Abstract
Diastolic dysfunction is a major cardiac dysfunction, and an important predisposing factor is age. Although exercise training is often used for the prevention and treatment of cardiovascular disease nowadays, little is currently known about whether exercise interventions associated with the slowing of cardiac aging are related to mtp‐related pathways. In the present study, the UAS/Tub‐Gal4 system was used to knockdown whole‐body mtp expression levels in Drosophila, which underwent 2 weeks of endurance training. By conducting different assays and quantifying different indicators, we sought to investigate the relationship between mtp, exercise, and age‐related diastolic dysfunction. We found that (1) Drosophila in the mtp RNAi youth group exhibited age‐related diastolic dysfunction and had a significantly shorter mean lifespan. (2) Endurance exercise could improve diastolic dysfunction and prolong lifespan in aged Drosophila. (3) Endurance exercise could increase the expression levels of apolpp and Acox3, and decrease the levels of TC, LDL‐C, and TG in the aged group. In summary, aging causes age‐associated diastolic dysfunction in Drosophila, and systemic knockdown of mtp causes premature age‐associated diastolic dysfunction in young Drosophila. Besides, endurance exercise improves age‐related diastolic dysfunction and prolongs lifespan.
Keywords: aging, cardiac function, Drosophila, endurance exercise, lifespan, mtp
Endurance exercise ameliorates age‐related diastolic dysfunction and prolongs average lifespan, which may be related to the upregulation of mtp expression and thus enhance lipid metabolism in aged Drosophila.
1. INTRODUCTION
Cardiovascular health is closely related to longevity, and cardiovascular disease (CVD) represents the leading cause of death in people aged 65 years and older. Diastolic dysfunction is a major cardiac dysfunction that can lead to diastolic heart failure, which impairs myocardial contractility as well as pumping capacity in the elderly, and an important predisposing factor is age (Kass et al., 2004). Aging, as a primary risk factor for cardiovascular disease, lowers the threshold for cardiovascular disease by promoting adverse changes in cardiac structure and function, with significant effects on the heart and vasculature, including atherosclerosis, hypertension, myocardial infarction, and stroke. Cardiac aging is associated with left ventricular remodeling, characterized by increased mass‐to‐volume ratio (Lakatta & Levy, 2003; North & Sinclair, 2012). The molecular mechanisms regulating cardiac aging remain incompletely understood, warranting further research.
The past few years have witnessed unprecedented progress in research on longevity genes. Microsomal triglyceride transfer protein (mtp), a direct homolog of human MTTP (microsomal triglyceride transfer protein), is a lipid transfer protein found in the liver and intestine. Given its role as a rate‐limiting enzyme in lipid metabolism, MTP has been associated with human longevity, coronary artery disease, and other vascular diseases caused by adverse lipid profiles (peripheral vascular disease, renal vascular disease, and stroke), which account for a significant portion of human mortality (Geesaman et al., 2003). Although mtp has been associated with many diseases, the link between mtp and age‐related diastolic dysfunction has not been elucidated.
Exercise is a fundamental health behavior that can prevent systemic cardiovascular disease and improve cardiovascular health through nontraditional mechanisms. Besides, it has been established that exercise and associated high levels of cardiorespiratory fitness can reduce cardiovascular mortality, the risk of heart failure and myocardial infarction, and age‐related arterial and cardiac stiffness. On the contrary, physical inactivity is a direct cause of organismal aging and a recognized risk factor for cardiovascular disease. Over the years, many epidemiological studies have reported that low activity and sedentary behavior lead to a 63% increase in the risk of developing cardiovascular disease (Jakovljevic, 2018). Accordingly, physical activity and exercise can attenuate age‐related cardiovascular changes by improving cardiovascular system function and metabolism. It has been reported that moderate‐intensity (≤70% of VOmax) dynamic exercise, which primarily involves aerobic energy pathways and large muscle mass (e.g., brisk walking and cycling), attenuates age‐related declines in cardiorespiratory fitness (Moore et al., 2012). In addition, elite athletes who maintain very high activity levels (e.g., former participants in the Tour de France or former Olympic marathon runners) typically exhibit greater longevity than the general population (Garatachea et al., 2014). Exercise to slow aging and reduce the risk of developing cardiovascular disease has been a hot topic of interest in medical and exercise science research, but it remains uncertain whether exercise amelioration of aging‐induced cardiovascular disease is linked to mtp and its molecular pathways.
The fruit fly Drosophila melanogaster has been shown to be an effective model for studying aging and adult cardiac function (Hughes & Reynolds, 2005; Piazza & Wessells, 2011). The Drosophila heart or dorsal blood vessel is a linear tube reminiscent of the primitive vertebrate embryonic heart tube, and although the final cardiac structure of Drosophila is very different from that of the vertebrate heart, the basic elements of cardiac development, function, and aging are very well conserved (Bodmer, 1995). The Drosophila heart model has proven to be an invaluable asset in elucidating the etiology of human cardiac disease, including dilated and restrictive cardiomyopathies, ion channelopathies, diabetic and congenital heart disease, and cardiac aging (Cammarato et al., 2008; Taghli‐Lamallem et al., 2008; Wessells et al., 2004). The Drosophila heart has also been used to identify new genes that may be involved in heart disease (Qian et al., 2011).
In the present study, after establishing a Drosophila exercise model, we found that cardiac systolic function and mtp expression levels decline with aging in Drosophila. Besides, there is a strong association between age‐related diastolic dysfunction and mtp expression levels. Importantly, endurance exercise improves age‐related diastolic dysfunction and prolongs lifespan, possibly related to the upregulation of mtp expression, thereby enhancing lipid metabolism in aged Drosophila.
2. MATERIALS AND METHODS
2.1. Drosophila strains and culture protocol
W 1118 wild‐type, tub‐Gal4 regulatory line (preserved by Hunan Key Laboratory of Physical Fitness and Sports Rehabilitation, Hunan Normal University, Hunan Province, China). TH02325.N (UAS‐mtpRNAi) was obtained from the Drosophila Centre, Tsinghua University. All Drosophila were reared using a standard medium (made from a combination of yeast, corn, and starch).
2.2. Drosophila hybridisation and grouping
W 1118 and TH02325.N virgin flies were crossed with tub‐Gal4 males, and the resulting virgin flies of F1 generation were randomly divided into the mtp normal expression group (Tub × W 1118 ) and the mtp systemic knockdown group (mtp RNAi ). Virgin flies were selected as experimental subjects since female flies store more triglycerides than males, and female flies are larger and easier to observe and dissect. The mtp normal expression group was further divided into a 7‐day‐old normal intervention group (7d‐N), a 14‐day‐old normal intervention group (14d‐N), a 21‐day‐old normal intervention group (21d‐N), a 28‐day‐old group (28d‐N), a 35‐day‐old normal intervention group (35d‐N), and 35‐day‐old exercise intervention group (35d‐E). The mtp systemic knockdown group was subdivided into a 14‐day‐old normal intervention group (14d‐N), a 35‐day‐old normal intervention group (35d‐N), and a 35‐day‐old exercise intervention group (35d‐E). Normal intervention is denoted by N, and exercise intervention is denoted by E, where 14‐day‐old (14d) Drosophila represented the young group and 35‐day‐old (35d) Drosophila represented the old group. The F1 generation produced by the two major groups was used as the experimental Drosophila 800 each, 20 flies/tube, and all Drosophila were put into the HWS intelligent constant temperature and humidity incubator, with a constant temperature of 25, constant humidity of 50%, and a 12‐h day/night cycle of rearing. The intervention was carried out on the 22nd day after Drosophila fledged in the old age group, where sampling was carried out on day 36, while sampling was carried out on day 15 after Drosophila fledged in the young age group.
2.3. Training programme
We used a Drosophila locomotor activity monitor to simulate the effects of locomotion on aging phenotypes. The monitor was designed to induce upward walking by taking advantage of the innate negative geotactic behavior of Drosophila. Flies were aged to 22 days post‐eclosion and exercised in vials with an inner diameter of 2.8 cm, rotating at 0.16 rpm,1.5 h per exercise for 2 weeks, with 5 days of training and 2 days of rest planned. Exercise sessions were conducted from 14:00 to 15:30. Most Drosophila responded consistently by climbing throughout the exercise period, and the few Drosophila that failed to climb were observed walking vigorously on the inner wall of the vials, which we termed as endurance exercise (Tinkerhess et al., 2012; Zheng et al., 2015).
2.4. Reverse transcription polymerase chain reaction
Ten flies were placed into 1 mL of Trizol reagent lysis solution (Invitrogen) for homogenization and RNA extraction. Trizol was used to extract the organic solvent, and 10 μg total RNA was purified using oligo (dT) synthesized from total RNA with superscript II reverse transcriptase (Invitrogen). qPCR amplification reactions were performed in triplicates by mixing 1 μL of RT product with 10 μL of SYBR qPCR Mastermix (TaKaRa) containing the appropriate PCR primers. Thermal cycling and fluorescence monitoring were performed in an ABI7300 (Applied Biosystems, United States) using the following PCR conditions: (30 s at 95°C, 5 s at 95°C, and 30 s at 60°C) × 40. Values were normalized with rp49. Primer purification was performed using polyacrylamide gel electrophoresis (PAGE). The primers (obtained from Beijing Tsingke Biotech Co., Ltd.) used were as follows:
- rp49
- F: 5′‐CTAAGCTAGTCGCACAAATAGG‐3′
- R: 5′‐AACTTCTTAGAATCCGGTAGGG‐3′
- mtp
- F:5′‐ACGGAAATCCAGCAGAACACT‐3′
- R:5′‐ATACGTAAAGCCAACGGCCA‐3′
- Acox3
- F: 5′‐ACTTCCGTAGCGGACCTTTAG‐3′
- R: 5′‐GCAGAAGATAGTAGGGGGTTCCA‐3′
- apoLpp
- F: 5′‐AATTCGC GGATGGTCTGTGTGT‐3′
- R: 5′‐GCCCCTTAGGGATA GCCTTT‐3′
2.5. Semi‐integrated Drosophila heart preparation and heartbeat analysis
After anesthetizing Drosophila, the head and ventral thorax were quickly removed and poured into oxygenated artificial haemolymph (AH), followed by the removal of the ventral abdominal cuticle and all viscera to expose the heart tube (Vogler & Ocorr, 2009). A 30‐s digital movie of high‐speed heartbeats was taken at 130 fps using an EM‐CCD high‐speed camera and recorded using HCImage software (Hamamatsu, Japan). Indicators of cardiac function, for example, heart period (HP), arrhythmia index (AI), diastolic interval (DI), systolic interval (SI), diastolic diameter (DD), systolic diameter (SD), fractional shortening (FS), and fibrillation (FL), were quantified using semiautomatic optical heartbeat analysis software (Fink et al., 2009).
2.6. Climbing test
The negative geotropic climbing ability test was adapted from a previously documented method (Coulom & Birman, 2004). The climbing device consisted of five 20 cm long glass tubes with an inner diameter of 2.8 cm (sponges are placed at the ends of the tubes to prevent escape but allow air exchange). The sponge plugs at the ends of the long glass tubes were 2 cm each, providing 16 cm of climbing space for the fruit flies. The long glass tubes were divided equally from bottom to top into 1, 2, 3, and 4 quadrants of 4 cm each. The drosophila flies were allowed to acclimatize to the vials for 30 min before their climbing ability was assessed. Negative geotropism was triggered by jolting the fruit flies to the bottom of the vials by rapidly and continuously vibrating the climbing device. The position of the fruit fly was taken from a digital image taken at the end of 10 s after the triggering behavior. This process was repeated five times with 20 flies per tube. These photographs were placed in Photoshop to analyze the climbing index, which was calculated using the following formula: Climbing index = number of fruit flies in quadrant 4/the total number of fruit flies in a glass jar.
2.7. Lifespan assay
Lifespan assays were carried out as described in a previous study (Zheng et al., 2015). Dead Drosophila were counted every 2 days. Ten replicates (approximately 200 Drosophila) were used per condition, and female Drosophila were used for all longevity experiments. Survival curves were plotted using GraphPad Prism software (San Diego, CA).
2.8. Protein blot analysis
A sample size of 10 Drosophila per group was homogenized in urea lysis buffer (PBS, pH 7.4, 5.0 M urea, 2.0 M thiourea, 50 mM DTT, 0.1% SDS) using a pestle and mortar and a sonicator at 4°C. The mixture was centrifuged at 12,000 g for 10 min at 4°C, and the resulting supernatant was used for protein blot analysis. Proteins were extracted from Drosophila and separated on a 10% SDS‐polyacrylamide (29:1) mini‐gel (Bio‐Rad) and subsequently electroblotted on a polyvinylidene difluoride membrane (0.45um). Primary antibodies were directed against microsomal triglyceride transfer protein (rat monoclonal anti‐myosin, ab75316, 1:500, Abcam, Massachusetts, USA). The secondary antibody was peroxidase‐coupled anti‐rat IgG (1:5000, Sigma). Proteins were visualized using Super Signal West Dura detection reagent (Thermo Scientific), and signals were detected using the ChemiGenius Bioimaging System. Analyses and quantitative assessments were performed using GraphPad Prism software (San Diego, CA). Experiments were performed in triplicate.
2.9. Cholesterol, low‐density lipoprotein, and triglyceride determination
Total cholesterol (TC), low‐density lipoprotein (LDL‐C), and triglyceride (TG) were determined using the corresponding assay kits (Nanjing Jiancheng Bioengineering Institution, China) according to the manufacturer's instructions (Diop et al., 2017). TC was measured at 500 nm, LDL‐C at 600 nm, and TG at 510 nm. The absorbance was measured at different wavelengths with a microplate reader (Shenzhen Huisong Technology Development Co., Ltd.), with the optical density (OD) value of the measured standard as the coordinate and the concentration of the standard as the ordinate. The standard curve was drawn on the coordinate paper or with relevant software, and the linear regression equation was obtained. The OD value of the sample was replaced in the equation to calculate the concentration of the sample. Ten flies were tested for each indicator.
2.10. Ghost pen cyclic peptide immunofluorescence staining
Semi‐intact Drosophila hearts were prepared and confirmed to show rhythmic beating in oxygenated ADH. ADH was quickly replaced by a relaxation buffer (containing 10 mM EGTA). The cardiac tissues were fixed in 4% formaldehyde for 20 min at room temperature, then washed three times (10 min per wash) with PBS at room temperature, and were stained with ghost cyclin (ghost cyclin‐iFluor 594,1:1000, Abcam) for 40 min followed by three washes with PBS at room temperature (10 min per wash). Fluorescence staining images were obtained with a confocal laser scanning microscope (Carl Zeiss; Oberkochen; Germany).
2.11. Statistical analysis
The data obtained were processed statistically using the Statistical Package for the Social Sciences for Windows (SPSS) version 24.0 (SPSSInc., Chicago, IL, USA), and the Shapiro–Wilk test was used to test the normal distribution of the samples in each group. If the data followed a normal distribution, we employed independent samples t‐test or ANOVA for analysis of variance. The mean ± standard deviation (SD) values are presented in figures for statistical descriptions. In cases where normal distribution assumptions were not met, the Kruskal‐Wallis H rank‐sum test was utilized. Data were analyzed using median and quartiles (M, P25, P75) for statistical description. The significance level was set at p < 0.05.
3. RESULTS
3.1. mtp knockdown induces age‐related diastolic dysfunction in young Drosophila similar to that in old Drosophila
Cardiac senescence is characterized by a progressive decline in cardiac function, intrinsically associated to the organ itself and correlated with the age of the organism (Ocorr et al., 2007). Compared with Drosophila in the Tub×W 1118 ‐14d‐N group, the Tub×W 1118 ‐35d‐N group showed a significant decline in cardiac function. Analysis of cardiac rhythms revealed that the heart period, arrhythmia index, and systolic interval were significantly increased, and there was no significant change in the diastolic interval (Figure 1a–d). On the contrary, cardiac systolic function was mainly manifested by a smaller diastolic diameter, a lower fractional shortening, and an increase in fibrillation. By contrast, there was no significant change in the systolic diameter (Figure 1e–j). Previous studies indicated a steady decline in the mean diastolic diameter of wild‐type Drosophila hearts over time, with a greater decline compared to systolic diameter, signifying age‐related diastolic dysfunction, as evidenced by a significant reduction in fractional shortening. Our results provide compelling evidence that diastolic performance is significantly diminished in aging (35d) Drosophila relative to juvenile (14d) Drosophila. This aging‐dependent response suggests impaired myocardial relaxation and possible chamber stiffness, commonly observed during age‐related human diastolic dysfunction. In mammals, aging is characterized by a decline in left ventricular diastolic function, including abnormal diastolic relaxation, chamber filling, and/or passive myocardial stiffness (Kaushik et al., 2012). The heart's pumping and filling properties affect cardiac output and overall cardiac function and can lead to heart failure when dysregulated (Borlaug & Redfield, 2011).
FIGURE 1.

(a–h) the M‐type electrocardiograms of Drosophila from the Tub×W 1118 ‐14d‐N group, the Tub×W 1118 ‐35d‐N group, the mtp RNAi ‐14d‐N group and mtp RNAi ‐35d‐N, respectively, and quantitatively analyzed for HP, AI, DI, SI, DD, SD, FS, and FL. In comparison with Drosophila from the Tub×W 1118 ‐14d group, Tub×W 1118 ‐35d‐N group HP, AI, SI, and FL (a, b, d, h) showed a significant increase; DD and FS (e, g) both showed a significant decrease, DI and SD (c, f) did not show a significant difference. Compared with Drosophila in the mtp RNAi ‐35d‐N group, Drosophila in the mtp RNAi ‐14d‐N group showed an increase in HP, AI, SI, and FL (a, b, d, h) and a decrease in DD (e); DI, SD, and FS (c, f, g) did not show a significant difference. Compared with Drosophila in the Tub×W 1118 ‐14d group, Drosophila in the mtp RNAi ‐14d‐N group showed a significant decrease in DD and FS (e, g), and a significant increase in FL (h). Compared with Drosophila in the Tub×W 1118 ‐35d group, Drosophila in the mtp RNAi ‐14d‐N group only showed a significant increase in SD (f). (i–l) cardiac cycle maps of Drosophila from the Tub×W 1118 ‐14d‐N group, the Tub×W 1118 ‐35d‐N group, the mtp RNAi ‐14d‐N group, and the mtp RNAi ‐35d‐N group, respectively, with an M‐mode ECG interception time of 10 s for each group. DD, SD, and FL are marked with a yellow line in the figures. (m) the climbing indices of Drosophila in the Tub×W 1118 ‐14d‐N group, the Tub×W 1118 ‐35d‐N group, the mtp RNAi ‐14d‐N group, and the mtp RNAi ‐35d‐N group. Climbing ability was significantly reduced in the Tub×W 1118 ‐35d‐N group compared to the Tub×W 1118 ‐35d‐N group; compared to the mtp RNAi ‐14d‐N group, climbing ability was also significantly reduced in the mtp RNAi ‐35d‐N group. The climbing ability of Drosophila in the mtp RNAi ‐14d‐N group and the Tub×W 1118 ‐35d group was both significantly lower than that of the Tub×W 1118 ‐14d group. (n) the mtp expression levels of Drosophila from 7d to 35d old in the Tub×W 1118 group, and the mtp expression levels gradually decreased with age. The sample sizes of the eight groups of Drosophila in the M‐mode ECG test were N = 17 (Tub×W 1118 ‐14d‐N), N = 17 (Tub×W 1118 ‐35d‐N), N = 18 (mtp RNAi ‐14d‐N), and N = 19 (mtp RNAi ‐35d‐N), respectively. The sample size for each group of Drosophila in the climbing test was N = 100. mtp mRNA expression levels were tested in groups with a sample size of N = 10. All Drosophila were virgin flies. T‐tests and ANOVA were used to compare differences between groups. LSD was used for post hoc testing. Data are expressed as mean ± standard deviation (SD). Statistical significance was set at p < 0.05.
Similar findings were observed in the mtp RNAi group. Compared to the mtp RNAi ‐14d‐N group, the mtp RNAi ‐35d‐N group showed significant increases in the heart period, arrhythmia index, systolic interval, and fibrillation and significant decreases in the diastolic diameter; there was no significant change in the diastolic interval, systolic diameter, and fractional shortening (Figure 1a–i,k). Interestingly, after systemic knockdown treatment of mtp, the mtp RNAi ‐14d‐N group also showed a significant reduction in cardiac systolic function compared to the Tub×W 1118 ‐14d‐N group, mainly in the form of a decrease in diastolic diameter and fractional shortening, and an increase in the frequency of fibrillation, while there was no significant difference in systolic diameter (Figure 1e–i,k). We next compared the cardiac systolic function of the Tub×W 1118 ‐35d‐N group with that of the mtp RNAi ‐14d‐N group and found that, except for a significant difference in the systolic diameter, no significant difference was observed in the indicators of diastolic diameter, fractional shortening, and fibrillation (Figure 1e–h,j,k). On the one hand, along with aging, the decline of cardiac contractile function also affected the climbing ability of Drosophila, which was significantly lower in the Tub×W 1118 ‐35d‐N group than in the Tub×W 1118 ‐14d‐N group, consistent with findings in the mtp RNAi ‐35d‐N group and mtp RNAi ‐14d‐N group (Figure 1m). On the other hand, the climbing ability of Drosophila in the mtp RNAi ‐14d‐N group was significantly lower than that in the Tub×W 1118 ‐14d‐N group but remained significantly higher than the Tub×W 1118 ‐35d‐N group (Figure 1m), which may be attributed to the knockdown of mtp only affecting the contractile function of the heart, with relatively insignificant effects on other physiological functions related to climbing ability. Meanwhile, the expression level of mtp gradually decreased with age and was significantly lower in Drosophila in the older group (35d) compared with the younger group (14d) (Figure 1n).
It is well‐established that the adult Drosophila heart resembles a simple linear tube and consists of two major cell types: cardioblasts (CBs), which form the cardiac tube and differentiate into contractile cardiomyocytes, and pericardial cells (PCs), which are irregularly arranged on both sides of the heart and undergo hemolymph filtration. To explore the myofibrillar changes associated with senescence and mtp knockdown, we further examined myofibrils in the myocardium, where F‐actin was labeled with ghost pen cyclic peptide. It was found that hearts in the Tub×W 1118 ‐35d‐N and mtp RNAi ‐14d‐N groups showed a reduction in myocardial diastolic diameter as well as flocculation of myofiber arrangement compared with those in the Tub×W 1118 ‐14d‐N group (Figure 2a–c). This suggests that both senescence and mtp knockdown can impair diastolic function and myofibril structure in Drosophila hearts.
FIGURE 2.

Tub×W 1118 ‐14d‐N group (a), Tub×W 1118 ‐35d‐N group (b) and mtp RNAi ‐14d‐N group (c) Drosophila cardiac ghost cyclic peptide staining plots; Drosophila cardiac ghost cyclic peptide staining plots, n = 5. Drosophila cardiac tubules were visualized with ghost cyclic peptide‐labeled F‐Actin using a confocal microscope with a scale bar = 250 μm. Blue arrows represent diastolic diameters, and white arrows point to myofiber arrangement. The Tub×W 1118 ‐35d‐N and mtp RNAi ‐14d‐N groups exhibited a reduction in myocardial diastolic diameter as well as flocculation of myofiber arrangement compared to the Tub×W 1118 ‐14d‐N group. (d) Survival curves and average lifespan of the Tub×W 1118 ‐N and mtp RNAi ‐N groups. The sample size of each group was 190–210 Drosophila. The average lifespan of Drosophila in the Tub×W 1118 ‐N group was significantly higher than that in the mtp RNAi ‐N group. All samples were virgins. p‐Values for the lifespan curves were calculated by log‐rank test. Statistical significance was set at p < 0.05.
Besides, we found that the average lifespan of Drosophila in the mtp RNAi group was significantly shortened compared with the Tub×W 1118 group (Figure 2d). The changes in the mtp RNAi ‐14d‐N group paralleled the decline in cardiac function and climbing ability due to senescence in Drosophila observed in the Tub×W 1118 ‐35d‐N group, suggesting that there is a close link between the level of mtp expression and Drosophila cardiac contractile function and lifespan are closely linked.
Our results showed that aging could induce age‐associated diastolic dysfunction in Drosophila accompanied by a significant decrease in mtp expression levels and systemic knockdown of mtp induced premature age‐associated diastolic dysfunction in young Drosophila similar to that in old Drosophila, suggesting that mtp is essential for cardiac systolic function and lifespan in Drosophila.
3.2. Endurance exercise upregulates the expression of mtp to improve cardiac contractile function and extend mean lifespan in aged Drosophila
Regular moderate‐intensity exercise improves all aspects of human health and is widely used as a preventive and therapeutic strategy for a variety of diseases (Qiu et al., 2023), especially playing an important role in reducing the risk of cardiovascular disease (Cattadori et al., 2018; Channon, 2020; Fiuza‐Luces et al., 2018). The contractile performance of the heart in wild‐type Drosophila deteriorates with age, as evidenced by a decrease in diastolic diameter, shortening fraction, and other indices (Kaushik et al., 2012). Drosophila from the 35d‐N group of Tub×W 1118 and mtp RNAi were found to have significantly higher levels of mtp expression after a 2‐week exercise intervention (Figure 3a,b). Correspondingly, the contractile function of Drosophila hearts in the Tub×W 1118 and mtp RNAi 's 35d‐N groups exhibited varying degrees of improvement, which, taken together, reflected an increase of diastolic diameter, an improvement in the shortening fraction as well as a decrease in fibrillation (Figure 3c,d), which indicated that endurance exercise exerted a significant ameliorative effect on Drosophila's aging‐induced diastolic dysfunction (Figure 4a,b). The improvement in cardiac systolic function also affected the climbing ability of Drosophila, and the climbing index was significantly improved in both Tub×W 1118 and mtp RNAi 's 35d‐N groups of Drosophila (Figure 3e). In addition, after endurance training, the average lifespan of both the Tub×W 1118 and mtp RNAi groups was extended to different degrees, with the mtp RNAi ‐E group showing a more significant extension of the average lifespan (Figure 4c). In human studies, regular exercise has been demonstrated to induce systemic adaptations in almost all organ systems, providing multiple health benefits. In this respect, exercise attenuates age‐related multisystem decline and helps maintain physical fitness, including cardiorespiratory fitness, muscle function, flexibility, and balance. For example, in a landmark 21‐year longitudinal study, participation in prolonged running and other vigorous exercise was reported to be associated with less disability and lower mortality in later life in older adults (Chakravarty et al., 2008).
FIGURE 3.
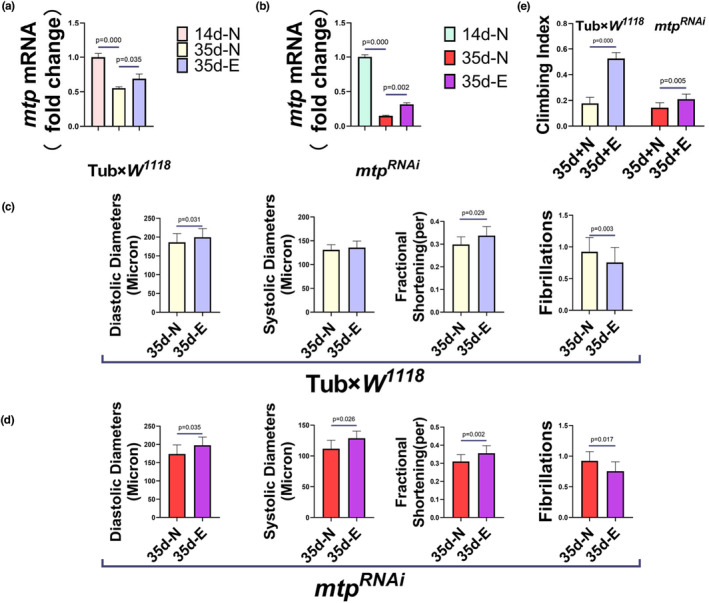
mRNA expression levels of mtp in Tub×W 1118 (a) and mtp RNAi (b) under different treatments. The mRNA expression level of mtp in Drosophila in the 35d‐N group of Tub×W 1118 and mtp RNAi was significantly decreased compared with the 14d‐N group. The mRNA expression level of mtp in Drosophila of the 35d‐E group of Tub×W 1118 and mtp RNAi increased after 2 weeks of endurance exercise compared to the 35d‐N group. M‐type ECGs and climbing tests were quantitatively analyzed for DD, SD, FS, and FL in Drosophila of the 35d‐N and 35d‐E groups of Tub×W 1118 and mtp RNAi . Quantitative analysis of DD, SD, FS, and FL was performed in the 35d‐E group of Tub×W 1118 and mtp RNAi : compared to the 35d‐N group, DD and FS appeared to increase and FL decreased significantly in the Tub×W 1118 ‐35d‐E group, and no significant difference was observed in SD (c); compared with the mtp RNAi ‐35d‐N group, DD, SD, and FS appeared to increase in the mtp RNAi ‐35d‐E group, and FL decreased (d). The climbing ability of Drosophila in the 35d‐E groups was significantly higher compared with the 35d‐N groups of Tub×W 1118 and mtp RNAi , and the sample sizes of all four groups of Drosophila in the climbing test were N = 100 (e). All Drosophila were virgin flies, and (a, b) were analyzed using one‐way ANOVA, with LSD used for post hoc testing. All P values in (c–e) are from independent samples t‐tests. Data are expressed as mean ± standard derivation (SD). Statistical significance was set at p < 0.05.
FIGURE 4.

mtp RNAi Group 35d‐N group (a) and 35d‐E group (b) Fluorescence images of Drosophila hearts, n = 5. Drosophila cardiac tubes were observed using a confocal microscope with ghost‐pen cyclic peptide‐labeled F‐Actin, scale bar = 250 μm. Blue arrows represent diastolic diameters, and white arrows point to myofiber arrangement. Compared with the mtp RNAi ‐35d‐N group, the mtp RNAi ‐35d‐E group exhibited increased myocardial diastolic diameter and tightly ordered myofibre arrangement. (c) Survival curves and average lifespan of the Tub×W 1118 ‐N, Tub×W 1118 ‐E, mtp RNAi ‐N, and mtp RNAi ‐E groups. The sample size of each group was 190–210. The average lifespan of Drosophila in the Tub×W 1118 ‐E group was slightly higher than that in the Tub×W 1118 ‐N group, and the average life span of Drosophila in the mtp RNAi ‐E group was significantly higher than that in the mtp RNAi ‐N group. All samples were virgins. p Values for the lifespan curves were calculated by log‐rank test. Statistical significance was set at p < 0.05.
3.3. Endurance exercise improves cardiac contractile function and prolongs lifespan in aged Drosophila in relation to lipid metabolic pathways downstream of mtp
The mitochondrial triglyceride transfer protein primarily performs a wide range of lipid transport functions required to maintain systemic lipid homeostasis (Shoulders & Shelness, 2005). We hypothesize that endurance exercise upregulates the expression level of mtp in aged Drosophila, thereby affecting lipid metabolism downstream of mtp, leading to improved cardiac contractile function and prolonged lifespan in aged Drosophila. In enterocytes and hepatocytes, MTP acts as an endoplasmic reticulum (ER)‐resident protein and is thought to transfer lipids to apolipoprotein B (apoB) when apoB transcripts are translated, thereby allowing apoB to fold correctly and assemble pristine lipoprotein particles (Davidson & Shelness, 2000; Gordon et al., 1995; Hussain, 2000). In addition to this, during the process of β‐oxidation catalyzing the production of acetyl cofactor from long‐chain fatty acids, MTPα, a subunit of MTP, possesses long‐chain 3‐enoyl‐coenzyme A hydratase and long‐chain 3‐hydroxyacyl‐coenzyme A dehydrogenase activities that catalyze the second step (hydration) and the third step (oxidation), respectively, while another subunit, MTPβ, possesses long‐chain 3‐ketoacyl‐coenzyme A thiolase activity that catalyzes the fourth step (sulpholysis). Several epidemiological studies have shown that high levels of LDL‐C and TC are important lipid risk factors for coronary heart disease (Anderson et al., 1987; Gordon et al., 1989; Law et al., 2003; Wetterau et al., 1997). Besides, elevated TG has been documented as an independent risk factor for coronary heart disease (Cui et al., 2001). Therefore, we examined the content of MTP and the mRNA expression levels of apolpp and Acox3 as well as TC, LDL‐C and TG in Drosophila from Tub×W 1118 and mtp RNAi in the youthful, elderly, and geriatric exercise groups. The content of MTP was significantly reduced in Drosophila in the Tub×W 1118 ‐35d‐N group compared with the 14d‐N group(Figure 5a). Consistently, the expression levels of apolpp and Acox3 were significantly downregulated in Drosophila 35d‐N in both the Tub×W 1118 and mtp RNAi groups compared with the 14d‐N group (Figure 6a,b), while the content of MTP in both the Tub×W 1118 ‐35d‐N and mtp RNAi ‐14d‐N groups showed no difference (Figure 5a). By contrast, after exercise intervention, the content of MTP and the expression levels of apolpp and Acox3 in the 35d‐E group showed different degrees of elevation compared with the 35d‐N group of Tub×W 1118 and mtp RNAi (Figure 5b,c; Figure 6a,b). These trends closely mirrored those of the mRNA expression levels of mtp (Figure 3a,b), indicating a significant influence of mtp expression on downstream related genes. MTP emerges as a crucial target in the overall lipid metabolic pathway, and its regulation through exercise training holds notable importance. The evaluation of LDL‐C, TC, and TG yielded similar results: in the Tub×W 1118 group, the TG content increased with age from 14 days (Figure 6c), contrary to the declining trend observed in mtp expression (Figure 1n). In the mtp RNAi ‐14d‐N group, compared to the Tub×W 1118 ‐14d‐N group, there was a significant increase in TC, LDL‐C, and TG levels (Figure 6d). The decrease in mtp expression compromised Drosophila's lipid metabolism ability, elevating the risk of cardiovascular diseases and reduced climbing ability (Figure 1m). Following the exercise intervention, along with an increase in the expression levels of MTP, apolpp, and Acox3, the levels of TC, LDL‐C, and TG were reduced (Figure 6e), and the systemic lipid metabolism ability was enhanced, which yielded a protective effect on cardiac function, improved the age‐associated diastolic dysfunction of the aged Drosophila, and prolonged lifespan.
FIGURE 5.
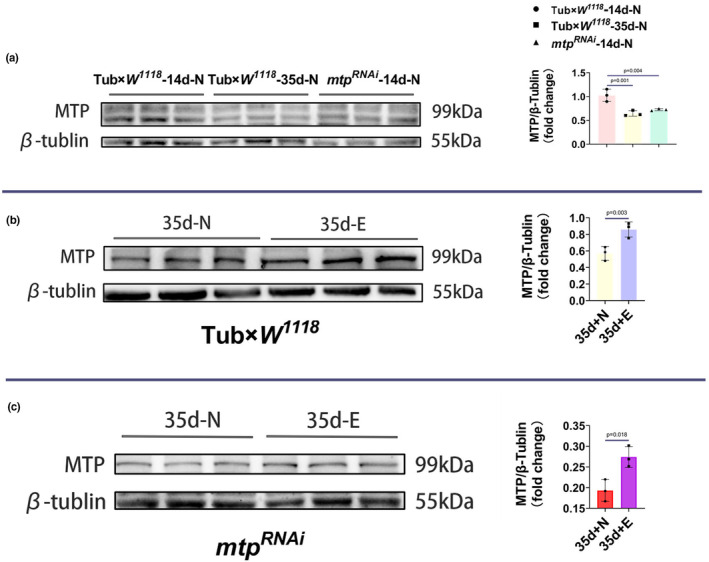
Protein blot analysis using antimicrosomal triglyceride transfer protein and antimicrotubule protein. Representative blots are shown, and the resulting bands were quantified and normalized to microtubule protein levels. (a) MTP levels were significantly higher in the Tub×W 1118 ‐14d‐N group than in the Tub×W 1118 ‐35d‐N and mtp RNAi ‐14d‐N groups, and there was no significant difference in MTP levels between the Tub×W 1118 ‐35d‐N and mtp RNAi ‐14d‐N groups. (b) The MTP content of the Tub×W 1118 ‐35d‐E group was higher than that of the Tub×W 1118 ‐35d‐N group. (c) The MTP content in the mtp RNAi ‐35d‐E group was higher than in the mtp RNAi ‐35d‐N group. All samples were virgins, N = 10, and measurements were repeated three times. (a) analyzed using ANOVA, and LSD was used for post hoc testing. (b, c) p values are from independent samples t‐tests. Data are expressed as mean ± standard deviation (SD). Statistical significance was set at p < 0.05.
FIGURE 6.

(a) mRNA expression levels of apolpp and Acox3 in 14d‐N, 35d‐N, and 35d‐E groups of Tub×W 1118 . The mRNA expression levels of apolpp and Acox3 in Drosophila in the 35d‐N group appeared to be reduced compared to the 14d‐N group; the mRNA expression levels of apolpp and Acox3 in Drosophila in the 35d‐E group increased significantly after exercise training compared to the 35d‐N. (B) mtp RNAi of the mRNA expression levels of apolpp and Acox3 in the 14d‐N, 35d‐N, and 35d‐E groups. The mRNA expression levels of apolpp and Acox3 were significantly reduced in Drosophila in the 35d‐N group compared to the 14d‐N group; the mRNA expression levels of apolpp and Acox3 were increased in the 35d‐E group compared to the 35d‐N after exercise training. (C) Whole‐body TG levels in Drosophila at different ages in the Tub×W 1118 group. From 14 days of age, the whole‐body TG levels of the Tub×W 1118 group increased significantly with age. (D) LDL‐C, TC, and TG levels in the 14d‐N groups of Tub×W 1118 and mtp RNAi , and the LDL‐C, TC, and TG levels in the mtp RNAi ‐14d‐N group were significantly higher than those in the Tub×W 1118 ‐14d‐N group. (E) LDL‐C, TC, and TG levels in the 35d‐N and 35d‐E groups of Tub×W 1118 were significantly lower in the Tub×W 1118 ‐35d‐E group compared with the Tub×W 1118 ‐35d‐N group. All samples were virgins, N = 10; measurements were repeated three times. (a–c) analyzed using ANOVA, and LSD was used for post hoc testing. (d, e) p values are from independent samples t‐tests. Data are expressed as mean ± standard deviation (SD). Statistical significance was set at p < 0.05.
4. DISCUSSION
Given that cardiovascular disease poses a significant threat to human health, especially with the accelerated aging of society, understanding the progression and control of age‐related changes in cardiac function has become increasingly crucial. Despite this urgency, our knowledge of the genetic mechanisms responsible for the decline in cardiac function with aging remains limited (Cullen, 2000). While endurance exercise improves cardiac function and is used as one of the therapeutic measures for many diseases (Sujkowski et al., 2022), there are no systematic studies on endurance exercise and age‐related diastolic dysfunction, and this knowledge gap suggests that exercise‐dependent molecular mechanisms that could be used to counter age‐related diastolic dysfunction are still largely unknown. Herein, we chose mtp as a key target to study the benefits of endurance exercise on age‐related diastolic dysfunction in a precisely controlled and meticulously quantified manner.
In our study, we found that the Tub×W 1118 aged group of Drosophila hearts exhibited a marked decline in diastolic diameter (rapid deterioration in diastolic diameter suggestive of age‐related diastolic dysfunction) as well as a significant reduction in shortening fraction, similar to symptoms in wild‐type aged Drosophila (Kaushik et al., 2012). Furthermore, an increase in fibrillation was noted, aligning with previous findings. In the mouse heart, mtp plays an essential role in myocardial lipid homeostasis, and blocking cardiac mtp expression resulted in an approximately 2‐fold increase in myocardial triglyceride stores (Geesaman et al., 2003). Nielsen and colleagues found that in patients with coronary artery disease, left ventricular mtp mRNA levels were negatively correlated with tissue triglyceride content, and it was hypothesized that upregulation of transcripts of mtp (and possibly apoB) in the ventricle could contribute to the reduction of triglyceride accumulation in hypoxic hearts (Bjorkegren et al., 2001). The West of Scotland Coronary Prevention Study (WOSCOPS) found that the mtp‐ 493 t allele or linked imbalanced alleles could increase the risk of coronary events by a mechanism associated with reduced cardiac mtp expression (Nielsen et al., 2002). In the present study, systemic inhibition of mtp revealed that the young group of mtp RNAi showed a significant increase in TC, LDL‐C, and TG and would exhibit diastolic dysfunction similar to that of aged Tub×W 1118 , as well as a substantial shortening of the average lifespan of mtp RNAi group, which suggests that mtp not only influences systemic lipid metabolism but also may be associated with the age‐related decline in cardiac function as well as lifespan. Since the subunits of mtp are involved in the β‐oxidation of fatty acids, there may be a correlation between changes in mtp and mitochondrial alterations. mRNA expression levels of mtp may affect OXPHOS genes, mitochondrial fusion/fission, and mitochondrial phagocytosis genes (Parra et al., 2014), leading to age‐related changes in mitochondria, which may ultimately affect lifespan, emphasizing the need for more research.
Exercise therapy is a cost‐effective therapy to reduce the risk of developing cardiovascular disease and mortality (Goenka & Lee, 2017). In aged Drosophila, exercise enhances cardiac function and improves cardiomyocyte ultrastructure and heart failure (Piazza et al., 2009). The CG9940 gene encodes a NAD(+) synthase protein in Drosophila. Low expression of CG9940 negatively affects lifespan, and exercise slows down the age‐related declines in cardiac function, mobility, and lifespan of these flies (Wen et al., 2016). Similar results were found in our study, where endurance exercise significantly improved cardiac systolic function, especially diastolic diameter and shortening fraction, in aged Drosophila and acted as a good therapeutic agent for age‐related diastolic dysfunction. In our previous study, exercise intervention was able to upregulate the cardiac expression of mtp and improve cardiac dysfunction and lipid metabolism abnormalities in high‐fat Drosophila (Peng et al., 2022). Meanwhile, we found that the climbing ability and average lifespan of old Drosophila increased, especially in the mtp RNAi group. This suggests that mtp may be an important target for exercise training to ameliorate age‐associated diastolic dysfunction and extend lifespan. Related studies have shown that exercise activates the cardiac dSir2/Foxo/SOD and dSir2/Foxo/bmm pathways and reduces the occurrence of diastolic dysfunction as well as enhances cardiac contractility (Rosenberg & Parkhurst, 2002; Wen et al., 2019). Based on our findings, it can be inferred that the regulatory pathways of mtp are complex and numerous, with the insulin pathway (Hagan et al., 1994), cholesterol (Sato et al., 1999), bile acids (Hirokane et al., 2004), endotoxin (LPS), cytokines tumor necrosis factor (TNF), IL‐1, and IL‐6 (Navasa et al., 1998), among others, all playing a role in the regulation of mtp. For example, in HepG2 cells, insulin inhibits mtp promoter activity in a dose‐ and time‐dependent manner, and insulin inhibits mtp transcription through activation of the MAPKerk cascade rather than through the phosphatidylinositol 3‐kinase pathway (Hagan et al., 1994). On the contrary, mtp promoter activity appears to be positively regulated by cholesterol in a dose‐dependent manner, and cholesterol regulation of mtp promoter activity is not dependent on the trans‐activating structural domain of the SREBP protein. Instead, the SRE‐binding protein (SREBP) binds to the ‐124 ‐116 SRE site and thus negatively regulates mtp gene expression (Sato et al., 1999). A clear relationship has been established between endurance exercise and the insulin pathway as well as cholesterol, and it is highly conceivable that the expression level of mtp may be indirectly regulated through the insulin pathway, cholesterol, or other molecular pathways, which will require more in‐depth studies in the future.
Studies on rats suggested that microvascular dysfunction and insufficient coronary perfusion could be the mechanism underlying diastolic dysfunction in aged rats (Hotta et al., 2017). In our study, we found that the decrease in mRNA and protein levels of mtp due to aging could impact systemic lipid metabolism, and the mRNA expression levels of apolpp and Acox3 downstream of mtp could be decreased, while the levels of TC, LDL‐C, and TG could be significantly increased. Dysregulation of lipid metabolism could lead to impaired cardiac function, with decreases in diastolic diameter and shortening fraction accompanied by an increase in fibrillation, which may account for the early onset of age‐related diastolic dysfunction in Drosophila in the mtp RNAi youth group. For example, individuals with higher levels of HDL (good cholesterol) and lower levels of LDL (bad cholesterol) have a relatively lower risk of heart disease and stroke compared to their peers (Barzilai et al., 2001; Terry et al., 2003).
In subjects without cardiovascular disease, regular physical activity reduces the risk of cardiovascular death by 30% (Nocon et al., 2008; Paffenbarger Jr et al., 1986), and adequate exercise leads to an additional 1–2 years of life compared to those with little or no physical activity (Franco et al., 2005; Kent‐Braun et al., 2000). Longevity studies have examined apolipoprotein E (ApoE), a gene involved in the metabolism of lipoproteins, and ApoE E3 and E2 have been associated with a lower risk of death in individuals who are regularly physically active compared to sedentary individuals (Dankner et al., 2020). After endurance exercise, Drosophila in the exercise group showed increased levels of mtp expression and showed varying degrees of prolongation of mean and maximum lifespan compared to Drosophila without exercise intervention. Lipid metabolism is crucial in regulating aging and longevity (Mutlu et al., 2021). According to our understanding, the prolonged lifespan may be attributed to the substantial mitigation of age‐induced declines in both mRNA and protein levels of mtp through endurance exercise. This effect, in turn, leads to increased mRNA expression levels of apolpp and Acox3, resulting in decreased levels of TC, LDL‐C, and TG. Consequently, there is an enhancement in whole‐body lipid metabolism, promoting an increase in systolic diameter and shortening fraction. This improvement in cardiac function contributes to reduced lipid accumulation in aging flies, ultimately serving as a mechanism to ameliorate age‐related diastolic dysfunction and extend the average lifespan. Our results can be summarized as follows: (1) the mtp RNAi youth group of Drosophila exhibits age‐related diastolic dysfunction similar to that of the Tub×W 1118 age group and has a significantly shorter lifespan, which may be related to the low level of mtp expression. (2) Endurance exercise improves diastolic dysfunction and prolongs lifespan in old Drosophila, which may be related to the upregulation of mtp expression by endurance exercise. (3) Relevant assays of lipid metabolism showed that compared with the Tub×W 1118 youth group, the mtp RNAi youth group exhibited higher TC, LDL‐C, and TG levels, while endurance exercise could reduce the TC, LDL‐C, and TG levels in the aged group, which may be related to the increased expression of mtp downstream of apolpp and Acox3.
5. CONCLUSION
Our findings suggest that endurance exercise ameliorates age‐related diastolic dysfunction and prolongs average lifespan, which may be related to the upregulation of mtp expression and thus enhances lipid metabolism in aged Drosophila (Figure 7). Importantly, our findings provide new insights for future research on cardiac aging and exercise interventions to improve age‐related diastolic dysfunction and prolong lifespan.
FIGURE 7.

Schematic diagram of the relationship between endurance exercise, mtp, and age‐related diastolic dysfunction.
AUTHOR CONTRIBUTIONS
Tianhang Peng was involved in conceptualisation and writing—original draft preparation. Tianhang Peng and Meng Ding were involved in methodology. Hanhui Yan, Ping Zhang and Rui Tian were involved in formal analysis and investigation. Yin Guo and Lan Zheng were involved in writing—review and editing. Lan Zheng was involved in funding acquisition, resources, and supervision. All authors have read and approved the final manuscript.
FUNDING INFORMATION
This research was funded by the National Natural Science Foundation of China, grant number 32071175.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We thank the Key Laboratory of Physical Fitness and Exercise Rehabilitation of Hunan Province and Tsinghua University for fly stocks. We also thank Karen Ocorr and Rolf Bodmer (Sanford Burnham Institute of Neuroscience and Aging Research Center) for supporting semiautomatic optical echocardiographic analysis software.
Peng, T. , Ding, M. , Yan, H. , Zhang, P. , Tian, R. , Guo, Y. , & Zheng, L. (2024). Endurance exercise upregulates mtp expression in aged Drosophila to ameliorate age‐related diastolic dysfunction and extend lifespan. Physiological Reports, 12, e15929. 10.14814/phy2.15929
DATA AVAILABILITY STATEMENT
Data are contained within the article.
REFERENCES
- Anderson, K. M. , Castelli, W. P. , & Levy, D. (1987). Cholesterol and mortality. 30 years of follow‐up from the Framingham study. Journal of the American Medical Association, 257, 2176–2180. [DOI] [PubMed] [Google Scholar]
- Barzilai, N. , Gabriely, I. , Gabriely, M. , Iankowitz, N. , & Sorkin, J. D. (2001). Offspring of centenarians have a favorable lipid profile. Journal of the American Geriatrics Society, 49, 76–79. [DOI] [PubMed] [Google Scholar]
- Bjorkegren, J. , Veniant, M. , Kim, S. K. , Withycombe, S. K. , Wood, P. A. , Hellerstein, M. K. , Neese, R. A. , & Young, S. G. (2001). Lipoprotein secretion and triglyceride stores in the heart. The Journal of Biological Chemistry, 276, 38511–38517. [DOI] [PubMed] [Google Scholar]
- Bodmer, R. (1995). Heart development in drosophila and its relationship to vertebrate systems. Trends in Cardiovascular Medicine, 5, 21–27. [DOI] [PubMed] [Google Scholar]
- Borlaug, B. A. , & Redfield, M. M. (2011). Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation, 123(18), 2006–2013. 10.1161/CIRCULATIONAHA.110.954388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarato, A. , Dambacher, C. M. , Knowles, A. F. , Kronert, W. A. , Bodmer, R. , Ocorr, K. , & Bernstein, S. I. (2008). Myosin transducer mutations differentially affect motor function, myofibril structure, and the performance of skeletal and cardiac muscles. Molecular Biology of the Cell, 19, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori, G. , Segurini, C. , Picozzi, A. , Padeletti, L. , & Anzà, C. (2018). Exercise and heart failure: An update. ESC Heart Fail, 5(2), 222–232. 10.1002/ehf2.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty, E. F. , Hubert, H. B. , Lingala, V. B. , & Fries, J. F. (2008). Reduced disability and mortality among aging runners: A 21‐year longitudinal study. Archives of Internal Medicine, 168(15), 1638–1646. 10.1001/archinte.168.15.1638 Erratum in: 2008 Dec 8;168(22):2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon, K. M. (2020). Exercise and cardiovascular health: New routes to reap more rewards. Cardiovascular Research, 116, e56–e58. [DOI] [PubMed] [Google Scholar]
- Coulom, H. , & Birman, S. (2004). Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. The Journal of Neuroscience, 24(48), 10993–10998. 10.1523/JNEUROSCI.2993-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Blumenthal, R. S. , Flaws, J. A. , Whiteman, M. K. , Langenberg, P. , Bachorik, P. S. , & Bush, T. L. (2001). Non–high‐density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Archives of Internal Medicine, 161, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Cullen, P. (2000). Evidence that triglycerides are an independent coronary heart disease risk factor. The American Journal of Cardiology, 86, 943–949. [DOI] [PubMed] [Google Scholar]
- Dankner, R. , Ben Avraham, S. , Harats, D. , & Chetrit, A. (2020). ApoE genotype, lipid profile, exercise, and the associations with cardiovascular morbidity and 18‐year mortality. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(10), 1887–1893. 10.1093/gerona/glz232 [DOI] [PubMed] [Google Scholar]
- Davidson, N. O. , & Shelness, G. S. (2000). APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annual Review of Nutrition, 20, 169–193. 10.1146/annurev.nutr.20.1.169 [DOI] [PubMed] [Google Scholar]
- Diop, S. B. , Birse, R. T. , & Bodmer, R. (2017). High fat diet feeding and high throughput Triacylglyceride assay in drosophila melanogaster. Journal of Visualized Experiments, 127, 56029. 10.3791/56029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, M. , Callol‐Massot, C. , Chu, A. , Ruiz‐Lozano, P. , Izpisua Belmonte, J. C. , Giles, W. , Bodmer, R. , & Ocorr, K. (2009). A new method for detection and quantification of heartbeat parameters in drosophila, zebrafish, and embryonic mouse hearts. BioTechniques, 46(2), 101–113. 10.2144/000113078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza‐Luces, C. , Santos‐Lozano, A. , Joyner, M. , Carrera‐Bastos, P. , Picazo, O. , Zugaza, J. L. , Izquierdo, M. , Ruilope, L. M. , & Lucia, A. (2018). Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nature Reviews. Cardiology, 15, 731–743. [DOI] [PubMed] [Google Scholar]
- Franco, O. H. , de Laet, C. , Peeters, A. , et al. (2005). Effects of physical activity on life expectancy with cardiovascular disease. Archives of Internal Medicine, 165(20), 2355–2360. [DOI] [PubMed] [Google Scholar]
- Garatachea, N. , Santos‐Lozano, A. , Sanchis‐Gomar, F. , Fiuza‐Luces, C. , Pareja‐Galeano, H. , Emanuele, E. , & Lucía, A. (2014). Elite athletes live longer than the general population: A meta‐analysis. Mayo Clinic Proceedings, 89, 1195–1200. [DOI] [PubMed] [Google Scholar]
- Geesaman, B. J. , Benson, E. , Brewster, S. J. , Kunkel, L. M. , Blanché, H. , Thomas, G. , Perls, T. T. , Daly, M. J. , & Puca, A. A. (2003). Haplotype‐based identification of a microsomal transfer protein marker associated with the human lifespan. Proceedings of the National Academy of Sciences of the United States of America, 100, 14115–14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka, S. , & Lee, I. M. (2017). Physical activity lowers mortality and heart disease risks. Lancet, 390(10113), 2609–2610. [DOI] [PubMed] [Google Scholar]
- Gordon, D. A. , Wetterau, J. R. , & Gregg, R. E. (1995). Microsomal triglyceride transfer protein: A protein complex required for the assembly of lipoprotein particles. Trends in Cell Biology, 5(8), 317–321. [DOI] [PubMed] [Google Scholar]
- Gordon, D. J. , Probstfield, J. L. , Garrison, R. J. , Neaton, J. D. , Castelli, W. P. , Knoke, J. D. , Jacobs, D. R., Jr. , Bangdiwala, S. , & Tyroler, H. A. (1989). High‐density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation, 79, 8–15. [DOI] [PubMed] [Google Scholar]
- Hagan, D. L. , Kienzle, B. , Jamil, H. , & Hariharan, N. (1994). Transcriptional regulation of human and hamster microsomal triglyceride transfer protein genes. Cell type‐specific expression and response to metabolic regulators. The Journal of Biological Chemistry, 269(46), 28737–28744. [PubMed] [Google Scholar]
- Hirokane, H. , Nakahara, M. , Tachibana, S. , Shimizu, M. , & Sato, R. (2004). Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor‐4. The Journal of Biological Chemistry, 279, 45685–45692. [DOI] [PubMed] [Google Scholar]
- Hotta, K. , Chen, B. , Behnke, B. J. , Ghosh, P. , Stabley, J. N. , Bramy, J. A. , Sepulveda, J. L. , Delp, M. D. , & Muller‐Delp, J. M. (2017). Exercise training reverses age‐induced diastolic dysfunction and restores coronary microvascular function. The Journal of Physiology, 595(12), 3703–3719. 10.1113/JP274172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, K. A. , & Reynolds, R. M. (2005). Evolutionary and mechanistic theories of aging. Annual Review of Entomology, 50, 421–445. 10.1146/annurev.ento.50.071803.130409 [DOI] [PubMed] [Google Scholar]
- Hussain, M. M. (2000). A proposed model for the assembly of chylomicrons. Atherosclerosis, 148(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Jakovljevic, D. G. (2018). Physical activity and cardiovascular aging: Physiological and molecular insights. Experimental Gerontology, 109, 67–74. 10.1016/j.exger.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Kass, D. A. , Bronzwaer, J. G. , & Paulus, W. J. (2004). What mechanisms underlie diastolic dysfunction in heart failure? Circulation Research, 94(12), 1533–1542. 10.1161/01.RES.0000129254.25507.d6 [DOI] [PubMed] [Google Scholar]
- Kaushik, G. , Zambon, A. C. , Fuhrmann, A. , Bernstein, S. I. , Bodmer, R. , Engler, A. J. , & Cammarato, A. (2012). Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. Journal of Cellular and Molecular Medicine, 16(8), 1656–1662. 10.1111/j.1582-4934.2011.01517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent‐Braun, J. A. , Ng, A. V. , & Young, K. (2000). Skeletal muscle contractile and noncontractile components in young and older women and men. Journal of Applied Physiology, 88(2), 662–668. [DOI] [PubMed] [Google Scholar]
- Lakatta, E. G. , & Levy, D. (2003). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises, part I: Aging arteries: A “set up” for vascular disease. Circulation, 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Law, M. R. , Wald, N. J. , & Rudnicka, A. (2003). Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: Systematic review and meta‐analysis. BMJ, 326, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. C. , Patel, A. V. , Matthews, C. E. , Berrington de Gonzalez, A. , Park, Y. , Katki, H. A. , Linet, M. S. , Weiderpass, E. , Visvanathan, K. , Helzlsouer, K. J. , Thun, M. , Gapstur, S. M. , Hartge, P. , & Lee, I. M. (2012). Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Medicine, 9, e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu, A. S. , Duffy, J. , & Wang, M. C. (2021). Lipid metabolism and lipid signals in aging and longevity. Developmental Cell, 56(10), 1394–1407. 10.1016/j.devcel.2021.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navasa, M. , Gordon, D. A. , Hariharan, N. , Jamil, H. , Shigenaga, J. K. , Moser, A. , Fiers, W. , Pollock, A. , Grunfeld, C. , & Feingold, K. R. (1998). Regulation of microsomal triglyceride transfer protein mRNA expression by endotoxin and cytokines. Journal of Lipid Research, 39, 1220–1230. [PubMed] [Google Scholar]
- Nielsen, L. B. , Perko, M. , Arendrup, H. , & Andersen, C. B. (2002). Microsomal triglyceride transfer protein gene expression andtriglyceride accumulation in hypoxic human hearts. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 1489–1494. [DOI] [PubMed] [Google Scholar]
- Nocon, M. , Hiemann, T. , Muller‐Riemenschneider, F. , et al. (2008). Association of physical activity with all‐cause and cardiovascular mortality: A systematic review and meta‐analysis. European Journal of Cardiovascular Prevention and Rehabilitation, 15(3), 239–246. [DOI] [PubMed] [Google Scholar]
- North, B. J. , & Sinclair, D. A. (2012). The intersection between aging and cardiovascular disease. Circulation Research, 110(8), 1097–1108. 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr, K. , Perrin, L. , Lim, H. Y. , Qian, L. , Wu, X. , & Bodmer, R. (2007). Genetic control of heart function and aging in drosophila. Trends in Cardiovascular Medicine, 17(5), 177–182. 10.1016/j.tcm.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger, R. S., Jr. , Hyde, R. T. , Wing, A. L. , & Hsieh, C. C. (1986). Physical activity, all‐cause mortality, and longevity of college alumni. New England Journal of Medicine, 314(10), 605–613. [DOI] [PubMed] [Google Scholar]
- Parra, V. , Verdejo, H. E. , Iglewski, M. , Del Campo, A. , Troncoso, R. , Jones, D. , Zhu, Y. , Kuzmicic, J. , Pennanen, C. , Lopez‐Crisosto, C. , Jaña, F. , Ferreira, J. , Noguera, E. , Chiong, M. , Bernlohr, D. A. , Klip, A. , Hill, J. A. , Rothermel, B. A. , Abel, E. D. , … Lavandero, S. (2014). Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt‐mTOR‐NFκB‐Opa‐1 signaling pathway. Diabetes, 63(1), 75–88. 10.2337/db13-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, T. , Ding, M. , Yan, H. , Li, Q. , Zhang, P. , Tian, R. , & Zheng, L. (2022). Exercise training upregulates cardiac mtp expression in Drosophila melanogaster with HFD to improve cardiac dysfunction and abnormal lipid metabolism. Biology (Basel)., 11(12), 1745. 10.3390/biology11121745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza, N. , Gosangi, B. , Devilla, S. , Arking, R. , & Wessells, R. (2009). Exercise‐training in young Drosophila melanogaster reduces age‐related decline in mobility and cardiac performance. PLoS One, 4(6), e5886. 10.1371/journal.pone.0005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza, N. , & Wessells, R. J. (2011). Drosophila models of cardiac disease. Progress in Molecular Biology and Translational Science, 100, 155–210. 10.1016/B978-0-12-384878-9.00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, L. , Wythe, J. D. , Liu, J. , Cartry, J. , Vogler, G. , Mohapatra, B. , Otway, R. T. , Huang, Y. , KIng, J. , Maillet, M. , Zheng, Y. , Crawley, T. , Taghli‐Lamallem, O. , Semsarian, C. , Dunwoodie, S. , Winlaw, D. , Harvey, R. P. , Fatkin, D. A. , Towbin, J. A. , … Bodmer, R. (2011). Tinman/Nkx2–5 acts via miR‐1 and upstream of Cdc42 to regulate heart function across species. The Journal of Cell Biology, 193, 1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Fernández‐García, B. , Lehmann, H. I. , Li, G. , Kroemer, G. , López‐Otín, C. , & Xiao, J. (2023). Exercise sustains the hallmarks of health. Journal of Sport and Health Science, 12(1), 8–35. 10.1016/j.jshs.2022.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. I. , & Parkhurst, S. M. (2002). Drosophila Sir2 is required for heterochromatic silencing and by euchromatic hairy/E(Spl) bHLH repressors in segmentation and sex determination. Cell, 109(4), 447–458. [DOI] [PubMed] [Google Scholar]
- Sato, R. , Miyamoto, W. , Inoue, J. , Terada, T. , Imanaka, T. , & Maeda, M. (1999). Sterol regulatory element‐binding protein negatively regulates microsomal triglyceride transfer protein gene transcription. The Journal of Biological Chemistry, 274, 24714–24720. [DOI] [PubMed] [Google Scholar]
- Shoulders, C. C. , & Shelness, G. S. (2005). Current biology of mtp: Implications for selective inhibition. Current Topics in Medicinal Chemistry, 5(3), 283–300. 10.2174/1568026053544560 [DOI] [PubMed] [Google Scholar]
- Sujkowski, A. , Richardson, K. , Prifti, M. V. , Wessells, R. J. , & Todi, S. V. (2022). Endurance exercise ameliorates phenotypes in drosophila models of spinocerebellar ataxias. eLife, 16(11), e75389. 10.7554/eLife.75389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghli‐Lamallem, O. , Akasaka, T. , Hogg, G. , Nudel, U. , Yaffe, D. , Chamberlain, J. S. , Ocorr, K. , & Bodmer, R. (2008). Dystrophin deficiency in drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell, 7, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, D. F. , Wilcox, M. , McCormick, M. A. , Lawler, E. , & Perls, T. T. (2003). Cardiovascular advantages among the offspring of centenarians. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58, M425–M431. [DOI] [PubMed] [Google Scholar]
- Tinkerhess, M. J. , Ginzberg, S. , Piazza, N. , & Wessells, R. J. (2012). Endurance training protocol and longitudinal performance assays for Drosophila melanogaster. Journal of Visualized Experiments, 61, 3786. 10.3791/3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler, G. , & Ocorr, K. (2009). Visualizing the beating heart in drosophila. Journal of Visualized Experiments, 31, 1425. 10.3791/1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, D. T. , Zheng, L. , Li, J. X. , Cheng, D. , Liu, Y. , Lu, K. , & Hou, W. Q. (2019). Endurance exercise resistance to lipotoxic cardiomyopathy is associated with cardiac NAD+/dSIR2/PGC‐1α pathway activation in old drosophila. Biology Open, 8(10), bio044719. 10.1242/bio.044719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, D. T. , Zheng, L. , Ni, L. , Wang, H. , Feng, Y. , & Zhang, M. (2016). The expression of CG9940 affects the adaptation of cardiac function, mobility, and lifespan to exercise in aging drosophila. Experimental Gerontology, 83, 6–14. 10.1016/j.exger.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Wessells, R. J. , Fitzgerald, E. , Cypser, J. R. , Tatar, M. , & Bodmer, R. (2004). Insulin regulation of heart function in aging fruit flies. Nature Genetics, 36, 1275–1281. [DOI] [PubMed] [Google Scholar]
- Wetterau, J. R. , Lin, M. C. , & Jamil, H. (1997). Microsomal triglyceride transfer protein. Biochimica et Biophysica Acta, 1345(2), 136–150. [DOI] [PubMed] [Google Scholar]
- Zheng, L. , Feng, Y. , Wen, D. T. , Wang, H. , & Wu, X. S. (2015). Fatiguing exercise initiated later in life reduces incidence of fibrillation and improves sleep quality in drosophila. Age (Dordrecht, Netherlands), 37, 9816. 10.1007/s11357-015-9816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.


