Abstract
Objectives
To inform an international task force about current evidence on Treat to Target (T2T) strategies in PMR and GCA.
Methods
A systematic literature research (SLR) was conducted in Medline, EMBASE, Cochrane Library, clinicaltrials.gov from their inception date to May 2022, and in the EULAR/ACR abstract database (2019–2021). Randomised clinical trials (RCTs) and non-randomised interventional studies published in English and answering at least one of the eleven PICO questions on T2T strategies, treatment targets and outcomes, framed by the taskforce, were identified. Study selection process, data extraction and risk of bias assessment were conducted independently by two investigators.
Results
Of 7809 screened abstracts, 397 were selected for detailed review and 76 manuscripts were finally included (31 RCTs, eight subgroup/exploratory analyses of RCTs and 37 non-randomised interventional studies). No study comparing a T2T strategy against standard of care was identified. In PMR RCTs, the most frequently applied outcomes concerned treatment (90.9% of RCTs), particularly the cumulative glucocorticoids (GC) dose and GC tapering, followed by clinical, laboratory and safety outcomes (63.3% each). Conversely, the most commonly reported outcomes in RCTs in GCA were prevention of relapses (72.2%), remission as well as treatment-related and safety outcomes (67.0% each).
Conclusions
This SLR provides evidence and highlights the knowledge gaps on T2T strategies in PMR and GCA, informing the task force developing T2T recommendations for these diseases.
Keywords: GCA, PMR, treat to target, T2T
Rheumatology key messages.
In PMR studies, glucocorticoid tapering and discontinuation were the most commonly used outcomes.
In GCA trials, disease activity parameters were prioritized as endpoints.
No randomized controlled trial investigated a treat to target strategy in PMR and GCA yet.
Introduction
PMR and GCA are overlapping inflammatory rheumatic disorders of the elderly [1–3]. Duration of glucocorticoid (GC) treatment and/or use of immunosuppressive drugs varies considerably among patients; however, many people with PMR or GCA are treated with GC for several years, particularly those with recurrent relapses [4]. Once remission has been achieved, an important goal is to minimize treatment toxicity and to balance dose reduction against the risk of relapse [5].
The treat to target (T2T) approach, implemented in several disciplines of medicine, has also been adopted in rheumatology. T2T recommendations are currently available for RA, PsA, axial spondylitis (axSpA) and SLE [6–8]. In RA, PsA and axSpA, regular monitoring with the aim to achieve a specific treatment target, and modification of treatment when the target has not been reached resulted in better clinical and structural outcomes than a conventional treatment strategy [9–13].
Although much progress has been made in the management of PMR and GCA, new unmet needs have emerged in terms of patients’ stratification, development of relevant treatment targets, and prevention of disease- and treatment-related complications. The objective of the present systematic literature review (SLR) was to inform an international task force developing new T2T recommendations in PMR and GCA about the evidence on treatment targets and outcomes in these conditions [14].
Methods
This SLR was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist [15]. At the first (virtual) meeting, the scientific committee. agreed on eleven key questions relevant to T2T in PMR and GCA (Table 1). The key clinical questions were eventually rephrased in the PICO format (Patients, Intervention, Comparator or Control, Outcome) which served as the basis for the SLR. A detailed description of the PICOs is depicted in Table 1.
Table 1.
Clinical key questions and PICOs used for the systematic literature review
Clinical key questions agreed upon by the scientific committee:
|
PICO questions used for the systematic literature review:
|
AEs: adverse events; PICO: patients, intervention, comparator, outcome; T2T: treat-to-target.
An experienced librarian (L.F.) developed the search strategy: Ovid Medline, EMBASE, the Cochrane Library and Epistemonikos were searched from their inception date until 13 March 2021 (Supplementary Table S1, available at Rheumatology online). An update of the SLR was performed on 3 May 2022. A manual search of abstracts from ACR and EULAR meetings from 2019 to 2021 (grey literature) was conducted. The SLR incorporated studies published solely as conference abstracts, but in cases where the related abstract's full text was accessible, only the latter was considered. Additional articles were retrieved searching the reference list of original and review articles and by contacting experts in the field.
All identified citations were downloaded to the Covidence software (Veritas Health Innovation, Australia), and duplicates were removed. Four researchers (E.H., M.B., L.E. and D.C.) conducted the SLR under the supervision of the methodologists (A.K. and D.A.). D.C. and L.E. independently performed screening and selection of articles but, due to the COVID-19 pandemic, they were not able to continue with the project. Therefore, the subsequent phases of the process (i.e. data extraction, data synthesis and quality appraisal) were performed by E.H. and M.B. Discordances between reviewers were discussed until agreement was achieved. When consensus was not achieved, one of the methodologists was consulted for a final decision.
We included full articles or research letters of interventional studies [randomized clinical trials (RCTs) as well as non-randomised interventional studies including >20 PMR and/or GCA patients (all subtypes)], published in English, and with no age restriction. Studies further required to have a control group receiving either placebo or an active treatment. Study details and results were extracted independently by E.H. and M.B. using a standardised data extraction sheet. Items of interest were: (i) population type (PMR, GCA with cranial and or large vessel involvement, PMR/GCA overlap) and demographics; (ii) number of patients included and proportion of those randomized to/receiving treatment; (iii) intervention and control treatment; (iv) outcomes and treatment targets; (v) strategies to monitor disease activity, adverse events (AEs) and comorbidities; (vi) predictors of disease course; (vii) the effect of different treatment regimens and (viii) the prognostic role of early vs established disease on outcomes.
Risk of bias (RoB) was assessed at study level (eventually considering multiple publications from one study) using the Cochrane Collaborations Risk of Bias tool for RCTs and the ROBINS-I tool for non-randomised interventional studies [16–18]. Due to the heterogeneity of the available studies, no meta-analysis was performed, and results are reported separately for each study.
The contents of this SLR were presented during the face-to-face meeting of the scientific committee and the task force in June 2022 and provided the scientific basis for the T2T recommendations in PMR and GCA [14].
Results
Included studies
The search identified 7809 references. In total, 76 of them were finally included in our SLR: 31 RCTs, eight post-hoc analyses of RCTs and 37 non-randomised interventional studies (see PRISMA flowchart in Supplementary Fig. S1, available at Rheumatology online). Several articles contributed data to more than one PICO: 73 articles (96.0%; 31 RCTs, eight post-hoc analyses of RCTs and 34 non-randomised studies) were assigned to PICO 1, one non-randomized study (1.3%) to PICO 6, 15 (19.7%; 2 RCTs, two post-hoc analyses, 11 non-randomized interventional studies) to PICO 8 and three (3.9%; one RCT sub-analysis and two non-randomized studies) to PICO 11. For the remaining PICOs (PICOs 2–5, 7, 9 and 10) no evidence was found.
Full data on quality assessment for RCTs and non-randomized interventional studies are depicted in Supplementary Table S2 and S3 (available at Rheumatology online), respectively.
Outcomes and treatment targets in polymyalgia rheumatica (PICO 1)
Twenty articles, 11 RCTs and nine non-randomized interventional studies were assigned to PICO 1 concerning outcomes and treatment targets (PICO 1).
Randomized controlled trials
Six out of the eleven (54.5%) RCTs were considered to have low RoB. Unclear and high RoB were assigned to 5/11 (45.4%) and 1/11 (9.1%) studies, respectively as shown in Supplementary Table S2, available at Rheumatology online. In RCTs, clinical improvement was always part of the study outcomes (11/11, 100%), being included in either the definitions of remission (6/11, 54.5%) or relapse (5/11, 45.4%), or independently, in terms of resolution of PMR specific signs and symptoms (6/11, 54.5%). Table 2 and Fig. 1 depict a summary of the outcomes used in RCTs; full details are shown in Supplementary Table S4, available at Rheumatology online.
Table 2.
Heat map of outcomes used in PMR RCTs
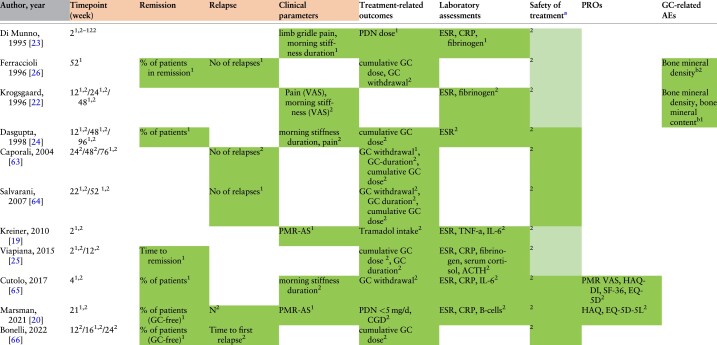
|
Overall, clinical and laboratory components were mainly used as outcomes and treatment targets. Assessment of clinical items was part of the study outcomes in all RCTs, in terms of the evaluation of either remission, relapse or resolution of specific signs and symptoms. Primary and secondary endpoints are expressed with numbers ‘1’ and ‘2’ inserted as exponential values in each outcome.
ACTH: adrenocorticotropic hormone; AE, adverse events; GC: glucocorticoids; IL-6: interleukin 6; Lab: laboratory component; MS: morning stiffness; N: number of relapses; PMR-AS: PMR activity score; PROs: patient reported outcomes; TNF-a: tumor necrosis factor alpha.
Safety of treatment was summarized by extracting the data related to AEs bassessed through DEXA.
Shading: AEs reported without being considered as a treatment target.
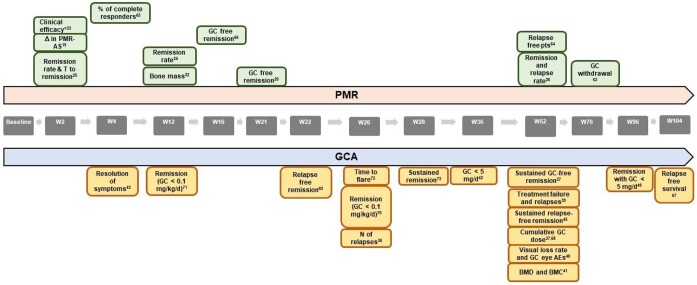
Figure 1.
Primary outcomes used in PMR and GCA RCTs and time points of their assessment. AEs: adverse events; BMC: bone mineral content; BMD: bone mineral density; GC: glucocorticoids; N: number; PMR-AS: PMR activity score; pts: patients; T: time; W: week; Δ, change in. * Clinical efficacy was defined as (reduction of limb gridle pain, morning stiffness, ESR, CRP, fibrinogen, steroid dosage) at W2, 4, 6 and 12. Each reference is inserted as an exponential number
Outcomes related to PMR treatment (10 out of 11 studies, 90.9%) included the GC cumulative dose (7/10 studies, 70.0%), GC discontinuation (4/10, 40.0%), GC duration (3/10, 30.0%) or a specific GC target dose (2/10, 20.0%). A single study considered the cumulative intake of tramadol as a secondary outcome [19].
Laboratory parameters (7 out of 11 studies, 63.3%). Among laboratory parameters, the erythrocyte sedimentation rate (ESR, 7/7 studies, 100%), C-reactive protein (CRP, 3/7, 42.9%) and/or fibrinogen serum concentrations (3/7, 42.9%) were most frequently considered as outcomes. Interestingly, among RCTs assigned to PICO 1, CRP was always part of the laboratory assessments from 2010 onwards, while earlier studies primarily evaluated the ESR. Interleukin-6 (IL-6) was considered in 2/7 (28.6%) studies, serum tumour necrosis factor alpha (TNFα), cortisol and adrenocorticotropic hormone (ACTH) levels in one (14.3%) RCT each. One recent study, assessing the efficacy of rituximab in PMR patients, also investigated B-cell depletion as an outcome [20].
Individual clinical parameters (6 out of 11 studies, 54.5%). Morning stiffness was the most frequent clinical component (6/6, 100%), being evaluated either separately (4/6, 66.7%), or as part of a composite score (i.e. the PMR activity score [21] in 2/6 studies, 33.3%). With the exception of one study who measured the intensity of morning stiffness (through a scale from 0 to 3) [22], it was mainly the duration of stiffness that was taken into account. Pain was assessed in 3/6 (50.0%) studies through either the pain VAS scale [23, 24] (100 mm; 0 = best, 100 = worst) or a 0–3 Likert scale [22] (0 = best, 3 = worst).
Safety of treatment (6 out of 11 studies, 54.5%). All RCTs reported AEs, none of them considered them as a primary end point, but 6/11 (54.5%) included AEs as a secondary outcome. No study addressed the reduction of GC-related AEs as a specific outcome.
Remission (6 out of 11 studies, 54.5%). Remission was evaluated as an outcome in 6/11 studies. Two of them referred to GC-free remission and one to the time to achieve remission. Remission was mostly defined by a combination of clinical and laboratory parameters, as shown in Fig. 2a. There was large heterogeneity between studies on when to measure remission, with a time span ranging from 2 weeks to 1 year after start of treatment [25, 26].
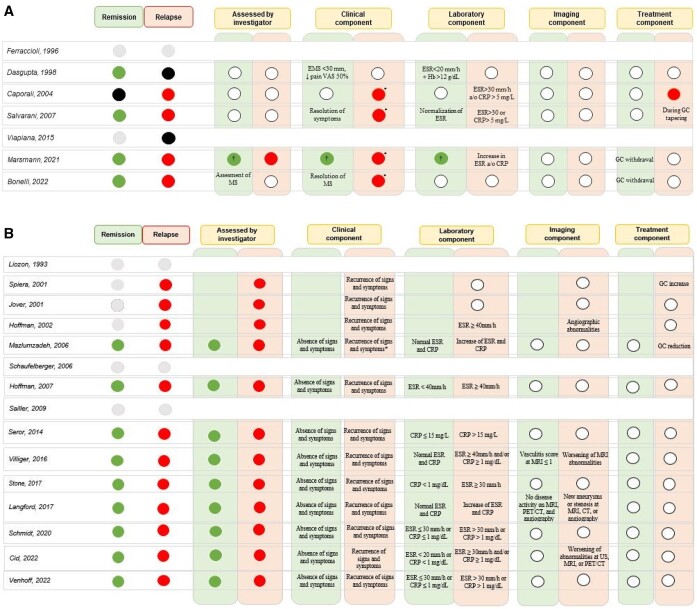
Figure 2.
Components used in defining remission and relapse in (A) PMR RCTs# and (B) GCA RCTs#. Grey circle: Remission and/or relapse used as outcomes but not defined in the study methods; Green circle: remission (or specific components) defined in the study methods; Red circle: relapse (or specific components) defined in the study methods; White circle: component not part of the definition of remission/relapse. Black circle: remission/relapse not used as an outcome. CRP, C-reactive protein; CT, computerized tomography; EMS, early morning stiffness; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; Hb, haemoglobin; MS, morning stiffness; MRI, magnetic resonance imaging; PET, positron emission tomography; US, ultrasound; VAS, visual analogue scale. *, signs and symptoms of active polymyalgia rheumatica; †, remission defined by PMR-AS (PMR Activity Score) <10; a/o, and/or. #Only RCTs considering remission and/or relapse as an outcome are listed. Overall, in (A) both remission and relapse were mainly defined as a combination of clinical and laboratory parameters. None of the studies defined sustained remission. In (B) relapse was defined as the return of signs and symptoms and/or an increase of ESR/CRP after reduction of prednisone dosage followed by an improvement of signs and symptoms when GC dosage was increased. Recurrence was defined as the reappearance of GCA signs and symptoms and/or increase of the inflammatory markers in a GCA patients not receiving GC therapy for at least 1 month. Overall, in GCA RCTs, both remission and relapse were mainly defined as a combination of clinical and laboratory parameters
Relapse (5 out of 11 studies, 45.4%). Relapse was an outcome in 5/11 RCTs; one of these evaluated the time to first relapse. A relapse was always defined by a combination of clinical and laboratory parameters as shown in Fig. 2a.
PROs (27.3%). PROs were part of the outcome measures in only 3/11 studies. The health assessment questionnaire disability index (HAQ-DI) (2/3, 66.7%), the EuroQol-5 dimension (EQ-5D) (2/3, 66.7%), the 36-Item Short-Form Health Survey (SF-36) (1/3, 33.3%), and pain visual analogue scale (VAS; 100 mm; 0 = best, 100 = worst) (1/3, 33.3% each) were used.
Other parameters. Two studies considered bone mineral content (BMC) or bone mineral density (BMD) either as a primary [22] or secondary outcome [26].
Outcomes and treatment targets in giant cell arteritis (PICO 1)
Forty-six articles were assigned to PICO 1: 18 RCTs, 20 non-randomised interventional studies and eight sub-analyses of RCTs. Among the latter, seven manuscripts were related to the GiACTA trial, a placebo-controlled phase III RCT to study the efficacy of tocilizumab in GCA [27] and one article was a post-hoc analysis of the phase II study on tocilizumab [28]. Among the eight papers related to GIACTA, the main publication analysed a number of pre-specified endpoints of the first double-blind phase of 52 weeks [27] whereas another reported the data from the second, open-label phase between weeks 52 and 104 [29]. The other six were subgroup analyses of the double-blind phase of GiACTA [30–35].
Randomized controlled trials
Relapses (13/18, 72.2%): In 3/13 RCTs (23%) and in 10/13 RCTs (77%), relapses were included as a primary or secondary end point, respectively. In 8/13 (61.5%) RCTs, the number of relapses was assessed whereas in 5/13 (38.5%) the time to first relapse was considered. All definitions of relapse used in these trials are summarized in Fig. 2b.
Remission (12/18, 66.7%): In 8 out of 12 RCTs (67.0%), remission was the primary end point. In seven (58.3%) and five RCTs (41.7%), remission combined with the achievement of a target GC dose ≤5 mg prednisone (PDN) equivalent per day or GC-free remission were used as outcomes, respectively. The time point when the achievement of remission was investigated was variable ranging from 3 to 24 months (See Table 3 for the time points of remission assessment, Fig. 2b for the definition of remission and Fig. 1 for the time points of evaluation of the primary endpoints in GCA).
Table 3.
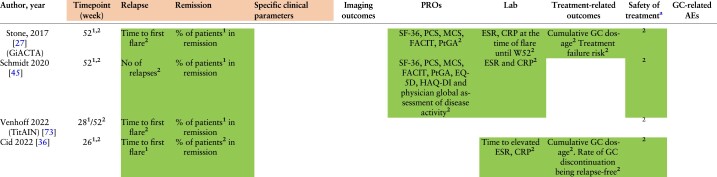
Heat map of outcomes used in GCA RCTs
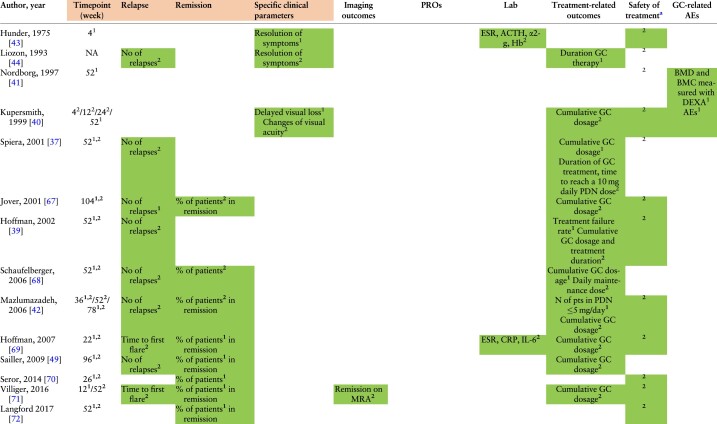
|
(continued)
Table 3.
(continued)
|
Overall, clinical and laboratory components were mainly used as outcomes and treatment targets. Assessment of clinical items was part of the study outcomes in 94.4% of the included RCTs, in terms of the evaluation of either remission, relapse or resolution of specific signs and symptoms.
Primary and secondary endpoints are expressed with numbers ‘1’ and ‘2’ inserted as exponential values in each outcome.
α2-g, alfa-2 globulins; ACTH, adrenocorticotropic hormone; AEs, adverse events; BMC, bone mineral content; BMD, bone mineral density; CRP, C-reactive protein; DEXA, dual-energy X-ray absorption; EQ-5D, EuroQol-5D; ESR, erythrocyte sedimentation rate; FACIT, Functional Assessment of Chronic Illness Therapy-Fatigue; GC, glucocorticoid; HAQ-DI, health assessment questionnaire disability index; IL-6, interleukin-6; MCS, Mental Component Summary scores and domains; MRA, magnetic resonance angiography; NA, not assessed; No, number; PCS, Physical Component Summary; PDN, prednisone; pts, patients; PtGA, Patient Global Assessment of Disease Activity; SF-36, 36-Item Short-Form Health Survey.
Shading: AEs reported without being considered part of the outcomes.
safety of treatment was summarized by extracting the data related to AEs.
Treatment-related outcomes (12/18, 66.7%): In 3/12 RCTs, treatment related outcomes were the primary end point. The most frequently reported outcome was the cumulative GC dose (11/18, 61.1%), followed by the risk of treatment failure (2/18, 11.1%), GC discontinuation (1/18, 5.5%) [36] and the time required to reach a target prednisone dose of 10 mg/day (1/18, 5.5%) [37]. In two trials, treatment failure was defined as the inability to achieve remission by week 12 or the occurrence of a relapse between weeks 12 and 52 [38] or as two distinct relapses or persistence of disease activity after the first relapse, in spite of increment of the PDN dose by ≥10 mg [39].
Safety of treatment (12/18, 66.7%): All RCTs reported AEs, safety of treatment was specified as the primary end point in two trials [40, 41] and as a secondary end point in 10/18 (55%) RCTs. Among the RCTs considering AEs as secondary endpoints, two of them focused on GC-related Aes [42, 43]. The RCTs including safety as primary endpoints evaluated ocular complications [40], or the changes of bone mineral density (BMD), measured with dual-energy X-ray absorptiometry (DEXA) [41] after one year of GC treatment.
Laboratory outcomes (27.8%): Among laboratory outcomes, the most frequently reported tests were ESR (5/5, 100%) and CRP (4/5, 80.0%), followed by serum concentrations of IL-6 (1/5, 20.0%), alpha2-globulins (1/5, 20.0%) and fibrinogen (1/5, 20.0%).
Specific clinical parameters, physician and patient-reported outcomes: Among the three trials reporting clinical parameters separately from remission/relapses as endpoints, two of them (75.0%) focused on the resolutions of symptoms as a primary [43] or secondary outcome [44]. Another RCT (33.3%) considered the rate of delayed visual loss in the first year as a primary end point [40]. Additionally, two trials assessed PROs as secondary outcomes [31, 45]; one of them also reporting the physician global assessment [45].
PROs included in both studies were the following: the SF-36, the Physical Component Summary (PCS), the Mental Component Summary scores (MCS), the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and the Patient Global Assessment of Disease Activity (PtGA). Additional scales, included in the research work of Schmidt et al. [45], were the EuroQoL-5D, the EuroQoL-5D visual analogue score, the HAQ-DI, the Physician’s Global Assessment of Disease Activity and a numeric pain rating scale.
Imaging outcomes (1/18, 5.5%): One RCT included imaging as a secondary outcome: more specifically, remission was defined according to magnetic resonance angiography (MRA) score ≤1 (range of the score from 0 to 3 with 0 indicating no mural thickening/enhancement and 3 suggesting strong mural thickening and perivascular enhancement) [28].
Detailed information about the outcomes and the main findings of the included trials are detailed in Supplementary Table S5, available at Rheumatology online.
PMR and GCA (mixed population). Seven studies assessing PMR and GCA as a single group reported data for PICO 1: two RCTs and five non-randomized studies. Both RCTs (both with either unclear or high RoB) evaluated treatment-related outcomes in terms of GC cumulative dose, change in GC dose and GC duration. Safety was reported as a secondary outcome in both studies (100%), referring either to MTX [46] or azathioprine [47]. None of the RCTs assessed the reduction of GC-related side effects (see Supplementary Table S6, available at Rheumatology online, for details). Remission (defined as treatment discontinuation) and relapse (defined as recurrence of original symptoms and increase of ESR or CRP in patients still receiving GC) were investigated in only one study (50%) [46].
The summarized data on outcomes and treatment targets for non-randomized studies in PMR and GCA are reported in the Supplementary Boxes S1–S3, available at Rheumatology online.
Impact of comorbidities on outcomes and treatment targets (PICO 6)
For GCA, only a single observational study provided evidence for PICO 6. In that study, the relapse rate of patients with biopsy-proven GCA was evaluated at one, two and five years. Hypertension (P = 0.007) and type 2 diabetes mellitus (P = 0.039) at the time of GCA diagnosis were associated with higher relapse rates, compared with those without these comorbidities. A higher proportion of patients with these comorbidities were present in the high relapse rate group (>0.5 relapses/year) compared with the group of no relapses or <0.5 relapses/year groups [48]. No data were available for patients with PMR.
Predictors of outcomes (PICO 8)
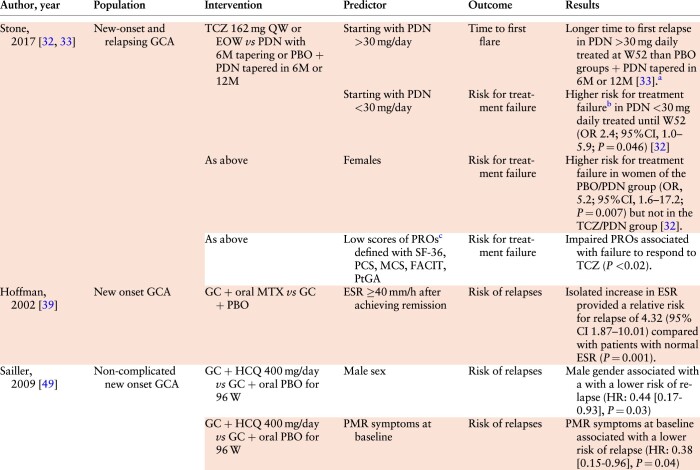
GCA. Eleven studies on GCA, including two RCTs with two subgroup analyses of the GIACTA trial and seven non-randomised interventional studies, were assigned to PICO 8. Among data resulting from RCTs, female sex, an initial PDN-dosage equivalent ≤30 mg daily, worse pre-treatment PROs inherent to the perception of disease activity (measured with the PtGA), fatigue (FACIT-Fatigue) and general health status (SF-36 or EQ-5D scores), and increased ESR levels after achieving remission were associated with a higher risk of relapses (Table 4) [32, 33, 39, 49]. Another RCT on GCA, published only as a conference abstract, identified male gender and PMR symptoms at baseline as protective factors against a relapse [49].
Table 4.
Predictors used in RCTs on GCA
|
These results are reported in the article as a Kaplan–Meier plot.
Treatment failure defined as the inability to achieve remission by week 12 or the occurrence of a relapse between weeks 12 and 52.
PROs were defined by standardised questionnaires self-reported by the patients.
EOW: every other week; FACIT: Functional Assessment of Chronic Illness Therapy-Fatigue; GC: glucocorticoid; HR: hazard ratio; M: month; MCS: Mental Component Summary scores and domains; OR: odds ratio; PBO: placebo; PCS: Physical Component Summary; PDN: prednisone; PtGA: Patient Global Assessment of Disease Activity; QW: every week; SF-36: 36-Item Short-Form Health Survey; TCZ: tocilizumab.
The systematic search for RCTs investigating prognostic factors for PMR did not yield any data.
Predictors of outcomes in non-randomized studies on PMR and GCA are reported, in summary, in Supplementary Boxes S4 and S5, available at Rheumatology online.
Outcomes in early vs established disease (PICO 11)
Three studies on GCA reported data for PICO 11, a post-hoc analysis of GiACTA [30] and two other non-randomized interventional studies [50, 51]. In the open-label extension of GiACTA, the number of flares at 3 years was compared between new-onset vs relapsing GCA. The authors reported that the relapse rate did not differ between these groups when the same treatment arm was analysed (TCZ every week, TCZ every other week or PBO). The disappearance of GCA signs and symptoms as well as changes in acute phase reactants after treatment with TCZ + GC or GC monotherapy were assessed in another observational study [50]. Among those who received TCZ, no difference was observed between patients with new-onset and established disease (defined as disease duration >6 months) concerning these outcomes. In another study of patients treated with leflunomide or MTX upon the occurrence of a (first) relapse, the rate of subsequent relapses was similar between groups with early and late GCA defined as disease duration less or more than one month, respectively [51].
No data were available from PMR studies on this PICO.
Discussion
In studies on PMR, GC-related outcomes (i.e. cumulative GC dose, GC discontinuation, GC duration, or a specific GC target dose) were the most common treatment targets (90.9%); remission and relapse were applied in only half of studies. In contrast, prevention of relapses, achievement of remission, and cumulative GC dosage were the most frequent targets in trials of GCA.
The preference of GC-related outcomes in PMR and outcomes related to disease activity in GCA remains subject to speculation. An explanation could be the fact that disease activity in PMR is difficult to assess because of the presence of comorbidities affecting or mimicking PMR symptoms [52, 53]. Another reason could be the observation that PMR does not cause long-term organ damage by itself (while certainly impacting quality of life when active) whereas the majority of patients experience AEs related to long-term GC therapy [54]. The reduction of GCs is therefore an important therapeutic goal in PMR. In GCA, disease activity potentially leads to vascular and organ damage, when not adequately treated [55].
In order to personalize treatment, it would be desirable to stratify patients according to disease severity, comorbidities and risk of developing GC-related toxicities. Unfortunately, data on this aspect are scarce, especially for PMR, whose predictors of clinical response (lower weight, age >60 years at diagnosis, extracapsular inflammatory pattern in MRI and increased musculoskeletal uptake in PET/CT) derive only from non-randomized studies of unclear or high risk of bias [56–59]. In GCA, female sex, a PDN-equivalent starting dosage <30 mg/day, absence of PMR symptoms, impaired pre-treatment PROs, and persistently raised acute phase reactants after achieving clinical remission predicted a higher relapse rate [32, 33, 39, 49].
We acknowledge that our SLR was limited to RCTs and non-randomised interventional studies, while studies without a control group were excluded. Therefore, some possible predictors might have been missed. Data from observational studies (not meeting our inclusion criteria) for example identified female sex, high acute-phase reactants levels, peripheral arthritis, higher starting GC dosage and fast tapering as possible predictors of PMR relapse and need for prolonged GC treatment [4]. In fact, despite mentioning that the quality of the evidence of the studies was low to moderate, female sex, high acute-phase reactants levels and peripheral arthritis are also reported as prognostic factors in the 2015 EULAR/ACR recommendations for the management of PMR [60]. In addition, a recent meta-analysis showed that female sex and large-vessel involvement are predictors of relapse in GCA [61]. Moreover, due to the inclusion criteria used in the present SLR, specific risk factors for ischaemic neuro-ophthalmic complications were not captured (i.e. jaw claudication, diplopia and temporal artery abnormalities as reported in the British Society of Rheumatology guidelines of GCA management [62], although originating mostly from observational studies).
We did not identify a study testing a T2T strategy in PMR and/or GCA. An obstacle to conduct such a trial might be the absence of internationally recognised remission criteria, which have been defined as the most relevant target for these diseases. Optimally, PMR and GCA trials adopting a T2T approach may be important for individualizing treatment, reducing side effects, and enhancing quality of life. Frequent monitoring aids in early detection of flares, enabling timely intervention to prevent severe exacerbations [10, 11, 13]. Moreover, this approach would foster standardized care, gather valuable research data, and support evidence-based decision-making, ultimately leading to improved long-term outcomes. Furthermore, the utilization of a T2T strategy calls for the introduction of new therapeutic interventions targeting individuals who exhibit limited responsiveness to the initial treatment regimen. We anticipate that this SLR and the T2T recommendations will stimulate further research in this regard [14].
We did not include studies without a control group in our SLR, which might be seen as a limitation; however, our objective was to identify targets and outcomes that might be valuable for a T2T strategy. Studies without an intervention were therefore considered less relevant for our purpose. Titles and abstract screening were performed by different fellows than data extraction and quality assessment; this is certainly not the standard approach and was a consequence of changed duties and personal developments during the COVID-19 crisis. However, every step was conducted under the supervision of the methodologists who guarantee the homogeneity of the different steps of this SLR.
In summary, our SLR synthetized the outcomes and treatment targets used in PMR and GCA RCTs and non-randomised interventional studies. GC cumulative dose and tapering were mostly considered as a target in PMR, while prevention of relapses and achievement of remission were mainly applied in GCA. This SLR informed the international task force developing the T2T recommendations for PMR and GCA.
Supplementary Material
Contributor Information
Elvis Hysa, Laboratory of Experimental Rheumatology and Academic Division of Clinical Rheumatology, Department of Internal Medicine, San Martino Polyclinic, University of Genoa, Genoa, Italy.
Milena Bond, Department of Rheumatology, Hospital of Bruneck (ASAA-SABES), Teaching Hospital of the Paracelsius Medical University, Bruneck, Italy.
Lisa Ehlers, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Dario Camellino, Division of Rheumatology, Local Health Trust 3, Genoa, Italy.
Louise Falzon, Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, England.
Christian Dejaco, Department of Rheumatology, Hospital of Bruneck (ASAA-SABES), Teaching Hospital of the Paracelsius Medical University, Bruneck, Italy; Department of Rheumatology, Medical University Graz, Graz, Austria.
Frank Buttgereit, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Daniel Aletaha, Division of Rheumatology, Department of Medicine III, Medical University of Vienna, Vienna, Austria.
Andreas Kerschbaumer, Division of Rheumatology, Department of Medicine III, Medical University of Vienna, Vienna, Austria.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data used in the preparation of this review are available on request from the corresponding author. E.H., M.B., L.E. and D.C. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contribution statement
E.H. and M.B. wrote the review, performed the data extraction and quality assessment of the included manuscripts; L.E. and D.C. performed the screening of the articles and full-text review; L.F. was involved in the search strategy; C.D. and F.B. conceived and wrote the review protocol; D.A. and A.K. provided the methodological support. All the authors contributed to design and/or interpretation, revised the manuscript for important intellectual content and approved the final version.
Funding
AbbVie provided an unrestricted grant to conduct the research and the task force meetings.
Disclosure statement: L.F.: nothing to disclose. E.H., M.B. and L.E. have received consulting fees from AbbVie. D.C. has received speaker fees from Abiogen, BMS and GSK. C.D. has received consulting/speaker’s fees from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Galapagos, Sparrow and Sanofi, all unrelated to this manuscript. F.B. has received consultancy fees, honoraria and travel expenses from Abbvie, Novartis, Pfizer, Roche and Sanofi, all unrelated to this manuscript. D.A. received grants, speaker fees, and/or consultancy fees from Abbvie, Amgen, Galapagos, Lilly, Janssen, Merck, Novartis, Pfizer, Sandoz and Sanofi. A.K. has received consultancy fees, honoraria and travel expenses from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Gilead, Janssen, Merck Sharp and Dohme, Novartis, UCB and Pfizer, all unrelated to this manuscript.
Acknowledgments
We thank the scientific committee (Eric L Matteson, Maria Cid, Peter Grayson, Andy Abril, Josef Smolen, Christina Duftner, Madeline Whitlock and Lorna Neill) for helping with the formulation of the clinical key questions.
References
- 1. Dejaco C, Brouwer E, Mason JC. et al. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat Rev Rheumatol 2017;13:578–92. [DOI] [PubMed] [Google Scholar]
- 2. Hysa E, Gotelli E, Sammorì S. et al. Immune system activation in polymyalgia rheumatica: which balance between autoinflammation and autoimmunity? A systematic review. Autoimmun Rev 2022;21:102995. [DOI] [PubMed] [Google Scholar]
- 3. Bond M, Tomelleri A, Buttgereit F, Matteson EL, Dejaco C.. Looking ahead: giant-cell arteritis in 10 years time. Ther Adv Musculoskelet Dis 2022;14:1759720x221096366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Floris A, Piga M, Chessa E. et al. Long-term glucocorticoid treatment and high relapse rate remain unresolved issues in the real-life management of polymyalgia rheumatica: a systematic literature review and meta-analysis. Clin Rheumatol 2022;41:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camellino D, Matteson EL, Buttgereit F, Dejaco C.. Monitoring and long-term management of giant cell arteritis and polymyalgia rheumatica. Nat Rev Rheumatol 2020;16:481–95. [DOI] [PubMed] [Google Scholar]
- 6. Aletaha D, Alasti F, Smolen JS.. Optimisation of a treat-to-target approach in rheumatoid arthritis: strategies for the 3-month time point. Ann Rheum Dis 2016;75:1479–85. [DOI] [PubMed] [Google Scholar]
- 7. Dures E, Shepperd S, Mukherjee S. et al. Treat-to-target in PsA: methods and necessity. RMD Open 2020;6:e001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parra Sánchez AR, Voskuyl AE, van Vollenhoven RF.. Treat-to-target in systemic lupus erythematosus: advancing towards its implementation. Nat Rev Rheumatol 2022;18:146–57. [DOI] [PubMed] [Google Scholar]
- 9. Smolen JS, Landewé RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 10. Grigor C, Capell H, Stirling A. et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 11. Verstappen SM, Jacobs JW, van der Veen MJ. et al. ; Utrecht Rheumatoid Arthritis Cohort Study Group. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molto A, López-Medina C, Van den Bosch FE. et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann Rheum Dis 2021;80:1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coates LC, Moverley AR, McParland L. et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dejaco C, Kerschbaumer A, Aletaha D. et al. Treat-to-target recommendations in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis 2023; ard-2022-223429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG.. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012;18:12–8. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 18. Sterne JA, Hernán MA, Reeves BC. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kreiner F, Galbo H.. Effect of etanercept in polymyalgia rheumatica: a randomized controlled trial. Arthritis Res Ther 2010;12:R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marsman DE, den Broeder N, van den Hoogen FHJ, den Broeder AA, van der Maas A.. Efficacy of rituximab in patients with polymyalgia rheumatica: a double-blind, randomised, placebo-controlled, proof-of-concept trial. Lancet Rheumatol 2021;3:e758–e66. [Google Scholar]
- 21. Leeb BF, Bird HA.. A disease activity score for polymyalgia rheumatica. Ann Rheum Dis 2004;63:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krogsgaard MR, Thamsborg G, Lund B.. Changes in bone mass during low dose corticosteroid treatment in patients with polymyalgia rheumatica: a double blind, prospective comparison between prednisolone and deflazacort. Ann Rheum Dis 1996;55:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Munno O, Imbimbo B, Mazzantini M. et al. Deflazacort versus methylprednisolone in polymyalgia rheumatica: clinical equivalence and relative antiinflammatory potency of different treatment regimens. J Rheumatol 1995;22:1492–8. [PubMed] [Google Scholar]
- 24. Dasgupta B, Dolan AL, Panayi GS, Fernandes L.. An initially double-blind controlled 96 week trial of depot methylprednisolone against oral prednisolone in the treatment of polymyalgia rheumatica. Br J Rheumatol 1998;37:189–95. [DOI] [PubMed] [Google Scholar]
- 25. Viapiana O, Gatti D, Troplini S. et al. Prednisone compared to methylprednisolone in the polymyalgia rheumatica treatment. Rheumatol Int 2015;35:735–9. [DOI] [PubMed] [Google Scholar]
- 26. Ferraccioli G, Salaffi F, De Vita S, Casatta L, Bartoli E.. Methotrexate in polymyalgia rheumatica: preliminary results of an open, randomized study. J Rheumatol 1996;23:624–8. [PubMed] [Google Scholar]
- 27. Stone JH, Tuckwell K, Dimonaco S. et al. Trial of tocilizumab in giant-cell arteritis. New Engl J Med 2017;377:317–28. [DOI] [PubMed] [Google Scholar]
- 28. Reichenbach S, Adler S, Bonel H. et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford) 2018;57:982–6. [DOI] [PubMed] [Google Scholar]
- 29. Stone JH, Han J, Aringer M. et al. Long-term effect of tocilizumab in patients with giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatol 2021;3:e328–e36. [DOI] [PubMed] [Google Scholar]
- 30. Stone JH, Spotswood H, Unizony SH. et al. New-onset versus relapsing giant cell arteritis treated with tocilizumab: 3-year results from a randomized controlled trial and extension. Rheumatology 2022;61:2915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strand V, Dimonaco S, Tuckwell K. et al. Health-related quality of life in patients with giant cell arteritis treated with tocilizumab in a phase 3 randomised controlled trial. Arthritis Res Ther 2019;21:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Unizony SH, Bao M, Han J. et al. Treatment failure in giant cell arteritis. Ann Rheum Dis 2021;80:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stone JH, Tuckwell K, Dimonaco S. et al. Glucocorticoid dosages and acute-phase reactant levels at giant cell arteritis flare in a randomized trial of tocilizumab. Arthritis Rheumatol 2019;71:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spiera R, Unizony SH, Bao M. et al. Tocilizumab vs placebo for the treatment of giant cell arteritis with polymyalgia rheumatica symptoms, cranial symptoms or both in a randomized trial. Semin Arthritis Rheum 2021;51:469–76. [DOI] [PubMed] [Google Scholar]
- 35. Mohan S, Han J, Stone JH.. FRI0220 efficacy of adjunctive methotrexate in patients with giant cell arteritis treated with tocilizumab plus prednisone tapering: subanalysis of the giacta trial. Ann Rheum Dis 2020;79:693.1– [Google Scholar]
- 36. Cid MC, Unizony SH, Blockmans D. et al. ; KPL-301-C001 Investigators. Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2022;81:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spiera RF, Mitnick HJ, Kupersmith M. et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001;19:495–501. [PubMed] [Google Scholar]
- 38. Unizony SH, Bao M, Han J. et al. Treatment failure in giant cell arteritis. Ann Rheum Dis 2021;80:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoffman GS, Cid MC, Hellmann DB. et al. ; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309–18. [DOI] [PubMed] [Google Scholar]
- 40. Kupersmith MJ, Langer R, Mitnick H. et al. Visual performance in giant cell arteritis (temporal arteritis) after 1 year of therapy. Br J Ophthalmol 1999;83:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nordborg E, Schaufelberger C, Andersson R, Bosaeus I, Bengtsson BA.. The ineffectiveness of cyclical oral clodronate on bone mineral density in glucocorticoid-treated patients with giant-cell arteritis. J Intern Med 1997;242:367–71. [DOI] [PubMed] [Google Scholar]
- 42. Mazlumzadeh M, Hunder GG, Easley KA. et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006;54:3310–8. [DOI] [PubMed] [Google Scholar]
- 43. Hunder GG, Sheps SG, Allen GL, Joyce JW.. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975;82:613–8. [DOI] [PubMed] [Google Scholar]
- 44. Liozon F, Vidal E, Barrier J.. Does dapsone have a role in the treatment of temporal arteritis with regard to efficacy and toxicity? Clin Exp Rheumatol 1993;11:694–5. [PubMed] [Google Scholar]
- 45. Schmidt WA, Dasgupta B, Luqmani R. et al. A multicentre, randomised, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of sirukumab in the treatment of giant cell arteritis. Rheumatol Ther 2020;7:793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Veen MJ, Dinant HJ, van Booma-Frankfort C, van Albada-Kuipers GA, Bijlsma JW.. Can methotrexate be used as a steroid sparing agent in the treatment of polymyalgia rheumatica and giant cell arteritis? Ann Rheum Dis 1996;55:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Silva M, Hazleman BL.. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis 1986;45:136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Labarca C, Koster MJ, Crowson CS. et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016;55:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sailler L, Lapeyire-Mestre M, Geffray L. et al. Adding hydroxychloroquine to prednisone does not improve the outcome in giant cell arteritis. A double blind randomized controlled trial. In: ACR/ARHP Scientific Meeting. 2009.
- 50. Calderón-Goercke M, Loricera J, Aldasoro V. et al. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin Arthritis Rheum 2019;49:126–35. [DOI] [PubMed] [Google Scholar]
- 51. Hocevar A, Rotar Z, Jese R. et al. Do early diagnosis and glucocorticoid treatment decrease the risk of permanent visual loss and early relapses in giant cell arteritis: a prospective longitudinal study. Medicine (Baltimore) 2016;95:e3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lundberg IE, Sharma A, Turesson C, Mohammad AJ.. An update on polymyalgia rheumatica. J Intern Med 2022;292:717–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hysa E, Ghorbannia A, Emamifar A, Milchert M, Manzo C.. Feasibility and usefulness of a fast-track clinic for patients suspected of polymyalgia rheumatica: notes for a work schedule through a narrative review of published literature. Reumatologia 2021;59:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Craig G, Knapp K, Salim B, Mohan SV, Michalska M.. Treatment patterns, disease burden, and outcomes in patients with giant cell arteritis and polymyalgia rheumatica: a real-world, electronic health record-based study of patients in clinical practice. Rheumatology 2021;8:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sugihara T, Hasegawa H, Uchida HA. et al. ; Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS). Associated factors of poor treatment outcomes in patients with giant cell arteritis: clinical implication of large vessel lesions. Arthritis Res Ther 2020;22:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mackie SL, Pease CT, Fukuba E. et al. Whole-body MRI of patients with polymyalgia rheumatica identifies a distinct subset with complete patient-reported response to glucocorticoids. Ann Rheum Dis 2015;74:2188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giraud N, Prati C, Wendling D, Verhoeven F.. Prognostic value of 18F-fluorodeoxyglucose PET-CT score at baseline on the therapeutic response to prednisone in patients with polymyalgia rheumatica. Joint Bone Spine 2021;88:105093. [DOI] [PubMed] [Google Scholar]
- 58. Charpentier A, Verhoeven F, Sondag M. et al. Therapeutic response to prednisone in relation to age in polymyalgia rheumatica: a comparison study. Clin Rheumatol 2018;37:819–23. [DOI] [PubMed] [Google Scholar]
- 59. Cimmino MA, Parodi M, Montecucco C, Caporali R.. The correct prednisone starting dose in polymyalgia rheumatica is related to body weight but not to disease severity. BMC Musculoskelet Disord 2011;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dejaco C, Singh YP, Perel P. et al. ; American College of Rheumatology. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015;74:1799–807. [DOI] [PubMed] [Google Scholar]
- 61. Moreel L, Betrains A, Molenberghs G, Vanderschueren S, Blockmans D.. Epidemiology and predictors of relapse in giant cell arteritis: a systematic review and meta-analysis. Joint Bone Spine 2023;90:105494. [DOI] [PubMed] [Google Scholar]
- 62. Dasgupta B, Borg FA, Hassan N. et al. ; BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010;49:1594–7. [DOI] [PubMed] [Google Scholar]
- 63. Caporali R, Cimmino MA, Ferraccioli G. et al. ; Systemic Vasculitis Study Group of the Italian Society for Rheumatology. Prednisone plus methotrexate for polymyalgia rheumatica: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2004;141:493–500. [DOI] [PubMed] [Google Scholar]
- 64. Salvarani C, Macchioni P, Manzini C. et al. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med 2007;146:631–9. [DOI] [PubMed] [Google Scholar]
- 65. Cutolo M, Hopp M, Liebscher S, Dasgupta B, Buttgereit F.. Modified-release prednisone for polymyalgia rheumatica: a multicentre, randomised, active-controlled, double-blind, parallel-group study. RMD Open 2017;3:e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bonelli M, Radner H, Kerschbaumer A. et al. Tocilizumab in patients with new onset polymyalgia rheumatica (PMR-SPARE): a phase 2/3 randomised controlled trial. Ann Rheum Dis 2022;81:838–44. [DOI] [PubMed] [Google Scholar]
- 67. Jover JA, Hernández-García C, Morado IC. et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;134:106–14. [DOI] [PubMed] [Google Scholar]
- 68. Schaufelberger C, Möllby H, Uddhammar A, Bratt J, Nordborg E.. No additional steroid-sparing effect of cyclosporine A in giant cell arteritis. Scand J Rheumatol 2006;35:327–9. [DOI] [PubMed] [Google Scholar]
- 69. Hoffman GS, Cid MC, Rendt-Zagar KE. et al. ; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007;146:621–30. [DOI] [PubMed] [Google Scholar]
- 70. Seror R, Baron G, Hachulla E. et al. Adalimumab for steroid sparing in patients with giant-cell arteritis: results of a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:2074–81. [DOI] [PubMed] [Google Scholar]
- 71. Villiger PM, Adler S, Kuchen S. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7. [DOI] [PubMed] [Google Scholar]
- 72. Langford CA, Cuthbertson D, Ytterberg SR. et al. ; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017;69:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Venhoff N, Schmidt WA, Bergner R. et al. OP0182 secukinumab in giant cell arteritis: the randomised, parallel-group, double-blind, placebo-controlled, multicentre phase 2 titain trial. Ann Rheum Dis 2022;81:121–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the preparation of this review are available on request from the corresponding author. E.H., M.B., L.E. and D.C. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.