Abstract
Although the exact etiology of inflammatory bowel diseases (IBD) is unknown, studies have shown that dysregulated immune responses, genetic factors, gut microbiota, and environmental factors contribute to their pathogenesis. Intriguingly, serotonin (5-hydroxytryptamine or 5-HT) seems to be a molecule with increasingly strong implications in the pathogenesis of intestinal inflammation, affecting host physiology, including autophagy and immune responses, as well as microbial composition and function. 5-HT may also play a role in mediating how environmental effects impact outcomes in IBD. In this review, we aim to explore the production and important functions of 5-HT, including its impact on the gut. In addition, we highlight the bidirectional impacts of 5-HT on the immune system, the gut microbiota, and the process of autophagy and how these effects contribute to the manifestation of intestinal inflammation. We also explore recent findings connecting 5-HT signalling and the influence of environmental factors, particularly diet, in the pathogenesis of IBD. Ultimately, we explore the pleiotropic effects of this ancient molecule on biology and health in the context of intestinal inflammation.
INTRODUCTION
The gastrointestinal (GI) tract represents a nexus of neuronal, immunological, digestive, endocrine, and microbial elements working in concert to maintain physiological homeostasis. Indeed, the gut represents the largest endocrine organ in the human body, plays host to nearly two-thirds of the body’s immune cells and houses trillions of microorganisms (1–4). Each of these factors, if perturbed, can disrupt the delicate homeostatic balance, resulting in pathophysiological conditions like inflammatory bowel disease (IBD).
Endocrine signals, including the biogenic monoamine serotonin (5-hydroxytryptamine or 5-HT), coordinate and perpetuate the necessary actions of the gut (5–7). Though best known for its neuronal effects (8,9), only a small portion of the body’s total 5-HT resides in the nervous system, including the brain, and the vast majority is produced in the intestines (10). This peripherally-produced 5-HT cannot cross the blood-brain barrier; thus, centrally-produced and peripherally-produced 5-HT represent distinct pools that are synthesized via different biochemical pathways (11–13).
In the gut, the vast majority of 5-HT, approximately 95%, is produced via enterochromaffin (EC) cells in the intestinal mucosa (11,12,14), with the remaining synthesized within enteric neurons and a small portion by resident microbes (15). Acting as sentinels in the GI tract, EC cells respond to various mechanical and chemical stimuli (16,17). Within these cells, 5-HT is synthesized from dietary tryptophan (18) via a pathway controlled by the rate-limiting enzyme, tryptophan hydroxylase 1 (TPH1) (19). First, dietary tryptophan is taken up into cells, where TPH1 converts this tryptophan to 5-hydroxytryptophan (5-HTP) (20–22). The enzyme, aromatic amino acid decarboxylase then converts 5-HTP to 5-HT (23). From here, 5-HT is shuttled to secretory storage vesicles via vesicular monoamine transporter 1 at the basolateral membrane of EC cells until primed for release (24,25). Once released via exocytosis (19,26,27), peripheral 5-HT mediates its effects largely through interaction with a multitude of receptors on various cells (8,11) including a variety of both innate and adaptive immune cells, afferent neurons, and smooth muscle cells (16,22). Recent evidence has expanded 5-HT’s known mode of action, showing the molecule has receptor-independent effects, including a direct influence on the gut microbiota (28) and can participate in post-translation epigenetic modifications known as serotonylation (29,30).
Reuptake of 5-HT is controlled by the serotonin reuptake transporter (SERT) (31) found on neighbouring enterocytes and immune cells, where it can be broken down via monoamine oxidase A (11,14,32). Released 5-HT can also be taken up by platelets via SERT, entering circulation where it acts at various sites throughout the body (33–35).
Neuronal 5-HT, representing approximately 10% of the body’s 5-HT, is synthesized via the rate-limiting enzyme, tryptophan hydroxylase 2 (TPH2) (3,36,37). The biosynthesis of 5-HT from EC cells and its targets in the GI tract is illustrated in Figure 1.
Figure 1.
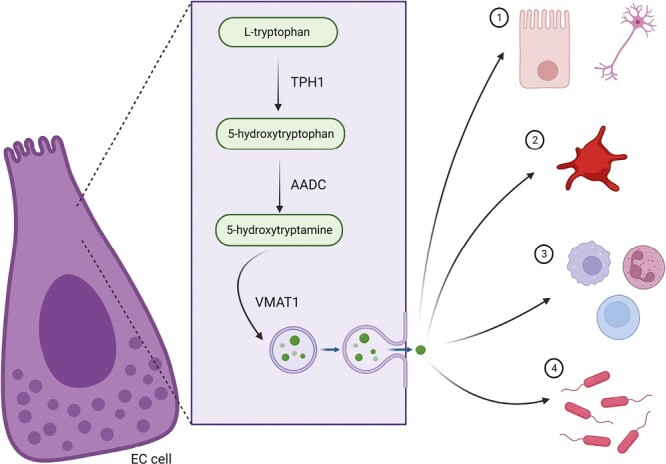
Gut-derived serotonin (5-HT) and its impact in the gut. In enterochromaffin (EC) cells, dietary tryptophan is converted by the rate-limiting enzyme tryptophan hydroxylase 1 (TPH1) to 5-hydroxytryptophan or 5-HTP. 5-HTP is then converted by aromatic amino acid decarboxylase (AADC) to serotonin or 5-hydroxytryptamine (5-HT). 5-HT is then sequestered by vesicular monoamine transport 1 (VMAT1) into storage vesicles at the basolateral membrane of the enterochromaffin (EC) cell until primed for release. Upon release by exocytosis, 5-HT can, in neighbouring enterocytes or neurons, interact via several receptors found in the gut, be taken up by the serotonin transporter, where it can be broken down, or contribute to post-translational modifications via serotonylation (1). 5-HT may also be taken up by platelets and distributed to various sites around the body, where it can elicit numerous physiological effects (2). 5-HT can also directly affect local immune cell activity and proliferation (3), and impact the composition and function of gut microbes (4).
FUNCTIONS OF 5-HT IN MAMMALIAN SYSTEMS
5-HT, acting as both a hormone and neurotransmitter, has myriad physiological effects across multiple body systems (8,38). The majority of these effects are carried out by at least 14 different subtypes of serotonin receptors (5HTRs), which are categorized into seven major families (8,39). Acting in concert or on their own, these receptors each have multiple physiological impacts, which are, in part, determined by local biological context. Though here the focus will be on the GI tract, an overview of these effects across various body systems can be seen in Figure 2.
Figure 2.
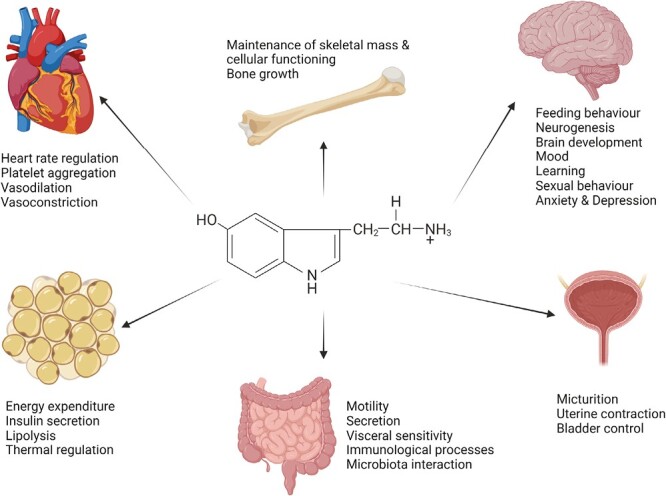
Physiological impacts of 5-HT. Both central nervous system-derived and gut-derived 5-HT can have a vast array of physiological impacts throughout the cardiovascular, skeletal, and genitourinary systems but also contribute to the functioning of the gut, metabolic processes, and to behaviour, development and mood in the brain.
In the gut, five of the seven 5-HTR families (5-HTR1, 2, 3, 4, and 7) are expressed, emphasizing serotonin’s influential role in this environment (40). As mentioned above, the vast majority of the body’s 5-HT is produced in the GI tract via EC cells and, to a smaller extent, enteric neurons (41). With that being said, 5-HT impacts myriad GI functions, including motility and transit, peristaltic reflexes, secretion, and visceral sensitivity (7,42).
Regulation of fluid, ion and mucus secretion is also under the purview of 5-HT, either by interaction with enterocytes via 5-HTR4 and 5-HTR2 or stimulation of reflexive neurons via 5-HTR1B, 5-HTR3, and 5-HTR4 (41,43–45).
In addition, 5-HT, both directly and indirectly, modulates the immune system and regulates various immune cells. In turn, factors released from these cells can influence the synthesis and actions of 5-HT. The role of serotonin on various immune cells has been extensively explored by Koopman et al. (46), Herr et al. (47), and Shajib et al. (16).
Within the nexus of the gut, 5-HT also modulates the microbiota and the immune system (36) and has a substantial impact on the pathogenesis of several GI disorders, including IBD, which is discussed in depth below (11,48).
ROLE OF 5-HT IN REGULATION OF GUT MICROBIAL COMPOSITION
Trillions of microorganisms occupy the digestive tract and play key roles in the processing of nutrients, competitive exclusion of pathogens, and the proper development of the gut and the immune system. These microbes, which include bacteria, fungi, archaea and viruses, hold great sway over the physiology of the gut, particularly the maintenance of immunological processes. Disruption in the delicate balance between host and microbiota can influence the propensity for inflammation and can also alter the serotonergic system. Though still an area of active investigation, building evidence suggests that 5-HT and the intestinal microbiota impact each other in a reciprocal manner.
Microbial Impact on 5-HT
Experiments in germ-free (GF) mice neatly illustrate the impact of the microbiota on 5-HT biology (49). Compared to conventionally raised mice, GF animals have reduced serum and colonic 5-HT concentrations (50–53). However, upon administration of either human or murine microbial samples, the restoration of the microbiota triggers increased expression of colonic TPH1 and elevated 5-HT concentrations (50,52). In a seminal work, Yano et al. (52) provided a mechanism of how these physiological changes may occur. Through a series of in vitro and in vivo studies, the authors identified key metabolites, including butyrate, cholate, and propionate, secreted by indigenous microbes, that directly interact with EC cells to increase the biosynthesis and release of 5-HT and amplify TPH1 expression in the colon of supplemented GF mice. These microbially-derived changes also had downstream effects, including altering GI motility and platelet aggregation.
We have also demonstrated a direct relationship between microbes and 5-HT production through toll-like receptors (TLRs) (54). Expressed on numerous colonic cells, including EC cells, TLRs act as immunological surveillance, recognizing microbial-associated molecular patterns and subsequently prompting an immune response (55). In this study, post-antibiotic treatment, C57BL/6 mice displayed significantly altered microbial compositions in conjunction with reduced colonic 5-HT levels, diminished EC cells and significantly downregulated expression of TLR2. These findings prompted investigation in TLR2 and TLR4 knockout mice. TLR2−/− mice, but not TLR4−/−, had significantly lower EC cell numbers and 5-HT concentrations in the colon compared to wild-type mice. Further, in both GF mice and 5-HT-producing BON-1 cells, manipulation of TLR2 signalling affected TPH1 expression and 5-HT production. Together, these findings suggest that TLR2, a key microbial sensor, is an important factor in not only EC cell biology but also the generation of 5-HT in the colon (54).
5-HT’s Impact on Microbes
In vitro and in vivo studies have demonstrated that 5-HT manipulation can affect the microbiota. For instance, Turicibacter sanguinis, a spore-forming bacteria, expresses a protein structurally similar to mammalian SERT (56), suggesting a direct interaction with 5-HT. Further, administering either 5-HT or the selective serotonin reuptake inhibitor (SSRI), fluoxetine, affects the ability of this microbe to compete against other microbes in the intestinal milieu (56). In addition, several bacterial species capable of synthesizing 5-HT include but are not limited to Klebsiella pneumoniae, Staphylococcus aureus, Escherichia coli K-12, and Lactobacillus plantarum (46). In vitro work has also demonstrated that 5-HT can affect the growth of certain bacterial species, including E. coli, Enterococcus faecalis, and Rhodospirillum rubrum, in a dose-dependent manner (15,57).
Recent work from our lab has also added to the growing body of research illustrating this reciprocal relationship, suggesting 5-HT can have both a direct and indirect impact on microbial composition and function (28). In this study, intrigued by our previous work implicating 5-HT in the pathogenesis of colitis (58), we investigated microbial differences between in Tph1−/− mice that have significantly reduced 5-HT in the gut compared to heterozygous Tph1+/− littermates. Deep sequencing revealed distinct compositional and functional alterations in the microbiota between Tph1−/− and Tph1+/− mice, the effects of which were tied with colitis severity in fecal transplant studies. Isolated commensal microbes, including Bacteroides thetaiotaomicron, B. fragilis, E. coli and Ruminicoccus gnavus were directly affected by 5-HT, showing diminished growth in a concentration-dependent manner. We also uncovered in both in vivo and in vitro work that 5-HT can impact the microbiota indirectly by modulating the production of antimicrobial peptides, particularly β-defensins, through 5-HTR7 signalling. In parallel, work by Singhal et al. (59) illustrated that increased intestinal 5-HT by way of SERT−/− also impacted murine microbial composition.
ROLE OF 5-HT IN GUT INFLAMMATION
Alterations in the 5-HT signalling, including modifications in EC cell number and 5-HT production, are associated with altered severity of intestinal inflammation as seen across both clinical studies and experimental models of IBD (48,58,60–62).
Clinical Evidence
The precise role 5-HT plays within the context of IBD is still under investigation. With that being said, the current state of research suggests that alterations, whether positive or negatively associated with disease severity, are present in the serotonergic system in IBD. It should be noted, however, that between studies, these changes are not always consistent, and alterations in sample size and severity of disease at the time of sampling may, in part, account for the discrepancies.
In UC patients, decreased levels of 5-HT (60,61) and TPH expression (60) have been documented, as well as downregulation of SERT (63). However, both increased (64) and decreased (60,62) EC cell number have also been reported in this condition.
In contrast, in CD patients, increased TPH1 expression, as well as downregulated expression of SERT (64), have been reported. EC cell number has been shown to be increased in CD (65,66). Moreover, mucosal 5-HT content in CD patients seems to vary with symptom status; CD patients who experience irritable bowel syndrome-like symptoms had significantly higher colonic 5-HT than those without (67). Inflamed intestinal tissue of CD patients also showed significantly increased 5-HTR7, 5-HTR4, and 5-HTR3 expression, suggesting that alterations in 5-HT signalling may influence inflammation in CD patients (64,68).
Experimental Evidence
Across several experimental models of colitis, including dextran sulphate sodium (DSS), trinitrobenzene sulfonic acid (TNBS), dinitrobenzene sulfonic acid (DNBS), as well as genetically susceptible models such as IL10−/− (69,70) and the CD4+ T cell transfer model, changes in the serotonergic system have been reported.
In DSS-treated rats, the density of EC cells was increased in both proximal and distal colonic tissue. Colonic levels of 5-HT were also increased (71). In guinea pigs administered TNBS, the resultant inflammation was correlated with increased 5-HT content and diminished SERT expression (72). Further, TNBS-treated SERT−/− mice manifested more severe intestinal inflammation than wild-type controls (70). In fact, SERT expression and functioning are, in part, controlled by local pro- and anti-inflammatory cytokines (73,74), demonstrating the bidirectional relationship between serotonergic and inflammatory processes. Downregulation of SERT has also been reported in DSS and CD4+ T cell models of colitis (63).
In addition, when treated with either DSS or DNBS, Tph1−/− mice or mice with pharmacologically reduced 5-HT displayed less severe colitis characterized by decreased levels of the pro-inflammatory cytokines, Interleukin(IL)-1β, IL-6 and tumour necrosis factor (TNF)-α, as well decreased macrophage infiltration in comparison to their wild-type counterparts (58). Further, Tph1−/− mice, when treated with the immediate precursor to serotonin, 5-HTP, restoring 5-HT levels, displayed colitis severity similar to that found in wild-type counterparts.
ROLE OF 5-HT IN THE REGULATION OF AUTOPHAGY IN THE CONTEXT OF GUT INFLAMMATION
Autophagy is the process of cellular ‘recycling’, a catabolic process that comprises packaging and shuttling damaged cellular components, debris and infectious agents to sequestered areas like the autophagosome and autolysosome where degradation and recycling can occur (75,76). It is a process crucial in the homeostatic balance of the gut, playing roles in antimicrobial defence and barrier function as well as combating the effects of local stressors such as hypoxia, infection, and cell death (77,78).
Genome-wide association studies have linked disrupted autophagy via genetic polymorphisms, such as ATG16L1 and IRGM, as a potential force in the pathogenesis of IBD (76). In animal models, mice lacking the autophagy genes, ATG16L1 and ATG7, develop more severe colitis when exposed to DSS than their wild-type counterparts (79–81). Moreover, treatment of IL-10−/− mice, which develop spontaneous colitis, with the potent autophagy inducer, rapamycin, ameliorated colitis severity, helping to restore disrupted intestinal permeability and diminish levels of the pro-inflammatory cytokines, IL-17, Interferon (IFN)-γ, and TNF-α, in the colon (82).
We have recently uncovered a key interaction between 5-HT and autophagic processes in the context of colitis. In mice, elevated 5-HT via 5-HTP or in SERT−/− mice, was found to impair autophagy, whereas reduced 5-HT, in Tph1−/− mice, was linked with upregulation of autophagic process (83). These findings were respectively linked with enhanced and diminished colitis susceptibility. Work in double knockout mice with both genetically disrupted autophagy in intestinal epithelial cells and diminished 5-HT, revealed that the protective effect of reduced 5-HT in the Tph1−/− mice in the context of DSS colitis was abrogated when Tph1−/− mice also had a deficiency in the key autophagosome formation enzyme, autophagy related 7 (ATG7). These double-knockout mice also exhibited a colitogenic microbial composition. Taken together, these findings suggest a key role of the 5-HT-autophagy axis in the processes of intestinal inflammation.
ROLE OF ENVIRONMENTAL AND DIETARY FACTORS IN THE REGULATION OF COLITIS VIA 5-HT
Environmental factors, including microbial exposure, antibiotic use, hygiene practices, diet and smoking, can impact the pathogenesis of IBD (84–88). One such factor at the forefront of research is the pattern of eating deemed as the ‘Western’ diet, which consists of low fibre, high salt, high fat, high-caloric intake, and elevated consumption of highly processed foods (89). Emerging research suggests that additives used within the processed foods common in the Western diet, including those utilized to improve colour, flavour or texture, may contribute to the pathogenesis of IBD (89–91).
For instance, dietary emulsifiers (91–95) have been implicated in the pathogenesis of colitis and the rising incidence of IBD. In particular, the common emulsifier, carrageenan, in both in vivo and in vitro studies, has been shown to promote pro-inflammatory cytokine expression, intestinal permeability, bacterial translocation, microbial changes and contribute to the severity of intestinal inflammation (91,92,96–98).
In addition to emulsifiers, the consumption of synthetic food colourants, such as Allura Red (AR or Red 40), Brilliant Blue (BB or Blue 1), and Sunset Yellow (SY or Yellow 6), have been increasing steadily since the 1950s (99,100). Studies show that Allura Red and Sunset Yellow are connected with fibrosis and leukocyte infiltration in both the liver and kidneys of rats by the promotion of oxidative stress (101), AR induces significant DNA damage in colonic tissue (102), and synthetic food dye consumption may also play a role in early-onset colorectal cancer (103). Work by He et al. (104) has furthered the notion that consumption of these dyes has a negative impact on colonic homeostasis. In a 2021 study, the authors found that the dye, Red 40, contributes to the development of colitis in mice which overexpress IL-23. These findings contribute to the idea that in genetically susceptible individuals, the interactions between the host immune system, microbiota, and environmental factors can create a ‘perfect storm’ in the GI tract to prompt colitis.
Recent work from our lab has supported this idea and linked the chronic consumption of Allura Red AC (FD&C Red 40) to the pathogenesis of colitis via alterations in the serotonergic system through both microbiota-dependent and independent mechanisms (105). Allura Red AC is a common ingredient in candies, soft drinks, dairy products and some cereals and is used to add colour and texture to foodstuffs, often to attract children. We uncovered that C57BL/6 mice exposed to AR, in food or water over the course of 12 weeks, displayed exacerbated intestinal inflammation, impaired barrier function and a perturbed microbiota across various models of colitis, including DSS and the CD4+ T cell-induced model of colitis (105). In addition, chronic exposure to AR was associated with increased levels of colonic 5-HT, and notably, in Tph1−/− mice, with significantly reduced levels of 5-HT in the colon, this chronic exposure to AR did not impact colitis susceptibility suggesting that the effects of AR are mediated through 5-HT. Further, AR significantly altered microbial composition and germ-free mice administered cecal content from these AR-exposed mice also exhibited heightened colitis susceptibility and elevated 5-HT.
Though extensive future work is needed, particularly within the human population, a growing body of evidence suggests that environmental factors are key contributors to the pathogenesis of IBD.
SUMMARY AND PERSPECTIVES
The mammalian gut is the nexus of immunological, endocrine, neuronal, and microbial factors. Emerging evidence suggests that 5-HT may serve as the intermediatory between host, microbiota, and environmental factors, and evidence continues to advocate that this molecule is a crucial mediator of gastrointestinal physiology and homeostasis. More and more environmental factors, including dietary composition and food additives, are becoming a significant area of concern with regard to intestinal inflammation. Recent work from our lab and from others has suggested that 5-HT plays a key role in modulating immune responses, gut microbiota, and autophagy, and in integrating environmental factors in the pathophysiology of colitis. A summary of recent work on the impact of serotonin in the context of intestinal inflammation can be seen in Figure 3. The complexity of IBD, as a multifactorial disease, is continuously being uncovered, and current research suggests manipulation of the serotonergic system in both direct and indirect ways may prove an exciting possibility for future therapies in IBD.
Figure 3.
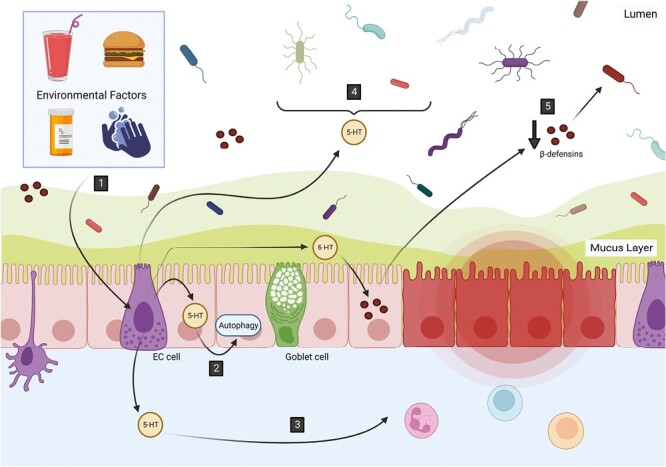
Serotonin (5-HT) and intestinal inflammation. 5-HT is a major mediator of gut physiology and can contribute to the pathogenesis of intestinal inflammation. Recent works have highlighted how serotonin signalling is impacted by environmental factors, including processed foods, hygiene practices, antibiotic exposure and food dyes, such as Allura Red (1). Altered 5-HT production and functioning can also affect the cellular recycling process of autophagy and ultimately impact both microbiota and the severity of intestinal inflammation (2). Additionally, 5-HT can prime various immune cells toward a pro-inflammatory profile and contribute to the exacerbated immune responses characteristic of inflammatory bowel diseases (3). 5-HT can also directly (4) and indirectly, through the production of host antimicrobial peptides, including β-defensins (5), impact the gut microbiota and contribute to a colonic environment predisposed to colitis.
Acknowledgements
J.A.G. wrote and edited the manuscript and designed and created the figures. W.I.K. supervised the project and edited the manuscript. Figures were created with BioRender.com.
Contributor Information
Jensine A Grondin, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON, Canada; Department of Pathology and Molecular Medicine, McMaster University, Hamilton, ON, Canada.
Waliul I Khan, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON, Canada; Department of Pathology and Molecular Medicine, McMaster University, Hamilton, ON, Canada.
Funding
This work was supported by a grant from the Canadian Institute of Health Research (CIHR) to W.I.K. (PJT 156262, PJ9-175373, and PJT-178305).
Conflict Interest
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
There are no data associated with this manuscript.
References
- 1. May CL, Kaestner KH.. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323(1):70. doi: 10.1016/J.MCE.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F.. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(Suppl. 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan WI, Ghia JE.. Gut hormones: Emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161(1):19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins SM, Surette M, Bercik P.. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–42. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 5. Keating DJ, Spencer NJ.. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol Res. 2019;140:50–5. doi: 10.1016/j.phrs.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 6. Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19(Suppl. 2):25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 7. Gershon MD. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 2001;13:15–30. doi: 10.1046/j.1365-2036.1999.00002.x-i2. [DOI] [PubMed] [Google Scholar]
- 8. Berger M, Gray JA, Roth BL.. The expanded biology of serotonin. Annu Rev Med. 2009;60(1):355–66. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lesch KP, Araragi N, Waider J, van den Hove D, Gutknecht L.. Targeting brain serotonin synthesis: Insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos Trans R Soc B: Biol Sci. 2012;367(1601):2426–43. doi: 10.1098/rstb.2012.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T.. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 11. El-Merahbi R, Löffler M, Mayer A, Sumara G.. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589(15):1728–34. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 12. Spohn SN, Mawe GM.. Non-conventional features of peripheral serotonin signalling-the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14(7):412–20. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthes S, Bader M.. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol Sci. 2018;39(6):560–72. doi: 10.1016/j.tips.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 14. Gershon MD. Review article: Serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 15. Haq S, Grondin JA, Khan WI.. Tryptophan-derived serotonin-kynurenine balance in immune activation and intestinal inflammation. FASEB J. 2021;35(10):e21888. doi: 10.1096/FJ.202100702R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shajib MS, Khan WI.. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015;213(3):561–74. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 17. Mawe GM, Hoffman JM.. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):564. doi: 10.1038/nrgastro.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keszthelyi D, Troost FJ, Masclee AAM.. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21(12):1239–49. doi: 10.1111/j.1365-2982.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 19. Walther DJ, Bader M.. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66(9):1673–80. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 20. Schäfermeyer A, Gratzl M, Rad R, Dossumbekova A, Sachs G, Prinz C.. Isolation and receptor profiling of ileal enterochromaffin cells. Acta Physiol Scand. 2004;182(1):53–62. doi: 10.1111/j.1365-201X.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 21. Fidalgo S, Ivanov DK, Wood SH.. Serotonin: From top to bottom. Biogerontology. 2012;14(1):21–45. doi: 10.1007/s10522-012-9406-3. [DOI] [PubMed] [Google Scholar]
- 22. Shajib MS, Baranov A, Khan WI.. Diverse effects of gut-derived serotonin in intestinal inflammation. ACS Chem Neurosci. 2017;8(5):920–31. doi: 10.1021/acschemneuro.6b00414. [DOI] [PubMed] [Google Scholar]
- 23. Walther DJ, Peter JU, Bashammakh S, et al. . Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 24. Hill SJ. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990;42(1):45–83. [PubMed] [Google Scholar]
- 25. Racké K, Reimann A, Schwörer H, Kilbinger H.. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1995;73(1-2):83–7. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 26. Tamir H, Gershon MD.. Serotonin-storing secretory vesicles. Ann N Y Acad Sci. 1990;600(1):53–66; discussion 67. doi: 10.1111/j.1749-6632.1990.tb16872.x. [DOI] [PubMed] [Google Scholar]
- 27. Bertrand PP, Bertrand RL.. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153(1-2):47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28. Kwon YH, Wang H, Denou E, et al. . Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol Gastroenterol Hepatol. 2019;7(4):709–28. doi: 10.1016/j.jcmgh.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walther DJ, Peter JU, Winter S, et al. . Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003;115(7):851–62. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 30. Bader M. Serotonylation: Serotonin signaling and epigenetics. Front Mol Neurosci. 2019;12:288. doi: 10.3389/fnmol.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martel F, Monteiro R, Lemos C.. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther. 2003;306(1):355–62. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- 32. Gershon MD, Ross LL.. Radioisotopic studies of the binding, exchange, and distribution of 5-hydroxytryptamine synthesized from its radioactivity precursor. J Physiol. 1966;186(2):451–76. doi: 10.1113/jphysiol.1966.sp008046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morrissey JJ, Walker MN, Lovenberg’ W.. The absence of tryptophan hydroxylase activity in blood platelets. Proc Soc Exp Biol Med. 1977;154:496–9. [DOI] [PubMed] [Google Scholar]
- 34. Holmsen H. Physiological functions of platelets. Ann Med. 1989;21(1):23–30. doi: 10.3109/07853898909149178. [DOI] [PubMed] [Google Scholar]
- 35. Mercado CP, Kilic F.. Molecular mechanisms of SERT in platelets: Regulation of plasma serotonin levels. Mol Interv. 2010;10(4):231–41. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banskota S, Ghia JE, Khan WI.. Serotonin in the gut: Blessing or a curse. Biochimie Published online 2018;161:56–64. doi: 10.1016/j.biochi.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 37. Erspamer V, Asero B.. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169(4306):800–1. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 38. Garfield AS, Heisler LK.. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587(1):49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaspar P, Cases O, Maroteaux L.. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 40. Kwon YH, Khan WI.. Peripheral serotonin: Cultivating companionship with gut microbiota in intestinal homeostasis. Am J Physiol Cell Physiol. 2022;323(2):C550–5. doi: 10.1152/ajpcell.00433.2021. [DOI] [PubMed] [Google Scholar]
- 41. Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141(8):1285–93. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. http://www.biomed.cas.cz/physiolres (Accessed April 25, 2023). [PubMed] [Google Scholar]
- 43. Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915(1):77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 44. Budhoo MR, Harris RP, Kellum JM.. 5-Hydroxytryptamine-induced Cl− transport is mediated by 5-HT3 and 5-HT4 receptors in the rat distal colon. Eur J Pharmacol. 1996;298(2):137–44. doi: 10.1016/0014-2999(95)00752-0. [DOI] [PubMed] [Google Scholar]
- 45. Hansen MB, Witte AB.. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. 2008;193(4):311–23. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 46. Koopman N, Katsavelis D, Ten Hove AS, Brul S, de Jonge WJ, Seppen J.. The multifaceted role of serotonin in intestinal homeostasis. Int J Mol Sci. 2021;22(17):9487. doi: 10.3390/ijms22179487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Herr N, Bode C, Duerschmied D.. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48. doi: 10.3389/FCVM.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manocha M, Khan WI.. Serotonin and GI disorders: An update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3(4):e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wikoff WR, Anfora AT, Liu J, et al. . Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reigstad CS, Salmonson CE, Rainey JF, et al. . Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sjögren K, Engdahl C, Henning P, et al. . The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yano JMM, Yu K, Donaldson GPP, et al. . Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Vadder F, Grasset E, Holm LM, et al. . Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–63. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang H, Kwon YH, Dewan V, et al. . TLR2 plays a pivotal role in mediating mucosal serotonin production in the gut. J Immunol. 2019;202(10):3041–52. doi: 10.4049/jimmunol.1801034. [DOI] [PubMed] [Google Scholar]
- 55. Bogunovic M, Davé SH, Tilstra JS, et al. . Enteroendocrine cells express functional toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):1770–83. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fung TC, Vuong HE, Luna CDG, et al. . Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019;4(12):2064–73. doi: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oleskin AV, Kirovskaya TA, Botvinko IV, Lysak LV.. Effects of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms. Microbiology. 1998;67(3):251–7. [PubMed] [Google Scholar]
- 58. Ghia JE, Li N, Wang H, et al. . Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137(5):1649–60. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 59. Singhal M, Turturice BA, Manzella CR, et al. . Serotonin transporter deficiency is associated with dysbiosis and changes in metabolic function of the mouse intestinal microbiome. Sci Rep. 2019;9(1):2138. doi: 10.1038/S41598-019-38489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coates MD, Mahoney CR, Linden DR, et al. . Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 61. Magro F, Vieira-Coelho MA, Fraga S, et al. . Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47(1):216–24. doi: 10.1023/a:1013256629600. [DOI] [PubMed] [Google Scholar]
- 62. Ahonen A, Kyosola K, Penttila O.. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8(1):1–7. https://europepmc.org/article/med/937988 (Accessed April 25, 2023). [PubMed] [Google Scholar]
- 63. Tada Y, Ishihara S, Kawashima K, et al. . Downregulation of serotonin reuptake transporter gene expression in healing colonic mucosa in presence of remaining low-grade inflammation in ulcerative colitis. J Gastroenterol Hepatol. 2016;31(8):1443–52. doi: 10.1111/jgh.13268. [DOI] [PubMed] [Google Scholar]
- 64. Shajib MS, Chauhan U, Adeeb S, et al. . Characterization of serotonin signaling components in patients with inflammatory bowel disease. J Can Assoc Gastroenterol. 2019;2(3):132–140. doi: 10.1093/jcag/gwy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. El-Salhy M, Danielsson A, Stenling R, Grimelius L.. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242(5):413–9. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 66. Bishop AE, Pietroletti R, Taat CW, Brummelkamp WH, Polak JM.. Increased populations of endocrine cells in Crohn’s ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410(5):391–6. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 67. Minderhoud IM, Oldenburg B, Schipper MEI, ter Linde JJM, Samsom M.. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin Gastroenterol Hepatol. 2007;5(6):714–20. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 68. Guseva D, Holst K, Kaune B, et al. . Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20(9):1516–29. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 69. Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC.. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil. 2010;22(7):826–e229. doi: 10.1111/J.1365-2982.2010.01479.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bischoff SC, Mailer R, Pabst O, et al. . Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G685–G695. doi: 10.1152/AJPGI.90685.2008. [DOI] [PubMed] [Google Scholar]
- 71. Oshima SI, Fujimura M, Fujimiya M.. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112(4):257–63. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- 72. Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM.. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):207–16. doi: 10.1152/ajpgi.00488.2002 [DOI] [PubMed] [Google Scholar]
- 73. Zhu CB, Blakely RD, Hewlett WA.. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–31. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 74. Latorre E, Mendoza C, Matheus N, et al. . IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 2013;61(3):778–84. doi: 10.1016/j.cyto.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 75. Kaur J, Debnath J.. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16(8):461–72. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 76. Haq S, Grondin J, Banskota S, Khan WI.. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J Biomed Sci. 2019;26(1):1–14. doi: 10.1186/S12929-019-0512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Elshaer D, Begun J.. The role of barrier function, autophagy, and cytokines in maintaining intestinal homeostasis. Semin Cell Dev Biol. 2017;61:51–9. doi: 10.1016/j.semcdb.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 78. Boya P, Reggiori F, Codogno P.. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–20. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsuboi K, Nishitani M, Takakura A, Imai Y, Komatsu M, Kawashima H.. Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J Biol Chem. 2015;290(33):20511–26. doi: 10.1074/jbc.M114.632257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saitoh T, Fujita N, Jang MH, et al. . Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456(7219):264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 81. Zhang H, Zheng L, McGovern DPB, et al. . Myeloid ATG16L1 facilitates host-bacteria interactions in maintaining intestinal homeostasis. J Immunol. 2017;198(5):2133–46. doi: 10.4049/jimmunol.1601293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao J, Wang H, Yang H, Zhou Y, Tang L.. Autophagy induction by rapamycin ameliorates experimental colitis and improves intestinal epithelial barrier function in IL-10 knockout mice. Int Immunopharmacol. 2020;81:105977. doi: 10.1016/j.intimp.2019.105977. [DOI] [PubMed] [Google Scholar]
- 83. Haq S, Wang H, Grondin J, et al. . Disruption of autophagy by increased 5-HT alters gut microbiota and enhances susceptibility to experimental colitis and Crohn’s disease. Sci Adv. 2021;7(45):6442. doi: 10.1126/sciadv.abi6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Danese S, Fiocchi C.. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12(30):4807–12. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rogler G. Update in inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2004;20(4):311–7. doi: 10.1097/00001574-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 86. Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT.. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2018;15(9):525–535. doi: 10.1038/s41575-018-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 88. Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S.. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647–59.e4. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 89. Statovci D, Aguilera M, MacSharry J, Melgar S.. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Antonio Uranga J, Ló pez-Miranda V, Lombó F, Abalo R.. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. doi: 10.1016/j.pharep.2016.05.002 [DOI] [PubMed]
- 91. Bancil AS, Sandall AM, Rossi M, Chassaing B, Lindsay JO, Whelan K.. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: Mechanistic insights in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):1068–79. doi: 10.1093/ecco-jcc/jjaa254. [DOI] [PubMed] [Google Scholar]
- 92. Viennois E, Merlin D, Gewirtz AT, Chassaing B.. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77(1):27–40. doi: 10.1158/0008-5472.CAN-16-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chassaing B, Van De Wiele T, De Bodt J, Marzorati M, Gewirtz AT.. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66(8):1414–27. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chassaing B, Koren O, Goodrich JK, et al. . Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–6. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Roberts CL, Rushworth SL, Richman E, Rhodes JM.. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohns Colitis. 2013;7(4):338–41. doi: 10.1016/j.crohns.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 96. Shang Q, Sun W, Shan X, et al. . Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol Lett. 2017;279:87–95. doi: 10.1016/j.toxlet.2017.07.904. [DOI] [PubMed] [Google Scholar]
- 97. Borthakur A, Bhattacharyya S, Dudeja PK, Tobacman JK.. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(3). doi: 10.1152/AJPGI.00380.2006. [DOI] [PubMed] [Google Scholar]
- 98. Jiang HY, Wang F, Chen HM, Yan XJ.. κ-Carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol Med Rep. 2013;8(6):1635–42. doi: 10.3892/mmr.2013.1726. [DOI] [PubMed] [Google Scholar]
- 99. Stevens LJ, Kuczek T, Burgess JR, Stochelski MA, Arnold LE, Galland L.. Mechanisms of behavioral, atopic, and other reactions to artificial food colors in children. Nutr Rev. 2013;71(5):268–81. doi: 10.1111/nure.12023. [DOI] [PubMed] [Google Scholar]
- 100. Vojdani A, Vojdani C.. Immune reactivity to food coloring. Altern Ther Health Med. 2015;21(Suppl. 1):52–62. [PubMed] [Google Scholar]
- 101. Khayyat LI, Essawy, Sorour AE, Soffar JM A., Sunset Yellow and Allura Red modulate Bcl2 and COX2 expression levels and confer oxidative stress-mediated renal and hepatic toxicity in male rats. PeerJ. 2018;2018(9):e5689. doi: 10.7717/PEERJ.5689/SUPP-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tsuda S, Murakami M, Matsusaka N, Kano K, Taniguchi K, Sasaki YF.. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol Sci. 2001;61(1):92–9. doi: 10.1093/TOXSCI/61.1.92. [DOI] [PubMed] [Google Scholar]
- 103. Hofseth LJ, Hebert JR, Chanda A, et al. . Early-onset colorectal cancer: Initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64. doi: 10.1038/s41575-019-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. He Z, Chen L, Catalan-Dibene J, et al. . Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 2021;33(7):1358–71.e5. doi: 10.1016/j.cmet.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kwon YH, Banskota S, Wang H, et al. . Chronic exposure to synthetic food colorant Allura Red AC promotes susceptibility to experimental colitis via intestinal serotonin in mice. Nat Commun. 2022;13(1):1–18. doi: 10.1038/s41467-022-35309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data associated with this manuscript.


