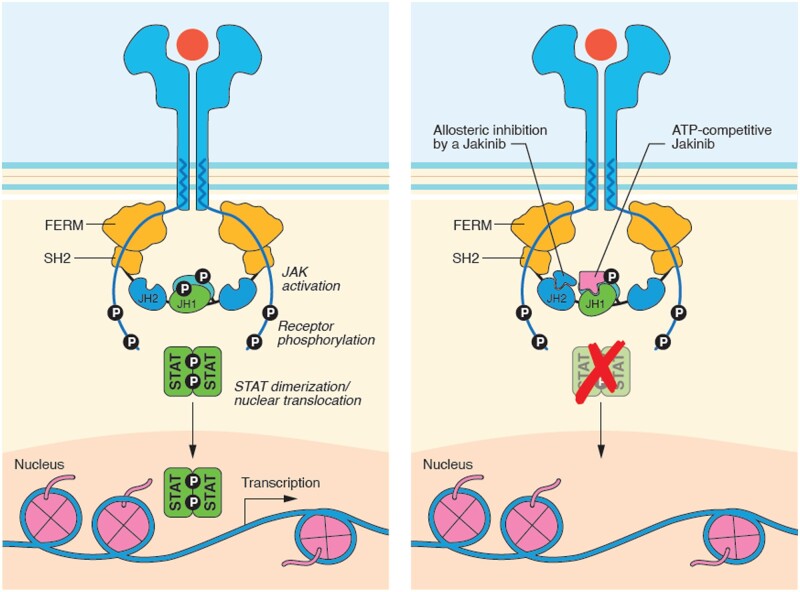
Cytokine binding induces conformational changes in the cognate receptor. Phosphorylation of receptor subunits creates docking sites for the latent transcription factors signal transducers and activators of transcription (STATs), which are also phosphorylated by activated JAKs [5, 13]. Activated STATs dimerize and translocate to the nucleus to regulate target gene expression (Fig. 2) [14–16].
Figure 2.
General mechanism of JAK inhibition. Adapted from Alexander et al. (2021) [2]. ATP: adenosine-5′-triphosphate; FERM: (4.1 protein, ezrin, radixin, moesin); JAK: Janus kinase; JH: JAK homology; P: phosphate; SH2: Src homology 2-like; STAT: signal transducer and activator of transcription
The pivotal role of JAKs in cytokine signalling and immune function was highlighted by critical JAK mutations in animals and humans, leading to development of small-molecule JAK inhibitors (JAKis) as pharmacotherapies for immune-mediated inflammatory disorders [2, 16–18]. This review specifically summarizes published evidence on the differential properties of six JAKis (abrocitinib, baricitinib, filgotinib, peficitinib, tofacitinib and upadacitinib) approved by relevant regulatory authorities for the treatment of immune-mediated inflammatory disorders, including RA, psoriasis, atopic dermatitis (AD) and IBD (Supplementary Table S1, available at Rheumatology online), and discusses how their distinct properties may translate into unique clinical profiles. Peficitinib is currently only approved for the treatment of RA in Japan and South Korea and available pharmacological data are limited. The TYK2-targeted JAKi deucravacitinib was also recently approved in the USA [19], but is not discussed herein due to its different mode of action (allosteric inhibition of receptor-mediated TYK2 activation) vs other JAKis, and limited published pharmacological data.
General mechanism of action of JAKis
JAKs contain four structurally conserved domains: N-terminal FERM (4.1 protein, ezrin, radixin, moesin) and Src homology 2 (SH2)-like domains that facilitate JAK association with cytokine receptors; pseudokinase domain [JAK homology 2 (JH2)], which has important regulatory functions in controlling JAK activity; and C-terminal JAK catalytic domain (JH1) [16, 20, 21]. The six JAKis covered herein are competitive, reversible adenosine-5'-triphosphate (ATP) inhibitors targeting the ATP-binding pocket in the JH1 domain, thereby suppressing JAK/STAT signalling [2, 22–25] (Fig. 2). Importantly, owing to the highly conserved structure of the ATP-binding pocket in each of the four JAKs, developing JAK-selective competitive ATP inhibitors is challenging [26].
Distinct chemical structures and modes of binding to target JAKs
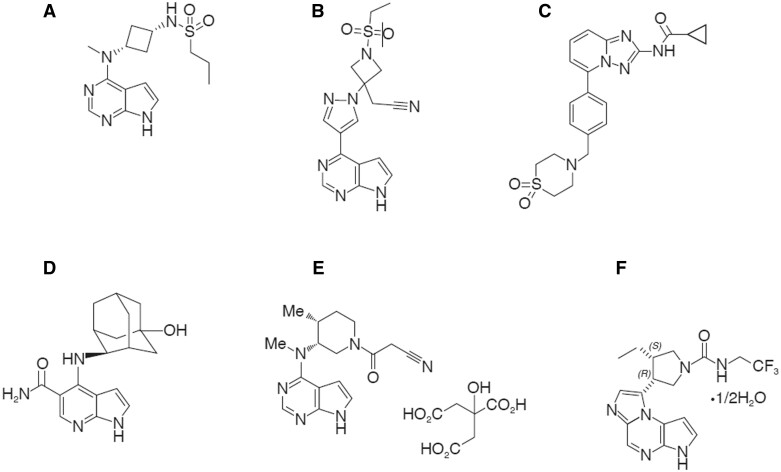
Each JAKi has a unique chemical structure (Fig. 3) and distinctive binding mode within the catalytic cleft of target JAKs [24, 25]. Binding modes of each JAKi are shown in Supplementary Table S1 and Fig. S1 (available at Rheumatology online), based on crystal structures of JAKis complexed with target JAKs submitted to the Protein Data Bank (https://www.rcsb.org/) [24].
Figure 3.
JAK inhibitor chemical structures. (A) Abrocitinib [23]. (B) Baricitinib [55]. (C) Filgotinib [24]. (D) Peficitinib [24]. (E) Tofacitinib [53]. (F) Upadacitinib [73]
Differential selectivity for JAK isoforms
Low selectivity may explain certain adverse effects of JAKis, including anaemia, thrombocytopenia and upper respiratory tract infections, prompting recent research to focus on developing more JAK-specific, highly selective JAKis to increase therapy precision and decrease off-target activity [24, 26]. Inhibition of specific JAKs may improve JAKi safety, which is particularly relevant for chronic, non-life-threatening diseases requiring long-term treatment. Furthermore, a favourable safety profile may permit higher dosing and improve efficacy [24, 26].
Notably, developing selective JAKis also poses challenges. The active JH1 domains of JAKs share high structural similarity around the ATP-binding site. Due to the involvement of multiple JAKs in certain cytokine signalling pathways, the dominant role of one JAK over another leads to differential selectivity profiles across pathways, with reported discrepancies between biochemical and cellular potencies [26]. Furthermore, JAK isoform selectivity is dose- and tissue-dependent and is thus relative rather than absolute [27].
Enzymatic and cellular assays
JAKi selectivity is assessed by determining inhibitory activity for each JAK isoform and may be determined in vitro (enzymatic biochemical assays) or ex vivo (cellular assays) by measuring cytokine release and/or phosphorylated STAT (pSTAT) activation [27], with low half-maximal inhibitory concentration (IC50) indicating high inhibitory activity. Assay data should be interpreted with respect to the fact that they are dose- and substrate/cell type-dependent [27], and may not reflect biologically relevant clinical effects.
Enzymatic assay results for individual isoform selectivity
Enzymatic assays are useful for describing inhibitory potency for individual JAK isoforms, although potency across JAKis should be compared only when using the same assay conditions. General quantitative comparisons across assays for each JAKi are possible, as outlined in Table 1. Briefly, abrocitinib and upadacitinib are selective JAK1 inhibitors; filgotinib is also considered a selective JAK1 inhibitor but, in biochemical isolated enzyme assays, it preferentially inhibits JAK1 and JAK2 (also inhibits JAK3 and TYK2—to a lesser degree, but at concentrations that may be clinically relevant); baricitinib is a selective JAK1 and JAK2 inhibitor; tofacitinib is a potent JAK1 and JAK3 inhibitor (less active against JAK2 and TYK2); and peficitinib is a pan-JAKi [27].
Table 1.
In vitro JAK isoform selectivity of the JAK inhibitors based on enzymatic assays
| Classification | IC50 (nM) |
||||
|---|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | ||
| Abrocitinib [47] | Selective JAK1 inhibitor | 29.2 | 803 | >10 000 | 1250 |
| Baricitinib [68] | Selective JAK1/2 inhibitor | 5.9 | 5.7 | >400 | 53 |
| Filgotinib [49] | JAK1/2 inhibitor | 10–53 | 28–29 | 311–810 | 116–177 |
| Peficitinib [39] | Pan-JAK inhibitor | 3.9 | 5.0 | 0.7 | 4.8 |
| Tofacitinib [72] | JAK1/3 inhibitor | 3.2 | 4.1 | 1.6 | 34 |
| Upadacitinib [54] | Selective JAK1 inhibitor | 43 | 120 | 2300 | 4700 |
IC50: half maximal inhibitory concentration; JAK: Janus kinase; TYK: tyrosine kinase.
Cellular assay results for selectivity across JAKis
Baricitinib, tofacitinib and upadacitinib
Peripheral blood mononuclear cells from healthy donors were incubated with clinically relevant concentrations of baricitinib, tofacitinib and upadacitinib, and STAT phosphorylation was measured following cytokine stimulation [28]. IL-6 inhibition was similar among JAK1/2-dependent cytokines, while tofacitinib was the least potent on IFN-γ. Tofacitinib and upadacitinib inhibited JAK1/3 signalling (IL-2, IL-4, IL-15, IL-21) more than baricitinib. Inhibition of JAK1/TYK2 signalling in the context of IL-10 was more potent for upadacitinib and tofacitinib vs baricitinib, while upadacitinib was the most potent and tofacitinib the least potent inhibitor of IFN-α signalling. Interestingly, upadacitinib was the most potent inhibitor of the JAK2/2 cytokines IL-3 and GM-CSF, while baricitinib was the most potent inhibitor of the JAK2/TYK2-dependent cytokine G-CSF. Average daily inhibition of STAT phosphorylation, based on pharmacologically relevant drug concentrations at approved daily doses, was consistent with IC50 data (Supplementary Table S2, available at Rheumatology online). Each JAKi displays different pharmacological profiles in ex vivo cellular assays, suggesting that they modulate target pathways to varying degrees and durations over a 24-h dosing interval. Notably, no JAKis completely or continuously inhibited an individual cytokine signalling pathway during this interval [28]. Due to the complex cooperative nature of JAKs, deriving cellular potency data on individual family members is difficult in native cells and as such, data employing engineered JAK-specific cell lines have been additionally generated for upadacitinib, showing markedly greater selectivity for JAK1 vs the other three isoforms [25].
Filgotinib vs baricitinib, tofacitinib and upadacitinib
In vitro JAK inhibitory activity of filgotinib was compared with baricitinib, tofacitinib and upadacitinib in whole blood from healthy donors and patients with RA [29]. Average daily inhibition of JAK-dependent pathways was modelled at plasma drug exposures equivalent to doses producing similar overall ACR responses [filgotinib 200 mg once daily (QD); baricitinib 4 mg QD; tofacitinib 5 mg twice daily; and upadacitinib 15 mg QD]. For JAK1/JAK2:IL-6/pSTAT1, all four JAKis showed similar predicted average daily inhibition at therapeutic doses. For JAK1/TYK2:IFN-α/pSTAT5, filgotinib showed average daily inhibition similar to baricitinib, but significantly lower than tofacitinib and upadacitinib. For JAK1/JAK2:IFN-γ/pSTAT1, filgotinib displayed significantly lower inhibition than baricitinib, tofacitinib and upadacitinib. Average daily inhibition of JAK1/JAK3:IL-4/pSTAT6 with filgotinib was similar to baricitinib, but significantly lower than tofacitinib and upadacitinib. For JAK2/TYK2:G-CSF/pSTAT3 and JAK2/JAK2:GM-CSF/pSTAT5, filgotinib showed significantly lower inhibition than the other three JAKis. Notably, specific JAK associations may display distinct properties in association with different cytokine receptors. For example, the inhibition hierarchy of JAK1/JAK2/pSTAT1 differs between IL-6 and IFN-γ receptors (Supplementary Table S3, available at Rheumatology online). Overall, filgotinib inhibited JAK1-dependent signalling similar to upadacitinib, tofacitinib and baricitinib, but exhibited the lowest inhibition of JAK2- and JAK3-dependent signalling. While these JAKis affect multiple type I/II cytokines, different inhibitors differentially modulate cytokine signalling in various immune cells throughout a 24-h dosing cycle. These observations may provide a mechanistic rationale for reported differences in JAKi clinical profiles across indications [28, 29]. Furthermore, IC50 is an artificial concept with uncertain relevance in the whole organism. In vivo, even 5–10% inhibition of multiple pathways integrated across a variety of immune cells will likely have a physiological effect. Thus, lower inhibition levels could be clinically relevant, and IC50 values may not accurately reflect differences in clinical effects among agents.
Tofacitinib, baricitinib, upadacitinib and filgotinib
To elucidate how the differential in vitro potencies of JAKis may translate to specific in vivo cytokine pathway inhibition, an integrated modelling approach was used [30]. Combined data from in vitro whole-blood cytokine assays and plasma pharmacokinetics were used to determine JAK-dependent cytokine receptor inhibition profiles of tofacitinib, baricitinib, upadacitinib and filgotinib, at exposure levels estimated to provide clinically meaningful responses in patients with RA [30]. Although in vitro potency differences between JAKis were apparent, cytokine receptor inhibition profiles across a broad range of pathways were similar after accounting for plasma protein binding, blood-to-plasma ratio and clinical exposure. However, there were small numerical differences between JAKis in cytokine receptor inhibition percentages. Tofacitinib showed numerically greater relative inhibition of most JAK1/3-mediated common γ chain cytokine receptors (IL-2, IL-4, IL-7 and IL-15) vs baricitinib, upadacitinib and filgotinib. Inhibition of JAK1/JAK2-mediated cytokine receptors (IFN-γ and G-CSF) and JAK2-mediated cytokine receptors (thrombopoietin, IL-3, GM-CSF) was numerically greater with upadacitinib vs other JAKis [30]. Minor differences in predicted cytokine receptor inhibition between JAKis suggested limited differences between their clinical profiles in RA. However, the study was not statistically powered for comparisons and, as the inhibitory activity of JAKis is non-linear over a concentration range, use of a single average plasma concentration may have minimized differences in pharmacodynamic profiles and obscured clinical implications [29].
Differential clinical toxicity profiles
As JAKis block cytokines that are involved in multiple pathways, they may have multi-system impacts. However, given the differences in inhibitory potency of individual JAKis for each JAK isoform, each molecule has a unique clinical toxicity profile, as supported by extensive pre-clinical and clinical findings. Long-term extension (LTE) and other post-marketing studies remain necessary to evaluate JAKi safety in the treatment of chronic inflammatory conditions, particularly considering that these diseases themselves are often associated with increased risk [2]. Recently raised concerns include increased risk of cardiovascular and thromboembolic events in certain higher-risk patient populations, highlighting the potential ramifications of JAK/STAT pathway inhibition, particularly with pan- or multi-JAKis [2]. However, there is no robust compelling evidence to date supporting that the mechanism of action of JAKis would be the reason for a potential increased risk for cardiovascular events or malignancies. Moreover, JAKis may decrease the risk of these events by decreasing disease activity, since chronic inflammation is a well-known independent risk factor for these events.
Haematological effects
As JAK signalling, particularly JAK2, is important in regulating erythropoiesis, thrombopoiesis and myelopoiesis [9–12], JAKis with greater JAK2 inhibitory activity (baricitinib, tofacitinib) would be expected to be associated with larger changes in haematological parameters. However, clinical trial data do not support this hypothesis. JAKis tend to be associated with small, transient changes, and no evidence suggests that baricitinib or tofacitinib are associated with larger changes in haematological parameters vs other JAKis [31–39] (Table 2). Improvements in disease activity may lead to increased haemoglobin level and reduced platelet count, particularly when IL-6 signalling is inhibited. The small, transient changes observed appear consistent with data showing that, at clinically relevant doses, a given JAKi inhibits target cytokine pathways for the minority of a 24-h dosing cycle [28–30]. However, the level of JAK inhibition that is clinically relevant is unclear.
Table 2.
Summary of haematological changes associated with JAK inhibitor treatment
| Platelet counts | Neutrophil counts | Lymphocyte counts | Haemoglobin levels | |
|---|---|---|---|---|
| Abrocitinib [31, 32] | Transient ↓ | No change | No change | No change |
| Baricitinib [33] | Transient ↑ | ↓ | Transient ↑ | Transient ↓ |
| Filgotinib [34, 35] | ↓ then stable weeks 4–24 (end of study) | ↓ then stable weeks 4–24 (end of study)a | No change; no correlations with treatment groups | ↑ then stable weeks 12–24 (end of study) |
| Peficitinib [39] | No change | Slight ↓ | ↓ | ↑ |
| Tofacitinib [36] | NR | ↓ then stabilized in LTEs | ↓ then stabilized at month 48 | ↑ then stabilized in LTEs |
| Upadacitinib [41, 42] | Small transient ↓ | ↓ in weeks 4–8 followed by stabilization at a lower value than baseline | Small transient ↑ up to week 36 | No change |
A transient change is defined here as a change from baseline followed by a return to baseline levels during the period of observation.
A further decrease from weeks 16–24 was seen with filgotinib 100 mg twice daily, with an overall decrease to week 24 observed in patients switched from filgotinib 50–100 mg. JAK: Janus kinase; LTE: long-term extension; NR: not reported.
Abrocitinib
An integrated analysis of long-term safety data from one phase 2b study, four phase 3 studies and one LTE in patients with AD found that abrocitinib reduced platelet counts transiently and dose-dependently [31]. Transient decreases in platelets were also reported in a population pharmacokinetic–pharmacodynamic modelling analysis of two phase 2 and three phase 3 studies in patients with psoriasis and AD, with the nadir occurring ∼24 days after continuous administration of abrocitinib 200 mg QD [32].
Baricitinib
In vitro, baricitinib reduced platelet adhesion to collagen, platelet aggregation, secretion and integrin αIIbβ3 activation, in response to the glycoprotein VI agonist collagen-related peptide (CRP-XL) [40]. Analysis of changes in haematological parameters after baricitinib treatment in eight randomized trials (four phase 3, three phase 2, one phase 1b) and one LTE in patients with RA found transient reductions in neutrophils and haemoglobin, with transient increases in lymphocytes and platelets [33]. Permanent baricitinib discontinuation due to abnormal neutrophil, lymphocyte, platelet or haemoglobin levels was rare (0.2–0.5%).
Filgotinib
A 24-week phase 2b study of filgotinib added to MTX in patients with RA and inadequate response to MTX found dose-dependent decreases in neutrophils and increases in haemoglobin in all filgotinib groups [34]. Similar findings were reported in a 24-week phase 2b study of filgotinib monotherapy in a similar patient population [35], with no apparent correlations between treatment groups and lymphocyte counts over time.
Peficitinib
The Japanese Pharmaceuticals and Medical Devices Agency (2019) reported low incidence of thrombocytopenia-related adverse events and no marked changes over time in platelet count in peficitinib clinical studies [39]. There was no evidence of dose-dependent increases in neutropenia or lymphopenia or differences between dosages, although haemoglobin levels tended to increase during peficitinib therapy.
Tofacitinib
In vitro, tofacitinib did not significantly affect platelet function [40]. Analysis of changes in haematological parameters in six phase 3 and two LTE studies of tofacitinib in patients with RA showed decreased mean neutrophil and lymphocyte counts, and increased haemoglobin in all tofacitinib groups [36]. Clinically meaningful reductions in haemoglobin levels (≤7 g/dl or ≥3 g/dl from baseline) occurred in <1.0% of patients in all treatment groups.
Upadacitinib
In vitro, upadacitinib did not significantly affect platelet adhesion, integrin activation or granule secretion induced by CRP-XL, although there was a significant effect on platelet spreading on fibrinogen [40]. In a phase 3 RA study, mean haemoglobin levels were stable over time, while initially slightly decreased platelet counts normalized over time [41]. Decreased neutrophil counts after 4–8 weeks of upadacitinib stabilized below the baseline, while small increases in lymphocyte counts were gradually restored with continued treatment [42]. Phase 3 data in PsA showed no clear exposure–response trends for haemoglobin <8 g/dl, or grade ≥3 lymphopenia or neutropenia after 24 weeks [43]. When combining upadacitinib with topical CSs for AD, neutropenia occurred more frequently with the 30 mg (4.4%) vs the 15 mg (1.1%) dose [37].
Off-target inhibition of non-JAKs
Due to the highly conserved structure of the catalytic sites of protein kinases, ATP-competitive inhibitors, including JAKis, may bind to kinases other than JAKs. This off-target binding may lead to increased toxicity and worse overall safety profile of individual JAKis [44–46]. However, laboratory changes that have been associated with JAKi treatment, such as creatine kinase elevation, do not necessarily translate to serious clinical effects [2].
Abrocitinib
In a ligand-binding assay, abrocitinib inhibited VEGF receptor 2 by 94% and monoamine oxidase A by 67% [47]. The clinical relevance of these findings is unknown.
Baricitinib
Baricitinib demonstrates a high binding affinity for adaptor-associated protein kinase 1 and cyclin G-associated kinase [48], the role of which in viral endocytosis was a key consideration when evaluating baricitinib for the treatment of COVID-19. These binding affinity characteristics are lacking for upadacitinib and tofacitinib.
Filgotinib
In vitro kinase activity-profiling studies with filgotinib found that some kinases display IC50 values below the maximum filgotinib plasma concentration. For some kinase targets, a high level of competitive inhibition of ATP binding was observed, including 95% inhibition for liver kinase B1 and 73% for serine/threonine protein kinase 1 [49]. The active metabolite of filgotinib, GS-829845, shows a much broader range of kinase binding vs filgotinib, binding to ∼20 non-JAK-associated kinases, although with substantially lower potency. Toxicity studies have not identified any GS-829845-related concerns, suggesting that off-target GS-829845 binding is not clinically relevant [49].
Tofacitinib
Quantitative analysis of kinase inhibitor selectivity for 38 inhibitors across a panel of 317 kinases (>50% of the predicted human protein kinome) found that tofacitinib demonstrated binding affinities <1 µM for protein kinase N1, sucrose nonfermenting 1/adenosine monophosphate-activated protein kinase-related kinase, and tyrosine kinase non-receptor 1 [50]. Furthermore, the binding potency for doublecortin-like kinase 3 (4.5 nM) was similar to that for JAK2 and -3 isoforms. The clinical relevance of these findings is unknown.
Upadacitinib
Upadacitinib demonstrated selectivity across a panel of >70 kinases, with only Rho-associated kinases (Rock)1 and 2 demonstrating IC50 <1 μM [25]. Binding potency for Rock1 and Rock2 was >20-fold and 10-fold lower, respectively, than for JAK1. Findings in isoform-specific knockout mice support a role of Rock1 and Rock2 in the pathogenesis of cardiac fibrosis and hypertrophy, suggesting that Rock inhibition may protect against cardiovascular disease [51].
Pre-clinical carcinogenicity
Pre-clinical carcinogenicity studies conducted on all JAKis during development have reported variable outcomes.
In a carcinogenicity study of abrocitinib, higher incidence of benign thymoma was noted in female rats [52]. Increased incidence of tumours in rats was also reported with filgotinib and tofacitinib [49, 53], but not with upadacitinib or baricitinib [54, 55]. A carcinogenicity study of peficitinib in mice found no effects on the incidence of hepatocellular adenoma or carcinoma [39].
DNA damage repair
Increasing evidence suggests DNA damage repair modulation by JAK/STAT signalling [56–60], with STAT3 in particular regulating genes controlling cell survival and proliferation, apoptosis and tumorigenesis [61]. Induction of JAK/STAT3 signalling by G-CSF in hepatocytes reduces DNA double-strand breaks (DSBs) caused by acetaminophen, whereas antagonism of G-CSF-mediated JAK/STAT3 signalling by the JAKi ruxolitinib abrogated the protective effect of G-CSF [58]. The impact of tofacitinib, baricitinib, filgotinib and upadacitinib on DNA DSB formation and radiation-induced DNA damage repair in vitro was evaluated in peripheral blood mononuclear cells [62]. Analyses of DNA DSB markers γH2AX and p53-binding protein 1 indicated enhanced levels of DNA damage in cells incubated with high filgotinib concentrations, and dose-dependent reduction in clearance of radiation-induced DSB foci in the presence of tofacitinib or baricitinib. Upadacitinib treatment did not cause increased levels of DSB foci [62]. These in vitro results indicated that JAKis may differentially impact DNA damage repair.
Reproductive toxicity
Filgotinib is the only JAKi for which adverse effects in the male reproductive system have been reported [49]. Impaired spermatogenesis and histopathological changes in the testis and epididymis were associated with filgotinib 60 mg/kg QD in pre-clinical animal studies, albeit at exposure levels considerably higher than the recommended human dose of 200 mg QD [49, 63]. Similar pre-clinical toxicity studies on other JAKis reported no observable effects on male reproductive organs [49]. Global regulatory authorities requested further research to elucidate the impact on humans [49, 64], and two studies on male reproductive function are ongoing: MANTA (NCT03201445) and MANTA-RAy (NCT03926195) [65]. The Committee for Medicinal Products for Human Use assessed interim results from MANTA and MANTA-RAy, and concluded that they were not suggestive of filgotinib-related effects on testicular function; however, overall conclusions will be based on the totality of the data, including secondary/exploratory measures (sperm motility/morphology, sex hormones, reversibility of any effects on semen parameters) [65].
Bone growth toxicity
Abrocitinib is the only JAKi for which adverse effects on bone growth have been reported. Toxicity studies of abrocitinib in rats at an age comparable to a human age of 12 years showed transient and reversible microscopic bone dystrophy. Furthermore, the exposure margins at which no bone finding was noted were 5.7–6.1 times the human area under the time–concentration curve (AUC) at the maximum recommended human dose (MRHD) of 200 mg. No bone findings were observed in rats at any dose in the 6-month toxicity study (up to 25 times the human AUC at the MRHD of 200 mg) or in any toxicity study in cynomolgus monkeys (comparable to human age of 8 years; up to 30 times the human AUC at the MRHD of 200 mg). However, the safety and efficacy of abrocitinib in children (<12 years) have not yet been established [52].
Abrocitinib use has been studied in adolescents (12–18 years). However, because of macroscopic and microscopic bone findings in juvenile rats (malrotated and/or impaired use of limbs or paws, fractures and/or femoral head abnormalities), additional long-term data are needed to conclude whether the benefits outweigh the risks in growing adolescents [52].
Metabolic and elimination profiles
Differences in JAKi metabolism and elimination result in differing recommendations regarding potential drug–drug interactions and dose modifications in patients with renal and hepatic impairment (Table 3) [39, 42, 52, 66–68]. Adjustment recommendations for different JAKis depend on primary clearance mechanism and extent of functional impairment, and vary from no adjustment in patients with mild impairment, to JAKis being not recommended or contraindicated in patients with severe impairment. Details are provided in Table 3 and below.
Table 3.
Summary of the metabolism and excretion of JAK inhibitors in patients with renal or hepatic impairment
| Primary metabolizing enzymes | Primary clearance mechanism | Renal impairment |
Hepatic impairment |
|||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild (Child Pugh A) | Moderate (Child Pugh B) | Severe (Child Pugh C) | |||
| Abrocitinib [52] | CYP2C19 and CYP2C9 | Urine | No dose adjustmenta | 50 or 100 mg QDb | 50 mg QDc | No dose adjustment | No dose adjustment | Contraindicated |
| Baricitinib [68] | CYP3A4 | Urine | No dose adjustmentd | 2 mg QDe | Not recommendedf | No dose adjustment | No dose adjustment | Not recommended |
| Filgotinib [67] | CES2 | Urine | No dose adjustmentd | 100 mg QDe | 100 mg QDf | No dose adjustment | No dose adjustment | Not recommended |
| Peficitinib [39, 70] | SULT2A1; NNMT | Urine/faeces | No dose adjustmenta | No dose adjustmentb | No dose adjustmentc | No dose adjustment | 50 mg QD | Contraindicated |
| Tofacitinib [66] | CYP3A4 | Hepatic metabolism | No dose adjustmentg | No dose adjustmenth | 5 mg QD when indicated dose is 5 mg BID; 5 mg BID when the indicated dose is 10 mg BIDf,i | No dose adjustment | 5 mg QD when indicated dose is 5 mg BID; 5 mg BID when indicated dose is 10 mg BID | Contraindicated |
| Upadacitinib [42] | CYP3A4 | Urine/faeces | No dose adjustmenta | No dose adjustmentb | 15 mg QD for rheumatological/dermatological indications; 30 mg induction dose and 15 mg maintenance dose for IBDc | No dose adjustment | No dose adjustment | Contraindicated |
eGFR 60 to <90 mL/min;
eGFR 30 to <60 mL/min;
eGFR 15 to <30 mL/min;
CrCL ≥60 mL/min;
CrCL 30 to <60 mL/min;
CrCL 15 to <30 mL/min;
CrCL 50–80 mL/min;
CrCL 30–49 mL/min;
patients with severe renal impairment should remain on a reduced dose even after haemodialysis. BID: twice daily; CES: carboxylesterase; CrCL: creatinine clearance; CYP: cytochrome P450; eGFR: estimated glomerular filtration rate; JAK: Janus kinase; NNMT: nicotinamide N-methyltransferase; QD: once daily; SULT: sulfotransferase.
Abrocitinib
Abrocitinib is primarily metabolized by cytochrome P450 (CYP)2C19 and CYP2C9, with contributions from CYP3A4 and CYP2B6. In a human radiolabelled study, abrocitinib was the most prevalent circulating species, with two active polar mono-hydroxylated metabolites identified as M1 (3-hydroxypropyl) and M2 (2-hydroxypropyl). M1 is less active, while M2 is as active as abrocitinib [69]. In patients receiving dual strong inhibitors of CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g. fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended abrocitinib dose should be halved (100 mg or 50 mg QD; Table 3) [52].
Baricitinib
In vitro, baricitinib is a CYP3A4 substrate (although <10% is metabolized via oxidation) and is also an in vitro substrate for organic anionic transporter (OAT)3 [68]. Therefore, the recommended dose is reduced from 4 mg to 2 mg QD in patients taking strong OAT3 inhibitors (e.g. probenecid). In clinical pharmacology studies, coadministration of baricitinib with a strong CYP3A inhibitor (e.g. ketoconazole) or inducer (e.g. rifampicin) resulted in no clinically meaningful changes to baricitinib pharmacokinetics. Renal elimination is the principal clearance mechanism, and renal function significantly affects baricitinib exposure; the mean ratios of exposure (AUC) in patients with mild/moderate renal impairment to patients with normal renal function are 1.41 and 2.22, respectively (Table 3) [55, 68]. Consequently, baricitinib should be used at the lower dose (2 mg QD) in moderate renal insufficiency and is not recommended in severe insufficiency.
Filgotinib
Filgotinib is mainly metabolized by carboxylesterase 2 [67], which is inhibited in vitro by fenofibrate, carvedilol, diltiazem and simvastatin; the clinical relevance of this interaction is currently unknown. Its primary metabolite, GS-829845, contributes to the overall efficacy with a 10-fold lower potency [49, 67].
Peficitinib
Peficitinib metabolism is mainly mediated by sulfotransferase 2A1 and nicotinamide N-methyltransferase [70]. Data from in vitro studies suggest that peficitinib may inhibit CYP2C8, but there were no marked differences in the incidence of adverse events between peficitinib treatment with and without a concomitant CYP2C8 substrate in clinical studies [39]. There is also evidence from in vitro studies that peficitinib may inhibit CYP3A.
Tofacitinib
Tofacitinib is primarily metabolized by CYP3A4 [66, 71]. The total daily dose should be halved in patients receiving potent CYP3A4 inhibitors, and in patients receiving concomitant medicinal products resulting in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g. fluconazole) (Table 3) [66].
Upadacitinib
Upadacitinib is primarily metabolized by CYP3A4 [42]. Therefore, as with baricitinib and tofacitinib, exposure can be affected by CYP3A4 inhibitors or inducers. Upadacitinib 15 mg QD should be used with caution in patients chronically treated with strong CYP3A4 inhibitors [42]. Upadacitinib 30 mg QD is not recommended for AD chronically treated with strong CYP3A4 inhibitors, while for ulcerative colitis or Crohn’s disease, the recommended induction and maintenance doses are 30 mg and 15 mg QD, respectively (Table 3).
Conclusions
The JAKis abrocitinib, baricitinib, filgotinib, peficitinib, tofacitinib and upadacitinib have unique chemical structures, which translate into different binding modes and affinities for JAK isoforms and variations in clinical pharmacology. As JAK enzymes work cooperatively, JAK1 can pair with any of the other three isoforms; therefore, even JAKis that are relatively JAK1-selective may have biological effects on all pairings involving JAK1. As JAK2 is the only JAK occurring as a homodimer, JAK2 blockade may lead to haematological effects. Overall, it is unclear which pathway blockade is most likely to translate into clinical efficacy, and whether the differential selectivity of JAKis translates into clinically meaningful differences in patient outcomes. The lack of head-to-head studies prevents direct comparison of the efficacy and safety of JAKis, and further research is required. Distinct differences in the metabolism and elimination profiles of different JAKis lead to dosing variations in patients with hepatic/renal impairment and potential drug–drug interactions with each JAKi, all of which have clinical significance. The evidence presented in this review indicates that each JAKi is a unique molecular entity, with multiple differential characteristics associated with a distinct pharmacological and clinical profile, which may help guide patient selection.
Search strategy and selection criteria
The references for this review were identified through searching PubMed for articles published between January 1990 and August 2022. Search criteria are listed in Supplementary Table S4, available at Rheumatology online. The final reference list was generated on the basis of originality and relevance to the scope of this review.
Supplementary Material
Acknowledgements
AbbVie and the authors thank the participants, study sites and investigators who participated in this real-world observational study. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Dan Booth, on behalf of 2 the Nth (Cheshire, UK), and was funded by AbbVie.
Contributor Information
Peter C Taylor, Botnar Research Centre, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Ernest Choy, Division of Infection and Immunity, School of Medicine, Cardiff University, Cardiff, UK.
Xenofon Baraliakos, Rheumazentrum Ruhrgebiet Herne, Ruhr-University Bochum, Bochum, Germany.
Zoltan Szekanecz, Faculty of Medicine, Department of Rheumatology, University of Debrecen, Debrecen, Hungary.
Ricardo M Xavier, Serviço de Reumatologia, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil.
John D Isaacs, Translational and Clinical Research Institute, Newcastle University and Musculoskeletal Unit, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Sander Strengholt, AbbVie B.V., Mijdrecht, Utrecht, The Netherlands.
Julie M Parmentier, Immunology Precision Medicine, AbbVie Bioresearch Center, Worcester, MA, USA.
Ralph Lippe, AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany.
Yoshiya Tanaka, First Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
No new data were generated or analysed in support of this review.
Funding
AbbVie funded this study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication.
Disclosure statement: P.C.T. has received research grants from Galapagos and has served as a consultant to AbbVie, Galapagos, Gilead, Lilly and Pfizer. E.C. reports personal fees from AbbVie, Amgen, Bristol Myer Squibb, Celgene, Chugai Pharma, Eli Lilly, Gilead, Galapagos, Janssen, ObsEva, Regeneron, Sanofi, SynAct Pharma and Tonix, and grants from Biogen; and grants and personal fees from BioCancer, Novartis, Novimmune, Pfizer, Roche and UCB Pharma. X.B. has received research grants and/or consulting fees from AbbVie, BMS, Galapagos, Lilly, MSD, Novartis, Pfizer, Sandoz and UCB. Z.S. has received research grants from Pfizer paid to institution, and personal consulting fees from AbbVie, Boehringer Ingelheim, Gedeon Richter, Lilly, MSD, Novartis, Pfizer and Roche. R.M.X. has received research grants and/or consulting/speaker’s fees from AbbVie, Janssen, Lilly, Novartis, Organon and Pfizer. J.D.I. is an employee of AbbVie, MA, USA. S.S. is an employee of AbbVie B.V., Mijdrecht, Utrecht, The Netherlands. J.M.P. is an employee of AbbVie Bioresearch Center, Worcester, MA, USA. R.L. is an employee of AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany. Y.T. has received speaking fees and/or honoraria from AbbVie GK, Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan KK, Janssen Pharmaceutical KK, Mitsubishi-Tanabe Pharma, Novartis, Pfizer Japan Inc., Takeda, Teijin and YL Biologics, and research grants from Asahi-Kasei Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Mitsubishi-Tanabe Pharma, Ono Pharmaceutical, Sanofi KK, Takeda and UCB Japan.
References
- 1. Yamaoka K, Saharinen P, Pesu M. et al. The Janus kinases (JAks). Genome Biol 2004;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander M, Luo Y, Raimondi G, O'Shea JJ, Gadina M.. Jakinibs of all trades: inhibiting cytokine signaling in immune-mediated pathologies. Pharmaceuticals (Basel) 2021;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris R, Kershaw NJ, Babon JJ.. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci 2018;27:1984–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kato M. New insights into IFN-gamma in rheumatoid arthritis: role in the era of JAK inhibitors. Immunol Med 2020;43:72–8. [DOI] [PubMed] [Google Scholar]
- 5. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM.. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017;77:521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin JX, Leonard WJ.. The common cytokine receptor gamma chain family of cytokines. Cold Spring Harb Perspect Biol 2018;10:a028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaffen SL, Jain R, Garg AV, Cua DJ.. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watford WT, Hissong BD, Bream JH. et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev 2004;202:139–56. [DOI] [PubMed] [Google Scholar]
- 9. Neubauer H, Cumano A, Muller M. et al. JAK2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998;93:397–409. [DOI] [PubMed] [Google Scholar]
- 10. Witthuhn BA, Quelle FW, Silvennoinen O. et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 1993;74:227–36. [DOI] [PubMed] [Google Scholar]
- 11. Varghese LN, Defour JP, Pecquet C, Constantinescu SN.. The thrombopoietin receptor: structural basis of traffic and activation by ligand, mutations, agonists, and mutated calreticulin. Front Endocrinol (Lausanne) 2017;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang Y, Liu W, Wang W. et al. Inhibition of JAK2 suppresses myelopoiesis and atherosclerosis in apoe(-/-) mice. Cardiovasc Drugs Ther 2020;34:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN.. The molecular regulation of Janus kinase (JAK) activation. Biochem J 2014;462:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villarino AV, Gadina M, O'Shea JJ, Kanno Y.. SnapShot: JAK–STAT signaling II. Cell 2020;181:1696–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Shea JJ, Schwartz DM, Villarino AV. et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015;66:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gadina M, Chisolm DA, Philips RL. et al. Translating JAKs to jakinibs. J Immunol 2020;204:2011–20. [DOI] [PubMed] [Google Scholar]
- 17. Jegatheeswaran J, Turk M, Pope JE.. Comparison of Janus kinase inhibitors in the treatment of rheumatoid arthritis: a systemic literature review. Immunotherapy 2019;11:737–54. [DOI] [PubMed] [Google Scholar]
- 18. McInnes IB, Szekanecz Z, McGonagle D. et al. A review of JAK-STAT signalling in the pathogenesis of spondyloarthritis and the role of JAK inhibition. Rheumatology (Oxford) 2022;61:1783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bristol Myers Squibb. SOTYKTU™ (deucravacitinib) tablets, for oral use. Prescribing information. 2022.
- 20. Ferrao R, Lupardus PJ.. The Janus kinase (JAK) FERM and SH2 domains: bringing specificity to JAK-receptor interactions. Front Endocrinol (Lausanne) 2017;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Min X, Ungureanu D, Maxwell S. et al. Structural and functional characterization of the JH2 pseudokinase domain of JAK family tyrosine kinase 2 (TYK2). J Biol Chem 2015;290:27261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu X, Li J, Fu M, Zhao X, Wang W.. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 2021;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfizer Inc. CIBINQO™ (abrocitinib) tablets, for oral use. Prescribing information. 2022.
- 24. Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM.. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics 2022;14:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parmentier JM, Voss J, Graff C. et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O.. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs 2019;33:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford) 2019;58:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McInnes IB, Byers NL, Higgs RE. et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther 2019;21:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Traves PG, Murray B, Campigotto F. et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis 2021;80:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dowty ME, Lin TH, Jesson MI. et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect 2019;7:e00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simpson EL, Silverberg JI, Nosbaum A. et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol 2021;22:693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wojciechowski J, Malhotra BK, Wang X. et al. Population pharmacokinetic-pharmacodynamic modelling of platelet time-courses following administration of abrocitinib. Br J Clin Pharmacol 2022;88:3856–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kay J, Harigai M, Rancourt J. et al. Changes in selected haematological parameters associated with JAK1/JAK2 inhibition observed in patients with rheumatoid arthritis treated with baricitinib. RMD Open 2020;6:e001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westhovens R, Taylor PC, Alten R. et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 2017;76:998–1008. [DOI] [PubMed] [Google Scholar]
- 35. Kavanaugh A, Kremer J, Ponce L. et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis 2017;76:1009–19. [DOI] [PubMed] [Google Scholar]
- 36. Schulze-Koops H, Strand V, Nduaka C. et al. Analysis of haematological changes in tofacitinib-treated patients with rheumatoid arthritis across phase 3 and long-term extension studies. Rheumatology (Oxford) 2017;56:46–57. [DOI] [PubMed] [Google Scholar]
- 37. Katoh N, Ohya Y, Murota H. et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (Rising Up): an interim 24-week analysis. JAAD Int 2022;6:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nader A, Mohamed MF, Winzenborg I. et al. Exposure-response analyses of upadacitinib efficacy and safety in phase II and III studies to support benefit-risk assessment in rheumatoid arthritis. Clin Pharmacol Ther 2020;107:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Japanese Ministry of Health, Labour and Welfare. Report on the deliberation results. 2019. https://www.pmda.go.jp/files/000233074.pdf (11 September 2023, date last accessed).
- 40. Parra-Izquierdo I, Melrose AR, Pang J. et al. Janus kinase inhibitors ruxolitinib and baricitinib impair glycoprotein-VI mediated platelet function. Platelets 2022;33:404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fleischmann R, Pangan AL, Song IH. et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. [DOI] [PubMed] [Google Scholar]
- 42. AbbVie Deutschland GmbH & Co. KG. Rinvoq (upadacitinib) [summary of product characteristics]. 2023. https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf (11 September 2023, date last accessed).
- 43. Muensterman E, Engelhardt B, Gopalakrishnan S, Anderson JK, Mohamed MF.. Upadacitinib pharmacokinetics and exposure-response analyses of efficacy and safety in psoriatic arthritis patients – analyses of phase III clinical trials. Clin Transl Sci 2022;15:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Faquetti ML, Grisoni F, Schneider P, Schneider G, Burden AM.. Identification of novel off targets of baricitinib and tofacitinib by machine learning with a focus on thrombosis and viral infection. Sci Rep 2022;12:7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Green MR, Newton MD, Fancher KM.. Off-target effects of BCR-ABL and JAK2 inhibitors. Am J Clin Oncol 2016;39:76–84. [DOI] [PubMed] [Google Scholar]
- 46. Rudolph J, Heine A, Quast T. et al. The JAK inhibitor ruxolitinib impairs dendritic cell migration via off-target inhibition of ROCK. Leukemia 2016;30:2119–23. [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency. Cibinqo (abrocitinib) assessment report. Procedure No. EMEA/H/C/005452/0000 (EMA/647846/2021). Amsterdam, The Netherlands: CHMP, 2021. https://www.ema.europa.eu/en/documents/assessment-report/cibinqo-epar-public-assessment-report_en.pdf (11 September 2023, date last accessed).
- 48. Tsai YC, Tsai TF.. Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review. Ther Adv Musculoskelet Dis 2020;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. European Medicines Agency. Jyseleca (filgotinib) assessment report. Procedure No. EMEA/H/C/005113/0000 (EMA/424374/2020). Amsterdam, The Netherlands: CHMP, 2020. https://www.ema.europa.eu/en/documents/assessment-report/jyseleca-epar-public-assessment-report_en.pdf (11 September 2023, date last accessed).
- 50. Karaman MW, Herrgard S, Treiber DK. et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 2008;26:127–32. [DOI] [PubMed] [Google Scholar]
- 51. Hartmann S, Ridley AJ, Lutz S.. The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol 2015;6:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfizer Europe. Cibinqo (abrocitinib) [summary of product characteristics]. Bruxelles, Belgium: Pfizer Europe MA EEIG, 2021. https://www.ema.europa.eu/en/documents/product-information/cibinqo-epar-product-information_en.pdf (11 September 2023, date last accessed).
- 53.Pfizer Inc. XELJANZ®. Prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf Accessed date can be today’s date (11 September 2023, date last accessed).
- 54. European Medicines Agency. Rinvoq (upadacitinib) assessment report. Procedure No. EMEA/H/C/004760/0000 (EMA/608624/2019 Corr. 1). 2020. https://www.ema.europa.eu/en/documents/assessment-report/rinvoq-epar-public-assessment-report_en.pdf (11 September 2023, date last accessed).
- 55.Eli Lilly and Company. OLUMIANT (baricitinib) tablets, for oral use. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf (11 September 2023, date last accessed).
- 56. Barry SP, Townsend PA, Knight RA. et al. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol 2010;91:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonner JA, Trummell HQ, Bonner AB. et al. Enhancement of cetuximab-induced radiosensitization by JAK-1 inhibition. BMC Cancer 2015;15:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gupta P, Sharma Y, Viswanathan P, Gupta S.. Cellular cytokine receptor signaling and ATM pathway intersections affect hepatic DNA repair. Cytokine 2020;127:154946. [DOI] [PubMed] [Google Scholar]
- 59. Nieborowska-Skorska M, Maifrede S, Dasgupta Y. et al. Ruxolitinib-induced defects in DNA repair cause sensitivity to PARP inhibitors in myeloproliferative neoplasms. Blood 2017;130:2848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang PL, Liu LX, Li EM, Xu LY.. STAT3, the challenge for chemotherapeutic and radiotherapeutic efficacy. Cancers (Basel) 2020;12:2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gu Y, Mohammad IS, Liu Z.. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol Lett 2020;19:2585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reddig A, Voss L, Guttek K. et al. Impact of different JAK inhibitors and methotrexate on lymphocyte proliferation and DNA damage. J Clin Med 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tanaka Y, Kavanaugh A, Wicklund J, McInnes IB.. Filgotinib, a novel JAK1-preferential inhibitor for the treatment of rheumatoid arthritis: an overview from clinical trials. Mod Rheumatol 2022;32:1–11. [DOI] [PubMed] [Google Scholar]
- 64.Gilead. Gilead receives complete response letter for filgotinib for the treatment of moderately to severely active rheumatoid arthritis. 2020. https://www.gilead.com/news-and-press/press-room/press-releases/2020/8/gilead-receives-complete-response-letter-for-filgotinib-for-the-treatment-of-moderately-to-severely-active-rheumatoid-arthritis (11 September 2023, date last accessed).
- 65. Hellstrom WJG, Dolhain R, Ritter TE. et al. MANTA and MANTA-RAy: rationale and design of trials evaluating effects of filgotinib on semen parameters in patients with inflammatory diseases. Adv Ther 2022;39:3403–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pfizer. Xeljanz (tofacitinib) [summary of product characteristics]. Bruxelles, Belgium: Pfizer, 2022. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf (11 September 2023, date last accessed).
- 67. Galapagos NV. Jyseleca (filgotinib) [summary of product characteristics]. Mechelen, Belgium: Galapagos NV, 2022. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf (11 September 2023, date last accessed).
- 68. Eli Lilly Nederland. Olumiant (baricitinib) [summary of product characteristics]. Utrecht, The Netherlands: Eli Lilly Nederland BV, 2021. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf (11 September 2023, date last accessed).
- 69. Wang X, Dowty ME, Wouters A. et al. Assessment of the effects of inhibition or induction of CYP2C19 and CYP2C9 enzymes, or inhibition of OAT3, on the pharmacokinetics of abrocitinib and its metabolites in healthy individuals. Eur J Drug Metab Pharmacokinet 2022;47:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tanaka Y, Izutsu H.. Peficitinib for the treatment of rheumatoid arthritis: an overview from clinical trials. Expert Opin Pharmacother 2020;21:1015–25. [DOI] [PubMed] [Google Scholar]
- 71. Dowty ME, Lin J, Ryder TF. et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab Dispos 2014;42:759–73. [DOI] [PubMed] [Google Scholar]
- 72.European Medicines Agency. Xeljanz (tofacitinib) assessment report. Procedure No. EMEA/H/C/002542/0000 (EMA/CHMP/425279/2013). 2013. https://www.ema.europa.eu/en/documents/assessment-report/xeljanz-epar-public-assessment-report_en-0.pdf (11 September 2023, date last accessed).
- 73.AbbVie Inc. RINVOQ® (upadacitinib) extended-release tablets, for oral use. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf (11 September 2023, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this review.