Abstract
Guided tissue regeneration (GTR) has been widely used in the periodontal treatment of intrabony and furcation defects for nearly four decades. The treatment outcomes have shown effectiveness in reducing pocket depth, improving attachment gain and bone filling in periodontal tissue. Although applying GTR could reconstruct the periodontal tissue, the surgical indications are relatively narrow, and some complications and race ethic problems bring new challenges. Therefore, it is challenging to achieve a consensus concerning the clinical benefits of GTR. With the appearance of stem cell-based regenerative medicine, mesenchymal stem/stromal cells (MSCs) have been considered a promising cell resource for periodontal regeneration. In this review, we highlight preclinical and clinical periodontal regeneration using MSCs derived from distinct origins, including non-odontogenic and odontogenic tissues and induced pluripotent stem cells, and discuss the transplantation procedures, therapeutic mechanisms, and concerns to evaluate the effectiveness of MSCs.
Keywords: Periodontal disease, Guided tissue regeneration, Periodontal regeneration, Mesenchymal stem/stromal cells, Induced pluripotent stem cells
1. Introduction
Periodontal disease, as one of the non‐communicable diseases, has been considered a global public health problem, with a high prevalence of up to 50% worldwide [1] and an estimated 1.1 billion severe periodontitis globally in 2019 [2]. With the invasion of periodontal pathogens, the accompanied production of cytokines results in soft and hard tissue degradation. Initial non-surgical periodontal therapy could control periodontal inflammation, including scaling, root planing, and oral hygiene instruction. Non-surgical periodontal treatment can reverse the periodontitis to a stable stage. However, in some cases, even after the inflammation is controlled by eliminating or decreasing subgingival biofilm, the persistence of deep pockets or furcation involvement indicates periodontal surgery [3].
Periodontal regenerative technologies have been applied to improve the short- and long-term clinical outcomes of periodontally compromised teeth. However, the authentic reconstruction of periodontal tissues is still a severe challenge. Starting in the last century, Langer et al. first proposed tissue engineering as a concept technique in the regeneration of lost tissues [4], and the concept of periodontal guided tissue regeneration (GTR) was promoted [5]. The basic idea of GTR is to improve the function of the associated cells and regulate the local microenvironment to facilitate regeneration. As the crucial component of the periodontium complex, the periodontal ligament (PDL) maintains the homeostasis of all periodontal tissues. Therefore, the aim of the GTR is the reformation of bone-PDL-cementum complex [6]. GTR has successfully succeeded in clinical practice by applying biomaterials and biological agents. However, the most frequently encountered complications include the collapse of the barrier membrane and soft tissue dehiscence, resulting in bacterial invasion and infection, and subsequent loss of the bone graft, leading to impaired regenerative outcomes. Besides, the surgical procedure results in hemorrhage, hematoma, and pain. Sometimes it takes a long time to recover, which is unfriendly to certain patients with dental anxiety. In addition, the potential of disease transmission and ethical concerns caused by applications of allogeneic and xenogeneic bone substitutes should be considered. However, the occurrence is infrequent, and limited cases are documented [7].
The undifferentiated mesenchymal cells in PDL are believed to differentiate into cementoblasts, osteoblasts, and fibroblasts. The investigations to regenerate periodontal tissues by stem cell transplantation have been wildly conducted over decades. Given the capacities of self-renewal, multipotentiality [8], immunomodulation, and tissue regeneration [9], mesenchymal stem/stromal cells (MSCs) are considered promising cell resources for cell-based regenerative medicine. This review highlights the different cell origins to generate MSCs and the MSC applications in preclinical and clinical research. It discusses the therapeutic mechanisms and concerns to evaluate the effectiveness of MSCs.
2. MSCs for periodontal regeneration
2.1. MSCs derived from non-odontogenic tissues
MSCs refer to a population with characteristics of secreting trophic factors [10], promoting angiogenesis [11], [12] and homing to inflamed or injured tissues [13], including fibroblasts, myofibroblasts, and even a small amount of stem/progenitor cells [14]. Given the capacity for self-renewal and multipotentiality, stroma cells derived from bone marrow were first described as stem cells [15]. Plastic adherence, expression of CD73, CD90, and CD105, lacking expression of CD11b, CD14, CD19, CD34, CD45, CD79a, HLA-DR, and capabilities to differentiate into adipocyte, chondrocyte, and osteoblast in vitro have been formulated as minimal criteria to define MSCs [16]. Notably, the markers to identify MSCs are occasionally variable in case of species differences, tissue sources, and culture conditions and even continuously evolving as our knowledge accumulates [8], [14].
In humans, MSCs can be isolated from multiple tissue resources, including bone marrow (BMMSCs) [17], [18], adipose tissue (ADMSCs) [19], [20], umbilical cord tissue (UCMSCs) [21], placenta [22], [23], umbilical cord blood [24], peripheral blood [25], [26], and skin [27]. The first two are the most frequently researched [28]. Hundreds of millions of MSCs can be yielded within a few weeks [18], [29], making the autologous MSCs applicable in clinical therapy. However, many preclinical and a portion of clinical trials are utilizing allogeneic MSCs (www.clinicaltrials.gov). One of the considerations may account for the declining number and overall fitness of MSCs obtained from donors with age [30]. Notwithstanding, the proprietary concerns are nonnegligible when determining allogeneic or autologous MSCs, or even distinct tissue resources [9]. Since 2012, MSCs have been conditionally approved in Canada, New Zealand, and Japan to treat children with graft versus host disease (GvHD) [9]. Besides, in 2018, the European Commission approved a marketing authorization for an MSC product (Alofisel) derived from the fat tissue to treat complex anal fistulas in adults with Crohn’s disease [31], [32].
Although there is not yet an MSC product approved in the clinic for periodontal disease, the preclinical and clinical studies showed that the transplantation of BMMSCs could improve periodontal regeneration. In a previously published review, Iwasaki et al. [33] extensively summarized the early preclinical studies of BMMSCs for cell-based periodontal regeneration. Herein we would like to supplement some preclinical studies in recent 5-year applying BMMSCs (Table 1). Collectively, the BMMSCs are likely to ameliorate periodontitis by regenerating periodontium-like tissues, including PDL fibers, cementum, and alveolar bone [34], [35], [36]. For clinic trials (Table 2), transplantation of UCMSCs with scaffolds and growth factor indicated clinic improvements and improved radiographic bone fill (RBF) on intrabony defects [37]. Transplantation of adipose tissue-derived multi-lineage progenitor cells (ADMPCs) with fibrin gel promoted probing depth (PD) reduction, clinical attachment level (CAL), and radiographic bone fill (RBF) [38]. However, The BMMSCs with autologous fibrin/platelet lysate (aFPL) showed no significant clinical or RBF improvement compared with the controls [39]. Thus, more sophisticated clinical trials should be carried out to convince the capability of non-odontogenic MSCs on periodontal regeneration.
Table 1.
BMMSCs in preclinical periodontal regeneration.
| Author/ year | Animal/ sample size | Cell source/ morphology | Carrier/Scaffold | Test | Control | Duration | Outcome measures | Results | Regenerated tissues |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. 2018[162] | Rat | Allogenic/ pellet-suspension | HyStem-HP hydrogel | ASA-BMMSCs with HP hydrogel | ASA-hydrogel, BMMSCs-hydrogel | 3 weeks | Histomorphometry, IHC, µCT | ASA-BMMSCs reduced inflammatory infiltration and alveolar bone loss | / |
| Vaquette et al. 2019[35] | Sheep | Autologous/ cell sheet | Biphasic scaffold | BMMSCs sheet on the biphasic scaffold | empty scaffold, GCs, PDLCs | 5, 10 weeks | Histomorphometry, µCT | Multiphasic construct with BMMSCs and PDLCs promote periodontal regeneration | Alveolar bone, cementum, PDL |
| Costa et al. 2022[36] | Rat | Allogenic/ pellet-suspension | / | BMMSCs in PBS | Defects left to spontaneously heal, BMAC in PBS | 30 days | Histomorphometry, µCT | BMMSCs promote early bone formation and maturation | Cementum-like tissue with new insertion of fibers |
| Jung et al. 2022[34] | Rat | Allogenic / pellet-suspension | / | BMP7-eBMSCs | eBMSC, hPDLSC | 8 weeks | µCT | Transplantation of BMP7-eBMSCs is a feasible for periodontal regeneration | Alveolar bone, PDL |
ASA: Acetylsalicylic acid; µCT: Micro-computed tomography; IHC: Immunohistochemistry; BMMSCs: Bone marrow mesenchymal stem/stromal cells; GCs: Gingival cells; PDLCs: Periodontal ligament cells; PDL: Periodontal ligament; PBS: Phosphate buffered saline; BMAC: Bone marrow aspirate concentrate; BMP: Bone morphogenetic protein; BMP7-eBMSCs: BMP-7-expressing engineered BMMSCs.
Table 2.
Non-odontogenic MSCs in clinical periodontal regeneration.
| Author/ year | RCT | Sample size | Cell source/ morphology | Carrier/Scaffold | Test | Control | Duration | Outcome measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| Dhote et al. 2015[37] | Yes | 14 patients, 24 infrabony defects | Allogenic, UCMSCs | β-TCP and rh-PDGF-BB | UCMSCs cultured on β-TCP combined with rh-PDGF-BB | OFD | 6 months | PI, PBI, PPD, R-CAL, DD, LBG | MSC on β-TCP with rh-PDGF-BB promoted CAL, PPD reduction, RBF and LBG |
| Apatzidou et al. 2021[39] | Yes | 27 patients in 3 groups | Autologous, BMMSCs (alveolar bone marrow) | Collagen scaffold enriched with aFPL | BMMSCs seeded on collagen scaffold enriched with aFPL | MAFS, collagen scaffold enriched with aFPL devoid of BMMSCs | 12 months | Clinical/ radiographical assessment | Similar clinic improvements among groups were found; RBF was not obvious using BMMSCs with aFPL |
| Takedachi et al. 2022[38] | No Single-arm | 12 patients | Autologous, adipose tissue-derived multi-lineage progenitor cells (ADMPCs) | Fibrin gel | ADMPCs mixed with fibrin gel | NA | 9 months | PPD, CAL, radiographical assessment | PPD reduction, CAL gain; new alveolar born formation |
RCT: Randomized controlled trial; UCMSCs: Umbilical cord-derived mesenchymal stem/stromal cells; TCP: Tricalcium phosphate, rh-PDGF: Recombinant human platelet-derived growth factor; OFD: Open flap debridement; PI: Plaque index; PBI: Papillary bleeding index; PPD: Probing pocket depth; R-CAL: Relative clinical attachment level; DD: Defect depth, LBG: Linear bone growth; RBF: Radiographic bone fill; BMMSCs: Bone marrow mesenchymal stem/stromal cells; aFPL: autologous fibrin/platelet lysate; MAFS: Minimal access flap surgery; ADMPCs: adipose tissue-derived multi-lineage progenitor cells; NA: Not available.
2.2. MSCs derived from odontogenic tissues
MSC-like cells have been isolated from a variety of human dental tissues, including dental pulp [40], exfoliated deciduous teeth [41], periodontal ligament [42], apical papilla [43], [44], dental follicle [45] and gingiva [46]. Compared with classic BMMSCs, dental tissue-derived stem/progenitor cells (DSCs) are supposed to be similar to neural crest cells due to ectomesenchyme origin [47]. DSCs can differentiate into osteogenic, adipogenic, and neurogenic lineages, with more tendency to odontogenic development rather than osteogenic one [48]. Thus, DSCs are widely investigated in tissue regeneration in dentistry (Table 3, Table 4).
Table 3.
Odontogenic tissue derived stem cells in preclinical periodontal regeneration.
| Author/year | Animals, sample size | Cell source/ morphology | Carrier/Scaffold | Test | Control | Duration | Outcome measures | Results | Regenerated tissues |
|---|---|---|---|---|---|---|---|---|---|
| Khorsand et al. 2013[191] | Mongrel dogs, 10 | Autologous, DPSCs | Bio-Oss | DPSCs+Bio-oss | Bio-oss | 8 weeks | Histomorphometry | DPSCs and Bio-Oss improved regeneration of periodontal tissues. | Cementum, bone, and PDL |
| Hu et al. 2016[192] | Miniature pigs, 12 | Xenogenic,hDPSCs | NA | hDPSCs cell injection /sheet implanatation | NA | 12 weeks | probing depth, attachment loss, µCT | hDPSCS cell injection /sheet transplantation significantly regenerated periodontal bone in swine | Bone |
| Fu et al. 2014[193] | Miniature pigs, 6 | Allogeneic, SHEDs and PDLSCs | HA/TCP | SHEDs+HA/TCP, PDLSCs +HA/TCP | HA/TCP | 12 weeks | PD, GR, AL, µCT, Histomorphometry | SHEDs and PDLSCs reduced PD, GR, and AL and induced hard tissue regeneration | Bone, cementum, and PDL |
| Gao et al. 2018[154] | Sprague Dawley rat, 28 | Xenogenic, hSHEDs (survive 7 days) | NA | SHED injection | Normal saline injection | 5 weeks | Histomorphometry, µCT | Multi‐dose SHED injection reduced inflammatory response and promoted bone regeneration | Bone |
| Yang et al. 2019[194] | Sprague Dawley rat, 9 | Xenogenic, cell sheets of hSHEDs and hDFSCs | TDM | TDM wrapped with SHEDs or DFCSs | TDM | 8 weeks | Histomorphometry | SHEDs or DFCSs with TDM regenerated periodontal tissues | blood vessels, PDL, bone |
| Yang et al. 2019[76] | Beagle dog, 4 | Xenogenic, cell sheets of hDFSCs | TDMP or HA/β-TCP | hDFSCs with TDMPs or HA/β-TCP | TDMP or HA/β-TCP | 8 weeks | Histomorphometry, µCT | DFSCs with TDMP improved regeneration of periodontal-like tissues | Cementum-lilke tissue, bone |
| Shi et al. 2018[67] | Beagle dog, 6 | Xenogenic, hPDLSCs | improved BCP | PDLSCs seeded on BCP | OFD | 4,8,12 weeks | Histomorphometry, µCT | PDLSCs seeded on BCP enhanced new bone formation and PDL-like collagen fibers | Bone and PDL |
| Li et al. 2018[71] | Miniature pigs | Xenogenic, hSCAPs from extracted wisdom teeth | NA | SCAP injection | Normal saline injection | 12 weeks | PD, GR, AL, histomorphometry, µCT | SCAPs restored periodontal tissue defect | Sharpey’s fibers, PDL, cementum, Bone |
| El-Sayed et al. 2012[195] | Miniature pigs | Autologous, GMSCs | DBCB, collagen | GMSCs loaded on DBCB/ collagen | DBCB, collagen, SRP, untreated | 12 weeks | CAL, PD, GR, RDV, HAL, JE, CTA | GMSCs loaded on scaffolds showed higher ΔCAL, ΔPD, ΔGR, HAL and lower JE and CTA | Bone, cementum, PDL |
| Sun et al. 2018[79] | Mouse | Xenogenic, GMSCs from human | NA | GMSCs suspends in a-MEM | a-MEM | 1, 2, 4 weeks | Histomorphometry | GMSCs increased bone height by homing to the periodontitis site | Bone, PDL |
| Qiu et al. 2020[80] | Rat | Xenogenic, GMSCs from human | Collagen | CM of GMSCs, PDLSCs/GFs loaded with collagen | Collagen | 1, 2, 4 weeks | Histomorphometry | CMSC- and PDLC-CM regenerated periodontal defects | Cementum-like tissue, bone, PDL |
(h)DPSCs: (human) Pulp tissue-derived dental pulp stem cells; PDL: Periodontal ligament; µCT: Micro computed tomography; SHEDs: Stem cells from human exfoliated deciduous teeth; PDLSCs: Periodontal ligament stem cells, HA/TCP: Hydroxyapatite/Tricalcium phosphate; DFSCs: Dental follicle cells; PD: Probing depth; GR: Gingival recession; AL: Attachment loss; TDM(P): Treated dentin matrix (particle); BCP: Biphasic calcium phosphate; OFD: Open flap debridement; SCAP: Stem cells from apical papilla; GMSCs: Gingiva-derived mesenchymal stem cells; DBCB: Deproteinized bovine cancellous bone; SRP: Scaling and root planing; RDV: Radiographic defect volume; HAL: Histological attachment level; JE: Junctional epithelium length; CTA: Connective tissue adhesion; CAL: Clinical attachment level; CM: Conditioned medium; GFs: Gingival fibroblast; NA: Not available.
Table 4.
Odontogenic tissue derived stem cells in clinical periodontal regeneration.
| Author/year | RCT | Sample size | Cell source/ morphology | Scaffold | Test | Control | Duration | Outcome measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. 2016[196] | No, Case | 2 patients, introbony defects | Autologous, DPSCs from inflammatory dental pulp tissues | β-TCP | DPSCs loaded on β-TCP | NA | 1, 3, 9 months | Dental plaque, sulcus bleeding index, GR, PD, furcation lesion, mobility | DPSC has both clinic and radiographic improvements |
| Aimetti et al. 2018[56] | No, Case Series | 11 patients, 11 introbony defects | Autologous, DPSCs from tooth requiring extraction for impaction or malpositioning | collagen sponge | DPSCs loaded on collagen sponge | NA | 6, 12 months | FMPS, FMBS, PD, CAL, REC, INFRA, periapical standardized radiographs | DPSCs improved complete pocket closure and RBF |
| Ferrarotti et al. 2018[197] | Yes | 29 patients, 29 introbony defects | Autologous, DPSCs from one vital tooth requiring extraction | collagen sponge | DPSCs seeded onto collagen sponge | collagen sponge | 6, 12 months | FMPS, FMBS, PD, CAL, REC, INTRA, IBD | DPSCs significantly improved PD, CAL, bone defect fill |
| Chen et al. 2016[70] | Yes | 29 patients, 41 teeth with periodontitis | Autologous, PDLSC sheets from third molars | Bio-oss | GTR and PDLSC sheets with Bio-oss | GTR with Bio-oss | 3, 6, 12 months | Alveolar bone height, CAL, PD, GR | Each group exerted improvement for alveolar bone height; no statistical difference for the two groups |
| Iwata et al. 2018[69] | No, single-arm | 10 patients | Autologous, PDLSC sheets from wisdom teeth | β-TCP | PDLSC sheets withβ-TCP | NA | 3, 6 months | Safety, GI, PI, PD, BOP, CAL, radiographic bone height (CBCT) | PDLSC sheets with β-TCP improved PD reduction, CAL gain and radiographic bone height |
RCT: Randomized controlled trial; DPSCs: Pulp tissue-derived dental pulp stem cells; β-TCP: beta tricalcium phosphate; GR: Gingival recession; PD: Probing depth; FMPS: Full-mouth plaque score; FMBS: Full-mouth bleeding score; PD: Probing depth; CAL: Clinical attachment level; REC: Gingival recession; INTRA: Intrasurgical intrabony defect depth; RBF: Radiographic bone fill; IBD: Intrabony defect depth; PDLSCs: Periodontal ligament stem cells; GTR: Guided tissue regeneration; GI: Gingival index; PI: Plaque index; BOP: Bleeding on probing; CBCT: Cone beam computed tomography; NA: Not available.
Pulp tissue-derived dental pulp stem cells (DPSCs) can differentiate into multilineages, including odontoblast-like cells, adipocytes, neurocytes [49], osteoblasts [50], chondrogenic and myogenic cells [51], and endotheliocytes [52]. It was indicated that the complex of DPSCs and Bio-Oss could improve the regeneration of periodontal tissues in a dog periodontitis model [53]. Likewise, Hu et al. found in a swine periodontitis model that cell sheet and local injection of human DPSCs clinically promoted periodontal tissue healing; moreover, the former improved bone regeneration more than the latter [54]. In an early clinical study, Li et al. found that DPSCs with β-tricalcium phosphate (β-TCP) had both clinical and radiographic improvements in treating introbony defects [55]. Aimetti et al. performed a case series study that transplantation of DPSCs with collagen sponge improved complete pocket closure and RBF [56]. In a randomized controlled trial (RCT), DPSCs seeded onto collagen sponge significantly benefited PD reduction, CAL gain, and bone defect fill [57].
Stem cells from human exfoliated deciduous teeth (SHEDs) have a higher proliferation rate than BMMSCs and DPSCs and possess capacities of osteogenic, adipogenic, neurogenic [58], myogenic, chondrogenic [59] and odontogenic [58], [60] differentiation. Local injection of SHEDs repressed periodontal tissue inflammation and promoted periodontal bone regeneration in a rat periodontitis model [61]. Besides, transplantation of SHEDs with treated dentin matrix (TDM) yielded PDL fiber, blood vessels, and alveolar bone [60]. There are no allogeneic clinical applications for periodontal regeneration using SHEDs. No autologous SHED application was reported after the donor had suffered from periodontitis since the SHEDs were established in 2003 [41].
Periodontal ligament stem cells (PDLSCs), expressing cementoblastic and osteoblastic markers, exhibit classic potentiality of osteogenic, adipogenic, and chondrogenic differentiation [62], [63], [64]. Furthermore, PDLSCs can be differentiated into cementoblast-like and collagen-generating cells [65]. In an alveolar bone dehiscence dog model, PDLSCs-seeded biphasic calcium phosphate (BCP) enhanced new bone formation and regenerated PDL-like collagen fibers with a location between cementum and bone [66], [67]. In a swine periodontitis model, PDLSCs or SHEDs with hydroxyapatite (HA)/TCP reduced PD, gingival recession (GR), and attachment level (AL) as well as inducing bone regeneration [68]. Although a single-arm clinical study conducted by Iwata et al. showed that PDLSC sheets with β-TCP improved PD reduction, CAL, and radiographic bone height (RBH) [69], Chen et al. in an earlier RCT indicated that there was no statistical difference for the improvement of RBH between PDLSC sheets with Bio-oss and Bio-oss alone[70].
Stem cells from apical papilla (SCAPs) derive from the apical soft tissue of developing permanent teeth, having the capability to differentiate into odontoblast, osteoblast, and adipocytes with a higher proliferation rate than DPSCs [43], [44]. Li et al. reported in a swine periodontitis model that local injection of SCAPs restored periodontal tissues by regenerating Sharpey’s fibers, PDL, cementum, and alveolar bone [71]. The same group also disclosed that SFRP2-expressing SCAPs increased clinical improvements and more tissue regeneration using the same swine model [72]. Up to now, the clinical application using SCAPs has still not been reported.
Dental follicle stem cells (DFSCs), derived from tissues surrounding the unerupted tooth germ, express multiple makers, including Nestin, Notch-1, collagen type Ⅰ, bone sialoprotein (NS), and osteocalcin (OCN) [45]. Osteogenic alkaline phosphatase activity and cementoblastic markers of DFSCs could be initiated by enamel matrix derivatives (EMD), BMP-2, and BMP-7 [73]. It was indicated in none-periodontitis animal models that DFSC sheets generated periodontal tissue-like structures [74], [75]. DFSCs with treated dentin matrix particles (TDMPs) improved the regeneration of periodontal-like tissues in a dog model with periodontal intrabony defects [76]. Recently, a parallel-group, prospective, randomized, controlled human multi-center clinical study is being performed to evaluate the safety and efficacy of allogeneic DFSCs in treating periodontal bone defects (ChiCTR2100054134).
Gingiva-derived mesenchymal stem cells (GMSCs), with classic multipotentiality and unique capabilities of higher proliferation, the propensity to differentiate into neural cell lineages, have been used in many preclinical models of human disorders, including inflammatory and autoimmune diseases, skin diseases, and peripheral nerve injuries [77]. El-Sayed et al. established a swine periodontitis model and found that GMSCs loaded on the deproteinized bovine cancellous bone (DBCB) or collagen showed reduced reductions of PD, GR, and increased CAL, histological attachment level (HAL) [78]. In a mouse periodontitis model, systematically delivered GMSCs increased bone height by homing to the periodontitis site to regenerate bone and PDL [79]. The conditioned medium (CM) of GMSCs promoted periodontal defect regeneration by activating host osteogenesis and inflammatory modulation[80]. Although one randomized and double-blind trial (NCT03638154) enrolled 20 patients with intrabony periodontal defects to evaluate the regenerative potential of GMSCs with β-TCP, being labeled as completed, no results were posted or published.
2.3. MSCs derived from induced pluripotent stem cells (iPSCs)
There are apparent disadvantages of MSCs that should be concerned, including inevitable invasive procedures for isolation, the limited amount in the single donor site, age-, tissue- and donor-associated heterogeneity, and finite proliferative capability[81], [82], [83], [84]. Additionally, MSCs are more likely to be senescent during cell culture [85], [86]. Moreover, the massive consumption of time and money for autologous MSCs and host immune rejection caused by allogenic MSCs hinder MSC-derived cell therapy.
iPSCs can be generated from adult somatic cells by exogenously delivering distinct combinations of transcription factors, including Oct3/4, Sox2, Klf4, c-Myc, Nanog, and Lin28 [87], [88], [89]. With fewer ethical issues and no signs of replicative senescence, iPSCs exhibit infinite expansion capability without attenuated proliferation, loss of differentiation capability, or telomere attrition [90], [91]. However, the infinite expansion of iPSCs is a double-edged sword, which may cause tumors after transplantation if undifferentiated and/or immature cells remain, reprogramming factors activate, or genetic mutations occur during in vitro expansion [92]. Strategies to reduce the tumorigenicity risk of iPSCs include substituting oncogenic reprogramming factors and differentiating iPSCs into lineage-specific progenitor cells before regenerative transplantation [93]. Zhao et al. reported that MSCs derived from transgene-free iPSCs (iMSCs) were readily scalable and underwent senescence, but did not form teratomas after long-term culture [94]. In addition, iMSCs had less potential to promote tumor-associated phenotypes than BMMSCs. Thus, alternative iMSCs have been a growing scientific interest to overcome the limitations of conventional MSCs.
2.3.1. Methodologies to produce iMSCs
Dupuis and Oltra well-reviewed and classified original approaches to generate iMSCs into five categories: MSC switch, embryoid body (EB) formation, specific differentiation, pathway inhibitor, and platelet lysate [95]. The MSC switch technique mainly depends on the spontaneous MSC conversion by using dishes with coatings and switching the iPSC culture medium to the one containing specific compounds with or without prior flow cytometry cell sorting (FACS) [96], [97], [98], [99]. EB formation technique briefly consists of steps dissociating iPSC colonies, formation of EBs, and induction using MSC-specific medium[100], [101], [102]. For specific differentiation techniques, the progenitors obtained from iPSCs are further induced into mesenchymal cells. These intermediate lineages consist of pro-mesoderm[103], paraxial mesoderm [104], lateral plate mesoderm [105], neuroepithelium [106], and neural crest cells (NCCs) [107], [108]. Pathway inhibitor technique overlaps the above-described methods and induces iPSCs into MSCs by adding small molecular inhibitors, including TGF-β inhibitor SB-431542 [109], [110], GSK-3 inhibitor CHIR, and p38 MAPK inhibitor SB-203580 [103]. The platelet lysate technique aims to produce iMSCs without animal-derived components (xeno-free), for example, fetal bovine serum (FBS), by using human platelet lysate to increase the safety in accordance with Good Manufacturing Practice (GMP) guidelines [111], [112], [113].
To generate GMP-compatible iMSCs, we recently developed a thorough xeno-free and more efficient technique[114]. Briefly, iPSCs are maintained with Stemfit AK03N medium in iMatrix (laminin-511 E8 fragment)-coated dishes. NCCs are induced from iPSCs with Stemfit Basic03 medium supplemented with SB and CHIR in iMatrix-coated dishes for 10 days. CD271high NCCs are enriched and expanded to passage number 4 in NCC expansion media containing SB and EGF, and FGF2 on fibronectin-coated dishes to proceed to MSC induction with PRIME-XV MSC Expansion XSFM medium. These xeno-free-induced MSCs (XF-iMSCs) are substantially similar in MSC marker expression, multipotentiality, and global gene expression profiles compared to BMMSCs, ADMSCs, and UCMSCs. The in vivo results suggest that XF-iMSCs could promote skull bone and muscle regeneration. Additionally, we recently found that Brequinar, one inhibitor of dihydroorotate dehydrogenase, could be used to eliminate undifferentiated iPSCs without affecting iMSCs on survival, three-lineage differentiation capacity, and gene expression, which benefits the purification of iMSCs and iMSC-based cell therapy [115].
2.3.2. Application of iMSCs in periodontal regeneration
Hynes et al. first transplanted the human iMSCs into periodontal defects in a rat fenestration model. They found that iMSCs treated with clotting factors significantly promoted the generation of PDL-like tissues and newly formed mineralized tissues [116]. They also generated mouse iPSC-MSC-like cells (miMSCs) and confirmed that systematically or locally delivered miMSCs inhibited inflammation and reduced alveolar bone loss in two periodontitis mouse models [117]. A pilot study in rats established experiment periodontitis by ligature and Porphyromonas gingivalis, and implied that intravenous and topical administration of iMSCs or TNFα-stimulated gene-6 (TSG-6) expressing iMSCs decreased the level of inflammation cytokines, IL-1β and TNF-a, and inhibited alveolar bone resorption [118]. Yin et al. revealed that recombinant human growth/differentiation factor-5 (rhGDF-5) enhanced the differentiation of iMSCs into osteogenic, fibrogenic, and cementogenic lineages. And the iMSCs with rhGDF-5 encapsulated in hyaluronic acid dramatically promoted specific differentiation into periodontal cells after subcutaneous implantation to athymic nude mice [119] (Table 5). Although a phase Ⅰ study has convinced the safety and efficacy of iMSC in acute steroid-resistant GvHD [105], the clinical application for periodontal regeneration using iMSCs has not been reported yet.
Table 5.
iMSCs in preclinical periodontal regeneration.
| Author/ year | Animal/ sample size | Cell source/ morphology | Carrier /Scaffold | Test | Control | Duration | Outcome measures | Results | Regenerated tissues |
|---|---|---|---|---|---|---|---|---|---|
| Hynes et al. 2013[116] | Rat | Xenogenic, human foreskin–derived iPSC-MSCs | / | iMSC treated with fibrinogen and thrombin | Clotting factors only or untreated | 2 weeks | Histomor-phometric analysis | iMSCs promoted generation of PDL-like tissues and newly formed mineralized tissues | PDL-like tissues, bone-like tissues |
| Hynes et al. 2018[117] | Mouse | Allogenic, tail-tip fibroblasts from NOD/Lt mice derived iPSC-MSCs | Sponge | Tail vein or subcutaneous injection of iMSCs | Tail vein or subcutaneous injection of PBS | 7 weeks | Histomorphometric analysis, µCT | iMSC inhibited inflammation and reduced alveolar bone loss in a periodontitis mouse model | / |
| Yang et al. 2014[118] | Rat, 30 | Allogenic, rat embryonic fibroblasts derived iPSC-MSCs | Matrigel for topical injection | Systemic or topical injection of iMSCs or TSG-6/iMSCs | Healthy control and untreated periodontitis | 3 months | Histomorphometric analysis, µCT, | iMSCs or TSG-6/iMSCs inhibited inflammation and alveolar bone absorption | / |
| Yin et al. 2017[119] | Athymic nude mice, 12 | Xenogenic, human peripheral blood mononuclear cells derived iPSC-MSCs | Hydrogel | iMSCs+rhGDF-5 +hydrogel, iMSCs+hydrogel | rhGDF-5 +hydrogel | 6 weeks | Immunohistological staining | iMSCs with rhGDF-5 promoted periodontal specific differentiation | / |
iPSC: induced pluripotent stem cells; iMSC: iPSC-derived mesenchymal stem/stromal cells; PDL: Periodontal dental ligament; PBS: Phosphate buffered saline; µCT: Micro computed tomography; TSG: Tumor necrosis factor alpha-stimulated gene; rhGDF: Recombinant human growth/differentiation factor.
3. Transplantation procedures
Transplantation procedures grafting the cells into the defect area are also critical to induce successful periodontal tissue regeneration. In general, administering a single-cell suspension into the defect tissue poses challenges due to the difficulties in ensuring cell adhesion. This often results in low cell survival and the diffusion of cells into unintended tissues. Thus, utilizing biomaterials as reliable cell carriers has become a common practice [120]. More specifically, various scientific endeavors have aimed to establish ideal scaffolds with biocompatibility, biodegradability, and low immunogenicity. Besides, recent advancements in the field have led to the development of scaffold-free cell transplantation procedures. These innovative methods offer an optimal cellular microenvironment and minimize disruption to the host’s physiological and metabolic processes. This fosters the functionality of both the grafted and host cells, thereby facilitating periodontal tissue regeneration. The following section will overview scaffold materials and scaffold-free cell transplantation procedures. Besides, future perspectives on transplantation procedures will be discussed.
3.1. Scaffold materials
There are several different types of scaffold materials, each with their distinct biological properties. For example, natural biomaterials, collagen and chitosan, exhibit excellent biocompatibility and low immunogenicity [121]. Besides, chitosan possesses osteoinductive and anti-inflammatory properties, potentially beneficial for bone or periodontal tissue formation [122].
In contrast, among the artificial scaffold, bioceramics, including such as HA, β-TCP, and carbonate apatite, that have been employed for current periodontal tissue regenerative therapy [123], have attracted attention as the MSCs carrier material. These bioceramic scaffolds offer substantial mechanical stability, outstanding osteoconductive capacities, and biocompatibility, which can support new alveolar bone formation by MSCs.
Besides, synthetic polyester-based polymers, including polylactic acid, polyglycolic acid poly (lactic-co-glycolic acid) (PLGA), poly (ethylene glycol) (PEG), and polycaprolactone (PCL), possess unique advantages as MSCs grafting materials. For instance, the biodegradation rates and mechanical stability can be easily tuned, and their straightforward manufacturing process makes them well-suited for cost-effective mass production, a critical factor for clinical applications [121].
Aside from biocompatibility and biodegradability, researchers have sought to enhance the biomedical activity of the materials by introducing beneficial chemicals or cytokines or by modifying their surface structure using electrospinning technologies to amplify the MSC function to induce robust periodontal tissue regeneration. The natural biopolymer chitosan can encapsulate various drugs, functioning as a local drug delivery system to facilitate periodontal tissue regeneration [124]. Thus, the transplantation of MSCs with those drug-loaded chitosan will show the additive or synergistic tissue regenerative effect. It has been reported that the surface of electrospun scaffolds, composed of well-aligned nanofibers, can enhance PDLSC proliferation [125]. Incorporation of the aligned PCL-PEG nanofibers into the porous chitosan scaffold can increase collagen fiber formation in PDL space [126]. Furthermore, as the other scaffold materials, chitosan, bioceramics, or polymers, are also applied to this electrospun nanofiber matrix to regulate its biological properties [127], [128], novel promising scaffolds will be developed for the MSCs transplantation procedures in the near future.
3.2. Scaffold-free procedures
Even though ideal scaffold materials that exert favorable bioactivity are becoming established, those should remain a “foreign substance” to the organisms. When MSCs are grafted with such artificial scaffolds, both the grafted and host cells need to not only form the new tissue but also metabolize such artificial materials to induce successful periodontal tissue regeneration. This metabolization process, non-existent in the natural healing process of the human body, could potentially burden the cells, leading to delayed tissue regeneration. To circumvent this issue, scaffold-free cell transplantation procedures using cell aggregates have been thoroughly explored. Notably, among the cell aggregate culture techniques, cell sheets produced with temperature-responsive intelligent polymer cell culture dishes have been well-tested in this field. These intact cell sheets, solely comprised of cells and extracellular matrix proteins (ECM), might better mimic physiological and cellular behaviors in the graft area than using artificial scaffolds. Indeed, subcutaneous transplantation of human PDLSCs sheets into immunodeficient mice formed ectopic periodontal tissue-like structures, including a cementum layer [129]. Excellent pre-clinical and clinical trials by Iwata et al. demonstrated that cell sheet technology could graft the PDLSCs onto a denuded tooth root surface without artificial scaffolds to induce successful periodontal tissue regeneration [130]. Nevertheless, their study design filled the bone defects with the artificial bioceramic β-TCP, suggesting that a single thin layer of the cell sheet may be insufficient for severe defect sites. Consequently, numerous attempts have been made to enlarge the cell sheet. Recent advancements in culture technology now allow for the production of multiple-layered cell sheets containing different cell types, such as MSCs and vascular endothelial cells [131], [132], [133]. However, current strategies of periodontal tissue regeneration by cell sheets often still involve combining bone graft materials.
To achieve fully scaffold-free MSCs transplantation procedures, clumps of MSCs/ECM complexes (C-MSCs), which consist of cells and self-produced ECM proteins, have been generated [134]. Three-dimensional (3D) C-MSCs, cell aggregates about 1 mm in diameter, can be grafted into bone defects without artificial scaffolds to induce rapid bone regeneration, outperforming MSC transplantation procedures using a collagen sponge as a scaffold. Besides, the implantation of multiple numbers of C-MSCs into inflamed furcation defects in beagle dogs led to successful periodontal tissue regeneration [135], whereas MSC implantation using β-TCP caused ankylosis, suggesting a disturbance in periodontal tissue metabolism [136]. Taken together, scaffold-free C-MSCs transplantation therapy can avoid the issues associated with the artificial materials’ metabolism and offer the ideal cellular microenvironment, composed of intact ECM proteins, for the grafted MSCs. This could then induce appropriate cellular differentiation and subsequent periodontal tissue regeneration. This novel scaffold-free cell therapy will need a further clinical trial to demonstrate its effectiveness and safety.
3.3. 3D printing technologies
As described above, despite the progress in developing ideal scaffold materials, cell sheets, or C-MSCs, larger defects in clinical settings remain a significant challenge. 3D printing technologies could provide tailor-made scaffold materials for irregularly shaped, severe periodontal defects. As early as 2014, the application of a 3D-printed scaffold as a dental stem cell carrier for periodontal tissue regenerative therapy was reported [137]. Besides, in 2015, the first clinical trial utilizing a 3D-printed PCL scaffold containing cytokines was conducted [138]. Subsequent studies have demonstrated the beneficial properties of 3D-printed scaffolds generated from PCL and PLGA for PDLSCs [139].
Moreover, advancements in material techniques have led to the emergence of “3D bio-printing”, which allows PDLSCs to be mixed into bioink and positioned in specific locations within the printed tissue. This process mimics the precision of periodontal tissue structure details [140], [141]. Remarkably, an artificial scaffold materials-free “3D bio-printing” approach has been recently established. A bio-3D printer can generate large, centimeter-sized tissue using cell aggregates, including cell spheroids or C-MSCs [142], [143], [144]. For example, a centimeter-sized human 3D nerve conduit was created from approximately 500 C-iMSCs using a bio-3D printer, successfully inducing peripheral nerve regeneration [145].
Applying these technologies, bio-3D grafts suitable for severe, complex periodontal tissue defects could be developed, demonstrating promising tissue regeneration capabilities. Although using a bio-3D printer remains nascent, 3D bio-printing could become a reliable and indispensable tool in cell transplantation procedures with further development and refinement.
4. Concerns on MSCs
Clinical trials on MSCs, being different from animal experiments, are rarely possible to evaluate the effect of periodontal regeneration at the histological or molecular level. Instead, it relies on clinical periodontal and radiological examination to assess the effectiveness objectively. Many clinical trials with different clinical settings have convinced the safety profile of MSCs in academia and industry. However, there is still no generally accepted consensus to proceed with standardized MSC cell therapy that is predictable, reproducible, and therapeutically efficient [146]. As a result, MSCs have just been conditionally approved in a few countries. Herein, we attempt to summarize the current concerns on MSCs for clinical application.
4.1. Effective mechanisms
It is widely accepted that transplanted MSCs exert therapeutic functions by three primary rationales: living cell expansion and multilineage differentiation, interactions with host cells and release of paracrine factors and exosomes, efferocytosis of apoptotic MSCs and immune cells and subsequent functional polarization of phagocytic cells [146].
4.1.1. Delivery and differentiation after transplantation
The regenerative phenomena were widely described in preclinical studies that MSCs promote the regeneration of host periodontal tissues via histomorphometry and µCT analysis [34], [35], [67], [76], [80]. To track transplanted MSCs, it is feasible to label culture-expanded MSCs before transplantation. The labeling techniques include radioactive labeling, labeling with fluorescent dyes, transfection with reporter genes, or donor cell-specific DNA microsatellites [147], [148], [149], [150].
Sun et al. reported that systemically injected human GFP+ GMSCs homed to the periodontal injury sites of mice and survived for at least 4 weeks, which promoted periodontal regeneration [79]. Yu et al. systemically delivered EGFP-labeled rat BMMSCs via intra-bone marrow transplantation into lethally irradiated rats with periodontal defects. They found that EGFP+ cells were localized in the newly formed bone, PDL, and cementum. Immunohistochemical staining confirmed osteoblast differentiation, implying the direct differentiation capability of BMMSCs into osteoblasts by homing to the host inflammatory or injured sites [151]. However, it was indicated that a majority of transfusional MSCs are trapped in the pulmonary vasculature and subsequently eliminated by the circulatory system [148], [152]. The number of engrafted MSCs at wound site by systemic administration is consistently low [153]. Therefore, the perspective that systemically injected MSCs home and function at sites of injury and inflammation is still far from being clarified.
Concerning periodontal regeneration, MSCs are more likely to be transplanted in situ alone or with biomaterials. Gao et al. transduced GFP and luciferase reporters into human SHEDs before topical injection in rat periodontal defect [154]. In vivo bioluminescence imaging demonstrated that the xenogenic SHEDs survived for approximately seven days in rat periodontal tissue with little tissue diffusion. Yang et al. transplanted GFP+ rat-BMMSCs into rat periodontal defect and found GFP+ and osteocalcin (a specific marker of mature bone) colocalized in partial areas of newly formed bone, cementum and PDL, which suggested MSCs directly differentiated into host tissues [155]. By labeling with PKH26 fluorescent cell linker to C-MSCs, the newly formed periodontal tissues, including cementum, PDL, and alveolar bone, primarily originated from the grafted cells [156]. The transplantation of human iMSCs into cranial bone-defect and muscle-injured mice was tracked through immunohistochemical staining by using anti-human-specific vimentin (h-Vimentin) and anti-human-specific lamin A/C (h-lamin A/C) antibodies [114]. Most areas of regenerated bone were lack of expression of h-Vimentin. Similarly, the regenerated MYH-4 (a mature muscle fiber maker)-positive muscle cells did not co-stain with h-lamin A/C.
The contradictions or differences among finite studies may account for distinct donors, cell origins, culture methodologies, animal models, etc. More standardized and sophisticated investigations should be complemented to convince the effectiveness of systemic and topical delivery and the substantial differentiation of transplanted MSCs into host cells.
4.1.2. Paracrine and immunomodulation
Accumulating evidence has uncovered that MSCs drive tissue regeneration by releasing trophic factors that are anti-apoptotic, anti-scarring, angiogenic, and mitotic instead of differentiation into the cells of the host [157]. Recently, we found that topically transplanted XF-iMSCs promote the regeneration of bone and skeletal muscle via paracrine factors rather than differentiation into the host cells [114]. Costa et al. reported that the level of osteopontin, osteocalcin, and sclerostin was increased after treatment with BMMSCs in rats with fenestration defects [36]. Qiu et al. indirectly illustrated the MSC paracrine effects on osteogenic differentiation of host bone progenitor cells in periodontal defects using the conditioned medium of GMSCs and PDLSCs [80]. Extracellular vesicles (EV), including exosomes and microvesicles, are known as regulators of the paracrine effects in tissue repair and immunomodulation [158], [159].
Through EVs, MSCs exert immunomodulation in several ways, including secretion of anti-inflammatory cytokines, M2 polarization, formation of immature dendritic cells (DCs), activation of regulatory T cells (Tregs), and suppression of effector T and B cells [146]. Chew et al. reported that MSC exosomes carried by collagen sponge promoted periodontal regeneration in an immunocompetent rat model with periodontal defect [160]. Nakao et al. illustrated that GMSC-derived exosomes decreased periodontal bone resorption and the number of osteoclasts, and the inhibitory effects were enhanced by pre-treatment with TNF-a [161]. Lei et al. demonstrated that PDLSC-derived exosomes loaded on Matrigel or β-TCP resulted in more bone formation in rats with alveolar bone defect around the first molar.
The injection of BMMSCs pretreated with ASA reduced the level of TNF-α and IL-17 in gingiva-mucosal tissues of rats with periodontitis [162]. DPSCs exert immunomodulatory functions by inhibiting the proliferation of peripheral blood mononuclear cells (PBMCs) [163], suppressing T cell proliferation, reducing IL-17, and activating Tregs differentiation [164], [165]. DFSCs infected with periodontitis-associated Prevotella intermedia and Tannerella forsythia, maintained their stem cell functionality and reduced tissue and bone degradation by inhibiting chemotaxis, phagocytic activity, and neutrophil extracellular traps (NET) formation of phagocytic polymorphonuclear leukocytes (PMNs) [166]. Topical injection of SHEDs repressed periodontal tissue inflammation in a rat periodontitis model, resulting from the induction of M2 macrophage polarization [61]. Both healthy and inflamed PDLSCs inhibited the proliferation of PBMCs, and the former suppressed associated cytokines, including IL-2, TNF-a, and IFN-γ [167]. Meanwhile, an STRO-1+ CD146+ subpopulation of PDLSCs inhibited T cell proliferation by decreasing non-classical major histocompatibility complex (MHC) glycoprotein CD1b on DCs [168].
Although the MSC-derived exosomes may pave the way for cell-free periodontal regeneration involving stem cells, the MSC secretome varies significantly due to different study settings. As a result, the MSC-EVs do not consistently reproduce the immunomodulatory functions as the parental MSCs do [158], [169].
4.1.3. Efferocytosis
Although MSCs can be well-expanded in vitro for many passages, the survival time of transplanted MSCs in vivo differs from one week to a few months due to distinct donor sites, application scenarios for varieties of diseases, and animal models (immunocompromised or immunocompetent) [79], [155], [170], [171]. The process of MSC apoptosis and phagocytosis by resident macrophages is called efferocytosis [172], implying MSCs may evoke a non-specific immune suppressive effect via phagocytosis derived from the host reticuloendothelial system [9]. Moreover, the immunosuppressive effect occurs irrespective of MSC viability and histocompatibility [146], [173]. Efferocytosis may explain the commonalities and contradictions among research that attempted to elucidate the MSC immunomodulation on the mechanisms of action.
4.2. Safety
4.2.1. Heterogeneity
Culture-expanded MSCs include a heterogeneous population of cells with distinct phenotypes and functional properties in terms of pluripotency, self-renewal, and gene expression profile, which account for internal and external factors, such as donor site, isolation technique, culture protocols, passage number, microenvironment, epigenetics [174], [175], [176]. Considering heterogeneity, it is suggested to characterize odontogenic MSC subpopulations with specific markers to address the distinct biological activities of regenerative therapy [177]. The iMSCs, derived from a single iPSC clone, are considered to hold lower heterogeneity [178], probably resulting from the gene expression patterns related to rejuvenation during expansion epigenetic and chromatin remodeling [174].
It is not well known how heterogeneity affects the therapeutic evaluation of MSCs between animals and humans in different diseases. Thus, it is indispensable to develop techniques to identify, decrease and control heterogeneity and deeply clarify the efficacy of both iMSCs and the primary MSCs.
4.2.2. Immunocompatibility
According to the literature summarized in the Tables, although allogenic and xenogenic MSCs were widely used in preclinical periodontal regeneration, most clinical trials used autologous MSCs. It comes to the problem of immune rejection. As above-described, MSCs mainly exert the preclinical or clinical values via the non-stem/progenitor cell capabilities by producing extracellular vesicles, cytokines, and growth factors that inhibit immune response. Thus, MSCs were assumed as immune privileged without MHC matching. Allogenic MSCs were applied in clinical trials to treat multiple diseases, including GvHD, lupus, bone fracture, heart failure, Crohn’s disease, and stroke [179]. For the pharmaceutical industry, allogenic MSC means one-size-fits-all and can be provided as an off-the-shelf product. The two approved MSC products to treat GvHD [9] and complex anal fistulas in adults with Crohn’s disease [180] were respectively derived from allogenic bone marrow and adipose tissue.
However, the results from many preclinical and clinical studies make the academic community doubt the immune-privileged status of MSCs. Through labeling techniques, most of the human MSCs in mice with severe combined immunodeficiency, mouse MSCs in syngeneic mice, and rat MSCs in allogenic rats died within 48 h after systemic infusion [181], [182], [183]. Similar results were observed in the tissue autopsy of 18 patients who received infusions of HLA-mismatched or haploidentical MSCs within one year before their death [184]. Only one patient who was severely ill at the time of MSC infusion (7 days before death) showed high levels of MSC donor DNA at levels > 1/1000 cells in multiple organs, including bone marrow, intestine, lung, and spleen. In reverse, no signs of ectopic tissue formation or malignant tumors derived from MSCs were found, which suggested a “hit and run” mechanism of MSCs instead of sustained engraftment and possibly reduced the long-term risks of MSC-based therapy [184]. It was revealed that allogenic MSCs-treated mice were involved in more CD4 + , CD122 + , CD44 + , and CD62low T cells, indicating that MSCs induce rejection via an immune memory [185]. Besides, human MSCs enhanced innate immune responses; thus, macrophage and neutrophil infiltration to the injection sites were observed in mice and rats [186], [187].
To extend the MSC persistence, immune rejection can be overcome by modifying the host or donor MSCs. Unfortunately, the fact that immunosuppressant drugs taken by the host enhance or interfere with the immunosuppressive properties of MSCs is not clear [175]. iPSCs from HLA homozygous donors are considered to cover most HLA haplotypes. However, it is time-consuming and expensive to recruit rare donors [188], [189]. Xu et al. generated HLA-pseudo-homozygous and HLA-C-retained iPSC via genome-editing approaches that could evade T cell and NK cell [190], which may be a promising cell resource to obtain iMSC with improved immune compatibility.
5. Concluding remarks and future perspectives
The rapid development of regenerative medicine, represented by stem cells, has opened a new chapter for periodontal treatments. Many preclinical and clinical studies have demonstrated the safety and clinical effectiveness of MSCs in inflammatory diseases, revealing the broad prospects of MSCs in the regeneration of periodontal disease. However, many problems should be solved, and the clinical application of MSCs still has a long way to go, such as quickly obtaining the mass-produced MSCs and the long-term storage of MSCs. The current technology cannot meet the demand for large-scale production of MSCs after they are launched on the market, and there is heterogeneity in MSCs from different individuals, which means the MSCs cannot obtain a unified quality standard even under the same conditions. iMSCs are likely to overcome some of these shortcomings. Cell transplantation procedures play a significant role in successful periodontal tissue regeneration. These factors pose challenges to evaluating the clinical efficacy of MSCs. More important, the interaction with the host and the effective mechanism and immune injection of MSCs should be further elucidated to realize the approved clinical application in periodontal regeneration. .
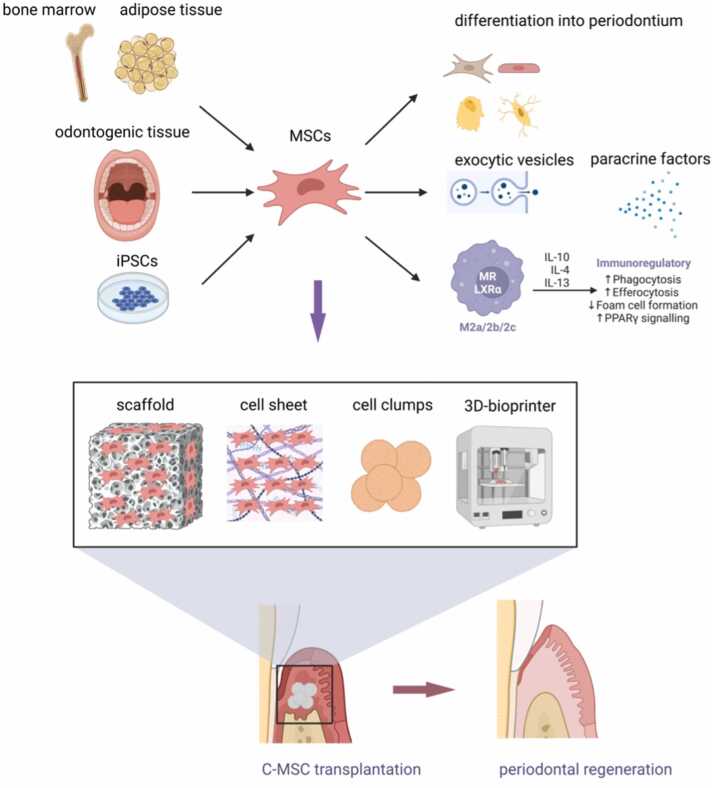
Fig. 1.
Potential periodontal regeneration using MSCs derived from multiple cell resources with various mechanisms and distinct Transplantation procedures. iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem/stromal cells; IL, Interleukin; M2, macrophage differentiation M2; C-MSCs, clumps of MSCs/ECM complexes.
Funding
The English editing and figure generation fees of this current work were supported by the China Scholarship Council (202008510007) for Pan Gao and the Sichuan Science and Technology Program (2022NSFSC1521) for Jingmei Yang.
Conflict of interest
None
Contributor Information
Makoto Ikeya, Email: mikeya@cira.kyoto-u.ac.jp.
Jingmei Yang, Email: yangjm@scu.edu.cn, yjm881222@hotmail.com.
References
- 1.Herrera D., Meyle J., Renvert S., Jin L. White Paper on Prevention and Management of Periodontal Diseases for Oral Health and General Health n.d.
- 2.Chen M.X., Zhong Y.J., Dong Q.Q., Wong H.M., Wen Y.F. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the Global Burden of Disease Study 2019. J Clin Periodo. 2021;48:1165–1188. doi: 10.1111/jcpe.13506. [DOI] [PubMed] [Google Scholar]
- 3.Matuliene G., Pjetursson B.E., Salvi G.E., Schmidlin K., Brägger U., Zwahlen M., et al. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodo. 2008;35:685–695. doi: 10.1111/j.1600-051X.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 4.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Karring T., Nyman S., Gottlow J., Laurell L. Development of the biological concept of guided tissue regeneration--animal and human studies. Periodontol 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- 6.de Jong T., Bakker A.D., Everts V., Smit T.H. The intricate anatomy of the periodontal ligament and its development: lessons for periodontal regeneration. J Periodontal Res. 2017;52:965–974. doi: 10.1111/jre.12477. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann G., Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011;42(Suppl 2):S16–S21. doi: 10.1016/j.injury.2011.06.199. [DOI] [PubMed] [Google Scholar]
- 8.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 9.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galipeau J., Krampera M., Barrett J., Dazzi F., Deans R.J., DeBruijn J., et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinnaird T., Stabile E., Burnett M. s, Shou M., Lee C. w, Barr S., et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 12.Gruber R., Kandler B., Holzmann P., Vögele-Kadletz M., Losert U., Fischer M.B., et al. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896–903. doi: 10.1089/ten.2005.11.896. [DOI] [PubMed] [Google Scholar]
- 13.Kallmeyer K., Pepper M.S. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015;15:477–479. doi: 10.1517/14712598.2015.997204. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., et al. Mesenchymal stem versus stromal cells: international Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Haynesworth S.E., Goshima J., Goldberg V.M., Caplan A.I. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Halvorsen Y.C., Wilkison W.O., Gimble J.M. Adipose-derived stromal cells—their utility and potential in bone formation. Int J Obes. 2000;24:S41–S44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 21.Liau L.L., Ruszymah B.H.I., Ng M.H., Law J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr Res Transl Med. 2020;68:5–16. doi: 10.1016/j.retram.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 22.He S., Gleason J., Fik-Rymarkiewicz E., DiFiglia A., Bharathan M., Morschauser A., et al. Human placenta-derived mesenchymal stromal-like cells enhance angiogenesis via T cell-dependent reprogramming of macrophage differentiation. Stem Cells. 2017;35:1603–1613. doi: 10.1002/stem.2598. [DOI] [PubMed] [Google Scholar]
- 23.In’t Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., de Groot-Swings G.M., Claas F.H., Fibbe W.E., et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A., et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 25.Potdar P.D., D’souza S.B. Isolation of Oct4+, Nanog+ and SOX2- mesenchymal cells from peripheral blood of a diabetes mellitus patient. Hum Cell. 2011;24:51–55. doi: 10.1007/s13577-011-0011-6. [DOI] [PubMed] [Google Scholar]
- 26.Potdar P., Subedi R. Defining molecular phenotypes of mesenchymal and hematopoietic stem cells derived from peripheral blood of acute lymphocytic leukemia patients for regenerative stem cell therapy. J Stem Cells Regen Med. 2011;7:29–40. doi: 10.46582/jsrm.0701004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeremias T. da S., Machado R.G., Visoni S.B.C., Pereima M.J., Leonardi D.F., Trentin A.G. Dermal substitutes support the growth of human skin-derived mesenchymal stromal cells: potential tool for skin regeneration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med. 2019;4(1):15. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger M.F., Mbalaviele G., Black M., Mosca J.D., Marshak D.R. Hum. Cell Cult. Springer; 2001. Mesenchymal stem cells; pp. 189–207. [Google Scholar]
- 30.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with crohn’s disease. Gastroenterology. 2018;154:1334–1342.e4. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki K., Peng Y., Kanda R., Umeda M., Ishikawa I. Stem cell transplantation and cell-free treatment for periodontal regeneration. Int J Mol Sci. 2022;23:1011. doi: 10.3390/ijms23031011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung Y.-H., Park J.-Y., Kim H.-J., Lee S.M., Kim S.-H., Yun J.-H. Regenerative potential of BMP7-engineered mesenchymal stem cells in ligature-induced periodontitis. Tissue Eng Part A. 2022 doi: 10.1089/ten.TEA.2022.0162. [DOI] [PubMed] [Google Scholar]
- 35.Vaquette C., Saifzadeh S., Farag A., Hutmacher D.W., Ivanovski S. Periodontal tissue engineering with a multiphasic construct and cell sheets. J Dent Res. 2019;98:673–681. doi: 10.1177/0022034519837967. [DOI] [PubMed] [Google Scholar]
- 36.Costa C.A., Deliberador T.M., Abuna R.P.F., Rodrigues T.L., Souza S.L.S. de, Palioto D.B. Mesenchymal stem cells surpass the capacity of bone marrow aspirate concentrate for periodontal regeneration. J Appl Oral Sci Rev FOB. 2022;30 doi: 10.1590/1678-7757-2021-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhote R., Charde P., Bhongade M., Rao J. Stem cells cultured on beta tricalcium phosphate (β-TCP) in combination with recombinant human platelet-derived growth factor - BB (rh-PDGF-BB) for the treatment of human infrabony defects. J Stem Cells. 2015;10:243–254. [PubMed] [Google Scholar]
- 38.Takedachi M., Sawada K., Sakura K., Morimoto C., Hirai A., Iwayama T., et al. Periodontal tissue regeneration by transplantation of autologous adipose tissue-derived multi-lineage progenitor cells. Sci Rep. 2022;12 doi: 10.1038/s41598-022-11986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apatzidou D.A., Bakopoulou A.A., Kouzi-Koliakou K., Karagiannis V., Konstantinidis A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J Clin Periodo. 2021;48:1111–1125. doi: 10.1111/jcpe.13474. [DOI] [PubMed] [Google Scholar]
- 40.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo B.-M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet Lond Engl. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 43.Sonoyama W., Liu Y., Yamaza T., Tuan R.S., Wang S., Shi S., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonoyama W., Liu Y., Fang D., Yamaza T., Seo B.-M., Zhang C., et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PloS One. 2006;1 doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morsczeck C., Götz W., Schierholz J., Zeilhofer F., Kühn U., Möhl C., et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol J Int Soc Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q., Shi S., Liu Y., Uyanne J., Shi Y., Shi S., et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol Balt Md. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang G.T.-J., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang G.T.-J., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gronthos S., Brahim J., Li W., Fisher L.W., Cherman N., Boyde A., et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 50.Laino G., d’Aquino R., Graziano A., Lanza V., Carinci F., Naro F., et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Min Res J Am Soc Bone Min Res. 2005;20:1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W., Walboomers X.F., Shi S., Fan M., Jansen J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 52.d’Aquino R., Graziano A., Sampaolesi M., Laino G., Pirozzi G., De Rosa A., et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 53.Khorsand A., Eslaminejad M.B., Arabsolghar M., Paknejad M., Ghaedi B., Rokn A.R., et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implant. 2013;39:433–443. doi: 10.1563/AAID-JOI-D-12-00027. [DOI] [PubMed] [Google Scholar]
- 54.Hu J., Cao Y., Xie Y., Wang H., Fan Z., Wang J., et al. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Zhao S., Nan X., Wei H., Shi J., Li A., et al. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aimetti M., Ferrarotti F., Gamba M.N., Giraudi M., Romano F. Regenerative treatment of periodontal intrabony defects using autologous dental pulp stem cells: a 1-year follow-up case series. Int J Periodontics Restor Dent. 2018;38:51–58. doi: 10.11607/prd.3425. [DOI] [PubMed] [Google Scholar]
- 57.Ferrarotti F., Romano F., Gamba M.N., Quirico A., Giraudi M., Audagna M., et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J Clin Periodo. 2018;45:841–850. doi: 10.1111/jcpe.12931. [DOI] [PubMed] [Google Scholar]
- 58.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerkis I., Kerkis A., Dozortsev D., Stukart-Parsons G.C., Gomes Massironi S.M., Pereira L.V., et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 60.Yang X., Ma Y., Guo W., Yang B., Tian W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics. 2019;9:2694–2711. doi: 10.7150/thno.31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao X., Shen Z., Guan M., Huang Q., Chen L., Qin W., et al. Immunomodulatory role of stem cells from human exfoliated deciduous teeth on periodontal regeneration. Tissue Eng Part A. 2018;24:1341–1353. doi: 10.1089/ten.tea.2018.0016. [DOI] [PubMed] [Google Scholar]
- 62.Xu J., Wang W., Kapila Y., Lotz J., Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487–496. doi: 10.1089/scd.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gay I.C., Chen S., MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149–160. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 64.Lindroos B., Mäenpää K., Ylikomi T., Oja H., Suuronen R., Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329–335. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 65.Seo B.-M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet Lond Engl. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 66.Liu J., Ruan J., Weir M.D., Ren K., Schneider A., Wang P., et al. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells. 2019;8:537. doi: 10.3390/cells8060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi H., Zong W., Xu X., Chen J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am J Transl Res. 2018;10:4030–4041. [PMC free article] [PubMed] [Google Scholar]
- 68.Fu X., Jin L., Ma P., Fan Z., Wang S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodo. 2014;85:845–851. doi: 10.1902/jop.2013.130254. [DOI] [PubMed] [Google Scholar]
- 69.Iwata T., Yamato M., Washio K., Yoshida T., Tsumanuma Y., Yamada A., et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets – a safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen F.-M., Gao L.-N., Tian B.-M., Zhang X.-Y., Zhang Y.-J., Dong G.-Y., et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li G., Han N., Zhang X., Yang H., Cao Y., Wang S., et al. Local injection of allogeneic stem cells from apical papilla enhanced periodontal tissue regeneration in minipig model of periodontitis. BioMed Res Int. 2018;2018 doi: 10.1155/2018/3960798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G., Han N., Yang H., Zhang X., Cao Y., Cao Y., et al. SFRP2 promotes stem cells from apical papilla-mediated periodontal tissue regeneration in miniature pig. J Oral Rehabil. 2020;47:12–18. doi: 10.1111/joor.12882. [DOI] [PubMed] [Google Scholar]
- 73.Kémoun P., Laurencin-Dalicieux S., Rue J., Farges J.-C., Gennero I., Conte-Auriol F., et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–294. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 74.Guo S., Guo W., Ding Y., Gong J., Zou Q., Xie D., et al. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transpl. 2013;22:1061–1073. doi: 10.3727/096368912X656036. [DOI] [PubMed] [Google Scholar]
- 75.Bai Y., Bai Y., Matsuzaka K., Hashimoto S., Fukuyama T., Wu L., et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011;48:1417–1426. doi: 10.1016/j.bone.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 76.Yang H., Li J., Hu Y., Sun J., Guo W., Li H., et al. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 2019;35:1238–1253. doi: 10.1016/j.dental.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Kim D., Lee A.E., Xu Q., Zhang Q., Le A.D. Gingiva-derived mesenchymal stem cells: potential application in tissue engineering and regenerative medicine - a comprehensive review. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.667221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fawzy El-Sayed K.M., Paris S., Becker S.T., Neuschl M., De Buhr W., Sälzer S., et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: an animal study. J Clin Periodo. 2012;39:861–870. doi: 10.1111/j.1600-051X.2012.01904.x. [DOI] [PubMed] [Google Scholar]
- 79.Sun W., Wang Z., Xu Q., Sun H., Liu X., Yang J., et al. The treatment of systematically transplanted gingival mesenchymal stem cells in periodontitis in mice. Exp Ther Med. 2019;17:2199–2205. doi: 10.3892/etm.2019.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu J., Wang X., Zhou H., Zhang C., Wang Y., Huang J., et al. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 2020;11 doi: 10.1186/s13287-019-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costa L.A., Eiro N., Fraile M., Gonzalez L.O., Saá J., Garcia-Portabella P., et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci CMLS. 2021;78:447–467. doi: 10.1007/s00018-020-03600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11 doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elahi K.C., Klein G., Avci-Adali M., Sievert K.D., MacNeil S., Aicher W.K. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016;2016 doi: 10.1155/2016/5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu L., Liu Y., Sun Y., Wang B., Xiong Y., Lin W., et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8 doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Ding Y., Liu Z., Liang X. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neri S., Borzì R.M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 2020;10:340. doi: 10.3390/biom10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 90.Koch C.M., Reck K., Shao K., Lin Q., Joussen S., Ziegler P., et al. Pluripotent stem cells escape from senescence-associated DNA methylation changes. Genome Res. 2013;23:248–259. doi: 10.1101/gr.141945.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lapasset L., Milhavet O., Prieur A., Besnard E., Babled A., Aït-Hamou N., et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 93.Gao P., Liu S., Wang X., Ikeya M. Dental applications of induced pluripotent stem cells and their derivatives. Jpn Dent Sci Rev. 2022;58:162–171. doi: 10.1016/j.jdsr.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Q., Gregory C.A., Lee R.H., Reger R.L., Qin L., Hai B., et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci USA. 2015;112:530–535. doi: 10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupuis V., Oltra E. Methods to produce induced pluripotent stem cell-derived mesenchymal stem cells: mesenchymal stem cells from induced pluripotent stem cells. World J Stem Cells. 2021;13:1094–1111. doi: 10.4252/wjsc.v13.i8.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao W.-X., Sun Y.-Q., Shi J., Li C.-L., Fang S.-B., Wang D., et al. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8 doi: 10.1186/s13287-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lian Q., Zhang Y., Zhang J., Zhang H.K., Wu X., Zhang Y., et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Goldberg A.J., Dennis J.E., Gronowicz G.A., Kuhn L.T. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lian Q., Zhang Y., Liang X., Gao F., Tse H.-F. Directed differentiation of human-induced pluripotent stem cells to mesenchymal stem cells. Methods Mol Biol Clifton NJ. 2016;1416:289–298. doi: 10.1007/978-1-4939-3584-0_17. [DOI] [PubMed] [Google Scholar]
- 100.Sheyn D., Ben-David S., Shapiro G., De Mel S., Bez M., Ornelas L., et al. Human induced pluripotent stem cells differentiate into functional mesenchymal stem cells and repair bone defects. Stem Cells Transl Med. 2016;5:1447–1460. doi: 10.5966/sctm.2015-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahfeldt T., Schinzel R.T., Lee Y.-K., Hendrickson D., Kaplan A., Lum D.H., et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villa-Diaz L.G., Brown S.E., Liu Y., Ross A.M., Lahann J., Parent J.M., et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wei, Tan H., Manasi G., Qiu S., Kong G., Yong P., et al. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012;9:87–100. doi: 10.1016/j.scr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Nakajima T., Shibata M., Nishio M., Nagata S., Alev C., Sakurai H., et al. Modeling human somite development and fibrodysplasia ossificans progressiva with induced pluripotent stem cells. Dev Camb Engl. 2018;145 doi: 10.1242/dev.165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bloor A.J.C., Patel A., Griffin J.E., Gilleece M.H., Radia R., Yeung D.T., et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med. 2020;26:1720–1725. doi: 10.1038/s41591-020-1050-x. [DOI] [PubMed] [Google Scholar]
- 106.Eto S., Goto M., Soga M., Kaneko Y., Uehara Y., Mizuta H., et al. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitsuzawa S., Ikeguchi R., Aoyama T., Ando M., Takeuchi H., Yurie H., et al. Induced pluripotent stem cell-derived mesenchymal stem cells prolong hind limb survival in a rat vascularized composite allotransplantation model. Microsurgery. 2019;39:737–747. doi: 10.1002/micr.30507. [DOI] [PubMed] [Google Scholar]
- 108.Fukuta M., Nakai Y., Kirino K., Nakagawa M., Sekiguchi K., Nagata S., et al. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y.S., Pelekanos R.A., Ellis R.L., Horne R., Wolvetang E.J., Fisk N.M. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Q., Gregory C.A., Lee R.H., Reger R.L., Qin L., Hai B., et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci. 2015;112:530–535. doi: 10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGrath M., Tam E., Sladkova M., AlManaie A., Zimmer M., de Peppo G.M. GMP-compatible and xeno-free cultivation of mesenchymal progenitors derived from human-induced pluripotent stem cells. Stem Cell Res Ther. 2019;10 doi: 10.1186/s13287-018-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frobel J., Hemeda H., Lenz M., Abagnale G., Joussen S., Denecke B., et al. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Rep. 2014;3:414–422. doi: 10.1016/j.stemcr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luzzani C., Neiman G., Garate X., Questa M., Solari C., Fernandez Espinosa D., et al. A therapy-grade protocol for differentiation of pluripotent stem cells into mesenchymal stem cells using platelet lysate as supplement. Stem Cell Res Ther. 2015;6 doi: 10.1186/scrt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kamiya D., Takenaka-Ninagawa N., Motoike S., Kajiya M., Akaboshi T., Zhao C., et al. Induction of functional xeno-free MSCs from human iPSCs via a neural crest cell lineage. Npj Regen Med. 2022;7(1):17. doi: 10.1038/s41536-022-00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Al-Akashi Z., Zujur D., Kamiya D., Kato T., Kondo T., Ikeya M. Selective vulnerability of human-induced pluripotent stem cells to dihydroorotate dehydrogenase inhibition during mesenchymal stem/stromal cell purification. Front Cell Dev Biol. 2023;11 doi: 10.3389/fcell.2023.1089945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hynes K., Menicanin D., Han J., Marino V., Mrozik K., Gronthos S., et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833. doi: 10.1177/0022034513498258. [DOI] [PubMed] [Google Scholar]
- 117.Hynes K., Bright R., Marino V., Ng J., Verma P.J., Gronthos S., et al. Potential of iPSC-derived mesenchymal stromal cells for treating periodontal disease. Stem Cells Int. 2018;2018 doi: 10.1155/2018/2601945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang H., Aprecio R.M., Zhou X., Wang Q., Zhang W., Ding Y., et al. Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: a pilot study. PloS One. 2014;9 doi: 10.1371/journal.pone.0100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yin X., Li P., Li Y., Cai Y., Wen J., Luan Q. Growth/differentiation factor-5 promotes in vitro/vivo periodontal specific differentiation of induced pluripotent stem cell-derived mesenchymal stem cells. Exp Ther Med. 2017;14:4111–4117. doi: 10.3892/etm.2017.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mooney D.J., Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 121.d’Avanzo N., Bruno M.C., Giudice A., Mancuso A., Gaetano F.D., Cristiano M.C., et al. Influence of materials properties on bio-physical features and effectiveness of 3D-scaffolds for periodontal regeneration. Mol Basel Switz. 2021;26:1643. doi: 10.3390/molecules26061643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niu X., Wang L., Xu M., Qin M., Zhao L., Wei Y., et al. Electrospun polyamide-6/chitosan nanofibers reinforced nano-hydroxyapatite/polyamide-6 composite bilayered membranes for guided bone regeneration. Carbohydr Polym. 2021;260 doi: 10.1016/j.carbpol.2021.117769. [DOI] [PubMed] [Google Scholar]
- 123.Woo H.N., Cho Y.J., Tarafder S., Lee C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact Mater. 2021;6:3328–3342. doi: 10.1016/j.bioactmat.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atia G.A.N., Shalaby H.K., Zehravi M., Ghobashy M.M., Attia H.A.N., Ahmad Z., et al. Drug-loaded chitosan scaffolds for periodontal tissue regeneration. Polymers. 2022;14:3192. doi: 10.3390/polym14153192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shang S., Yang F., Cheng X., Walboomers X.F., Jansen J.A. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur Cell Mater. 2010;19:180–192. doi: 10.22203/ecm.v019a18. [DOI] [PubMed] [Google Scholar]
- 126.Jiang W., Li L., Zhang D., Huang S., Jing Z., Wu Y., et al. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015;25:240–252. doi: 10.1016/j.actbio.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y., Liu Y., Zhang X., Liu N., Yu X., Gao M., et al. Engineering electrospun nanofibers for the treatment of oral diseases. Front Chem. 2021;9 doi: 10.3389/fchem.2021.797523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qian Y., Zhou X., Zhang F., Diekwisch T.G.H., Luan X., Yang J. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces. 2019;11:37381–37396. doi: 10.1021/acsami.9b07053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Washio K., Iwata T., Mizutani M., Ando T., Yamato M., Okano T., et al. Assessment of cell sheets derived from human periodontal ligament cells: a pre-clinical study. Cell Tissue Res. 2010;341:397–404. doi: 10.1007/s00441-010-1009-1. [DOI] [PubMed] [Google Scholar]
- 130.Iwata T., Yamato M., Tsuchioka H., Takagi R., Mukobata S., Washio K., et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30:2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 131.Raju R., Oshima M., Inoue M., Morita T., Huijiao Y., Waskitho A., et al. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci Rep. 2020;10 doi: 10.1038/s41598-020-58222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H., Liu S., Zhu B., Xu Q., Ding Y., Jin Y. Composite cell sheet for periodontal regeneration: crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panduwawala C.P., Zhan X., Dissanayaka W.L., Samaranayake L.P., Jin L., Zhang C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J Periodontal Res. 2017;52:408–418. doi: 10.1111/jre.12405. [DOI] [PubMed] [Google Scholar]
- 134.Kittaka M., Kajiya M., Shiba H., Takewaki M., Takeshita K., Khung R., et al. Clumps of a mesenchymal stromal cell/extracellular matrix complex can be a novel tissue engineering therapy for bone regeneration. Cytotherapy. 2015;17:860–873. doi: 10.1016/j.jcyt.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 135.Takewaki M., Kajiya M., Takeda K., Sasaki S., Motoike S., Komatsu N., et al. MSC/ECM cellular complexes induce periodontal tissue regeneration. J Dent Res. 2017;96:984–991. doi: 10.1177/0022034517708770. [DOI] [PubMed] [Google Scholar]
- 136.Nagahara T., Yoshimatsu S., Shiba H., Kawaguchi H., Takeda K., Iwata T., et al. Introduction of a mixture of β-tricalcium phosphate into a complex of bone marrow mesenchymal stem cells and type I collagen can augment the volume of alveolar bone without impairing cementum regeneration. J Periodo. 2015;86:456–464. doi: 10.1902/jop.2014.140384. [DOI] [PubMed] [Google Scholar]
- 137.Lee C.H., Hajibandeh J., Suzuki T., Fan A., Shang P., Mao J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20:1342–1351. doi: 10.1089/ten.TEA.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rasperini G., Pilipchuk S.P., Flanagan C.L., Park C.H., Pagni G., Hollister S.J., et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94:153S–157SS. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- 139.Peng C., Zheng J., Chen D., Zhang X., Deng L., Chen Z., et al. Response of hPDLSCs on 3D printed PCL/PLGA composite scaffolds in vitro. Mol Med Rep. 2018;18:1335–1344. doi: 10.3892/mmr.2018.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tian Y., Liu M., Liu Y., Shi C., Wang Y., Liu T., et al. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J Biomed Mater Res A. 2021;109:1209–1219. doi: 10.1002/jbm.a.37114. [DOI] [PubMed] [Google Scholar]
- 141.Thattaruparambil Raveendran N., Vaquette C., Meinert C., Samuel Ipe D., Ivanovski S. Optimization of 3D bioprinting of periodontal ligament cells. Dent Mater Publ Acad Dent Mater. 2019;35:1683–1694. doi: 10.1016/j.dental.2019.08.114. [DOI] [PubMed] [Google Scholar]
- 142.Yoshimatsu M., Ohnishi H., Zhao C., Hayashi Y., Kuwata F., Kaba S., et al. In vivo regeneration of rat laryngeal cartilage with mesenchymal stem cells derived from human induced pluripotent stem cells via neural crest cells. Stem Cell Res. 2021;52 doi: 10.1016/j.scr.2021.102233. [DOI] [PubMed] [Google Scholar]
- 143.Murata D., Fujimoto R., Nakayama K. Osteochondral regeneration using adipose tissue-derived mesenchymal stem cells. Int J Mol Sci. 2020;21:3589. doi: 10.3390/ijms21103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakamura A., Murata D., Fujimoto R., Tamaki S., Nagata S., Ikeya M., et al. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication. 2021;13 doi: 10.1088/1758-5090/ac1c99. [DOI] [PubMed] [Google Scholar]
- 145.Mitsuzawa S., Zhao C., Ikeguchi R., Aoyama T., Kamiya D., Ando M., et al. Pro-angiogenic scaffold-free Bio three-dimensional conduit developed from human induced pluripotent stem cell-derived mesenchymal stem cells promotes peripheral nerve regeneration. Sci Rep. 2020;10 doi: 10.1038/s41598-020-68745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krampera M., Blanc K.L. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell. 2021;28:1708–1725. doi: 10.1016/j.stem.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 147.Kraitchman D.L., Tatsumi M., Gilson W.D., Ishimori T., Kedziorek D., Walczak P., et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leibacher J., Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., et al. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 150.Devine S.M., Cobbs C., Jennings M., Bartholomew A., Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 151.Yu M., Ge S., Wang F., Wen Y., Yan X., Zeng Q., et al. The role of systemically delivered bone marrow-derived mesenchymal stem cells in the regeneration of periodontal tissues. Int J Oral Maxillofac Implants. 2013;28:e503–e511. doi: 10.11607/jomi.te31. [DOI] [PubMed] [Google Scholar]
- 152.Salvadori M., Cesari N., Murgia A., Puccini P., Riccardi B., Dominici M. Dissecting the pharmacodynamics and pharmacokinetics of MSCs to overcome limitations in their clinical translation. Mol Ther - Methods Clin Dev. 2019;14:1–15. doi: 10.1016/j.omtm.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rustad K.C., Gurtner G.C. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care. 2012;1:147–152. doi: 10.1089/wound.2011.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao X., Shen Z., Guan M., Huang Q., Chen L., Qin W., et al. Immunomodulatory role of stem cells from human exfoliated deciduous teeth on periodontal regeneration. Tissue Eng Part A. 2018;24:1341–1353. doi: 10.1089/ten.TEA.2018.0016. [DOI] [PubMed] [Google Scholar]
- 155.Yang Y., Rossi F.M.V., Putnins E.E. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials. 2010;31:8574–8582. doi: 10.1016/j.biomaterials.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 156.Sone H., Kajiya M., Takeda K., Sasaki S., Horikoshi S., Motoike S., et al. Clumps of mesenchymal stem cells/extracellular matrix complexes directly reconstruct the functional periodontal tissue in a rat periodontal defect model. J Tissue Eng Regen Med. 2022;16:945–955. doi: 10.1002/term.3343. [DOI] [PubMed] [Google Scholar]
- 157.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bazzoni R., Takam Kamga P., Tanasi I., Krampera M. Extracellular vesicle-dependent communication between mesenchymal stromal cells and immune effector cells. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.596079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bier A., Berenstein P., Kronfeld N., Morgoulis D., Ziv-Av A., Goldstein H., et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018;174:67–78. doi: 10.1016/j.biomaterials.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 160.Chew J.R.J., Chuah S.J., Teo K.Y.W., Zhang S., Lai R.C., Fu J.H., et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252–264. doi: 10.1016/j.actbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 161.Nakao Y., Fukuda T., Zhang Q., Sanui T., Shinjo T., Kou X., et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y., Xiong Y., Chen X., Chen C., Zhu Z., Li L. Therapeutic effect of bone marrow mesenchymal stem cells pretreated with acetylsalicylic acid on experimental periodontitis in rats. Int Immunopharmacol. 2018;54:320–328. doi: 10.1016/j.intimp.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 163.Wada N., Menicanin D., Shi S., Bartold P.M., Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 164.Özdemir A.T., Özgül Özdemir R.B., Kırmaz C., Sarıboyacı A.E., Ünal Halbutoğlları Z.S., Özel C., et al. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol. 2016;310:108–115. doi: 10.1016/j.cellimm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 165.Kwack K.H., Lee J.M., Park S.H., Lee H.W. Human dental pulp stem cells suppress alloantigen-induced immunity by stimulating T cells to release transforming growth factor beta. J Endod. 2017;43:100–108. doi: 10.1016/j.joen.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 166.Hieke C., Kriebel K., Engelmann R., Müller-Hilke B., Lang H., Kreikemeyer B. Human dental stem cells suppress PMN activity after infection with the periodontopathogens Prevotella intermedia and Tannerella forsythia. Sci Rep. 2016;6 doi: 10.1038/srep39096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tang H.-N., Xia Y., Yu Y., Wu R.-X., Gao L.-N., Chen F.-M. Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities: a patient-matched comparison. J Clin Periodo. 2016;43:72–84. doi: 10.1111/jcpe.12501. [DOI] [PubMed] [Google Scholar]
- 168.Shin C., Kim M., Han J.-A., Choi B., Hwang D., Do Y., et al. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 2017;52:135–146. doi: 10.1111/jre.12378. [DOI] [PubMed] [Google Scholar]
- 169.Gouveia de Andrade A.V., Bertolino G., Riewaldt J., Bieback K., Karbanová J., Odendahl M., et al. Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev. 2015;24:1374–1376. doi: 10.1089/scd.2014.0563. [DOI] [PubMed] [Google Scholar]
- 170.Takenaka-Ninagawa N., Kim J., Zhao M., Sato M., Jonouchi T., Goto M., et al. Collagen-VI supplementation by cell transplantation improves muscle regeneration in Ullrich congenital muscular dystrophy model mice. Stem Cell Res Ther. 2021;12 doi: 10.1186/s13287-021-02514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harada A., Goto M., Kato A., Takenaka-Ninagawa N., Tanaka A., Noguchi S., et al. Systemic supplementation of collagen VI by neonatal transplantation of iPSC-derived MSCs improves histological phenotype and function of Col6-deficient model mice. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.790341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boada-Romero E., Martinez J., Heckmann B.L., Green D.R. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21:398–414. doi: 10.1038/s41580-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Elliott M.R., Koster K.M., Murphy P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wruck W., Graffmann N., Spitzhorn L.-S., Adjaye J. Human induced pluripotent stem cell-derived mesenchymal stem cells acquire rejuvenation and reduced heterogeneity. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.717772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mabuchi Y., Okawara C., Méndez-Ferrer S., Akazawa C. Cellular heterogeneity of mesenchymal stem/stromal cells in the bone marrow. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.689366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y., Zhang Z., Yang X., Liu A., Liu S., Feng J., et al. Odontogenic MSC heterogeneity: challenges and opportunities for regenerative medicine. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.827470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang J., Chen M., Liao J., Chang C., Liu Y., Padhiar A.A., et al. Induced pluripotent stem cell-derived mesenchymal stem cells hold lower heterogeneity and great promise in biological research and clinical applications. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.716907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with crohn’s disease. Gastroenterology. 2018;154:1334–1342.e4. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 181.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells Dayt Ohio. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toma C., Wagner W.R., Bowry S., Schwartz A., Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.von Bahr L., Batsis I., Moll G., Hägg M., Szakos A., Sundberg B., et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells Dayt Ohio. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 185.Zangi L., Margalit R., Reich-Zeliger S., Bachar-Lustig E., Beilhack A., Negrin R., et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells Dayt Ohio. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 186.Grinnemo K.H., Månsson A., Dellgren G., Klingberg D., Wardell E., Drvota V., et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 187.Xia Z., Ye H., Choong C., Ferguson D.J.P., Platt N., Cui Z., et al. Macrophagic response to human mesenchymal stem cell and poly(epsilon-caprolactone) implantation in nonobese diabetic/severe combined immunodeficient mice. J Biomed Mater Res A. 2004;71:538–548. doi: 10.1002/jbm.a.30185. [DOI] [PubMed] [Google Scholar]
- 188.Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 189.Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 190.Xu H., Wang B., Ono M., Kagita A., Fujii K., Sasakawa N., et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24:566–578.e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 191.Khorsand A., Eslaminejad M.B., Arabsolghar M., Paknejad M., Ghaedi B., Rokn A.R., et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implant. 2013;39:433–443. doi: 10.1563/AAID-JOI-D-12-00027. [DOI] [PubMed] [Google Scholar]
- 192.Hu J., Cao Y., Xie Y., Wang H., Fan Z., Wang J., et al. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fu X., Jin L., Ma P., Fan Z., Wang S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodo. 2014;85:845–851. doi: 10.1902/jop.2013.130254. [DOI] [PubMed] [Google Scholar]
- 194.Yang X., Ma Y., Guo W., Yang B., Tian W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics. 2019;9:2694–2711. doi: 10.7150/thno.31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fawzy El-Sayed K.M., Paris S., Becker S.T., Neuschl M., De Buhr W., Sälzer S., et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: an animal study. J Clin Periodo. 2012;39:861–870. doi: 10.1111/j.1600-051X.2012.01904.x. [DOI] [PubMed] [Google Scholar]
- 196.Li Y., Zhao S., Nan X., Wei H., Shi J., Li A., et al. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther. 2016;7 doi: 10.1186/s13287-016-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ferrarotti F., Romano F., Gamba M.N., Quirico A., Giraudi M., Audagna M., et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J Clin Periodo. 2018;45:841–850. doi: 10.1111/jcpe.12931. [DOI] [PubMed] [Google Scholar]