Abstract
While the aetiology of inflammatory bowel disease (IBD) has been linked to genetic susceptibility coupled with environmental factors, the underlying molecular mechanisms remain unclear. Among the environmental factors, diet and the gut microbiota have been implicated as drivers of immune dysregulation in IBD. Indeed, epidemiologic studies have highlighted that the increase in incidence of IBD parallels the increase in dietary intake of omega-6 (n-6) polyunsaturated fatty acids (PUFAs) and the change in balance of intake of n-6 to n-3 fatty acids. Experimental evidence suggests that the increase in n-6 PUFA intake increases cell membrane arachidonic acid, which is accompanied by the production of pro-inflammatory mediators as well as increased oxidative stress; together, this contributes to the development of chronic inflammation. However, it is also increasingly clear that some of the n-6 PUFA-derived mediators exert beneficial effects depending on the settings and timing of ingestion. In contrast to n-6, when n-3 PUFA eicosapentaenoic acid and docosahexaenoic acid are incorporated into the cell membrane and are metabolized into less pro-inflammatory eicosanoids, as well as strong specialized pro-resolving mediators, which play a role in inflammation cessation. With a focus on preclinical models, we explore the relationship between dietary lipid, the gut microbiome, and intestinal inflammation.
Keywords: diet, fatty acids, n-3, n-6 fatty acids, IBD
Introduction
During the latter part of the 20th century, industrialized nations experienced a rapid rise in inflammatory bowel disease (IBD) incidence,1 which is now being followed by a rising incidence in developing countries. IBD is a multi-factorial disease linked to genetic susceptibility coupled with environmental factors. Since it is unlikely that the prevalence of genetic susceptibilities has increased, changes in environmental factors have been identified as key determinants, with diet and its impacts on intestinal microbiome ecology playing a key role. Diets high in refined sugars, animal fat, and complex carbohydrates have been associated with higher rates of IBD, whereas diets rich in omega-3 (n-3) fatty acids, vegetables, fruits, and fibre appear to protect against the development of IBD.2,3
Relationship between dietary fatty acid intake and IBD
The relationship between fatty acid intake and IBD in humans has not yet been well demonstrated in the literature; however, there has been extensive research on this relationship using animal models. Animal studies have demonstrated that fat, either alone or in combination with sugar, contributes to ileal inflammation, intestinal dysbiosis, altered barrier integrity, and a pro-inflammatory environment.4–6 Despite this, longitudinal cohort studies in humans have failed to show associations between total or specific fatty acids and Crohn’s disease (CD), except for one study reporting that docosahexaenoic acid (DHA, 22:6n-3) was protective against CD.7 In ulcerative colitis (UC), omega-6 (n-6), polyunsaturated fatty acids (PUFA), linoleic acid (LA, 18:2n-6), and arachidonic acid (ARA, 20:4n-6) have been associated with increased risk of UC, while the n-3 fatty acid docosahexaenoic acid (DHA, 22:6n-3) and monounsaturated omega-9 fatty acid, oleic acid (18:1n-9), has been associated with decreased risk.8,9 A recent systematic review and meta-analysis of six studies (563 CD, 260 UC) reported no association between fish consumption and overall risk of IBD;10 however, when stratified by IBD type and using a random-effects model, there was a significant inverse association between fish consumption and risk of CD (0.54; 95% CI 0.31–0.96). A two-sample Mendelian randomization study on the effects of fatty acids on IBD also reported a protective effect of n-3 PUFA against UC.11 These human studies emphasize the need for further evaluation of the potential benefits of n-3 PUFA and potential deleterious effects of n-6 fatty acids on IBD development.
In more recent studies, evidence of the benefits of the Mediterranean diet (MD) (high in monounsaturated fatty acids, 18:1n-9, and n-3 fatty acids) in potentially reducing the risk of IBD development was reported through the Genetic, Environmental, Microbial (GEM) Project.12 The study evaluated a cohort of 2289 healthy FDRs of patients with CD and reported that individuals consuming a diet resembling the MD had an increased abundance of fibre-degrading bacteria, such as Ruminococcus, as well as taxa such as Faecalibacterium and a significantly lower levels of subclinical gut inflammation (defined by faecal calprotectin), compared with other dietary patterns.12 Furthermore, a prospective study of two adult Swedish cohorts reported that greater adherence to an MD was associated with a significant lower risk of developing CD.13 Here, we will review the evidence and discuss the proposed mechanisms for the role of fatty acids in IBD.
Change in dietary fatty acid consumption and metabolic consequences
The dramatic changes in dietary fat content and composition during the 20th century have been linked to the rising incidence of IBD in Western countries. The association between plasma LDL cholesterol and atherosclerotic heart disease led to the replacement of dietary sources of saturated animal fats from around 18%–20% to 11%–12% dietary energy with vegetable oils and margarines high in n-6 PUFA, with an increase in LA from around 3% to 5%–7% of dietary energy.14–17 Currently, n-6 LA intake in the typical Western diet is about 5–15 times higher than n-3 α-linolenic acid (ALA; 18:3n-3) leading to LA as the dominant substrate.14,18 The increase in LA intake also leads to an increase in LA in breast milk, leading to an increase in milk LA to ALA ratio from about 4:1 to 10:1 (14) which in turn has influenced PUFA transfer to the newborn14,19,20). Similarly, infant formulas have had a high level of LA, providing 8%–10% dietary energy, and an LA to ALA ratio of about 8–10:1.21,22
LA and ALA are regarded as essential fatty acids and are not synthesized in animals and humans. As LA and ALA are synthesized in plants, they are mainly found in high amounts in foods of plant origin: LA rich in safflower, sunflower, soybean, corn oils (COs), and pumpkin seeds, and ALA-rich in canola, soybean, flax seeds and flaxseed oil, pumpkin seeds, and walnuts.18,23 The metabolic conversion of LA to ARA and of ALA to eicosapentaenoic acid (EPA, 20:5n-3) share the same enzymes (Figure 1A), with the result that the rate of synthesis of ARA and EPA and subsequently docosahexaenoic acid (DHA, 22:6n-3) is dependent upon the relative availability of the substrate.23 Early experimental studies have shown that increasing LA intake, over ALA, reduces tissue EPA and DHA14 and decreases downstream tissue n-3 fatty acid metabolites and regulatory molecules.18 EPA and DHA are essential for normal physiologic functions, and it has been demonstrated that the rate of biosynthesis of EPA and DHA from ALA is low and insufficient to meet the physiological demands, and therefore requires additional dietary intake of EPA and DHA.24,25 EPA and DHA in human diets are found mainly in marine animals and plant plankton and have either remained unchanged or decreased over time. The reliance on marine oil supplements has also increased significantly.26 Notably, n-3 fatty acids are chemically unstable and rapidly oxidize during storage to lipid peroxides and secondary oxidation products (potentially pro-inflammatory) resulting in reduced concentrations of unoxidized EPA and DHA.27 Moreover, the duration of storage, heat (commonly kept at room temperature), and light exposure also impact lipid peroxidation and lower EPA and DHA concentrations. Consequently, a significant number of Westernized societies and adults in North America, in particular, are not meeting the recommendations for n-3 fatty acid intake and are generally deficient in EPA and DHA.28
Figure 1.
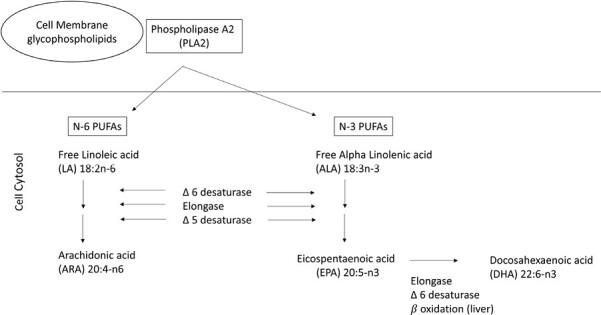
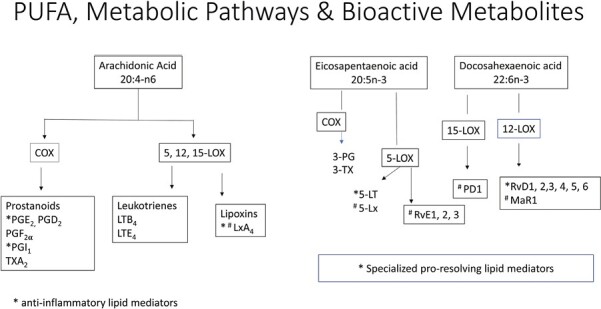
(A) Pathways of conversion of essential fatty acids linoleic acid (LA) and alpha-linolenic acid to their respective longer chain and more unsaturated derivatives. (B) Pathway of metabolism of arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) via the actions of cyclooxygenase (COX) and lipoxygenase (LOX) pathways to their respective bioactive metabolites. ARA metabolism leads to the formation of prostaglandins (PGs) and leukotrienes (LTs) generally exhibiting a pro-inflammatory role, although resolution of inflammation is also regulated via PGE2, prostacyclin (PGI2) and Lipoxins (Lx), with LxA4 considered a potent pro-resolving mediator. EPA and DHA and their downstream metabolites are generally considered to have lower pro-inflammatory potential and include 5-series LTs and specialized pro-resolving mediators (SPM), which play a role in inflammation cessation including 5-series lipoxins (5-Lx), E and D resolvins (RvE and RvD), protectins (PD1), and maresins (macrophage mediator in resolving inflammation, MaR1).
Overall, these dietary changes associated with the marked increase in the balance of n-6 to n-3 fatty acid in the Western diet has had important consequences on plasma membrane phospholipids (PLs) fatty acid composition; reflective of the abundance of ARA, and decreased presence of EPA and DHA, which has also resulted in alterations in inflammatory metabolites towards a pro-inflammatory environment.15,29
Bioactive lipids and mediators of inflammation
ARA is an integral constituent of cell membranes conferring it with fluidity and flexibility necessary for normal physiological function of all cells. Free ARA (released from cell membrane phospholipid) modulates the function of ion channels, several receptors, and enzymes. Despite these beneficial properties, free ARA levels are kept at very low levels in cells because uncontrolled accumulation is linked to inflammation, and can impair cell survival via induction of apoptosis.30,31 ARA is metabolized via the cyclooxygenase (COX) pathways to form prostaglandins (PGs) and thromboxanes (TXs) termed prostanoids, the lipoxygenase (LOX) pathways to form leukotrienes (LTs) and lipoxins (LXs) (Figure 1B), and the cytochrome P450 pathways to form various epoxy, hydroxy, and dihydroxy derivatives.32 While COX-1 is constitutively expressed in almost all tissue types, COX-2 is inducible by bacterial products such as lipopolysaccharide (LPS) and toll-like receptor-4 (TLR-4) activation and inflammatory cytokines.32–34 ARA has been reported to be over 10 times more effective than EPA as a substrate for COX-1 and 3.3 times more effective as a substrate for COX-2.35 In humans, 5-LOX is expressed predominantly in cells of myeloid origin including neutrophils, eosinophils, monocytes, macrophages, mast cells and dendritic cells36 and 12-LOX in platelets, and 15-LOX in endothelial cells.
Experimental and clinical evidence link ARA to inflammation through the metabolism of pro-inflammatory eicosanoids (Figure 1B), including PGE2, TxA2, and LTB4. Notably, the profile of eicosanoid production is defined by the type and duration of the inflammatory stimulus, the nature of cells and differential expression of enzymes within cells present at the sites of inflammation: mast cells predominantly generate PGD2, monocytes, and macrophages produce PGE2, TXA2, and prostacyclin (PGI2), activated platelets produce TXA237,38 and dendritic cells produce PGE2 and LTB4.39 LTB4 produced primarily by pro-inflammatory M1 macrophages is a potent activator and chemoattractant of polymorphonuclear leukocytes (PMNs), such as neutrophils and macrophages, and is important in the early phases of infection and inflammation.40 LTB4 enhances the production of reactive oxygen species and NF-κB-mediated activation of pro-inflammatory cytokines.32 Paradoxically, LTB4 plays a critical role in bacterial clearance but also contributes to long-term inflammatory disorders. In addition, the pro-inflammatory eicosanoids (PGE2, TxA2, and LTB4) promote IL-23 and inhibits IL-12 and IL-27 with a shift in Th1/Th17 balance.41–43 Furthermore, IL-17 synergizes with LPS to induce cyclooxygenase (COX) 2 expression in colonic sub-epithelial myofibroblasts34 maintaining a pro-inflammatory environment. However, there is also increasing evidence that despite the pro-inflammatory actions of ARA-derived eicosanoids, resolution of inflammation is also regulated via PGE2, PGI2, and Lx, with LxA4 considered a potent pro-resolving mediator.44,45 Notably, PGE2 limits inflammation by controlling cytokine levels (TNF-α, IL-1β, and IL-6),46 augmenting IL-10 secretion,47 suppressing 5-LOX and inhibiting synthesis of LTs and inducing 15-LOX to promote the synthesis of LXs.48,49 However, PGE2 has been reported to decrease the phagocytic activity of neutrophils, stimulate gastrointestinal mucus secretion,50 and ameliorate DSS-induced colitis in mice by increasing T reg cells.51 These unique functions of PGE2 emphasize its importance during the inflammatory process and in the resolution of inflammation. Similarly, PGI2 has an important role in maintaining gastrointestinal integrity by maintaining epithelial barrier intergrity, increasing mucosal blood flow, and stimulating mucus secretion.50 Indeed, treatment with PGI2 in DSS-induced colitis was shown to attenuate the disruption in epithelial barrier integrity by decreasing epithelial cell apoptosis, attenuating barrier disruption and ameliorating the colitis.52 Furthermore, the anti-inflammatory and pro-resolving actions of LXs have been reported to exert their effects via multiple pathways: by limiting PMN recruitment to the site of inflammation by targeting endothelial adhesion; by enhancing monocyte recruitment and M2-resolving macrophage phenotypes (express Lxs); by increasing neutrophil apoptosis; and by increasing macrophage efferocytosis of apoptotic neutrophils.53 However, the beneficial effects of LXs are limited by their rapid metabolic inactivation and degradation. LxA4 has also been reported to inhibit the production of PGE2 and LTs and suppress cytokine production.54 The various epoxy, hydroxy, and dihydroxy derivatives are also involved in inflammation with various actions. Their pro-inflammatory effects include 20 hydroxyeicosatetraenoic acid (20-HETE) acting to increase reactive oxygen species, expression of cellular adhesion molecules,55 and pro-inflammatory cytokines. Whereas, eicosatetraenoic acids (EETs) are generally anti-inflammatory acting to inhibit inflammatory cytokine production and leucocyte-endothelial adhesion by mechanisms involving inhibition of transcription factor NK-κβ.56 Indeed the timing and elaboration of the appropriate mediators with a switching from a predominantly pro to anti-inflammatory environment is essential for the resolution of the inflammatory process.
The preventative and therapeutic benefits of n-3 fatty acids are believed to involve both the reduction in cell membrane phospholipid ARA and partial replacement of ARA with EPA and DHA.57,58 EPA and DHA and their downstream metabolites (Figure 1B) are generally considered to have lower pro-inflammatory potential29,45 via multiple mechanisms: through activation of G-protein coupled receptors (GPR), activation of peroxisome proliferator-activated receptor-γ (PPAR-γ); decreasing activation of NF-KB signal transduction pathways;59 and production of various pro-inflammatory cytokines, chemokines, adhesion molecules; and reducing oxidative stress pathways. Notably, 15-deoxy-PGJ2, a metabolite of PGD2 also binds to PPAR-γ and inhibits NF-κB signalling and inflammasome activation to exert its immunoregulatory effects.60,61 Some of the additional benefits include the induction of Treg cells with an increase in IL-10 production, preventing overdevelopment of a Th17 response, a shift in macrophages towards an M2 phenotype to promote healing and enhancing epithelial barrier integrity.45,58,59,62–64
Further evidence has emerged that EPA and DHA can be metabolized via the LOX pathway to bioactive specialized pro-resolving mediators (SPMs, Figure 1B) including EPA derived 3 series resolvins (RVe1-3) and lipoxins and DHA-derived D-series resolvins (RvD: RvD1-6), macrophage mediator in resolving inflammation (maresins, MaR: Mar1-2), and protectins (PD: PD1, PDX).65–68 These bioactive lipids display potencies in the nanomolar range and signal through G-protein coupled receptors (GPR) with many of the anti-inflammatory benefits described above attributed to these bioactive lipids. The SPMs limit and/or terminate neutrophil recruitment, induce neutrophil apoptosis, shift macrophages towards an M2 phenotype,69 and increase IL-10.66,70,71 Other more recently described benefits include an increase in generation of Treg cells and preventing naïve CD4+ T cell differentiation into Th1 and Th17 cells72 and an increase in generation of type 2 innate lymphoid cells.73
PUFA exposure in preclinical models of IBD
The variable age of onset of IBD and duration of subclinical disease makes it exceedingly difficult to prove a causal relationship with diet. Moreover, the timing of dietary exposure is likely exceedingly important and probably precedes the onset of clinical disease possibly by many years. Collectively, experimental studies of chemically induced colitis or ileitis in adult rats and mice (Supplementary Tables 1A–C) induced by trinitrobenzene sulfonic acid (TNBS) or dinitronbenzene sulfonic acid (DNBS) (Supplementary Table 1A),74–81 dextran sodium sulfate (DSS) (Supplementary Table 1B)82–86, or acetic acid (87 and in infectious colitis with Citrobacter rodentium in mice (Supplementary Table 1C)88,89 show beneficial effects of n-3 PUFAs, most often given as fish oil, and given prior to the inflammatory insult. Diets high in n-3 PUFA have also been reported to decrease spontaneous colitis in IL-10 knockout mice90 and spontaneous ileitis in senescence-accelerated mice (SAM)P1/Yit mice.91 In addition, in transgenic fat-1 mice that endogenously produce n-3 PUFAs, protection against DSS-induced colitis has been reported.92,93
In chemically induced colitis and ileitis studies and C. rodentium infection, various sources of n-3 fatty acids were utilized, including fish oil, krill oil, cod liver oil, ALA-rich camelina oil, and omegaven (Supplementary Table 1A and C). The source of n-6 fatty acids was mostly soybean oil (54% LA, 23% oleic acid, 8% ALA) but also included CO and safflower oil. In these studies, percent dietary calories from fat was quite variable ranging from 8% to 20% as was n3:n6:n-9 fatty acid ratios (Table 1). All lipid diets but one were commenced prior to induction of intestinal inflammation with the timing ranging from -47 days to -7 days until the end of the experiment, which was also quite variable ranging from post-induction day 2 to 50 with various time points in between. The beneficial effects of the diets high in n-3 fatty acids in the various studies (Supplementary Tables 1A–C) included an attenuation of inflammation, mediated by a decrease in COX-2 expression, an induction of PPAR-γ expression and a reduction in NF-κB activation. There were resultant decrease in oxidative stress (decrease in colonic inducible nitric oxide, an increase in colonic glutathione levels, a decrease in urinary 8-isoprostane levels), and a decrease in colonic ARA-derived eicosanoids (PGE2 and LTB4), pro-inflammatory cytokines (TNF-α, INFγ, IL1β, IL-6, IL-8, IL17A, IL-23), chemokines (MCP1, MIP2, KC), and endothelial VCAM-1 and VEGFR2 expressions. Moreover, the high n-3 fatty acid diets were associated with an increase in anti-inflammatory cytokines (IL-10, and IL-22), an attenuation in intestinal epithelial barrier disruption, and an increase in goblet cells with mature mucin granules and MUC2 expression. Additionally, the high n-3 fatty acid diet prevented C. rodentium translocation.88 One study used a dual therapeutic approach combining fish oil (Omegaven) with a 5-ASA compound (75 mg/kg) and reported that the dual combination was more effective at reducing NF-κB activation and inducing PPARγ expression but added no benefit on ARA-derived eicosanoids or on TNF-α and IL-1β production.80 Similarly, in transgenic fat-1 mice protection against DDS colitis was associated with a reduction in COX-2 and PGE2 expression (92 70) and reduced expression of IL-17F, IL-21, and CCR6, whereas IL-27 was increased suppressing Th17 responses.93 In contrast, high dietary intake of LA prior to and during induction of colitis, enriched colon PLs with LA and ARA, and exacerbated host mucosal Th17 response with increased tissue damage.
Table 1.
TNBS and DSS colitis and dietary ratios of n3, n6, and n-9. Soybean oil contains approximately 54% LA (18:2n-6), 23% oleic acid (18:1n-9), 8% ALA (18:3n-3), and 6% saturated fat. Sunflower oil contains approximately 85% unsaturated fatty acids consisting of 44%–75% LA, 14%–43% oleic acid, and 15% saturated fat. Sunflower oil contains approximately 60% LA, 25% oleic acid, 15% saturated fat, and minimal ALA. Olive oil contains approximately 55%–83% oleic acid, 2.5%–21% LA, 0.0%–1.5% ALA, and 7.5%–20% saturated fat.
| Dietary fatty acids | Colitis model | n-3:n-6:n-9 | Ref |
|---|---|---|---|
| Sunflower—high n-6 fatty acid diet Cod liver oil—high n-3 fatty acid diet |
TNBS | 1:66:32 1:1:1.4 |
52 |
| Corn oil high n-6 fatty acid diet ALA-rich camelina oil—high n-3 fatty acid diet |
TNBS | 1:62:33 1:1:1.2 |
55 |
| Soybean oil (intralipid) —high n-6 fatty acid diet FO (omegaven)—high n-3 fatty acid diet |
TNBS | DHA/EPA: 1:1 1:0.15:0.3 |
56 |
| CTRL well-balanced diet High n-3 diet High n-6 diet (comparable to Western diet) High n-9 diet (comparable Mediterranean diet) |
TNBS | 1:4:16 1:1:4 1:16:16 1:4:24 |
59 |
| Soybean oil (SO) high n-6 fatty acid diet Virgin olive oil- FO (fat 96% virgin OO and 4% refined FO) |
DSS | 1:15.7:5.9 1:15:146 1:4.4:39 |
60 |
| SO (soybean oil) FO (fat 96% virgin OO and 4% refined FO) FO + oral flavonoid quercitrin (QR)* |
DSS | 1:16:6 1:4.4:39 1:4.4:39 |
61 |
| SO (soybean oil) FO (100% fish oil) SF (50% soybean oil and 50% FO) |
DSS | 1:10 1:6 1:2 |
62 |
| Low-dose Krill oil (L-KO)—0.25 g/kg KO High-dose Krill (H-KO)—0.5 g/kg KO |
DSS | 1:0.8:4.79 EPA:DHA 2:1 |
64 |
Abbreviations: ALA, α-linolenic acid; DSS, dextran sulfate sodium; LA, linoleic acid; TNBS, trinitrobenzene sulfonic acids.
A more recent study by Charpentier et al.81 (Table 1, Supplementary Table 1a) included: a control group with a “well balanced” diet, a high n-3 diet, based on a Japanese clinical trial for IBD patients94; a high n-6 diet, based on a Western diet (a typical western diet has an n-3:n-6 ratio of 1:15) and a n-9 enriched diet based on the MD95 reinforced the benefits of the high n-3 diet as described. In addition, the n-9 diet was also associated with a reduction in colon IL-6 production and an increase in MUC2 expression. Unfortunately, this study only continued the diet for 2 days after the instillation of TNBS, thus missing out on evaluating the longer-term benefits and comparisons between the high n-3 and high n-9 diets.
Despite the positive anti-inflammatory effects of n-3 fatty acid diets, some studies have drawn correlation to some negative systemic effects of a diet high in n-3 fatty acids (Supplementary Table 1C)).88 An infectious colitis study reported increased mortality in the mice fed the high n-3 diet (not observed in the other studies), which the authors linked to the suboptimal induction of intestinal IFN-γ, TNF-α, IL-17A, IL-22, IL-23, and Relmβ compared to pre-infection expression. Moreover, they reported a reduction in expression of intestinal alkaline phosphatase (IAP) in infected mice (also reported in other studies, Supplementary Table 1A and B), a key endogenous mucosal defense factor inducible by microbiota96 that dephosphorylates LPS during infection and prevent sepsis.97,98 Furthermore, IL-15, a cytokine shown to induce sepsis, was increased as was serum lipopolysaccharide-binding protein (LBP) levels, a clinical biomarker of sepsis in the n-3 PUFA treated and infected mice,.99 Indeed, adverse outcomes with n-3 PUFA intake have also been observed in several murine models exposed to viral, bacterial, and fungal pathogens associated with impaired pathogen clearance and increased mortality.100
Timing of PUFA exposure and bowel inflammation
Epidemiological studies also suggest that the early dietary experience wherein nutrient deficiencies or imbalances occur has the potential to alter the normal developmental trajectory and lead to long-lasting effects on cell function and disease susceptibility. Indeed, in our experimental model, maternal dietary fat intake during gestation and lactation influenced the fatty acid composition of milk and was associated with altered colonic membrane fatty acids in the newborn and nursing rat.101,102 In 15-day old suckling pups examined 12 h post-instillation of DNBS, pups in the cohort of high maternal n-6 intake developed a severe early colitis.101 This was in contrast to the high n-3 diet, which abrogated the inflammatory response. On the other hand, the maternal diet high in oleic acid, resulted in an inflammatory response between that of the other two diet groups. In this study, we reported an absence in the differences in colonic phospholipid ARA between pups in the high n-6 and n-3 groups suggesting that the enhanced susceptibility to the colitis may have involved the significant increase in colonic LA or reduced levels of long-chain n-3 fatty acids. However, despite the short-term benefit of the n-3 fatty acids in this model we also observed that the maternal diet high in n-3 fatty acids with the induced changes in colonic membrane n-6 and n-3 fatty acid content had a negative effect on colonic barrier integrity (shorter crypts, increased intestinal permeability) in 15-day-old rat pups.102 Additionally, the altered intestinal development noted in 15-day-old rat pups persisted post weaning, priming the offspring to an exaggerated inflammatory response at 3 months of age to DNBS-induced colitis in the absence of long-lasting effects on colonic lipids.102 These observations lend support to the double hit theory wherein changes in colonic membrane n-6 and n-3 fatty acids in early life alter the intestinal developmental trajectory, predisposing, or priming the host to an exaggerated immunological response to an inflammatory insult in later life.
PUFA and the microbiome
The interplay between gut bacteria and inflammation continues to be explored, and dietary intake plays a critical role in the microbiome. Animal models have demonstrated a correlation between microbiome and dietary PUFA intake. In the study by Gosh et al.88 as discussed previously, mice fed a high n-3 diet had an attenuated colitis, which was associated with an increase in benificial microbes, including Bifidobacteria spp., Lactobacillus spp., and Clostridia coccoides; this was in contrast to those fed a high n-6 PUFA diet (CO), who had increased potential pathobionts including Enterobacteriaceae,103,104 segmented filamentous bacteria (SFB) and Clostridia spp. Notably in the maternal-offspring diet study, 15-day-old pups in both the high maternal n-3 and n-6 groups had a decrease in overall bacterial density, a decreased ratio of Firmicutes to Bacteroidetes, and a decrease in several dominant microbes, compared to a standard chow-fed group (n-3:n-6 ratio 1:8).105 Offspring in the n-3 dietary group had enriched populations of bacteria from the phyla Bacteroidetes, while n-6 groups had enriched populations from the phyla Firmicutes; in this study, both groups had a decrease in Enterobacteriaceae, Bifidobacteria and Lactobacillus spp., C. coccoides, Bacillus spp., and SFB. In addition, pups in the n-3 group were noted to harbour intestinal pathobionts including Bilophila wadsworthia, Enterococcus faecium, and Bacteroides fragilis. Offspring in both n-3 and n-6 diet groups had delayed development of T cells in colonic tissues (reduction of CD3+, CD4+, and CD4+FOXp3+ T cells). In contrast, only the n-3 diet group had reduced colonic CD8+ T cells, likely leading to an imbalance in host defensive cells and contributing to the blooms of opportunist pathogens and reduced M2 macrophages resulting in a skewed M1 to M2 ratio, important for resolution of acute inflammation. Taken together these data suggest that the balance of n-6 to n-3 fatty acids in gestation and lactation is likely different to what is required in later life with further studies needed to evaluate these observations.
Mediterranean diet
To date, much of the dietary research in IBD has focused on the potential negative impact of high fat intake8,106; however, one diet higher in fat intake than is recommended in the national guidelines, is the Mediterranean diet (MD) (40% by energy derived from fat). The MD is characterized by a high intake of plant-based foods (vegetables, fruits, breads, and cereals (traditionally minimally refined), nuts, legumes, and seeds); minimally processed; seasonally fresh and locally grown foods; a high intake of unsaturated fat such as olive oil (especially virgin and extra-virgin olive oil); a medium intake of fish, dairy products, and a low consumption of saturated fat, meat, and sweets.107,108 This diet has been associated with beneficial effects in IBD.109,110
Haskey et al. recently reinforced the benefits of the MD.111 In this study, 40.8% of total calories were derived from fat; the MD fat blend (high mono unsaturated fatty acids (MUFA), n-3:n-6 1:2) was compared to CO (n-6 PUFA), olive oil, (OO high MUFA), and anhydrous milk fat (MF, high saturated FA) on spontaneous colitis development in Muc2−/− mice. Overall, the MD was most effective at attenuating the colitis, followed by the OO diet, and in contrast to the CO and MF diets that were associated with severe colitis. The MD also induced tolerogenic CD103+ CD11b+ dendritic, Th22 and IL-17+ IL-22+ cells necessary for intestinal barrier repair. The MD and MF diets showed increased IAP activity, suggesting that saturated fat might play a key role in IAP function. Moreover, the MD was associated with beneficial microbes including Lactobacillus animalis, and was associated with higher acetic acid levels, which negatively correlated with colitogenic microbes like Akkermansia muciniphila. In contrast, CO showed a higher prevalence of mucin-degraders including A. muciniphila and potential pathobionts Enterobacteriaceae. Notably, SFA was needed in some amounts in the MD with several beneficial responses reported including epithelial barrier function and insulin tolerance. Indeed, these findings support the concept that not a single type of fat is important in its ability to completely protect against colitis and that different fat diets correspond to unique immunological responses, though this requires further evaluation. Hasket et al. also recently reported the benefit of the Mediterranean dietary pattern (MDP) in a 12-week randomized controlled trial in adult patients with mild-moderate UC in remission compared to the Canadian Habitual Diet Pattern (CHD).112 The MDP was associated with improved bowel symptoms (gas and tenesmus), maintenance of remission in the majority of patients, (defined as faecal calprotectin levels <100 μg/g; 87% MDP vs. 25% CHD), a significant increase in faecal secretory IgA (sIgA) and higher levels of total faecal SCFAs (including acetic acid and butyric acid). Several other studies in both paediatric and adult IBD patients have shown benefits.110 In another recent study in adult patients with CD with mild to moderate symptoms, the MD was reported to be similarly effective at achieving symptomatic remission at 6 and 12 weeks compared to the specific carbohydrate diet.113 The findings together with the relative ease of implementation of the MD and the added general health benefits of the MD suggest that the MD might be the preferred diet. It is important to note that, in addition to MUFAs, water-soluble fibre (including inulin, pectin, gums, and beta-glucans),114,115 vegetables and fruit,116 legumes,117,118 yogurt and other fermented dairy products,118 and spices and herbs (particularly turmeric) have been reported to provide added benefit in the prevention and management of IBD119,120 While these studies have had shown beneficial effects associated with the MD, there has yet to be definitive studies demonstrating that the MD solely modifies disease course or outcomes in IBD patients. Moreover, the Canada food guide has a number of similarities to the traditional MD also promoting a diet rich in grains, fruits, and vegetables but also recommending limited alcohol intake, suggesting that this might be another dietary approach in IBD care.121,122
Conclusion
Animal models suggest multiple mechanisms by which dietary lipid intake influences inflammation and colitis. The contrasting differences in dietary lipid intake and outcomes in animal models and patient with IBD make it difficult to make substantive conclusions. In animal models of IBD, the lipid diet is most often given as fish oil, and provided prior the onset of the inflammatory insult; is provided in a controlled environment, in the absence of other confounding dietary variables, and conducted in specific, pathogen-free environments. This environment does not factor in the role of microbes in causality, as well as the differences in gut microbiota composition between humans and rodents.
N-3 PUFAs have been shown to be beneficial in comparison to N-6 PUFAs in animal studies; however, despite these animal results, this has not been convincingly demonstrated in human trials. A primary reason for the lack of response of fish oil supplements in IBD patients may relate to studying standardized concentrations of n-3 fatty acids as encapsulated dietary supplements which differs substantially from the oil in fresh fish with its higher bioavailability. Moreover, as n-3 fatty acids are chemically unstable and are subject to lipid peroxidation with the result that the composition of a fish oil supplement cannot be simply inferred from the labelled EPA and DHA concentrations.
The benefits of fish oil, and more particularly fresh marine food, may lie in reducing the risk of developing IBD in susceptible individuals; however, further studies are required to evaluate this taking into account the balance of n-3 and n-6 fatty acids and their bioactive metabolites, the timing of exposure (different in the developing foetus and infant vs. older children and adults) and on potentially reducing host resistance to infection. Moreover, further studies are required to evaluate the complementary benefits of fish oil taking into account the variables identified above and fresh marine food in known IBD patients. The MD high in MUFA might also be beneficial in modifying progression to IBD and is promising as a complementary approach to pharmacotherapy in IBD patients.
Supplementary Material
Contributor Information
Matthew Smyth, Department of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, Faculty of Medicine, British Columbia Children’s Hospital, University of British Columbia, B.C., Vancouver, British Columbia, Canada, V6H 3V4.
Genelle Lunken, Department of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, Faculty of Medicine, British Columbia Children’s Hospital, University of British Columbia, B.C., Vancouver, British Columbia, Canada, V6H 3V4; British Columbia Children Hospital Research Institute,Vancouver, British Columbia, Canada, V5Z 4H4.
Kevan Jacobson, Department of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, Faculty of Medicine, British Columbia Children’s Hospital, University of British Columbia, B.C., Vancouver, British Columbia, Canada, V6H 3V4; British Columbia Children Hospital Research Institute,Vancouver, British Columbia, Canada, V5Z 4H4; Department of Cellular and Physiological Sciences, University of British Columbia, Vancouver, British Columbia, Canada, V6T 2A1.
Authors contributions
Literature review: KJ, Drafting of the manuscript: MS and KJ, Critical review of the manuscript: MS and GL, Revision of the manuscript: MS and KJ, Final approval of the manuscript: MS, GL, KJ, and Overall guarantor of the work: KJ.
Funding
None declared.
Conflicts of interest
Kevan Jacobson has been on Advisory boards of Abbvie Canada, Janssen Canada, Amgen; Merck Canada and Mylan Pharmaceuticals, Viatris; Mckesson Canada. He has been on the speaker’s bureau of Abbvie Canada and Janssen Canada. He has received investigator-initiated research support from Abbvie Canada and Janssen Canada; Stock options: Engene. The other two authors declare that they have no conflicts of interest.
Data availability
This is a review article and all the data included and discussed have been published and referenced accordingly.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 2. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–977. 10.1053/j.gastro.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan J, Wang L, Gu Y, Hou H, et al. Dietary patterns and gut microbiota changes in inflammatory bowel disease: current insights and future challenges nutrients. Nutrients. 2022;14(19):4003. 10.3390/nu14194003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma X, Torbenson M, Hamad ARA, et al. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151(1):130–138. 10.1111/j.1365-2249.2007.03530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gruber L, Kisling S, Lichti P, et al. High fat diet accelerates pathogenesis of murine Crohn’s disease-like Ileitis independently of obesity. PLoS ONE. 2013;8(8):e71661. 10.1371/journal.pone.0071661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Medina M, Denizot J, Dreux N, et al. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63(1):116–124. 10.1136/gutjnl-2012-304119 [DOI] [PubMed] [Google Scholar]
- 7. Chan SSM, Luben R, Olsen A, et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment Pharmacol Ther. 2014;39(8):834–842. 10.1111/apt.12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. IBD in EPIC Study Investigators; Tjonneland A, Overvad K, Bergmann MM, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58(12):1606–1611. 10.1136/gut.2008.169078 [DOI] [PubMed] [Google Scholar]
- 9. de Silva PSA, Luben R, Shrestha SS, et al. Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: a prospective cohort study using 7-day food diaries. Eur J Gastroenterol Hepatol. 20114;26(1):11–18. [DOI] [PubMed] [Google Scholar]
- 10. Mozaffari H, Daneshzad E, Larijani B, et al. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2020;59(1):1–17. 10.1007/s00394-019-01901-0 [DOI] [PubMed] [Google Scholar]
- 11. He J, Luo X, Xin H, et al. The effects of fatty acids on inflammatory bowel disease: a two-sample Mendelian randomization study. Nutrients. 2022;14(14):2883. 10.3390/nu14142883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turpin W, Dong M, Sasson G, et al. Mediterranean-like dietary pattern associations with gut microbiome composition and sub-clinical gastrointestinal inflammation. Gastroenterology. 2022;163(3):685–695. 10.1053/j.gastro.2022.05.037 [DOI] [PubMed] [Google Scholar]
- 13. Khalili H, Håkansson N, Chan SS, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut. 2020;69(9):1637–1644. 10.1136/gutjnl-2019-319505 [DOI] [PubMed] [Google Scholar]
- 14. Innis SM, Pinsk V, Jacobson K.. Dietary lipids and intestinal inflammatory disease. J Pediatr. 2006;149(5 Suppl):S89–S–96.. 10.1016/j.jpeds.2006.06.058 [DOI] [Google Scholar]
- 15. Jacobson K and Calder PC.. Role of omega-6 and omega-3 fatty acids in inflammatory bowel disease. springer book series—advances in the pharmaceutical sciences series. Pharma-Nutrition. 2014;12: 75–89. Cham: Springer. 10.1007/978-3-319-06151-1_5 [DOI] [Google Scholar]
- 16. Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, proetin, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322(12):1178–1187. 10.1001/jama.2019.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber C, Harnack L, Jihnson A, et al. Nutrient comparisons of margarine/ margarine-like productsand butter in the US marketplace in 202 Post-FDA ban on partial hydogenated oils. Public Health Nutr. 2022;25(5):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker EJ, Miles EA, Burdge GC, et al. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016;64:30–56. 10.1016/j.plipres.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 19. Popkin BM, Siega-Riz AM, Haines PS, Jahns L.. Where’s the fat? trends in U.S. diets 1965-1996. Prev Med 2001;32(3):245–254. 10.1006/pmed.2000.0807 [DOI] [PubMed] [Google Scholar]
- 20. Elias SL, Innis SM.. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acid are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr 2001;73(4): 807–814. 10.1093/ajcn/73.4.807 [DOI] [PubMed] [Google Scholar]
- 21. Innis, SM. Human milk and formula fatty acids. J Pediatrics 1992;120(4 Pt 2): S56–S61. 10.1016/s0022-3476(05)81237-5 [DOI] [PubMed] [Google Scholar]
- 22. Innis SM. Polyunsaturated fatty acid in human milk: an essential role in infant development. Adv Exp Med Biol 2004;554:27–43. 10.1007/978-1-4757-4242-8_5 [DOI] [PubMed] [Google Scholar]
- 23. Djuricic I, Calder PC.. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2012. Nutrients 2021;13(7):2421. 10.3390/nu13072421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burdge GC, Calder PC.. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45(5):581–597. 10.1051/rnd:2005047 [DOI] [PubMed] [Google Scholar]
- 25. Chen CT, Bazinet RP.. β-Oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot Essent Fatty Acids. 2015;92:33–40. 10.1016/j.plefa.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 26. Fenton JL, Hord NG, Gosh S, Gurzell EA.. Immunomodulation by dietary long chain omega-3 fatty acids and the potential adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids. 2013;89(6):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albert BB, Cameron-Smith D, Hofman PL, Cutfield WS.. Oxidation of marine omega-3 supplements and human health. BioMed Res Int. 2013;2013:464921. 10.1155/2013/464921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papanikolaou Y, Brooks J, Reidre C, Fulgoni VL.. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008.Nutr J.2014;13: 31. 10.1186/1475-2891-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75(3): 645–662. 10.1111/j.1365-2125.2012.04374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pérez R, Matabosch X, Llebaria A, et al. Blockade of arachidonic acid incorporation into phospholipids induces apoptosis in U937 promonocytic cells. J Lipid Res. 2006;47(3):484–491. 10.1194/jlr.M500397-JLR200 [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez-Perilli L, Prolo C, Alvarez MN.. Arachidonic acid and nitroarachidonic: effects on NADPH oxidase activity. Adv Exp Med Biol. 2019;1127:85–95. 10.1007/978-3-030-11488-6_6 [DOI] [PubMed] [Google Scholar]
- 32. Calder PC. Eicosanoids. Ess Biochem. 2020;64(3):423–441. 10.1042/EBC20190083 [DOI] [PubMed] [Google Scholar]
- 33. Vane JR, Bakhle YS, Botting RM.. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. 10.1146/annurev.pharmtox.38.1.97 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Z, Andoh A, Inatomi O, et al. Interleukin-17 and lipopolysaccharides synergistically induce cyclooxygenase-2 expression in human intestinal myofibroblasts. J Gastroenterol Hepatol. 2005;20: 619–627. 10.1111/j.1440-1746.2004.03748.x [DOI] [PubMed] [Google Scholar]
- 35. Wada M, DeLong CJ, Hong YH, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282(31): 22254–22266. 10.1074/jbc.M703169200 [DOI] [PubMed] [Google Scholar]
- 36. Radmark O, Werz O, Steinhilber D, Samuelsson B.. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851(4):331–339. 10.1016/j.bbalip.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 37. Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediators. 2022;68–69:165–175. 10.1016/s0090-6980(02)00029-1 [DOI] [PubMed] [Google Scholar]
- 38. Uchiya K-I, Nikai T.. Salmonella enterica Serovar typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella Pathogenicity Island 2. Inf Immun. 2004;72(12);6860–6869. 10.1128/IAI.72.12.6860-6869.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harizi H, Gualde N.. Dendritic cells produce eicosanoids, which modulate generation and function of antigen-presenting cells. Prostoglandins Leukot Essent Fatty Acids. 2002;66(5):459–466. [DOI] [PubMed] [Google Scholar]
- 40. Nakamura M, Shimizu T.. Recent advances in function and structure of two leukotriene B4 receptors: BLT1 and BLT2. Biochem Pharmacol. 2022;203:115178. 10.1016/j.bcp.2022.115178 [DOI] [PubMed] [Google Scholar]
- 41. Jupp J, Hillier K, Elliott DH, et al. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(5):537–546. 10.1002/ibd.20094 [DOI] [PubMed] [Google Scholar]
- 42. Sheibanie AF, Yen JH, Khayrullina T, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23 → IL-17 axis. J Immunol. 2007;178(12):8138–8147. 10.4049/jimmunol.178.12.8138 [DOI] [PubMed] [Google Scholar]
- 43. Chen H, Qin J, Wei P, et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80(4):195–200. 10.1016/j.plefa.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 44. Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013; 5(174):10.1126–1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esser-von Bieren, J. Immune-regulation and-functions of eicosanoid lipid mediators. Biol Chem. 2017;398(11):1177–1191. 10.1515/hsz-2017-0146 [DOI] [PubMed] [Google Scholar]
- 46. Miles EA, Allen E, Calder PC.. In vitro effects of eicosanoids derived from different 20-carbon fatty acids on production of monocyte-derived cytokines in human whole blood cultures. Cytokine. 2002;20(5):215–223. 10.1006/cyto.2002.2007 [DOI] [PubMed] [Google Scholar]
- 47. Cheon H, Rho YH, Choi SJ, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177(2):1092–1100. 10.4049/jimmunol.177.2.1092 [DOI] [PubMed] [Google Scholar]
- 48. Levy BD, Clish CB, Schmidt B, et al. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2(7):612–619. 10.1038/89759 [DOI] [PubMed] [Google Scholar]
- 49. Vachier I, Chanez P, Bonnans C, et al. Endogenous anti-inflammatory mediators from arachidonate in human neutrophils. Biochem Biophys Res Commun. 2002;290(1):219–224. 10.1006/bbrc.2001.6155 [DOI] [PubMed] [Google Scholar]
- 50. Takeuchi K, Amagase K.. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr Pharm Des. 2018;24(18):2002–2011. 10.2174/1381612824666180629111227 [DOI] [PubMed] [Google Scholar]
- 51. An JY, Song WJ, Li Q, et al. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviates DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pouchard C, Gonzales J, Bessard A, et al. PGI2 inhibits intestinal epithelial permeability and apoptosis to alleviate colitis. Cell Mol Gastroenterol Hepatol. 2021;12(3):1037–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Godson C, Guiry P, Brennan E.. Lipoxin mimetics and the resolution of inflammation. Annu Rev Pharmacol Toxicol. 2023; 63:429–488. 10.1146/annurev-pharmtox-051921-085407 [DOI] [PubMed] [Google Scholar]
- 54. Gundala NKV, Naidu VGM, Das UN.. Arachidonic acid and lipoxinA4 attenuate streptozotocin-induced cytotoxicity to RIN5 F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition. 2017;35:61–80. 10.1016/j.nut.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 55. Medhora M, Chen Y, Gruenloh S, et al. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L902–L911. 10.1152/ajplung.00278.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1. 10.1126/science.285.5431.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oppedisano F, Macrì R, Gliozzi M, et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection. Biomedicines. 2020;8(9):306. 10.3390/biomedicines8090306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Calder PC. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr Soc. 2020;79(4):404–416. 10.1017/s0029665120007077 [DOI] [PubMed] [Google Scholar]
- 59. Babcock TA, Kurland A, Helton WS, et al. J. Inhibition of activator protein-1 transcription factor activation by omega-3 fatty acid modulation of mitogen-activated protein kinase signaling kinases. J Parent Enteral Nutrition. 2016;27(3):176–180. [DOI] [PubMed] [Google Scholar]
- 60. Li J, Guo C, Wu J.. 15-Deoxy-∆-12,14-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-γ: function and Mechanism. PPAR Res. 2019;2019:7242030. 10.1155/2019/7242030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maier NK, Leppla SH, Moayeri M.. The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J Immunol. 2015;194(6): 2776–2785. 10.4049/jimmunol.1401611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams-Bey Y, Boularan C, Vural Aet al. , Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS One. 2014;9(6):article e97957. 10.1371/journal.pone.0097957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim J, Lim K, Kim KH, et al. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLos One. 2018;13(3):e0194331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fu Y, Wang Y, Gao H, et al. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm. 2021;2021:8879227. 10.1155/2021/8879227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Serhan CN. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature. 2014;510(7503):92–101. 10.1038/nature13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwanke RC, Marcon R, Bento AF, Calixto JB.. EPA-and DHA-derived resolvins’ actions in inflammatory bowel disease. European Journal of Pharmacology. 2016;785:156–164. 10.1016/j.ejphar.2015.08.050 [DOI] [PubMed] [Google Scholar]
- 67. Chiang N, Serhan CN.. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–129. 10.1016/j.mam.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barnig C, Bezemma T, Calder PC, et al. Activation of resolution pathways to prevent and fight chronic inflammation: lessons from asthma and inflammatory bowel disease. Front Immunol. 2019;10(1718):1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herova M, Schmid M, Gemperle C, Hersberger M.. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol. 2015;194(5):2330–2337. 10.4049/jimmunol.1402166 [DOI] [PubMed] [Google Scholar]
- 70. Schwab JM, Chiang N, Arita M, Serhan CN.. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. 10.1038/nature05877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ohira T, Arita M, Omori K, et al. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285(5):3451–3461. 10.1074/jbc.M109.044131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chiurchiu V, Leuti A, Dalli J, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8(353):3533ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krishnamoorthy N, Burkett PR, Dalli J, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194(3):863–867. 10.4049/jimmunol.1402534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vilaseca J, Salas A, Guarner F, et al. Dietary fish oil reduces progression of chronic inflammatory lesions in a rat model of granulomatous colitis. Gut. 1990;31(5):539–544. 10.1136/gut.31.5.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nieto N, Torres MI, Rios A, Gil A.. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J. Nutr. 2002;132(1); 11–19. 10.1093/jn/132.1.11 [DOI] [PubMed] [Google Scholar]
- 76. Andoh A, Tsujikawa T, Ishizuka I, et al. N-3 fatty acid-rich diet prevents early response of interleukin- 6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int J Mol Med. 2003;12:721–725. [PubMed] [Google Scholar]
- 77. Hassan A, Ibrahim K, Mbodji K, et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-ĸB in rats with TNBS-induced colitis. J Nutr. 2010;140(10):1714–1721. [DOI] [PubMed] [Google Scholar]
- 78. Ibrahim A, Mbodji K, Hassan A, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. 2011;30(5):678–687. 10.1016/j.clnu.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 79. Ibrahim A, Aziz M, Hassan A, et al. Dietary α-linolenic acid–rich formula reduces adhesion molecules in rats with experimental colitis. Nutrition. 2012;28(7–8):799–802. 10.1016/j.nut.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 80. Mbodji K, Charpentier C, Guérin C, et al. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-κB in rats with TNBS-induced colitis. J Nutr Biochem. 2013;24(4):700–705. 10.1016/j.jnutbio.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 81. Charpentier C, Chan R, Salameh E, et al. Dietary n-3 PUFA may attenuate experimental colitis. Mediators Inflamm. 2018;2018:8430614. 10.1155/2018/8430614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Camuesco D, Galvez J, Nieto A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135(4):687–694. 10.1093/jn/135.4.687 [DOI] [PubMed] [Google Scholar]
- 83. Camuesco D, Comalada M, Concha A, et al. Intestinal anti-inflammatory activity of combined quercitrin and dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, in rats with DSS-induced colitis. Clin Nutr. 2006;25(3):466–476. 10.1016/j.clnu.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 84. Barros KV, Xavier RA, Abreu GG, et al. Soybean and fish oil mixture increases IL-10, protects against DNA damage and decreases colonic inflammation in rats with dextran sulfate sodium (DSS) colitis. Lipids Health Dis. 2010;9(68):1–9. 10.1186/1476-511X-9-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Turk HF, Monk, JM, Fan YY, et al. , Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing, Am J Physiol Cell Physiol. 2013;304(9):C905–C917. 10.1152/ajpcell.00379.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou X, Xiang X, Zhou Y, et al. Protective effects of Antarctic krill oil in dextran sulfate sodium-induced ulcerative colitis mice. J Functional Foods. 2021;79(1):104394. [Google Scholar]
- 87. Empey LR, Jewell LD, Garg ML, Thomson AB, Clandinin MT, Fedorak RN.. Fish oil-enriched diet is mucosal protective against acetic acid-induced colitis in rats. Can J Physiol Pharmacol. 1991;69(4):480–487. 10.1139/y91-072 [DOI] [PubMed] [Google Scholar]
- 88. Ghosh S, DeCoffe D, Brown K, et al. , Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis, PLoS One. 2013;8(2):e55468. 10.1371/journal.pone.0055468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hekmatdoost A, Wu X, Morampudi V, et al. Dietary oils modify the host immune response and colonic tissue damage following Citrobacter rodentium-infection in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304(10):G917–G928. 10.1152/ajpgi.00292.2012 [DOI] [PubMed] [Google Scholar]
- 90. Mane J, Pedrosa E, Loren V, et al. Partial replacement of dietary (n-6) fatty acids with medium-chain triglycerides decreases the incidence of spontaneous colitis in interleukin-10-deficient mice. J Nutr 2009;139(3):603–610. 10.3945/jn.108.101170 [DOI] [PubMed] [Google Scholar]
- 91. Matsunaga H, Hokari R, Kurihara C, et al. KuriharaOmega-3 polyunsaturated fatty acids ameliorate the severity of ileitis in the senescence accelerated mice (SAM)P1/Yit mice model. Clin Exp Immunol. 2009:158(3):325–333. 10.1111/j.1365-2249.2009.04020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gravaghi C, La Perle KM, Ogrodwski P, et al. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22(4):360–365. 10.1016/j.jnutbio.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 93. Monk JM, Jia Q, Callaway E, et al. RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142(1):117–124. 10.3945/jn.111.147058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Uchiyama K, Nakamura M, Odahara S, et al. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(10):1696–1707. 10.1002/ibd.21251 [DOI] [PubMed] [Google Scholar]
- 95. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. 10.1161/01.cir.99.6.779 [DOI] [PubMed] [Google Scholar]
- 96. Bates JM, Akerlund J, Mittge E, Guillemin K.. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2(6):371–382. 10.1016/j.chom.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Goldberg RF, AustenWG, Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. PNAS. 2008;105(9):3551–3556. 10.1073/pnas.0712140105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen KT, Malo MS, Moss AK, et al. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G467–G475. 10.1152/ajpgi.00364.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Orinska Z, Maurer M, Mirghomizadeh F, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med, 2007;13(8):927–934. 10.1038/nm1615 [DOI] [PubMed] [Google Scholar]
- 100. Fenton JI, Hord NG, Gosh S, Gurzell EA.. Long chain omega-3 fatty acid immunmodulation and the potential for adverse health outcomes. Prostaglandins, Leukot Essent Fatty Acids. 2013;89(2013):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jacobson K, Mundra H, Innis SM.. Intestinal responsiveness to experimental colitis in young rats is altered by maternal diet. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G13–G20. 10.1152/ajpgi.00459.2004 [DOI] [PubMed] [Google Scholar]
- 102. Innis SM, Dai C, Wu X, et al. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am J Physiol Gastrointest Liver Physiol. 2010 Dec;299(6):G1376–G1385. 10.1152/ajpgi.00258.2010 [DOI] [PubMed] [Google Scholar]
- 103. Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS. 2007;104(34):13780–13785. 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mondot S, Kang S, Furet JP, et al. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm Bowel Dis. 2011;17(1):185–192. 10.1002/ibd.21436 [DOI] [PubMed] [Google Scholar]
- 105. Gibson DL, Gill SK, Brown N, et al. Maternal exposure to fish oil primes offspring to harbor intestinal pathobionts associated with altered immune cell balance. Gut Microbes. 2015;6(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014;63(5):776–784. 10.1136/gutjnl-2013-305304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–1406S. 10.1093/ajcn/61.6.1402S [DOI] [PubMed] [Google Scholar]
- 108. Guasch-Ferre M, Willett WC.. The Mediterranean -diet and health: a comprehensive overview. J Intern Med 2021;290(3):549–566. 10.1111/joim.13333 [DOI] [PubMed] [Google Scholar]
- 109. Sasson AN, Ingram RJM, Zhang Z, et al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2021;6(9):754–769. 10.1016/S2468-1253(21)00097-2 [DOI] [PubMed] [Google Scholar]
- 110. Ratajczak AE, Festa S, Aratari N, et al. Should the Mediterranean diet be recommended for inflammatory bowel diseases patients? A narrative review. Front Nutr 2023;9(1088693):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Haskey N, Ye J, Estaki M, et al. A Mediterranean-like fat blend protects against the development of severe colitis in the mucin-2 deficient murine model. Gut Microbes. 2022;14(1):2055441. 10.1080/19490976.2022.2055441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Haskey N, Estaki M, Ye J, et al. A mediterranean diet pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: a randomized controlled trial. J Crohns Colitis 2023;17(10):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lewis JD, Sandler R, Brotherton C, et al. A randomized trial comparing the specific carbohydrate diet to a mediterranean diet in aduls with Crohn’s disease. Gastroenterology. 2021;161(3):837–852.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Merra G, Noce A, Marrone G, et al. Influence of mediterranean diet on human gut microbiota. Nutrients 2020;13(1):7. 10.3390/nu13010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Healey GR, Tsai K, Lisko DJ, et al. Prebiotic enriched exclusive enteral nutrition suppresses colitis via gut microbiome modulation and expansion of anti-inflammatory T cells in a mouse model of colitis. Cell Mol Gastroenterol Hepatol. 2021;12(4):1251–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li F, Liu X, Wang W, et al. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(6):623–630. [DOI] [PubMed] [Google Scholar]
- 117. Duarte M, Vasconcelos M, Pinto E.. Pulse consumption among portuguese adults: potential drivers and barriers towards a sustainable diet. Nutrients 2020;12(11):3336. 10.3390/nu12113336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rondanelli M, Lamburghini S, Faliva MA, et al. A food pyramid, based on a review of the emerging literature, for subjects with inflammatory bowel disease. Endocrinol Diabetes Nutr. 2021;68(1):17–46. 10.1016/j.endinu.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 119. Masoodi, M, Mahdiabadi MA, Mokhtare M, et al. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: a randomized double-blind controlled trial. J Cell Biochem. 2018;119(11):9552–9559. 10.1002/jcb.27273 [DOI] [PubMed] [Google Scholar]
- 120. Banerjee R, Pal P, Penmetsa A, et al. Novel bioenhanced curcumin with mesalamine for induction of clinical and endoscopic remission in mild-to-moderate ulcerative colitis: a randomized double-blind placebo-controlled pilot study. J Clin Gastroenterol. 2021;55(8):702–708. 10.1097/MCG.0000000000001416 [DOI] [PubMed] [Google Scholar]
- 121. Downs SM, Willows ND.. Should Canadians eat according to the traditional Mediterranean diet pyramid or Canada’s food guide? Appl Physiol Nutr Metab. 2008;33(3):527–535. 10.1139/H08-030 [DOI] [PubMed] [Google Scholar]
- 122. Brassard D, Elvidge Munene LA, St-Pierre G, et al. Development of the Healthy Eating Food Index (HEFI)-2019 measuring adherence to Canada’s Food Guide 2019 recommendations on healthy food choices. Appl Physiol Nutr Metab. 2022;47(5):595–610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a review article and all the data included and discussed have been published and referenced accordingly.


