Abstract
Clinical use of trastuzumab (TZM), has been widely associated with increased incidence of cardiotoxicity. Ocimum gratissimum Linn. is a household medicinal plant popularly used for treating inflammatory conditions. In this study, we investigated the abrogative potential of 100 mg/kg/day of the ethanol leaf extract of Ocimum gratissimum Linn. (OG) and its petroleum ether (PEOG), ethyl acetate (EAOG) and ethanol (EOG) fractions in TZM intoxicated Wistar rats for 7 days using anthropometric, biochemical, histopathological and immunohistochemical endpoints. In addition, secondary metabolite constituents in OG and its fractions were determined through Gas Chromatography-Mass Spectrometry (GC-MS). The study results showed that oral pretreatments with OG and OG fractions as well as the fixed dose valsartan-lisinopril (VAL-LSP) combination effectively ameliorated and restore nearly normal levels the TZM-altered plasma cardiac troponin I and antioxidant profile which were corroborated by histopathological and immunohistochemical findings as indicated by the inhibition of TZM-induced activation of caspases-3 and − 9 and profound upregulation of BCL-2 expression. Phytoscan of OG and its fractions showed the presence of thymol and in high amount. Overall, our findings revealed the cardioprotective potentials of OG, OG fractions and fixed dose VAL-LSP combination against TZM-induced cardiotoxicity which probably was mediated via abrogation of cardiomyocyte apoptosis and antioxidant mechanisms.
Keywords: Wistar rats, Ocimum gratissimum Linn., Ethanol leaf extract and solvent fractions, Oxidative stress markers, Apoptosis markers, Trastuzumab-induced cardiotoxicity
Graphical Abstract
Highlights
-
•
The anti-cancer drug, trastuzumab (TZM), presents with cardiotoxicity.
-
•
The Ocimum gratissimum ethanolic leaf extract (OG), its ethylacetate (EAOG) fraction, and valsartan-lisinopril (VAL-LSP) fixed dose combination pretreatments attenuated and reversed the TZM-associated cardiotoxicity in rats, probably mediated via anti-apoptosis and antioxidant mechansisms.
1. Introduction
Trastuzumab (TZM), since it was first approved by the Food and Drug Administration in September 1998 for the clinical management of patients with HER2-positive metastatic breast cancer, has remain the gold standard treatment strategy [48], [61], [99]. TZM, a purified recombinant DNA-based humanized monoclonal antibody, elicits its cytotoxic action by binding with high affinity and specificity to the extracellular domain IV of the tyrosine kinase human epidermal growth factor receptor 2 (HER2) expressed by solid tumors such as early and metastasized breast cancer [21], [56], gastro-esophageal adenocarcinoma, gastric cancer and salivary gland tumors [83], [52], [33].
TZM induces anticancer action through multiple mechanisms that include HER2 internalization and degradation, antibody-dependent cellular cytotoxicity, and MAPK and PI3K/Akt interference [101], [97], [34]. However, despite TZM’s high efficacy in cancer treatment, its use has been limited by its off-target cardiotoxicity and treatment resistance. TZM cardiotoxicity is postulated to be mediated via multiple mechanisms that include HER2 signaling dysregulation, cardiomyocyte autophagy suppression, and cardiomyocyte reactive oxygen species (ROS) accumulation [65], [22] with all these culminating in accelerated cardiomyocyte apoptosis [65], [66]. TZM, like doxorubicin (an anthracycline cytotoxic agent), also binds to the DNA topoisomerase IIB (TOP2B) protein to cause DNA breakage, activation of DNA damage response pathway, and cardiomyocytes apoptosis [66], [34]. To prevent cardiotoxicity, several strategies have been developed: (i) angiotensin-converting enzyme inhibitors, sartans, beta-blockers, aldosterone antagonists, and statins; (ii) iron chelators; and (iii) by development of new anthracycline derivatives with little or no cardiotoxicity [19], although their effectiveness remain debatable.
Ocimum gratissimum Linn. (Lamiaceae) is an aromatic perennial medicinal and ornamental plant that is widely distributed across tropical and subtropical regions of the world ([4], [79] where it is commonly known as clove basil, African basil and wild basil [88]. It is a common food seasoning and condiment popularly used in the West African and Asian cuisines [4], [40]. Ocimum gratissimum Linn. is known by different names and these include “Scent leaves” (Nigeria), “Nunu Bush” (Jamaica), “Fobazen” (Haiti), “Mujaaja” (Uganda) and “Maduruthala” (Sri Lanka) [70].
In the Asian traditional medicine, Ocimum especially Ocimum basilicum have the ancestral history of being used in the local treatment of heart diseases including hypertension and coronary heart disease [95]. Reports have it that Ocimum gratissimum Linn. possesses different biological activities and these include anti-stress, antidiarrheal [1], [27], [43], [72], anti-helminthic, anti-inflammatory, antipyretic [84], [3], [85], antioxidant and free radical scavenging [42], [3], antimutagenic and anticancer [17], anti-ulcerative and gastroprotective, antibacterial [70], fungicidal [92], hepatoprotective [18], antiapoptotic [53]. Its other biological activities include male anti-fertility and aphrosidiac [71], [74], [69], and sedative activities [30], [75]. Polyphenol-rich fraction of Ocimum gratissimum Linn. leaves have reportedly protected against cyclophosphamide-induced nephrotoxicity [5], gentamicin-induced hepatotoxicity [73] as well as lead acetate-induced cerebellar dysfunction [94] in rats. Other studies have also reported the safety and male fertility-enhancing effect of both the ethyl acetate and n-butanol fractions of Ocimum gratissimum Linn. leaf extract in Wistar rats [69]. While Ocimum gratissimum has been reported to possess antihyperlipidemic, anti-ischemic, and antihypertensive activities [91], a bioactive compound isolated from the plant leaves. gratissinol, is documented to be responsible for its cardioprotective activities [81].
There have been extensive reports and documentations on the TZM-associated left ventricular (LV) dysfunction (LVD) and heart failure (LVHF) but emerging and accumulating reports showed that TZM treatment equally often induces subtle but significant deleterious and fatal effects on the right ventricle (RV) structure and function that are mostly unrecognized [11], [10]. Indeed, RV heart failure (RVHF) has been documented to co-exist with LVFH [14] and may be irreversible even after the discontinuation of chemotherapy unlike LVHF [44]. Other studies have demonstrated that TZM blocks the HER2 receptors in the cardiomyocytes rendering both RV and LV vulnerable to type II or reversible cardiotoxicity [64], [93]. However, the thinner structure of the RV with lesser myofibrils may make it more vulnerable to the TZM toxicity [35].
In our quest to provide effective and affordable therapeutic option(s) for treating TZM-induced cardiotoxicity, this study was designed at evaluating the possible therapeutic potential of Ocimum gratissimum Linn. leaf extract and fractions in acute TZM-induced cardiotoxicity in Wistar rats, using biochemical markers (cardiac enzyme, complete lipids profile, cardiac oxidative stress, apoptosis), as well as histopathological and immunohistochemical endpoints.
2. Materials and methods
2.1. Drugs and chemicals
The drugs and chemicals used for this study are as reported by [77].
2.2. Collection of plant materials
10 kg of the fresh whole plant of Ocimum gratissimum L. was collected from the thick forest of Moro Local Government Area of Kwara State, Nigeria in the month of June 2020. The plant was botanically identified and authenticated by Mr. Bolu Ajayi, a Senior Herbarium Curator at the University of Ilorin Herbarium. A voucher specimen with reference number UILH/001/984/2002 was prepared and deposited in the herbarium. The rest of the collected plant samples were gently rinsed under flowing tap water, de-stalked and air-dried under shade in the laboratory at room temperature of 28–32 ℃ for 3 weeks. The completely air-dried plant leaves were pulverized using Laboratory Hammer mill and stored in air- and water-tight containers and kept at room temperature in the laboratory until needed for extraction.
2.3. Extraction processes and calculation of % yield
Pulverized sample of Ocimum gratissimum Linn. dried leaves (2.6 kg) was soaked in 5.2 L of 99.7% ethanol for 72 h after which it was continuously stirred for 1 h before it was filtered using 180 mm of filter paper. The filtrate was then concentrated at 40°C to complete dryness using rotary evaporator. The deep brown, sweet-smelling solid residue left behind was weighed, stored in air- and water-proof container which was kept in a refrigerator at 4 ºC. This procedure was repeated for four more times. From this stock, fresh solutions were made whenever required.
The average percentage yield of the Ocimum gratissimum L. ethanol leaf extract (OG) obtained was 11.52%.
2.4. Solvent fractionation of Ocimum gratissimum Linn. ethanol leaf extract (OG)
OG was solvent-solvent portioned using procedure earlier described by Njan et al. [69] with slight modifications in the solvents and volumes used.
Briefly described, OG solution was made with 150 ml of ethanol to which 250 ml of distilled water was added to give a hydroethanolic solution which was transferred into a 2 liter separating funnel. 1 liter of n-hexane was added and the mixture was rigorously shaken for 15 min to ensure complete dissolution. The solution was then left to stand for 4 h to obtain two immiscible layers. The lower aqueous layer was gently drained into a clean container while the upper n-hexane fraction was drained into another container. This separation procedure was repeated for the hydroethanolic extract residue with further addition of n-hexane until a clear upper layer was obtained. The entire process was repeated with the extract residue using ethyl acetate followed by ethanol to obtain the ethyl acetate and ethanol fractions, respectively. The residue left behind, “marc”, was kept in a clean container at the end of the experiment. Individual fraction obtained was concentrated in vacuo at 40 °C using the rotary evaporator using the process described by Adeneye et al. [2]. The concentrated fractions were further freeze-dried using a freeze drier (LTE LYOTRAP.LTE Scientific Ltd, Greenfield, England) and stored in both air- and water-tight containers and kept in the refrigerator at 4 °C as previously reported by Adeneye et al. [2]. The petroleum ether, ethyl acetate and ethanol fractions were denoted as PEOG, EAOG and EOG, respectively and used for the experiment.
2.5. Gas chromatography-mass spectrophotometer (GC-MS) analysis of OG and OG fractions
The procedure for conducting the gas chromatography-mass spectrophotometer (GC-MS) analysis for OG and its solvent fractions to determine the relative abundance of its secondary metabolites was strictly in compliance with that earlier described by Olorundare et al. [76].
2.6. Experimental animals
A total of seventy-five (75) of adult male Wistar Albino rats (aged 10–12 weeks old and body weight: 180–200 g) were obtained from the Animal Farm in Ogbomoso, Oyo State, Nigeria and housed in the Animal House of the Department of Veterinary Medicine, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria, after an ethical approval (UERC Approval number: UERC/ASN/2022/2327) was obtained from the University of Ilorin Ethical Review Committee for Staff and Postgraduate Research. The rats were acclimatized for 2 weeks in the facility, cared for and handled in line with global best practices guiding the Use and Handling of Experimental Animals as stipulated by the National Research Council [68]. Experimental rats were freely fed with standard rat feed and potable water and maintained at standard laboratory conditions throughout the period of the study.
Prior to the commencement of the experiment, rats whose body weight differences within and between groups do not exceed ± 20% of the average weight of all the experimental rats were randomly allotted to twelve (12) treatment groups and used for the study.
2.7. Body weight measurement
The body weights of the experimental rats were taken at the beginning and end of the experiment using a digital rodent weighing scale (®Virgo Electronic Compact Scale, New Delhi, India) as previously described by Olorundare et al. [77], [78]. The values obtained were expressed in grams (g).
2.8. Experimental induction of TZM-mediated cardiotoxicity and drug treatments
TZM-mediated cardiotoxicity was successfully induced in all the TZM-treated rats using the method earlier described by Olorundare et al. [77], [78] but with slight modifications. Briefly described, the experimental rats based on their treatment groups were orally pretreated with OG, PEOG, EAOG, EOG and VAL-LSP-VAL fixed dose combination therapy 1 h prior to injection with 2.25 mg/kg of TZM given via the intraperitoneal route. This was done on daily basis for 7 consecutive days. The treatment protocol adopted is as follows:
Veh. – 10 ml/kg/day distilled water p.o. + 1 ml/kg/day distilled water i.p.
Veh. + TZM – 10 ml/kg/day distilled water p.o. + 2.25 mg/kg/day TZM i.p.
OG – 100 mg/kg/day OG p.o. + 1 ml/kg/day distilled water i.p.
OG + TZM – 100 mg/kg/day OG p.o. + 2.25 mg/kg/day TZM i.p.
PEOG – 100 mg/kg/day PEOG p.o. + 1 ml/kg/day distilled water i.p.
PEOG + TZM - 100 mg/kg/day PEOG p.o. + 2.25 mg/kg/day TZM i.p.
EAOG - 100 mg/kg/day EAOG p.o. + 1 ml/kg/day distilled water i.p.
EAOG + TZM - 100 mg/kg/day EAOG p.o. + 2.25 mg/kg/day TZM i.p.
EOG - 100 mg/kg/day EOG p.o. + 1 ml/kg/day distilled water i.p.
EOG + TZM - 100 mg/kg/day EOG p.o. + 2.25 mg/kg/day TZM i.p.
VAL-LSP – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 1 ml/kg/day distilled water i.p.
VAL-LSP + TZM – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 2.25 mg/kg/day TZM i.p.
The choice of 100 mg/kg/day of OG as the treatment dose was made based on the results of the preliminary studies previously conducted which showed it to be the most effective dose with the no toxicity.
2.9. Blood sample collection
24 h after the last TZM injection, the overnight fasted rats were humanely sacrificed under controlled, light inhaled halothane anesthesia and whole blood samples were collected directly from the heart with fine 21 G injectable needle and 5 ml syringe. The rat heart was carefully identified, harvested and weighed.
2.10. Plasma cardiac troponin I and lipids measurements
Blood samples obtained via cardiac puncture was collected into 10 ml heparinized sample bottles and centrifuged at 5000 rpm to separate out the plasma that were used for the assays of cardiac troponin I (cTnI), triglyceride (TG), total cholesterol (TChol.) and cholesterol fractions: high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), very low density lipoprotein cholesterol (VLDL-c)] using standard bioassay procedures and commercial kits.
2.11. Calculation of atherogenic and coronary artery risk indices (AI and CRI)
AI was calculated using the Castelli’s risk indices:
and
while CRI was calculated as:
as described by Lemieux et al. [54], Ren et al. [86] and Azeez [8].
2.12. Determination of cardiac tissue antioxidant profile
The cardiac tissue was obtained, preserved and the activities of oxidative stress markers such as superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), Malondialdehyde (MDA) and reduced glutathione (GSH) were determined using methods earlier described by Olorundare et al. [78].
2.13. Histopathological studies of cardiac tissues
This was done using the right half of the heart dissected out and the choice of right ventricle being the most susceptible of the heart chambers to TZM toxicity [10], [44]. Tissue slide preparations and readings were also done as earlier reported by Olorundare at al. (2021).
2.14. Enzyme-linked immunosorbent assay (ELISA) to determine cardiac tissue caspases-3 and − 9 levels
The levels of cardiac caspases-3 and − 9 were determined using the commercial enzyme linked immunosorbent assay (ELISA) kits (Wuhan Elabscience Biotechnology Company Limited, No. 1 Shizishan Street, Hongshan District, Wuhan, Hubei, China) following the Manufacturers’ instruction and in line with the procedure earlier described by Olorundare et al. [78].
2.15. Immunohistochemical analysis of caspase-3 and BCL-2 expressions in cardiac tissues
The immunohistochemical staining of cardiac to determine the levels of expression of caspase-3 and BCL-2 as previously described by Olorundare et al., [78]. The immunoreactive positive expression of caspase-3 and BCL-2 indicated by brown staining on the prepared sample slides were viewed using a photo microscope (Olympus) and a digital camera (Toupcam®, China). Photomicrographs were analyzed for intensity of protein expression using image J Fiji software application.
2.16. Data analysis
Data were presented as mean ± S.E.M. of three observations for the immunohistochemical assays and mean ± S.D. of five observations for the in vivo studies. Statistical analysis was done using One-way analysis of variance followed by Tukey’s post hoc test so as to compare within and between groups on GraphPad Prism Version 5. Statistical significance was considered at p < 0.05, p < 0.001, and p < 0.0001.
3. Results
3.1. Gas chromatography-mass spectrometry to determine secondary metabolites in OG, PEOG, EAOG and EOG fractions
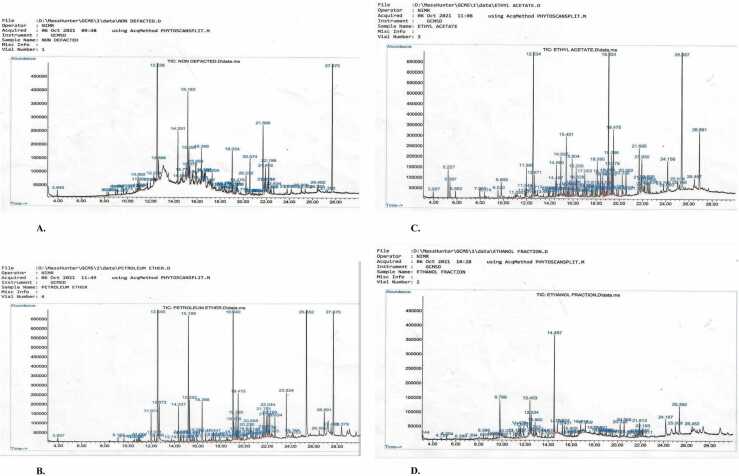
GC-MS analysis of OG shows the presence and relative high abundance of thymol, β-caryophyllene, naphthalene, octahydronaphthalene, phenol isocaryophyllene naphthalene, neophytadiene, pentadecanoic acid, hexadecenoic acid ethyl ester, phytol, octadecatrienoic acid, octadecatrienoate, squalene and supraene although other secondary metabolites were also present but in relatively negligible amount {Table 1 and Fig. 1(a)}. The PEOG shows the presence of thymol, phenol esters, β-caryophyllene esters, guaiene, naphthalene esters, neophytadiene, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 1,1′-di-n-butylferrocene, 4 H-1-benzopyran-4-one, methoxy-THC, phthalic acid esters, 1,3-benzenedicarboxylic acid bis(2-ethylhexyl) ester and squalene {Table 2 and Fig. 1(b)}. The EAOG shows the presence and relative high abundance of cyclohexene, thymoquinone, thymol, phenol derivatives, N-ethyl-4-nitroaniline, 4-amino-1-butanol, 2,4-di-tert-butylphenol, p-cymene-2,5-diol, bicyclo[4.4.0]dec-1-ene, bicyclosequiphellandrene, neophytadiene, hexadecen-1-ol derivatives, phthalic acids, neophytadiene, tetradecanoic acid, 1-docosene, phytol, phthalate phthalic acid, octadecatrienoic acid and 1,3-benzenedicarboxylic acid amongst other secondary metabolites that in relatively low quantity in the fraction {Table 3 and Fig. 1(c)}. Similarly, EOG is abundant in 2(1 H)-pyridinone, 6-hydroxy-1 H-pyrrole, benzofuran, 2,3-dihydrobenzofuran, 5-hydroxymethylfurfural, furan, 2,3-dihydro-5-methyl-2-butenal, 2,5-hexanediol, 2,5-dimethylpyrrolidine, 2-methoxy-4-trifluoromethyl-allopurinol, imidazole 2-trifluoromethyl-allopurinol, cycloheptasiloxane, 9-oxabicyclo[6.1.0]nonane isomers, hexadecenoic acid ethyl ester, octadecanoic acid ethyl ester, 9,12,15-octadecatrienoic acid esters, thymol, phenol esters, and phthalic acid ester {Table 4 and Fig. 1(d)}.
Table 1.
GC-MS Analysis showing the secondary metabolites and their relative abundance in Ocimum gratissimum Linn. ethanol leaf extract (OG).
| S/No. | RT | Sec. Metab. | compound | CAS Library ID/Ref# | % Purity |
|---|---|---|---|---|---|
| Peak Area (%) | name | ||||
| 1. | 12.528 | 40.59 | Thymol | 000089-83-8 | 94 |
| 2. | 14.331 | 2.65 | β-Caryophyllene | 000087-44-5 | 99 |
| 3. | 15.183 | 4.07 | Naphthalene | 017066-67-0 | 99 |
| 4. | 15.286 | 1.42 | 2-Isopropenyl-4a,8-dimethy-1, 2,3,4,4a,5,6,8a-octahydronaphthalene | 1000193-57-0 | 98 |
| 5. | 15.372 | 1.44 | 1,2,3,5,6,8a-hexahydro-4,7-dimethy-1-(1-methyl ethyl) | 000483-76-1 | 90 |
| 6. | 15.893 | 1.49 | Phenol, 4-(methoxymethyl)− 2,6 dimethylbicyclo[4.3.0]nona-3,7-diene | 005048-02-2 | 53 |
| 7. | 16.368 | 1.64 | isocaryophillenenaphthalene | 1000140-07-2 | 90 |
| 8. | 17.295 | 1.07 | 1.6-dimethyl-4-(1-methyl ethyl)-Naphthalene | 000483-78-3 | 98 |
| 9. | 19.034 | 1.89 | Neophytadiene | 000504-96-1 | 90 |
| 10. | 20.230 | 1.29 | Pentadecanoic acid | 001002-84-2 | 91 |
| 11. | 20.574 | 1.78 | Hexadecanoic acid,ethyl ester | 000628-97-7 | 97 |
| 12. | 21.695 | 5.26 | Phytol | 000150-86-7 | 53 |
| 13. | 21.918 | 1.41 | 9,12,15-octadecatrienoicacid | 001191-41-9 | 91 |
| 14. | 22.199 | 1.94 | ethyl 9,12,15Octadecatrienoate | 1000336-77-4 | 99 |
| 15. | 27.675 | 13.51 | Squalene | 000111-02-4 | 99 |
| Supraene | 007683-64-9 | 97 |
Fig. 1.
GC-MS fingerprints/phytoscan showing the relative abundance of the secondary metabolites identifiable in OG(1 A), PEOG(1B), EAOG(1 C) and EOG(1D).
Table 2.
GC-MS Analysis showing the secondary metabolites and their relative abundance in the petroleum ether fraction of Ocimum gratissimum Linn. leaf extract (PEOG).
| S/No. | RT | Sec. Metab. | compound | CAS Library ID/Ref# | % Purity |
|---|---|---|---|---|---|
| Peak Area (%) | name | ||||
| 1. | 12.546 | 46.07 | Thymol | 000089-83-8 | 94 |
| Phenol, 2-methyl-5(1-methylethyl) | 000499-75-2 | 93 | |||
| 2. | 14.337 | 1.09 | β-Caryophyllene | 000087-44-5 | 99 |
| Bicyclo[5.2.0]nonane | 242794-76-9 | 99 | |||
| 2-methylene-4,8,8-Trimethyl-4-vinyl-caryophyllene | 017066-67-0 | 99 | |||
| 3. | 15.189 | 4.85 | Naphthalene, decahydro4a-methyl-1-methylene-7-(1-methylethyl)-[4aR-(4a.alpha.,7.alpha,8a.beta)]- | 017066-67-0 | 99 |
| Naphthalene,decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-[4aR-(4a.alpha.,7.alpha.,8a.beta)].alpha.-Guaiene | 003691-12-1 | 96 | |||
| 4. | 15.292 | 1.38 | 2-isopropenyl-4a,8-dimethy-1, 2,3,4,4a,5,6,8a-octahydro,naphthalene | 1000193-57-0 | 83 |
| Naphthalene,decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-[4aR-(4a.alpha.,7.alpha,8a.beta)] | 017066-67-0 | 81 | |||
| 2-isopropenyl-4a,8-dimethyl- | 1000192-43-5 | 81 | |||
| 1,2,3,4,4a,5,6,7-octahydro- | |||||
| naphthalene | |||||
| 5. | 16.368 | 1.40 | Caryophyllene oxide | 001139-30-6 | 80 |
| 6. | 19.040 | 5.51 | Neophytadiene | 000504-96-1 | 94 |
| 7. | 19.475 | 1.56 | 3,7,11,15-tetramethyl-2- | 102608-53-7 | 72 |
| hexadecen-1-ol | |||||
| methyl)− 2,6 dimethyl | |||||
| bicyclo[4.3.0]nona-3,7-diene | |||||
| 8. | 21.701 | 1.08 | Phenol | 000585-34-2 | 38 |
| - | m-tert-butyl-(z)− 2,6-dimethylocta | 033746-71-3 | 38 | ||
| 2,5,7-trien-4-one | |||||
| 2,5-diethylphenol | 000876-20-0 | 35 | |||
| 9. | 22.044 | 1.05 | 1,1′-di-n-butylferrocene | 001274-08-4 | 46 |
| 4 H-1-benzopyran-4-one | 005128-44-9 | 43 | |||
| 10. | 23.624 | 1.72 | Methoxy-THC | 1000380-01-1 | 64 |
| 11. | 25.352 | 9.77 | Bis(2-ethylhexyl) phthalate | 000117-81-7 | |
| Phthalic acid, 2ethylhexyl | 1000308-98-5 | 90 | |||
| isohexyl ester | |||||
| 12. | 26.891 | 1.15 | 1,3-benzenedicarboxylic acid, | 000137-89-3 | 91 |
| bis(2-ethylhexyl) ester | |||||
| 13. | 27.675 | 6.38 | squalene | 000111-02-4 |
Table 3.
GC-MS Analysis showing the secondary metabolites and their relative abundance in the ethyl acetate fraction of Ocimum gratissimum Linn. leaf extract (EAOG).
| S/No. | RT | Sec. Metab. | compound | CAS Library ID/Ref# | % Purity |
|---|---|---|---|---|---|
| Peak Area (%) | name | ||||
| 1. | 11.968 | 1.08 | cyclohexene | 000499-03-06 | 92 |
| Thymoquinone | 000490-91-5 | 90 | |||
| 2. | 12.534 | 8.66 | Thymol | 000089-83-8 | 94 |
| Phenol, 2-methyl-5-(1-methylethyl) | 000499-75-2 | 93 | |||
| 3. | 14.560 | 1.09 | N-ethyl-4-nitroaniline | 003665-80-3 | 72 |
| 4. | 14.937 | 1.48 | 4-amino-1-butanol | 100352-30-2 | 96 |
| 5. | 15.401 | 2.05 | 2,4-di-tert-butylphenol | 000096-76-4 | 97 |
| 6. | 15.904 | 1.45 | p-cymene-2,5-diol | 002217-60-9 | 91 |
| 7. | 16.259 | 1.33 | Bicyclo[4.4.0]dec-1-ene | 150320-52-8 | 95 |
| 8. | 17.003 | 1.29 | 2-isopropyl-5-methyl-9-methylene- | 054274-73-6 | 92 |
| (+)-epi-Bicyclosesquiphellandrene | |||||
| .tau. | |||||
| 9. | 18.090 | 1.43 | 1-(1 S,3aR,4 R,7 S,7aS)− 4- | 001911-78-0 | 98 |
| Hydroxy-7isopropyl-4-methyl | |||||
| octahydro-1 H-inden-1-yl) ethanone | |||||
| 10. | 19.034 | 9.60 | Neophytadiene | 000504-96-1 | 91 |
| 11. | 19.286 | 1.56 | 3.7,11,15-tetramethyl-2- | 102608-53-7 | 68 |
| hexadecen-1-ol | |||||
| 12. | 19.378 | 1.15 | Phthalic acid | 100356-84-3 | |
| 13. | 19.475 | 2.46 | 3,7,11,15-Tetramethyl-2- | 102608-53-7 | 80 |
| Hexadecen-1-ol | |||||
| 14. | 20.236 | 1.06 | Tetradecanoic acid | 000544-63-8 | 86 |
| 15. | 20.562 | 1.07 | 1-Docasene | 001599-67-3 | 91 |
| 16. | 21.695 | 2.27 | Phytol | 001599-67-3 | 91 |
| 17. | 21.930 | 1.76 | 9,12,15-octadecatrienoic acid | 000463-40-1 | 91 |
| 18. | 24.156 | 1.97 | 3-methyl-4-isopropylphenol | 003228-02-2 | 60 |
| 19. | 25.351 | 28.45 | Bis(2-ethylhexyl)phthalate | 000117-81-7 | 91 |
| Phthalic acid | |||||
| 20. | 26.891 | 3.42 | 1,3-benzenedicarboxylic acid | 000137-89-3 | 62 |
Table 4.
GC-MS Analysis showing the secondary metabolites and their relative abundance in the ethanol fraction of Ocimum gratissimum Linn. leaf extract (EOG).
| S/No. | RT | Sec. Metab. | compound | CAS Library ID/Ref# | % Purity |
|---|---|---|---|---|---|
| Peak Area (%) | name | ||||
| 1. | 3.144 | 1.01 | 5-(prop-2-enoyloxy)pentadecane | 1000245-67-6 | 35 |
| 2. | 9.799 | 9.96 | 2(1 H)-pyridinone | 000626-06-2 | 72 |
| 6-hydroxy-1 H-pyrrole | |||||
| 3. | 11.190 | 1.16 | catechol | 000120-80-9 | 87 |
| 4. | 11.470 | 2.79 | benzofuran | 000496-16-2 | 87 |
| 2,3-dihydrobenzafuran | |||||
| 5. | 11.613 | 2.85 | 5-hydroxymethylfurfural | 000067-47-0 | 64 |
| 6. | 11.876 | 2.64 | furan 2,3-dihydro-5-methyl-2- | 001487-15-6 | 64 |
| butenal | |||||
| 7. | 12.260 | 1.12 | 2-furancarboxaldehyde | 000620-02-0 | 30 |
| 5-methyl-2-furancarboxaldehyde | |||||
| 8. | 12.403 | 4.71 | 2,5-hexanediol | 000110-03-2 | 53 |
| 9. | 12.534 | 2.39 | Thymol | 000089-83-8 | 93 |
| 3-methyl-4-isopropylphenol | 003228-02-2 | 53 | |||
| 10. | 12.717 | 1.42 | 1,1-dimethyl-1-silacyclo-3-pentene | 016054-12-9 | 43 |
| Thiophene | |||||
| 2-ethyl thiophene | 001795-01-3 | 43 | |||
| 3-ethyl thiophene | 001795-01-3 | 43 | |||
| 11. | 12.860 | 2.02 | 2-ethoxy-4-vinylphenol | 007786-61-0 | 87 |
| 12. | 13.976 | 1.16 | 1,3-disilacyclobutane | 001627-98-1 | 38 |
| 13. | 14.497 | 16.85 | Imidazole,2-trifluoromethyl- | 066675-22-7 | 53 |
| allopurinol | |||||
| 14. | 14.789 | 1.71 | benzene,1,2-dimethyl-4- | 000099-51-4 | 60 |
| nitrobenzenemethanol | |||||
| 15. | 15.224 | 2.07 | cycloheptasiloxane | 000107-50-6 | 90 |
| 16. | 15.401 | 1.77 | phenol | 000128-39-2 | 94 |
| 2,6-bis(1,1-dimethylethyl) | 000128-39-2 | 92 | |||
| phenol | |||||
| 17. | 16.814 | 1.87 | 1-(4-tert-butylphenyl)propan-2-one | 081561-77-5 | 35 |
| 18. | 17.209 | 1.32 | N-(trifluoroacetyl)− 0,0′,0″-tris | 1000072-26-3 | 47 |
| trimethylsilyl)norepinephrine | |||||
| 19. | 20.225 | 2.62 | 9-oxabicyclo[6.1.0]nonane | 000286-62-4 | 25 |
| 20. | 20.568 | 2.58 | hexadecanoic acid, ethyl ester | 000628-97-7 | 89 |
| 21. | 21.913 | 2.53 | 9,12,15-octadecatrienoic acid | 000463-40-1 | 91 |
| (z,z,z)− 9,12,15-octadecatrienoicacid, methyl ester | 000463-40-1 | 91 | |||
| 22. | 22.193 | 1.36 | bicyclo[4.3.0]nonane,2-methylene | 040954-37-8 | 62 |
| 23. | 24.167 | 5.50 | Thymol | 000089-83-8 | 90 |
| Phenol,2,3,4,6-tetramethyl-phenol | 003238-38-8 | 87 | |||
| Phenol,2-methyl-5-(1-methylethyl) | 000499-75-2 | 87 | |||
| 24. | 25.008 | 1.82 | Myristoyl chloride | 000112-64-1 | 49 |
| Undecanoyl chloride | 017746-05-3 | 43 | |||
| Octadecanedioic acid | 000871-70-5 | 43 | |||
| 25. | 25.352 | 5,26 | Phthalic acid, di(2-propylpentyl)ester | 1000377-93-5 | 90 |
| 26. | 26.462 | 1.00 | cyclooctane | 002213-60-7 |
3.2. Effect of OG and OG fractions on body weight gain pattern in TZM-treated rats
Daily treatment with Veh. + TZM administered via intraperitoneal route for 7 consecutive days did not produce any significant (p > 0.05) average body weight changes in the treated rats when compared to the Veh.only-treated group (Table 5). Similarly, repeated oral pre-treatments with OG and its fractions as well as VAL-LSP did not significantly (p > 0.05) alter the average body weight change in the treated rats (Table 5).
Table 5.
Effect of Ocimum gratissimum Linn. leaf extract (OG), OG petroleum ether fraction (PEOG), OG ethyl acetate fraction (EAOG) and OG ethanol fraction (EOG) treatments on weight gain pattern in TZM-intoxicated rats.
| Treatment Groups | Average body weights on: | %weight changes | |
| Day 1 | Day 7 | ||
| Veh. | 216.00 ± 28.97 | 202.40 ± 72.22 | 04.49 ± 07.49 |
| Veh. + TZM | 222.90 ± 26.44 | 209.70 ± 36.05 | -03.22 ± 04.62 |
| OG | 211.40 ± 21.71 | 212.60 ± 20.20 | -03.25 ± 08.45 |
| OG + TZM | 217.20 ± 33.53 | 216.50 + 33.10 | -00.22 ± 05.15 |
| PEOG | 216.80 ± 19.53 | 212.00 ± 30.02 | -01.92 ± 14.09 |
| PEOG + TZM | 217.30 ± 31.15 | 207.80 ± 21.21 | -03.19 ± 12.17 |
| EAOG | 215.30 ± 23.42 | 216.90 ± 21.20 | 00.91 ± 03.76 |
| EAOG + TZM | 218.20 ± 34.17 | 217.30 ± 42.63 | -00.72 ± 04.20 |
| EOG | 217.10 ± 28.34 | 220.90 ± 29.21 | 01.90 ± 05.79 |
| EOG + TZM | 218.10 ± 16.65 | 221.40 ± 13.33 | -00.14 ± 04.61 |
| VAL-LSP | 218.70 ± 17.54 | 223.10 ± 20.63 | 02.00 ± 03.52 |
| VAL-LSP + TZM | 216.30 ± 23.22 | 206.30 ± 18.17 | -04.40 ± 03.07 |
Veh. – 10 ml/kg/day distilled water p.o. + 1 ml/kg/day distilled water i.p.
Veh. + TZM – 10 ml/kg/day distilled water p.o. + 2.25 mg/kg/day TZM i.p.
OG – 100 mg/kg/day OG p.o. + 1 ml/kg/day distilled water i.p.
OG + TZM – 100 mg/kg/day OG p.o. + 2.25 mg/kg/day TZM i.p.
PEOG – 100 mg/kg/day PEOG p.o. + 1 ml/kg/day distilled water i.p.
PEOG + TZM - 100 mg/kg/day PEOG p.o. + 2.25 mg/kg/day TZM i.p.
EAOG - 100 mg/kg/day EAOG p.o. + 1 ml/kg/day distilled water i.p.
EAOG + TZM - 100 mg/kg/day EAOG p.o. + 2.25 mg/kg/day TZM i.p.
EOG - 100 mg/kg/day EOG p.o. + 1 ml/kg/day distilled water i.p.
EOG + TZM - 100 mg/kg/day EOG p.o. + 2.25 mg/kg/day TZM i.p.
VAL-LSP – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 1 ml/kg/day distilled water i.p.
VAL-LSP + TZM – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 2.25 mg/kg/day TZM i.p.
3.3. Effects of OG and its fractions on the plasma lipids in TZM-treated rats
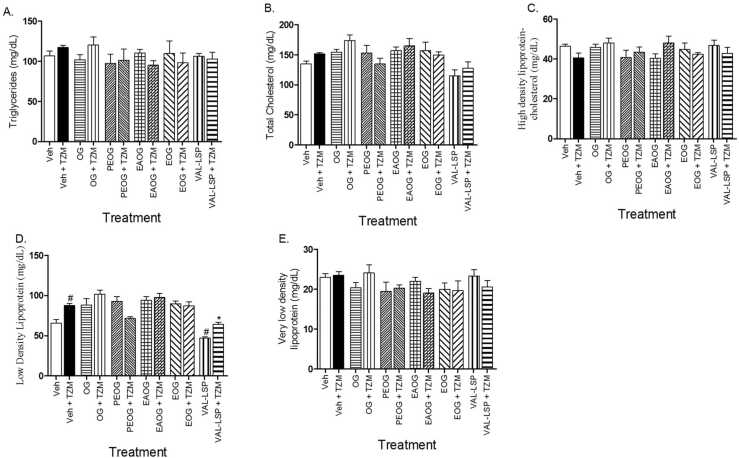
Treatment with Veh. + TZM caused significant (p < 0.05) increase in the LDL-c level (Fig. 2D) and this elevation was markedly lowered only in rats treated with VAL-LSP + TZM when compared to other TZM-treated groups (Fig. 2D). However, in the treatment groups on OG + TZM, PEOG + TZM, EAOG + TZM, EOG + TZM and VAL-LSP + TZM, there were no significant (p > 0.05) alterations in the circulating levels of TG (Fig. 2A), TChol. (Fig. 2B), HDL-c lipoprotein (Fig. 2C), and VLDL-c lipoprotein fractions (Fig. 2E).
Fig. 2.
Depicts the effect of oral treatments with TZM, OG, PEOG, EAOG and EOG on plasma TG (2 A), TChol. (2B), HDL-c (2 C), LDL-c (2D) and VLDL-c (2E) in TZM-intoxicated rats. Bar represents mean ± SEM (n = 5), significant difference denoted by #p < 0.05 vs Group I or *p < 0.05 vs Group II by one-way ANOVA followed by Tukey’s post hoc test.
3.4. OG and OG fractions on the plasma AI and CRI in TZM-treated rats
The effects of OG and its fractions on the plasma atherogenicity (AI, HDL-c ÷ LDL-c ratio) and coronary artery index (CRI) in TZM-treated rats are depicted in Fig. 3(A)-3 C. Treatment with Veh. + TZM was associated with significant (p < 0.05) increases in the AI and CRI values (Figs. 3A and 3(C), respectively) while it caused a significant (p < 0.05) reduction in the HDL-c ÷ HDL-c ratio value (Fig. 3B). However, in the treatment groups on PEOG + TZM, EAOG + TZM, EOG + TZM and VAL-LSP + TZM, there were significant (p < 0.05) reversal and attenuation in the increase induced by Veh. + TZM treatment (Fig. 3A). On the CRI value, while Veh. + TZM caused a significant (p < 0.05) increase in its value, VAL-LSP + TZM treatment significantly attenuated the increase while OG and fractions did not significantly (p > 0.05) alter the CRI values (Fig. 3C). Similarly, OG and fractions did not significantly (p > 0.05) alter the HDL-c ÷ LDL-c ratio which was significantly (p < 0.05) decreased by Veh. + TZM treatment (Fig. 3B). However, this ratio was significantly (p < 0.05) increased by VAL-LSP + TZM treatment (Fig. 3B).
Fig. 3.
Effect of OG and fractions on plasma atherogenicity {AI (3 A) and HDL-c ÷ LDL-c ratio (3B)} and plasma coronary artery index (CRI) (3 C) in TZM-treated rats. Bar represents mean ± SEM (n = 5), significant difference denoted by #p < 0.05 vs Veh.only-treated group or *p < 0.05 vs Veh. + TZM-treated group by one-way ANOVA followed by Tukey’s post hoc test.
3.5. OG and OG fractions on the plasma cardiac troponin I (cTnI) levels in TZM-treated rats
Repeated daily treatments with Veh. + TZM caused a marked (p < 0.001) increase in the plasma cTnI when compared to the Veh.-only treated group (Table 6). However, oral pretreatments with OG and its fractions as well as fixed-dose VAL-LSP combination significantly attenuated (p < 0.05, p < 0.001) increases in the plasma cTnI level when compared to the Veh. + TZM-treated group (Table 6).
Table 6.
Effect of Ocimum gratissimum Linn. leaf extract (OG), OG petroleum ether fraction (PEOG), OG ethyl acetate fraction (EAOG), OG ethanol fraction (EOG) and valsartan-lisinopril fixed dose combination on the plasma cardiac troponin I (cTnI) levels in TZM-intoxicated rats.
| Treatment Groups | Plasma cardiac troponin I levels (ng/ml) |
| Veh. | 04.62 ± 01.35 |
| Veh. + TZM | 13.21 ± 01.80c+ |
| OG | 01.77 ± 00.84c- |
| OG + TZM | 08.85 ± 04.51a+ |
| PEOG | 03.99 ± 01.07a- |
| PEOG + TZM | 02.86 ± 01.87a- |
| EAOG | 03.18 ± 00.93a- |
| EAOG + TZM | 02.32 ± 01.21c- |
| EOG | 01.75 ± 00.36c- |
| EOG + TZM | 02.16 ± 01.23a- |
| VAL-LSP | 01.39 ± 00.26c- |
| VAL-LSP + TZM | 01.46 ± 00.51c- |
c+ represents a significant increase at p < 0.05 when compared to values for the Veh.only-treated group while a- and c- represent significant decreases at p < 0.05 and p < 0.001 when compared to values for the Veh. + TZM-treated group
Veh. – 10 ml/kg/day distilled water p.o. + 1 ml/kg/day distilled water i.p.
Veh. + TZM – 10 ml/kg/day distilled water p.o. + 2.25 mg/kg/day TZM i.p.
OG – 100 mg/kg/day OG p.o. + 1 ml/kg/day distilled water i.p.
OG + TZM – 100 mg/kg/day OG p.o. + 2.25 mg/kg/day TZM i.p.
PEOG – 100 mg/kg/day PEOG p.o. + 1 ml/kg/day distilled water i.p.
PEOG + TZM - 100 mg/kg/day PEOG p.o. + 2.25 mg/kg/day TZM i.p.
EAOG - 100 mg/kg/day EAOG p.o. + 1 ml/kg/day distilled water i.p.
EAOG + TZM - 100 mg/kg/day EAOG p.o. + 2.25 mg/kg/day TZM i.p.
EOG - 100 mg/kg/day EOG p.o. + 1 ml/kg/day distilled water i.p.
EOG + TZM - 100 mg/kg/day EOG p.o. + 2.25 mg/kg/day TZM i.p.
VAL-LSP – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 1 ml/kg/day distilled water i.p.
VAL-LSP + TZM – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 2.25 mg/kg/day TZM i.p.
3.6. OG and OG fractions on the cardiac tissue antioxidant activities in TZM-treated rats
Repeated Veh. + TZM treatment for 7 days caused significant (p < 0.001) decreases in the activities of cardiac tissue CAT, SOD, GST and GSH levels when compared to the Veh.only-treated group (Table 7). Conversely, repeated TZM treatment markedly (p < 0.05) increased MDA activity in the Veh. + TZM group when compared with Veh.only-treated group (Table 7). However, in groups pretreated with OG + TZM, PEOG + TZM, EAOG + TZM, EOG + TZM and VAL-LSP + TZM, there were significant (p < 0.05) restoration of the activities of the measured cardiac tissue antioxidant enzymes to near normal values (Table 7).
Table 7.
Effect of Ocimum gratissimum Linn. leaf extract (OG), OG petroleum ether fraction (PEOG), OG ethyl acetate fraction (EAOG), OG ethanol fraction (EOG) and valsartan-lisinopril (VAL-LSP) fixed dose combination on the cardiac antioxidant enzyme activities in TZM-treated Wistar rats.
| Treatment Groups | GSH (µmoles/mg protein) |
MDA (ηmoles/mg protein) |
CAT (U/mg protein) |
SOD (U/mg protein) |
GST (U/min/mg protein) |
| Veh. | 63.58 ± 1.56 | 13.25 ± 0.61 | 40.20 ± 0.73 | 1.42 ± 0.04 | 0.53 ± 0.04 |
| Veh. + TZM | 25.57 ± 1.22#- | 27.87 ± 0.74# | 16.97 ± 1.19#- | 0.75 ± 0.08#- | 0.23 ± 0.05#- |
| OG | 40.58 ± 1.83 * | 13.64 ± 0.58 * - | 31.17 ± 1.77 * | 1.18 ± 0.04 * | 0.51 ± 0.03 * |
| OG + TZM | 38.32 ± 0.92 * | 15.96 ± 0.56 * - | 28.51 ± 1.34 * | 1.32 ± 0.05 * | 0.42 ± 0.03 * |
| PEOG | 47.94 ± 3.50 * | 17.93 ± 1.36 * - | 27.54 ± 1.11 * | 1.48 ± 0.10 * | 0.48 ± 0.07 * |
| PEOG + TZM | 44.19 ± 2.12 * | 17.56 ± 2.76 * - | 26.61 ± 2.34 * | 1.60 ± 0.16 * | 0.41 ± 0.08 * |
| EAOG | 42.41 ± 1.26 * | 15.07 ± 0.44 * - | 25.57 ± 0.41 * | 1.38 ± 0.08 * | 0.44 ± 0.05 * |
| EAOG + TZM | 39.31 ± 1.07 * | 17.12 ± 1.08 * - | 23.73 ± 0.44 * | 1.78 ± 0.11 * | 0.44 ± 0.02 * |
| EOG | 39.73 ± 2.57 * | 14.45 ± 0.42 * - | 28.23 ± 1.49 * | 1.45 ± 0.08 * | 0.51 ± 0.06 * |
| EOG + TZM | 32.97 ± 1.68 * | 16.82 ± 0.96 * - | 25.12 ± 1.48 * | 1.56 ± 0.20 * | 0.48 ± 0.05 * |
| VAL-LSP | 39.92 ± 1.11 * | 14.30 ± 0.63 * - | 27.28 ± 0.44 | 1.44 ± 0.37 * | 0.45 ± 0.07 * |
| VAL-LSP+ TZM | 38.82 ± 1.99 * | 18.72 ± 0.79 * - | 25.75 ± 2.03 * | 1.69 ± 0.14 * | 0.41 ± 0.03 * |
Values represents mean ± SEM (n = 5)
# and #-represents a significant increase and decrease at p < 0.05, respectively, when compared to values for the Veh.only-treated group while * and *- represent a significant increase and decrease at p < 0.05, respectively, when compared to values for the Veh. + TZM-treated group.
Veh. – 10 ml/kg/day distilled water p.o. + 1 ml/kg/day distilled water i.p.
Veh. + TZM – 10 ml/kg/day distilled water p.o. + 2.25 mg/kg/day TZM i.p.
OG – 100 mg/kg/day OG p.o. + 1 ml/kg/day distilled water i.p.
OG + TZM – 100 mg/kg/day OG p.o. + 2.25 mg/kg/day TZM i.p.
PEOG – 100 mg/kg/day PEOG p.o. + 1 ml/kg/day distilled water i.p.
PEOG + TZM - 100 mg/kg/day PEOG p.o. + 2.25 mg/kg/day TZM i.p.
EAOG - 100 mg/kg/day EAOG p.o. + 1 ml/kg/day distilled water i.p.
EAOG + TZM - 100 mg/kg/day EAOG p.o. + 2.25 mg/kg/day TZM i.p.
EOG - 100 mg/kg/day EOG p.o. + 1 ml/kg/day distilled water i.p.
EOG + TZM - 100 mg/kg/day EOG p.o. + 2.25 mg/kg/day TZM i.p.
VAL-LSP – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 1 ml/kg/day distilled water i.p.
VAL-LSP + TZM – 5 mg/kg/day VAL-0.035 mg/kg/day LSP fixed-dose combination p.o. + 2.25 mg/kg/day TZM i.p.
3.7. Histopathological studies of OG and OG fractions on the TZM-treated cardiac tissues
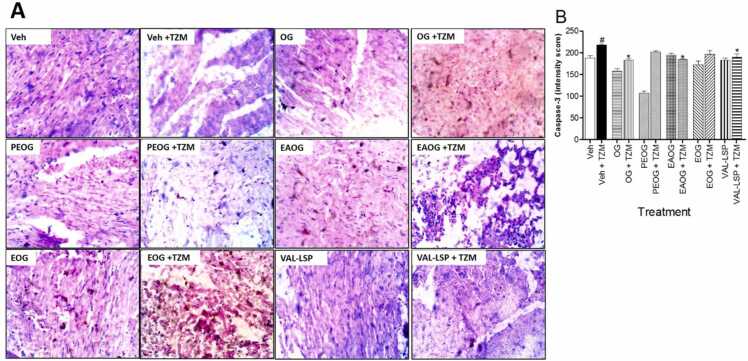
Treatments with Veh. + TZM for 7 days resulted in severe vascular congestion and cardiomyocyte degeneration and cartilaginous degeneration (Fig. 4B) when compared to normal cardiac histoarchitecture of Veh.-only treated group (Fig. 4A). These cardiac histopathological changes were markedly improved in OG + TZM (Fig. 4D), EAOG + TZM (Fig. 4H) and EOG + TZM (Fig. 4J), as well as VAL-LSP + TZM (Fig. 4L) treated groups. These improvements were essentially marked by normal cardiomyocytes with varying degree of coronary endothelial hyperplasia and cartilaginous degeneration. However, the PEOG + TZM treatment group was associated with diffuse myocardial scarring (Fig. 4F). In the groups treated with Veh. + OG (Fig. 4C), Veh. + PEOG (Fig. 4E), Veh. + EAOG (Fig. 4G), Veh. + EOG (Fig. 4I) and Veh. + VAL-LSP (Fig. 4K), there were no associated significant cardiac histological changes.
Fig. 4.
A representative photographic section of (i). Veh.only-treated cardiac tissue showing normal coronary vasculature (indicated in brown thick arrow) and normal cardiomyocytes (indicated in purple thick arrows) (x100 magnification, Hematoxylin & Eosin stains) (4 A); (ii). Veh. + TZM-treated rat cardiac tissue showing myocardiocyte degeneration with cartilaginous degeneration (indicated in black thick arrow) and severe coronary arteriolar congestion indicated in red thick arrow (x100 magnification, Hematoxylin & Eosin stains) (4B); (iii). OG-only pretreated cardiac tissue showing normal cardiomyocytes (indicated in purple thick arrows) (x100 magnification, Hematoxylin & Eosin stains) (4 C); (iv). OG + TZM-treated rat cardiac tissue showing moderate coronary arteriolar congestion and coronary artery tunica media thickening indicated by red thick and blue thick arrows, respectively (x100 magnification, Hematoxylin & Eosin stains) (4D); (v). PEOG-only pretreated rat cardiac tissue showing coronary artery tunica media hyperplasia resulting in its narrowing (x100 magnification, Hematoxylin & Eosin stain) (4E); (vi). PEOG + TZM-treated rat cardiac tissue showing myocardial scarring (indicated in blue thin arrow) (x400 magnification, Hematoxylin & Eosin stain) (4 F); (vii). EAOG-only pretreated cardiac tissue showing normal coronary vessels and cardiomyocytes (x100 magnification, Hematoxylin & Eosin stains) (4 G); (viii). EAOG + TZM-treated rat heart tissue showing coronary artery tunica media hyperplasia and cartilaginous deposits in blue thick and black thick arrows, respectively (x100 magnification, Hematoxylin & Eosin stains) (4 H); EOG-only pretreated cardiac tissue showing diffuse cardiomyocyte hypertrophy indicated in yellow thick arrow (x100 magnification, Hematoxylin & Eosin stains) (4I); EOG + TZM-treated rat heart tissue showing mild coronary artery tunica media thickening/hyperplasia indicated in blue thick arrow (x100 magnification, Hematoxylin & Eosin stains) (4 J); VAL + LSP fixed dose combination-only pretreated cardiac tissue showing normal coronary vessels (indicated in the brown thick arrows) and normal cardiomyocytes (indicated in purple thick arrows) ((x100 magnification, Hematoxylin & Eosin stains) (4 K); VAL-LSP fixed dose combination + TZM-treated rat heart tissue showing coronary artery cystic medial degeneration indicated in orange thin arrow (x400 magnification, Hematoxylin & Eosin stains) (4 L).
3.8. Immunohistochemical studies of OG and OG fractions on TZM-treated cardiac tissues
3.8.1. OG and OG fractions on TZM-treated cardiac tissue caspase-3 and capsase-9 levels
Using ELISA technique, Veh. + TZM treatment caused marked (p < 0.05) elevation in the total cardiac caspase-3 and caspase-9 levels when compared with Veh. only-treated (untreated normal) cardiac tissue (Figs. 5A and 5(B), respectively). Treatments with Veh. + OG, Veh. + PEOG, Veh. + EAOG, Veh. + EOG and Veh. + VAL-LSP was associated with no significant (p > 0.05) changes in the cardiac tissue caspase-3 levels (Fig. 5A). However, with EAOG + TZM and EOG + TZM treatments, there were significant (p<0.05) attenuation in the elevated caspase-3 levels (Fig. 5A). Similarly, in groups treated with OG + TZM, PEOG + TZM, EAOG + TZM, EOG + TZM and VAL-LSP + TZM, there was profound (p < 0.05) decreases in caspase-9 levels when compared with the Veh. + TZM-treated values (Fig. 5B).
Fig. 5.
Effect of oral pretreatment with OG, PEOG, EAOG and EOG on cardiac caspase-3 (5 A) and cardiac caspase-9 (5B) in TZM-intoxicated rats.
3.8.2. OG, OG fractions and VAL-LSP fixed dose combination on TZM-treated cardiac tissue caspase-3 expression and intensity score
Caspase-3 expressions in the TZM intoxicated cardiac tissues and cardiac tissues subsequently pretreated with OG, PEOG, EAOG, EOG and VAL-LSP fixed dose combination are depicted in Fig. 6. Veh. + TZM-treated cardiac tissue showed enhanced expression of caspase-3 when compared with Veh. group (Fig. 6 A). Quantification of the immunohistochemical intensity showed profound (p < 0.05) increase in intensity score suggesting profound enhanced caspase-3 expression in the Veh. + TZM-treated cardiac tissue compared with the Veh.only-treated group (Fig. 6B). However, there were substantial (p < 0.05) decreases in intensity scores for the groups treated with OG +TZM, PEOG +TZM, EAOG + TZM, EOG +TZM and VAL-LSP +TZM (Fig. 6B).
Fig. 6.
. (A) Representative section of photomicrographs of immunohistochemical staining of cardiac tissue caspase-3 expression of cardiac tissues pretreated with OG and fractions (magnification x 400), and (B) Intensity score of expressions. Bar represents mean ± SEM (n = 3), significant difference denoted by #p < 0.05 vs Veh.only-treated group or *p < 0.05 vs Veh + TZM-treated group by One-way ANOVA followed by Tukey’s post hoc test.
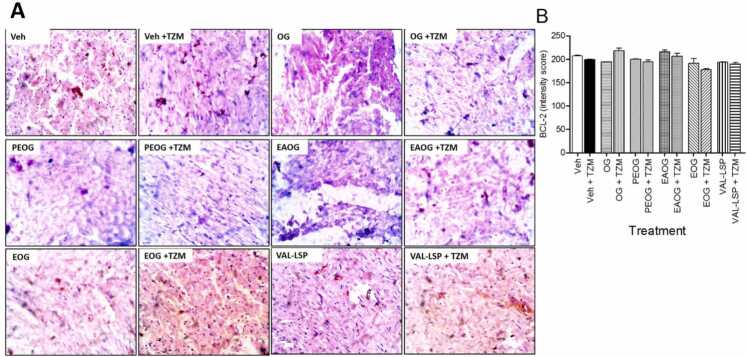
3.8.3. OG, OG fractions and VAL-LSP fixed dose combination on TZM-treated cardiac tissue BCL-2 expression and intensity scoring
The immunohistochemical staining for BCL-2 expression and intensity scores for the treated cardiac tissues are depicted in Fig. 7 A and 7B, respectively. Veh. + TZM treatment caused a statistically non-significant (p˃0.05) reduction in the BCL-2 intensity score (Fig. 7B) while in the OG + TZM, PEOG + TZM, EAOG + TZM, EOG + TZM and VAL-LSP +TZM treatment groups, the BCL-2 expression was not significantly (p˃0.05) improved (Fig. 7B). Similar patterns were recorded in the BCL-2 expressions in Fig. 7 A.
Fig. 7.
. (A) Representative section of photomicrographs of immunohistochemical staining of cardiac tissue BCL-2 expression (magnification x 400), and (B) Intensity score of expressions. Bar represents mean ± SEM (n = 3), significant difference denoted by #p < 0.05 vs Veh.-only treated or *p < 0.05 vs Veh. + TZM–treated group by One-way ANOVA followed by Tukey’s post hoc test.
4. Discussion
Experimental induction of drug-induced cardiotoxicity with repeated administration of TZM is well reported [77], [58], [26], [51]. In this study, repeated daily treatment of rats with 2.25 mg/kg of TZM administered via the intraperitoneal route for 7 consecutive days successfully established cardiotoxicity which was positively associated with profound elevation in the plasma cTnI. Generally, cTnI is considered to be a reliable and specific marker of acute myocardial injury due to its confinement and specificity to cardiac muscles [9], [82], [63]. Thus, myocardial injury from whatever cause is often associated with cardiomyocyte necrosis with attendant rise in the blood cTnI and cardiac troponin T (cTnT) levels [49], [60], [6]. However, there are emerging evidences that there could be increased blood levels of these cardiac enzyme markers without corresponding cardiomyocyte necrosis and apoptosis, usually from cardiac perfusion ischemia [38], [36], [98], [32]. Of these two mechanisms, the former appeared to be the likely mechanism by which TZM mediated its cardiotoxicity in our study as evidenced by the profound increase in the plasma cTnI levels and cardiomyocyte and cartilaginous degeneration. Also, the fact that there was an increased cardiac tissue caspase-3 protein expressions coupled with reduced SOD and CAT activities as well as the accumulation of GSH and MDA levels following repeated intraperitoneal injections of TZM, all showed that TZM-induced cardiotoxicity was fully established in this experiment. However, these biochemical, immunohistochemical and histopathological alterations were effectively abrogated by oral pretreatments with OG and OG fractions, especially the EAOG fractions which exhibited the most effective abrogation.
TZM is notorious for inducing accelerated cardiomyocytes apoptosis which is marked by the upregulation of cardiac tissue caspase‐9 and caspase‐3 expressions while down-regulating the cardiac tissue BCL2 expression [13], [57], [62]. In the present study, we observed that repeated TZM treatment was similarly associated with increased caspases-9 and − 3 expressions indicating cardiomyocyte apoptosis in line with previous reports although the BCL2 expression was unaltered. The reason for this variant effect on the BCL2 expression in our present study remains unknown but this could constitute an area of further studies in the nearest future. However, oral pretreatment with OG and OG fractions effectively reversed the TZM -mediated caspases-9 and − 3 upregulation without altering cardiac tissue BCL-2 expression suggesting anti-apoptosis mechanism as a possible cardioprotective mechanism of OG and its fractions.
Increases in TChol, TG, and LDL-c and reduction in HDL-c are reliable key cardiovascular disease risk predictors which could be elevated in various heart diseases including drug (TZM)-mediated cardiotoxicity [8], [15], [50], [55]. The fact that there were attenuations in the TZM-induced elevations in the plasma AI by the oral pretreatments with OG and OG fractions suggested the possible cardioprotective potential of these bioactive agents in TZM cardiotoxicity. Also, the fact that EAOG and EOG, profoundly lowered the CRI is indicative of their cardioprotective potentials. Similar cardioprotective potential was offered by VAL-LSP combination and this is in strong support of the earlier report of the cardioprotective potential of VAL-LSP combination against TZM-mediated cardiotoxicity [78].
The presence of secondary metabolites such as thymol, phenol, squalene, phthalic acid and neophytadiene that are present in OG, EAOG and EOG in high amounts could be responsible for the observed protective effects and mechanisms against TZM-mediated cardiotoxicity, although this remains speculative. These secondary metabolites are known to elicit various biological effects and these include antihyperglycemic and antihyperlipidemic [90], anti-obesity [37], antihypertensive and vasorelaxant [67], [89], gastroprotective [87], [31], hepatoprotective [31], nephroprotective [24], [46], neuroprotective [47], antioxidant, free radicals scavenging, anti-inflammatory and antiapoptosis activities [80], [23], and antiparasitic [39] effects.
Thymol, chemically known as 2-isopropyl-5-methylphenol, is a colorless crystalline dietary monoterpene phenol with diverse biological actions that include anti-oxidative [67], [23], antihyperlipidemic and anti-atherosclerotic effects [100], [45], anti-apoptotic and anti-inflammatory [12], [29]. Thymol and carvacrol were also reported to have exhibited synergistic cardioprotective effect against doxorubicin-induced cardiotoxicity that was attributed to their antioxidant, anti-inflammatory, and antiapoptotic activities [25]. Although, both carvacrol and thymol share the same chemical formula (C10H14O) and appear closely similar even with their m/z value on GC-MS because they are isomers. However, the key difference lies in the fact that carvacrol contains a hydroxyl group at the ortho position of the benzene ring whereas thymol contains a hydroxyl group at the meta position of the benzene ring. Therefore, thymol is more hydrophobic than carvacrol [16], [20]. Equally, squalene has been documented to elicit cardioprotective effect via inhibition of lipids accumulation and antioxidant [28], anti-inflammatory [41], anti-atherosclerotic and antineoplastic [59] mechanisms. The fact that thymol was abundantly present in OG, EAOG and EOG as well as the fact that thymol has antioxidant and anti-apoptosis activities acting alone or in combination with other secondary metabolites strongly suggest it could be the active principle in OG and its fractions responsible for the observed anti-apoptosis observed in this study, although the EAOG appeared to be most effective in this respect going by its remarkable inhibition of the cardiac tissue caspase-3 and caspase-9 expressions. Similarly, OG and its fractions, especially EAOG are relatively abundant in β-caryophyllene, a finding that is similar to that previously reported by Olugbade et al. [79]. β-caryophyllene, a dietary phytocannabinoid sequiterpene, has previously been reported to attenuate oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in both in vitro and in vivo studies [7]. Thus, the presence of this secondary in high relative abundance in OG and PEOG could also be responsible for the recorded anti-apoptotic and antioxidant activities in the present study. β-caryophyllene was also reported to inhibits Fas-receptor (FasR) and caspase-mediated apoptosis signaling pathway and endothelial dysfunction in experimental myocardial injury [96]. Thus, it is plausible that β-caryophylline in OG and PEOG was responsible either in part or wholly for the extracts’ cardioprotective effects against TZM-cardiotoxicity via these same mechanisms.
In conclusion, this study has highlighted the promising therapeutic potentials of OG and its fractions (especially EAOG fraction) in ameliorating TZM-induced cardiotoxicity that were probably mediated via anti-apoptosis and antioxidant mechanisms. However, bioassay-guided isolation and structural elucidation of the bioactive compounds in each of the OG fractions will be required in the near future.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Olufunke Esan OLORUNDARE reports financial support was provided by Tertiary Education Trust Fund. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are grateful to Tertiary Education Trust Fund (TETFUND) Nigeria, for the grant (TETFUND//NRF/UIL/ILORIN/STI/VOL.1/B2.20.12) awarded to Prof Olorundare and her research team for this study. Authors also appreciate the technical assistance provided by the Laboratory Manager, Mr. Emmanuel Ekoh and other staff of the Laboratory Services, AFRIGLOBAL MEDICARE, Mobolaji Bank Anthony Branch Office, Ikeja, Lagos, Nigeria in assaying for the plasma cardiac troponin I and lipid profile. Similarly, the authors are grateful to the technical staff of the Department of Pharmacology, Therapeutics and Toxicology, LASUCOM as well as staff of LASUCOM Animal House, for the care of the Experimental Animals used for this study. The technical expertise of Dr. Theophilus Aghogho Jarikre, Department of Veterinary Pathology, Faculty of Veterinary Medicine, University of Ibadan, in the area of immunohistochemical staining is also deeply appreciated.
Author contributions
OEO designed the experimental protocol for this study and was involved in the manuscript writing and editing; AAA also designed, supervised the research, analyzed data and wrote the manuscript; AOA is a postgraduate student in OEO’s Laboratory and assisted throughout the experiment; OA was involved in the extraction and solvent partitioning of OG; AMA conducted the lipids, antioxidant markers and apoptosis markers assays and was also involved in data analysis; IIO prepared the cardiac tissue slides for histopathological studies; SSS and AIM independently interpreted the prepared histology slides of the treated cardiac tissues; RMA is our collaborator in the U.S.A. who was involved in the conceptualization, proof-read and edited the manuscript.
6. Contribution to the field statement
Clinical use of TZM has been widely limited by its cumulative but reversible off-target cardiotoxicity. In this study therapeutic potential of OG and its solvent fractions in mitigating TZM-mediated cardiotoxicity is proposed. This theory relies on cardiac injury enzyme markers, cardiac oxidative stress markers, histological examination and immunohistochemical markers as the therapeutic evaluation endpoints of OG, OG fractions and VAL-LSP fixed dose combination in TZM-intoxicated rats.
Results of the study showed that that repeated TZM treatment caused remarkable elevations in the plasma LDL-c levels, atherogenicity and coronary artery indices, cardiac troponin I levels, and cardiac tissue caspases-3 and − 9 levels but decreased BCL-2 expression. TZM also significantly reduced CAT, SOD, GST and GPx activities and increased MDA levels in the treated cardiac tissues. In addition, TZM cardiotoxicity was characterized by severe vascular congestion and cardiomyocyte degeneration and cartilaginous degeneration. Oral pretreatments with OG, OG fractions (especially EAOG fraction) and VAL-LSP fixed dose combination significantly prevented and reversed the biochemical, histopathological and immunohistochemical alterations. Based on our novel findings which we are reporting for the first time, we establish a case for the therapeutic potentials of OG, EAOG fractions and VAL-LSP fixed dose combination in TZM-mediated cardiotoxicity which is probably mediated via anti-apoptosis and antioxidant mechanisms.
Ethics approval
Ethical approval (UERC Approval number: UERC/ASN/2022/2327) for the study was obtained from the University of Ilorin Ethical Review Committee for Staff and Postgraduate Research, University of Ilorin, Kwara State, Nigeria.
Handling editor: Prof. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Adebolu T.T., Oladimeji S.A. Antimicrobial activity of leaf extracts of Ocimum gratissimum on selected diarrhea causing bacteria in Southwestern Nigeria. Afr. J. Biotechnol. 2005;4:682–684. [Google Scholar]
- 2.Adeneye A.A., Crooks P.A., Miller A.-F., Goodman J., Adeyemi O.O., Agbaje E.O. Isolation and structure elucidation of a new indole alkaloid, erinidine, from Hunteria umbellata seeds. Pharmacologia. 2012;3(7):204–214. [Google Scholar]
- 3.Ajayi A.M., Ologe M.O., Ben-Azu B., Okhale S.E., Adzu B., Ademowo O.G. Ocimum gratissimum Linn. leaf extract inhibits free radical generation and suppressed inflammation in carrageenan-induced inflammation models in rats. J. Basic Clin. Physiol. Pharm. 2017;28(6):531–541. doi: 10.1515/jbcpp-2016-0096. [DOI] [PubMed] [Google Scholar]
- 4.Ajibola M.I., Ibrahim R.B., Imam A.M., Mustapha A., Safiriyu A., Etibor A.T. Neurodegenerative potential of the aqueous leaf extract of Ocimum gratissimum: A histological and biochemical study. Anat. J. Afr. 2017;4(2):563–570. [Google Scholar]
- 5.Alabi Q.K., Akomolafe R.O., Omole J.G., Aturamu A., Ige M.S., Kayode O.O., Kajewole-Alabi D. Polyphenol-rich extract of Ocimum gratissimum leaves prevented toxic effects of cyclophosphamide on the kidney function of Wistar rats. BMC Complement Altern. Med. 2021;21 doi: 10.1186/s12906-021-03447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandraki A., Papageorgiou E., Zacharia M., Keramida K., Papakonstantinou A., Cipolla C.M., Tsekoura D., Naka K., Mazzocco K., Mauri D., Tsekoura D., Tsiknakis M., Manikis G.C., Marias K., Marcon Y., Kakouri E., Konstantinou I., Daniel M., Galazi M., Kampouroglou E., Ribnikar D., Brown C., Karanasion G., Antoniades A., Fotiadis D., Filippatos G., Constantinidou A. New insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers. 2023;15:3290. doi: 10.3390/cancers15133290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Taee H., Azimullah S., Nagoor Meeran M.F., Almheiri M.K.A., Al-Jasmi R.A., Tariq S., Khan M.A.B., Adeghate E., Ojha S. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: an in vitro and in vivo study. Eur. J. Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.172467. [DOI] [PubMed] [Google Scholar]
- 8.Azeez T.A. Association between lipid indices and 10-year cardiovascular risk of a cohort of black Africans living with type 2 diabetes mellitus. J. Ayurveda Integr. Med. 2021;10(1):38–42. [Google Scholar]
- 9.Babuin L., Jaffe A.S. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173(10):1191–1202. doi: 10.1503/cmaj.050141. Corrected version CMAJ 174(3): 353 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthur A., Brezden-Masley C., Connelly K.A., Dhir V., Chan K.K.W., Haq R., Kirpalani A., Barfett J.J., Jimenex-Juan L., Karur G.R., Deva D.P., Yan A.T. Longitudinal assessment of right ventricular structure and function by cardiovascular magnetic resonance in breast cancer patients treated with trastuzumab: a prospective observational study. J. Cardiovasc Magn. Res. 2017;19 doi: 10.1186/s12968-017-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayar N., Küçükseymen S., Göktaş S., Arslan Ş. Right ventricle failure associated wıth trastuzumab. Ther. Adv. Drug Saf. 2015;6(3):98–102. doi: 10.1177/2042098615582162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayatmakoo R., Rashtchizadeh N., Yaghmaei P., Farhoudi M., Karimi P. Thymol decreases apoptosis and carotid inflammation induced by hypercholesterolemia through a discount in oxidative stress. Crescent J. Med. Biol. Sci. 2017;4(4):186–193. [Google Scholar]
- 13.Bulut G., Atmaca H., Karaca B. Trastuzumab in combination with AT-101 induces cytotoxicity and apoptosis in HER2 positive breast cancer cells. Future Oncol. 2020;16(3):4485–4495. doi: 10.2217/fon-2019-0521. [DOI] [PubMed] [Google Scholar]
- 14.Calleja A., Poulin F., Khorolsky C., Shariat M., Bedard P.L., Amir E., Rakowski H., McDonald M., Delgado D., Thavendiranathan P. Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J. Oncol. 2015;2015 doi: 10.1155/2015/609194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caliskan Z., Demircioglu K., Sayar S., Kahraman R., Caklili O., Ozcan F.B., Kostek O., Baycan O.F., Doganay H.L., Caliskan M. Lipid profile, atherogenic indices, and their relationship with epicardial fat thickness and carotid intima–media thickness in celiac disease. North Clin. Istanb. 2019;6(3):242–247. doi: 10.14744/nci.2019.54936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemistry On Stack Exchange (2022). Retrieved online on 20 June 2022 via 〈https://chemistry.stackexchange.com/questions/13944/whats-the-difference-between-carvacrol-and-thymol〉.
- 17.Chen H.-M., Lee M.-J., Kao S.-H., Kuo C.-Y., Tsai P.-L., Liu J.-Y. Ocimum gratissimum aqueous extract induces apoptotic signaling in lung adenocarcinoma cell A549. Evid. Based Complementary Altern. Med. 2011 doi: 10.1155/2011/739093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu C.-C., Huang C.-Y., Tzy-Yen Chen T.-Y., Kao S.-H., Liu J.-Y., Wang Y.-W., Tzang B.-S., Hsu T.-C. Beneficial effects of Ocimum gratissimum aqueous extract on rats with CCl4-induced acute liver injury. Evid. Based Complementary Altern. Med. 2012. Artic. ID. 2012 doi: 10.1155/2012/736752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempke W.C.M., Zielinski R., Winkler C., Silberman S., Reuther S., Priebe W. Anthracycline-induced cardiotoxicity - are we about to clear this hurdle? Eur. J. Cancer. 2023;185:94–104. doi: 10.1016/j.ejca.2023.02.019. [DOI] [PubMed] [Google Scholar]
- 20.DifferenceBetween.com (2022). Difference between carvacrol and thymol. Retrieved online on 20 June 2022 via: 〈https://www.differencebetween.com/difference-between-carvacrol-and-thymol/#:~:text=Both%20these%20compounds%20have%20the,position%20of%20the%20benzene%20ring〉.
- 21.Early Breast Cancer Trialists’ Collaborative group Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–1150. doi: 10.1016/S1470-2045(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton H., Timm K.N. Mechanisms of trastuzumab induced cardiotoxicity – is exercise a potential treatment? Cardio-Oncol. 2023;9 doi: 10.1186/s40959-023-00172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Marasy S.A., El Awdan S.A., Hassan A., Abdallah H.M.I. Cardioprotective effect of thymol against adrenaline-induced myocardial injury in rats. Heliyon. 2020;6(7) doi: 10.1016/j.heliyon.2020.e04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sayed E.M., Abd-Allah A.R., Mansour A.M., El-Arabey A.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2015;29(4):165–172. doi: 10.1002/jbt.21681. [DOI] [PubMed] [Google Scholar]
- 25.rEl-Sayed M.E.-S., Mansour A.M., Abdul-Hameed A.S. Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2016;30(1):37–44. doi: 10.1002/jbt.21740. [DOI] [PubMed] [Google Scholar]
- 26.Erbaş O., Altuntaş İ., Çağlar Ö., Özyilmaz E., Sari E., Üzümcü İ., Erbakan K. In: Risk factors for cardiovascular disease [Internet] Chahine J., editor. (London, UK: IntechOpen); 2022. Experimental model of cardiotoxicity.〈https://www.intechopen.com/chapters/79957〉 [Google Scholar]
- 27.Ezekwesili C.N., Obiora K.A., Ugwu O.P. Evaluation of anti-diarrhoeal property of crude aqueous extract of Ocimum gratissimum L. (Labiatae) in rats. Biokemistri. 2004;16:122–131. [Google Scholar]
- 28.Farvin K.H., Anandan R., Kumar S.H., Shiny K.S., Mathew S., Sankar T.V., Nair P.G. Cardioprotective effect of squalene on lipid profile in isoprenaline-induced myocardial infarction in rats. J. Med. Food. 2006;9(4):531–536. doi: 10.1089/jmf.2006.9.531. [DOI] [PubMed] [Google Scholar]
- 29.Fouad A.A., Moussa N.A., Kareem M.M.A., Akl U.I., Abdelghany M.I., Abdel-Aziz A.M. Thymol exerts antioxidant, anti-inflammatory, and anti-apoptotic protective effects against gentamicin nephrotoxicity in rats. Pharmacia. 2022;69(1):181–186. doi: 10.3897/pharmacia.69.e77338. [DOI] [Google Scholar]
- 30.Freire C.M., Marques M.O., Costa M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J. Ethnopharmacol. 2006;105:161–166. doi: 10.1016/j.jep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Geyikoglu F., Yilmaz E.G., Erol H.S., Koc K., Cerig S., Ozek N.S., Aysin F. Hepatoprotective role of thymol in drug-induced gastric ulcer model. Ann. Hepatol. 2020;17(6):980–991. doi: 10.5604/01.3001.0012.7198. [DOI] [PubMed] [Google Scholar]
- 32.Giannitsis E., Mueller C., Katus H.A. Skeletal myopathies as a non-cardiac cause of elevations of cardiac troponin concentrations. Diagn. (Berl. ) 2019;6(3):189–201. doi: 10.1515/dx-2019-0045. [DOI] [PubMed] [Google Scholar]
- 33.Gibo T., Sekiguchi N., Gomi D., Noguchi T., Fukushima T., Kobayashi T., Ozawa T., Yamada S.-I., Koizumi T. Targeted therapy with trastuzumab for epidermal growth factor receptor 2 (HER2)-positive advanced salivary duct carcinoma: a case report. Mol. Clin. Oncol. 2019;11:111–115. doi: 10.3892/mco.2019.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goulas K., Farmakis D., Constantinidou A., Kadoglou N.P.E. Cardioprotective agents for the primary prevention of trastuzumab-associated cardiotoxicity: a systematic review and meta-Analysis. Pharmaceuticals. 2023;16:983. doi: 10.3390/ph16070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grover S., Leong D.P., Chakrabarty A., Joerg L., Kotasek D., Cheong K., Joshi R., Joseph M.X., DePasquale C., Koczwara B., Selvanayagam J.B. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int. J. Cardiol. 2013;168:5465–5467. doi: 10.1016/j.ijcard.2013.07.246. [DOI] [PubMed] [Google Scholar]
- 36.Hammarsten O., Mair J., Möckel M., Lindahl B., Jaffe A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers. 2018;23(8):725–734. doi: 10.1080/1354750X.2018.1490969. [DOI] [PubMed] [Google Scholar]
- 37.Haque M.R., Ansari S.H., Najmi A.K., Ahmad M.A. Monoterpene phenolic Compound thymol prevents high fat diet induced obesity in murine model. Toxicol. Mech. Methods. 2014;24(2):116–123. doi: 10.3109/15376516.2013.861888. [DOI] [PubMed] [Google Scholar]
- 38.Hickman P.E., Potter J.M., Aroney C., Koerbin G., Southcott E., Wu A.H., Roberts M.S. Cardiac troponin may be released by ischemia alone, without necrosis. Clin. Chim. Acta. 2010;411(5-6):318–323. doi: 10.1016/j.cca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Hikal W., Tkachenko K., Said-Al Ah H., Sany H., Sabra A., Baeshen R., Bratovcic A. Chemical composition and biological significance of thymol as antiparasitic. Open J. Ecol. 2021;11:240–266. [Google Scholar]
- 40.Ibironke S.I., Akinola E.A., Adepeju A.B. ). Comparative study of condiment vegetable basil leaf (Ocimum gratissimum) and bitter leaf (Vernonia. amygdalina). J. Food Nutr. Res. 2017;5(3):95–98. [Google Scholar]
- 41.Ibrahim N., Naina-Mohamed I. Interdependence of anti-inflammatory and antioxidant properties of squalene-implication for cardiovascular health. Life (Basel) 2021;11(2):103. doi: 10.3390/life11020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ighodaro O.M., Ebuehi O.A. Aqueous leaf extract of Ocimum gratissimum potentiates activities of plasma and hepatic antioxidant enzymes in rats. Nig. Q. J. Hosp. Med. 2009;19(2):106–109. [PubMed] [Google Scholar]
- 43.Ilori M., Sheteolu A.O., Omonigbehin E.A., Adeneye A.A. Antidiarrheal activities of Ocimum gratissimum (Lamiaceae) J. Diarrhoeal Dis. Res. 1996;14(4):283–285. [PubMed] [Google Scholar]
- 44.Jahangir E., Harinstein M.E., Murthy V.L., Moslehi J. The forgotten right ventricle in cardio-oncology. J. Nucl. Cardiol. 2020;27:2164–2166. doi: 10.1007/s12350-019-01602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamhiri M., Hafizibarjin Z., Ghobadi M., Moradi A., Safari F. Effect of thymol on serum antioxidant capacity of rats following myocardial hypertrophy. J. Arak Uni. Med. Sci. 20(4) 2017:10–19. [Google Scholar]
- 46.Jamshidi H.R., Taheri F. The effect of thymol on renal toxicity induced by mercury chloride in rats. Int. J. Med. Lab. 2021;8(3):223–232. [Google Scholar]
- 47.Javed H., Azimullah S., Nagoor Meeran M.F., Ansari S.A., Ojha S. Neuroprotective effects of thymol, a dietary monoterpene against dopaminergic neurodegeneration in rotenone-induced rat model of Parkinson’s disease. Int. J. Mol. Sci. 2019;20:1538. doi: 10.3390/ijms20071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeyakumar A., Younis T. Trastuzumab for HER2-positive metastatic breast cancer: clinical and economic considerations. Clin. Med. Insights Oncol. 2012;6:179–187. doi: 10.4137/CMO.S6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katrukha I.A., Kogan A.E., Vylegzhanina A.V., Kharitonov A.V., Tamm N.N., Filatov V.L., Bereznikova A.V., Koshkina E.V., Katrukha A.G. Full-size cardiac troponin I and its proteolytic fragments in blood of patients with acute myocardial infarction: Antibody selection for assay development. Clin. Chem. 2018;64(7):1104–1112. doi: 10.1373/clinchem.2017.286211. [DOI] [PubMed] [Google Scholar]
- 50.Kazemi T., Hajihosseini M., Moossavi M., Hemmati M., Ziaee M. Cardiovascular risk factors and atherogenic indices in an Iranian population: Birjand East of Iran. Clin. Med. Insights Cardiol. 2018;12:1–6. doi: 10.1177/1179546818759286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khuanjing T., Maneechote C., Ongnok B., Prathumsap N., Arinno A., Chunchai T., Arunsak B., Chattipakorn S.C., Chattipakorn N. Acetylcholinesterase inhibition protects against trastuzumab-induced cardiotoxicity through reducing multiple programmed cell death pathways. Mol. Med. 2023;29 doi: 10.1186/s10020-023-00686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lameire N. Nephrotoxicity of recent anti-cancer agents. Clin. Kidney J. 2015;7(1):11–22. doi: 10.1093/ckj/sft135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee, M.J., Chen, H.M., and Tzang, B.S. (2011). Ocimum gratissimum aqueous extract protects H9c2 myocardiac cells from H2O2-induced cell apoptosis through akt signaling. Evid. Based Complementary Altern. Med. 2011, Article ID 578060. [DOI] [PMC free article] [PubMed]
- 54.Lemieux I., Lamarche B., Couillard C., Pascot A., Cantin B., Beergeron J., Dagenais G.R., Després J.-P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec cardiovascular study. Arch. Intern. Med. 2001;161(22):2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 55.Li Y.W., Kao T.W., Chang P.K., Chen W.-L., Wu L.-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin M., Xiong W., Wang S., Li Y., Hou C., Li C., Li G. The research progress of trastuzumab-induced cardiotoxicity in HER-2-positive breast cancer treatment. Front. Cardiovasc. Med. 2022;8 doi: 10.3389/fcvm.2021.821663. (Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G., Meng P., Xie Y., Qi X. The effects of Ginsenoside Rg2 on trastuzumab-induced cardiotoxicity. J. Altern. Complement. Integr. Med. 2021;7:179. [Google Scholar]
- 58.Liu G., Zhang J., Sun F., Ma J., Qi X. Ginsenoside Rg2 attenuated trastuzumab-induced cardiotoxicity in rats. Biomed. Res. Int. 2022;2022 doi: 10.1155/2022/8866660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lou-Bonafonte J.M., Martínez-Beamonte R., Sanclemente T., Surra J.C., Herrera-Marcos L.V., Sanchez-Marco J., Arnal C., Osada J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018;62(15) doi: 10.1002/mnfr.201800136. [DOI] [PubMed] [Google Scholar]
- 60.Mahmud Z., Zahran S., Liu P.B., Reiz B., Chan B.Y.H., Roczkowsky A., McCartney C.E., Davies P.L., Li L., Schulz R., Hwang P.M. Structure and proteolytic susceptibility of the inhibitory c-terminal tail of cardiac troponin I. Biochim. Biophys. Acta Gen. Subj. 2019;1863:661–671. doi: 10.1016/j.bbagen.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Maximiano S., Magalhães P., Guerreiro M.P., Morgado M. Trastuzumab in the treatment of breast cancer. BioDrugs. 2016;30(2):75–86. doi: 10.1007/s40259-016-0162-9. [DOI] [PubMed] [Google Scholar]
- 62.Méndez-Valdés G., Gómez-Hevia F., Bragato M.C., Lillo-Moya J., Rojas-Solé C., Saso L., Rodrigo R. Antioxidant protection against trastuzumab cardiotoxicity in breast cancer therapy. Antioxidants. 2023;12:457. doi: 10.3390/antiox12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng C., Fan L., Wang X., Wang Y., Li Y., Pang S., Lv S., Zhang J. Preparation and evaluation of animal models of cardiotoxicity in antineoplastic therapy. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3820591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milano G., Raucci A., Scopece A., Daniele R., Guerrini U., Sironi L., Cardinale D., Capogrossi M.C., Pompilio G. Doxorubicin and trastuzumab regimen induce biventricular failure in mice. J. Am. Soc. Echocardiogr. 2014;27(5):568–579. doi: 10.1016/j.echo.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Mohan N., Shen Y., Endo Y., Endo Y., ElZarrad M.K. Trastuzumab, but not pertuzumab dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol. Cancer Ther. 2016;15:321–331. doi: 10.1158/1535-7163.MCT-15-0741. [DOI] [PubMed] [Google Scholar]
- 66.Mohan N., Jiang J., Dokmanovic M., Wu W.J. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib. Ther. 2018;1(1):13–17. doi: 10.1093/abt/tby003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagoor Meeran M.F., Hayate J., Hasan A.-T., Sheikh A., Ojha S.K. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Font. Pharm. 2017;2017:00388. doi: 10.3389/fphar.2017.00380. 〈https://www.frontiersin.org/article/10.3389/fphar.2017.00380〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011). 8th edition. Washington DC, USA: National Academies Press, 〈https://www.ncbi.nlm.nih.gov/books/NBK54050/〉. [PubMed]
- 69.Njan A.A., Olaoye S.O., Afolabi S.O., Ejimkonye B.C., Soje A., Olorundare O.E., Iwalewa E.O. Safety effect of fractions from methanolic leaf extract of Ocimum gratissimum on reproduction in male Wistar rats. Toxicol. Rep. 2019;6:496–504. doi: 10.1016/j.toxrep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nweze E.I., Eze E.E. Justification for the use of Ocimum gratissimum L in herbal medicine and its interaction with disc antibiotics. BMC Complement. Altern. Med. 2009;9(37) doi: 10.1186/1472-6882-9-37. Article Number 1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Obianine A.W., Aprioku J.S. Ocimum gratissimum Linn reverses cadmium-induced toxicity in the testicular structure and function of the male guinea-pig. West Afr. Soc. Pharmacol.: Book Abstr. ETP. 2008;11:17. [Google Scholar]
- 72.Offiah V.N., Chikwendu U.A. Antidiarrheal effects of Ocimum gratissimum leaf extract in experimental animals. J. Ethnopharmacol. 1999;68(1-3):327–330. doi: 10.1016/s0378-8741(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 73.Ogundipe O.J., Akinpelu O.F., Oyerinde A., Oluwakemi O.R. Ocimum gratissimum (Linn) leaves extract attenuates oxidative stress and liver injury in gentamicin-induced hepatotoxicity in rats. Egypt. J. Basic Appl. Sci. 2021;8(1):146–155. [Google Scholar]
- 74.Ojo O.A., Ojo A.B., Oyinloye B.E., Ajiboye B.O., Anifowose O.O., Akawa A., Olaiya O.E., Olasehinde O.R., Kappo A.P. Ocimum gratissimum Linn. leaves reduce the key enzymes activities relevant to erectile dysfunction in isolated penile and testicular tissues of rats. BMC Complement. Altern. Med. 2019;19:71. doi: 10.1186/s12906-019-2481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okoli C.O., Ezike A.C., Agwagah O.C., Akah P.A. Anticonvulsant and anxiolyticevaluation of leaf extracts of Ocimum gratissimum, a culinary herb. Pharmacogn. Res. 2010;2:36–40. doi: 10.4103/0974-8490.60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olorundare E.O., Adeneye A.A., Akinsola A.O., Sanni D.A., Koketsu M., Mukhtar H. Clerodendrum volubile ethanol leaf extract: a potential antidote to doxorubicin-induced cardiotoxicity in rats. J. Toxicol. 2020;2020 doi: 10.1155/2020/8859716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olorundare O., Adeneye A., Akinsola A., Soyemi S., Mgbehoma A., Okoye I., Ntambi J.M., Mukhtar H. African vegetables (Clerodendrum volubile leaf and Irvingia gabonensis seed extracts) effectively mitigate trastuzumab-induced cardiotoxicity in Wistar rats. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/9535426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olorundare O.E., Adeneye A.A., Akinsola A.O., Ajayi A.M., Agede O.A., Soyemi S.S., Mgbehoma A.I., Okoye I.I., Albrecht R.M., Ntambi J.M., Crooks P.A. Therapeutic potentials of selected antihypertensive agents and their fixed-dose combinations against trastuzumab-mediated cardiotoxicity. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.610331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olugbade T.A., Kolipha-Kamara M.I., Elusiyan C.A., Onawunmi G.O., Ogundaini A.O. Essential oil chemotypes of three Ocimum species found in Sierra Leone and. Niger. J. Med. Aromat. Plants. 2017;6:284. [Google Scholar]
- 80.Ozkan A., Erdogan A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat. Prod. Comm. 2012;7(12):1557–1560. [PubMed] [Google Scholar]
- 81.Parcha V., Dobhal Y., Dhasmana D.C. Isolation, characterization and cardioprotective potential of gratissinol from chloroform extract of Ocimum gratissimum (Linn.) Leaves. J. Biol. Act. Prod. Nat. 2019;9(4):260–268. [Google Scholar]
- 82.Patibandla, S., Gupta, K., and Alsayouri, K. (2021). Cardiac enzymes. StatPearls [Internet] NBK545216; 〈https://www.ncbi.nlm.nih.gov/books/NBK545216/〉. [PubMed]
- 83.Poon K.A., Flagella K., Beyer J., Tibbitts J., Kaur S., Saad O., Yi J.-H., Girishi S., Dybdal N., Reynolds T. Preclinical safety profile of trastuzumab emtansine (T-DM1): Mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol. Appl. Pharmacol. 2013;273:298–313. doi: 10.1016/j.taap.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Prabhu K.S., Lobo R., Shirwaikar A.A., Shirwaikar A. Ocimum gratissimum: a review of its chemical, pharmacological and ethnomedicinal properties. Open Complement. Med. J. 2009;1:1–15. [Google Scholar]
- 85.Priyanka C., Shivika S., Vikas S. In: In Biotechnological approaches for medicinal and aromatic plants. Kumar N., editor. Springer; Singapore: 2018. Ocimum gratissimum: a review on ethnomedicinal properties, phytochemical constituents, and pharmacological profile. [DOI] [Google Scholar]
- 86.Ren X.Y., Shi D., Ding J., Cheng Z.Y., Li H.Y., Li J.S., Pu H.Q., Yang A.M., He C.L., Zhang J.P., Ma Y.B., Zhang Y.W., Zheng T.Z., Bai Y.N., Cheng N. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 2019;18 doi: 10.1186/s12944-019-0984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribeiro A.R., Diniz P.B., Pinheiro M.S., Albuquerque-Júnior R.L., Thomazzi S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: the role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem. -Biol. Interact. 2016;244:121–128. doi: 10.1016/j.cbi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Sakuntala P., Selva Raju R., Jaleeli K.A. FTIR and energy dispersive x-ray analysis of medicinal plants, Ocimum gratissimum and Ocimum tenuiflorum. Int. J. Sci. Res. Phys. Appl. Sci. 2019;7(3):6–10. [Google Scholar]
- 89.Sampaio R.D., Oliveira G.A., Vasconcelos L.H.C., Ferreira P.B., Silva M.D.C., Tavares J.F., Cavalcante F.D., da Silva B.A. Vasorelaxation in rat pulmonary artery induced by the monoterpene thymol: evaluation of the endothelium derived relaxant factors dependence. Res. Soc. Dev. 2021;10(4) [Google Scholar]
- 90.Saravanan S., Pari L. Role of thymol on hyperglycemia and hyperlipidemia in high fat diet-induced type 2 diabetic C57BL/6J mice. Eur. J. Pharmacol. 2015;761:279–287. doi: 10.1016/j.ejphar.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 91.Sharma V., Chanda D. In: In The Ocimum genome. Compendium of Plant Genomes. Shasany A., Kole C., editors. Springer Nature Switzerland AG); Switzerland: 2018. Ocimum: “The holy basil against cardiac anomalies”. [DOI] [Google Scholar]
- 92.Silva M.R.M., Oliveira J.G., Jr, Fernades O.F.L., Passos X.S., Costa C.R., Souza L.K.H., Lemos J.A., Paula J.R. Antifungal activities of Ocimum gratisimum towards dermatophytes. J. Ethnopharmacol. 2005;48:72–86. doi: 10.1111/j.1439-0507.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- 93.Tan-Chiu E., Yothers G., Romond E., Geyer C.E., Jr., Ewer M., Keefe D., Shannon R.P., Swain S.M., Brown A., Fehrenbacher L., Vogel V.G., Seay T.E., Rastogi P., Mamounas E.P., Wolmark N., Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. 2015;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 94.Udi O.A., Oyem J.C., Ebeye O.A., Chris-Ozoko L.E., Igbigbi P.S., Olannye D.U. The effects of aqueous extract of Ocimum gratissimum on the cerebellum of male Wistar rats challenged by lead acetate. Clin. Nutr. Open Sci. 2022;44:28–41. [Google Scholar]
- 95.Umar A., Imam G., Yimin W., Kerim P., Tohti I., Berké B., Moore N. Antihypertensive effects of Ocimum basilicum L. (OBL) on blood pressure in renovascular hypertensive rats. Hypertens. Res. 2010;33:727–730. doi: 10.1038/hr.2010.64. [DOI] [PubMed] [Google Scholar]
- 96.Yovas A., Ponnian S.M.P. β-caryophyllene inhibits Fas- receptor and caspase-mediated apoptosis signaling pathway and endothelial dysfunction in experimental myocardial infarction. J. Biochem. Mol. Toxicol. 2021;35(12) doi: 10.1002/jbt.22907. [DOI] [PubMed] [Google Scholar]
- 97.Vu T., Claret F.X. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012;2 doi: 10.3389/fonc.2012.00062. Article 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weil B.R., Suzuki G., Young R.F., Iyer V., Canty J.M.Jr. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J. Am. Coll. Cardiol. 2018;71(25):2906–2916. doi: 10.1016/j.jacc.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wynn C.S., Tang S.-C. Anti-HER2 therapy in metastatic breast cancer: many choices and future directions. Cancer Metastas-.-. Rev. 2022;41:193–209. doi: 10.1007/s10555-022-10021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu Y.-M., Chao T.-Y., Chang W.-C., Chang M.J., Lee M.-F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016;24(3):556–563. doi: 10.1016/j.jfda.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S., Huang W.C., Li P., Guo H., Poh S.B., Brady S.W., Xiong Y., Tseng L.M., Li S.H., Ding Z., Sahin A.A., Esteva F.J., Hortobagyi G.N., Yu D. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.