Summary
We present a protocol for the rapid postmortem bedside procurement of selected tissue samples using an endoscopic endonasal surgical technique that we adapted from skull base surgery. We describe steps for the postmortem collection of blood, cerebrospinal fluid, a nasopharyngeal swab, and tissue samples; the clean-up procedure; and the initial processing and storage of the samples. This protocol was validated with tissue samples procured postmortem from COVID-19 patients and can be applied in another emerging infectious disease.
For complete details on the use and execution of this protocol, please refer to Khan et al. (2021)1 and Khan et al. (2022).2
Subject areas: Clinical Protocol, Neuroscience
Graphical abstract
Highlights
-
•
Protocol for postmortem bedside procurement of tissue and non-tissue samples
-
•
Endoscopic endonasal surgical technique adapted from skull base surgery
-
•
Description of clean-up procedure and initial processing and storage of samples
-
•
Protocol validated with tissue samples procured postmortem from COVID-19 patients
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
We present a protocol for the rapid postmortem bedside procurement of selected tissue samples using an endoscopic endonasal surgical technique that we adapted from skull base surgery. We describe steps for the postmortem collection of blood, cerebrospinal fluid, a nasopharyngeal swab, and tissue samples; the clean-up procedure; and the initial processing and storage of the samples. This protocol was validated with tissue samples procured postmortem from COVID-19 patients and can be applied in another emerging infectious disease.
Before you begin
The human olfactory mucosa is located high up within the nasal cavity, in the olfactory cleft covering the medial aspects of the middle and superior turbinates, the cribriform plate, and the superior part of the nasal septum.3 The olfactory region does not comprise a contiguous stretch of olfactory mucosa but consists of an archipelago of olfactory mucosa islands of various sizes scattered amidst respiratory mucosa. As the olfactory mucosa cannot be distinguished visually from the respiratory mucosa, the anatomical boundaries of the olfactory mucosa cannot be determined macroscopically, and samples are therefore best referred to as olfactory cleft mucosa samples. At the microscopic level, the olfactory epithelium also features aneuronal areas, which are devoid of mature olfactory sensory neurons.4,5 For all these reasons, sampling a small piece of tissue from the olfactory region has a variable success rate and the sampling error for a given human case is unknown. Resecting wide parts of the olfactory cleft mucosa would be unethical in living human beings given the potential impact on the sense of smell and can therefore only be performed postmortem or during specific surgical settings. Procuring olfactory bulb samples from living human beings would have debilitating consequences given their intracranial position and has only been performed in patients of whom a whole olfactory bulb needed to be removed for medical reasons during a surgical procedure.6,7
We have developed a protocol for postmortem bedside sampling that is based on an endoscopic endonasal approach commonly used in skull base surgery. Our design of a mobile endoscopy unit allows for rapid bedside sampling in various hospital settings to consistently achieve a very short postmortem interval (PMI), making the tissue samples consistently suitable for histological staining and confocal imaging. Compared to an autopsy with opening of the skull to access the brain, the endoscopic procedure leaves no visible incisions and allows for a faster return of the body to the next of kin or the hospital authorities. The bedside procedure is simpler than a typical autopsy: it can be performed with fewer staff, fewer procedural steps, and fewer resources, and is therefore suitable in an emerging infectious disease such as was the case during the early phase of the COVID-19 pandemic. The protocol can be adapted for application outside a hospital environment. The required equipment is relatively compact, would fit in a small van or light aircraft, and can be operated on battery power. The protocol could thus be applied during an outbreak of an emerging infectious disease by a mobile team working autonomously in remote regions of the world where little or no infrastructure is available.
Institutional permissions
The protocol involves the collection of biological material from deceased human subjects: tissue samples (respiratory mucosa, olfactory cleft mucosa, olfactory bulb, and frontal lobe of the brain), blood samples, cerebrospinal fluid (CSF) samples, and nasopharyngeal swabs.
All research must be approved in advance by the relevant local or institutional ethical committees. All applicable laws, rules, and regulations must be followed. Informed consent must have been obtained from the patient or the next of kin prior to tissue procurement, as per the stipulations of the ethical approval and usually via the treating physician of the patient. Samples must be handled with the utmost respect and caution.
The multicentric ANOSMIC-19 project is registered on clinicaltrials.gov (NCT04445597). We obtained ethical approval from the Ethical Committee of the University Hospitals Leuven in Leuven, Belgium (S64042), the General Hospital Sint-Jan Brugge-Oostende AV in Bruges, Belgium (2736) and the Universitair Ziekenhuis Brussel in Brussels, Belgium (EC-2021-360).
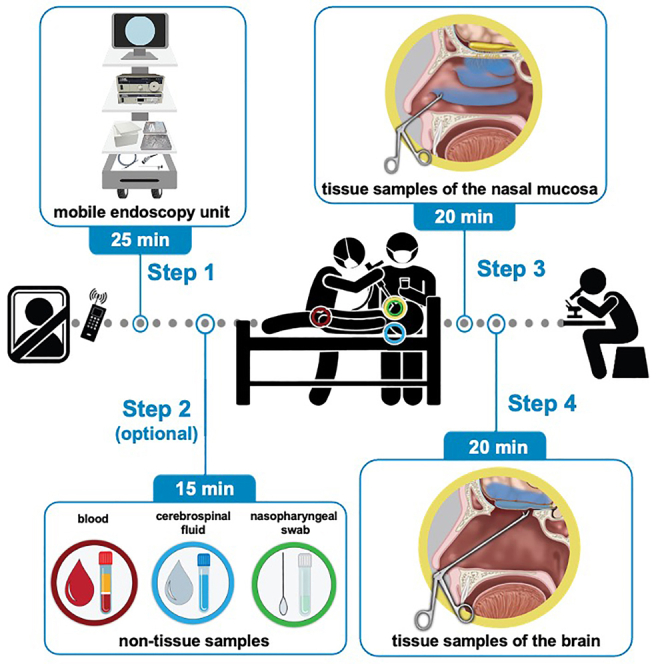
Preparation of mobile endoscopy unit
Timing: 30 min
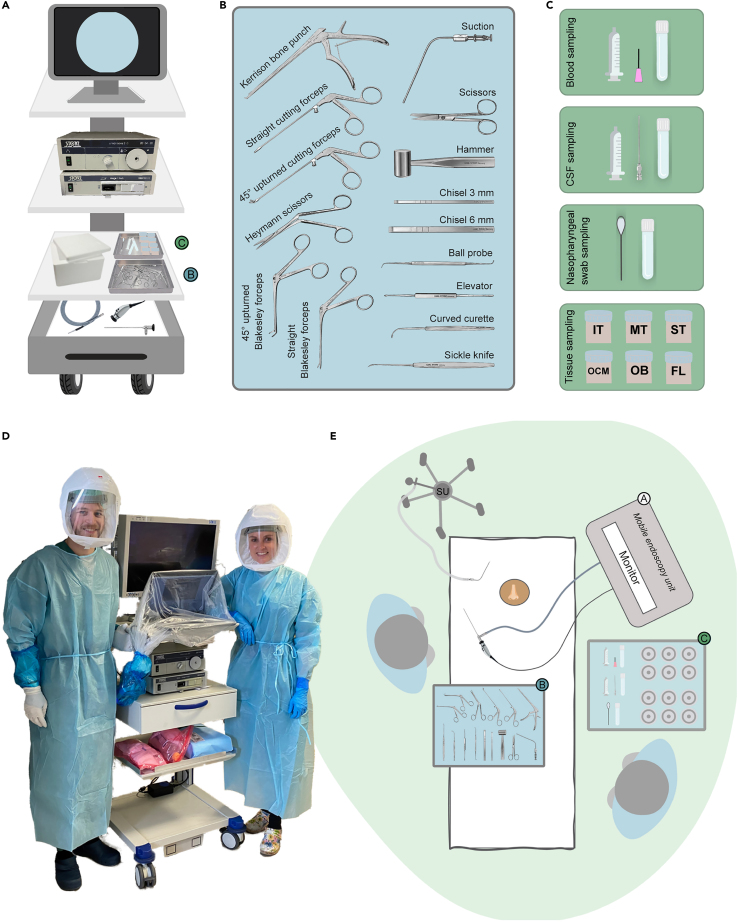
The mobile endoscopy unit (Figure 1A) is equipped with endoscopic equipment (monitor, camera hub, light source, endoscope, camera, light cable), surgical instruments (Figure 1B), and consumables and reagents to collect the tissue samples (Figure 1C). Prepare the unit ahead of time, such that it is ready-to-go at short notice for a sampling procedure.
-
1.
Prepare the endoscopic equipment and surgical instruments.
Note: Two sets of surgical instruments offer more logistic flexibility, to carry out procedures simultaneously or consecutively within a short period of time, or to have a spare set readily available when a set cannot be used (e.g., due to contamination or instrument malfunction).
-
2.Prepare consumables and reagents. Assemble new consumables and reagents and pre-label all consumables used for sample collection.
-
a.At least two syringes and the adequate needles (e.g., 18G needle and atraumatic spinal needle, respectively), for blood sampling and CSF sampling, along with the appropriate collection tubes, such as Serum Separator Tubes and cryotubes.
-
b.Disposable nasopharyngeal flock swab and appropriate collection tube (e.g., Sigma MM tube).
-
c.Formalin pots for collection of tissue samples.
-
a.
Figure 1.
Equipment and room set-up
(A) Mobile endoscopic unit containing the endoscopic equipment – endoscope, light source and cable, camera, and camera hub connected to a monitor to display the endoscopic image – as well as the surgical instruments and the consumables for the collection of the tissue and non-tissue samples.
(B) Surgical instruments.
(C) Material for collection of a blood sample, a CSF sample, a nasopharyngeal swab, and tissue samples.
(D) Photo of the mobile endoscopy unit with surgical instruments and consumables. The two operators are equipped with powered air-purifying respirator masks and are about to enter the room of a deceased COVID-19 patient to carry out the postmortem bedside surgical procedure.
(E) Room setup (shown here for a right-handed operator), with the lead operator on the opposite side of the endoscopy tower with the monitor. The table with surgical instruments is placed close to the lead operator. The table with the collection material is within reach of the assisting operator. The suction unit trolley is typically placed by the head end of the bed. Abbreviations: CSF, cerebrospinal fluid; FL, frontal lobe of the brain; IT, inferior turbinate; MT, middle turbinate; OB, olfactory bulb; OCM, olfactory cleft mucosa; ST, superior turbinate; SU, suction unit.
Equipment and room set-up
Timing: 25 min
The equipment and room set-up are depicted in Figure 1. The procedure is performed by two operators.
-
3.
The workflow is initiated by a phone call from an on-site health care worker shortly after the death of a hospitalized patient.
-
4.
Change into appropriate working clothes (scrub suit, surgical gown) and wear additional protective equipment when applicable (Figure 1D).
-
5.Set up the room (Figure 1E).
-
a.Transport the mobile endoscopy unit to the bedside of the deceased patient.
-
b.Clear the bedside area when necessary: remove medical devices such as ECMO and dialysis equipment, chairs, carts.
-
c.Set up the area next to the deathbed as depicted in Figure 1E (for a right-handed operator).
-
a.
-
6.Prepare the endoscopic equipment for endoscopy.
-
a.Connect the endoscope (typically a 4 mm, 0° endoscope) to the camera, monitor, and light source.
-
b.White-balance before the start of the procedure.
-
c.When video documentation of the procedure is desired, start the recording.
-
a.
-
7.Prepare the surgical instruments and collection material.
-
a.Place the surgical instruments on an overbed table or table near the bed.
-
b.Place the consumables for collection on a separate table within reach of the assisting operator.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Other | ||
| Extra fine Graefe forceps | Fine Science Tools | Cat#11150-10 |
| Disposable scalpel, #11 | Electron Microscopy Sciences | Cat#72042-11 |
| Razor blade | Electron Microscopy Sciences | Cat#72000 |
| 18G needle | Becton Dickinson | Cat#304622 |
| Spinal needle with Quincke point | Becton Dickinson | Cat#405256 |
| Atraumatic spinal needle (90 mm, 22G) | Pajunk | Cat#001151-30C |
| Syringe | Becton Dickinson | Cat#307736 |
| Disposable nasopharyngeal flock swab | Copan | Cat#553C |
| Serum tube 5 mL | BD Vacutainer | Cat#367955 |
| Cryotube | Thermo Fisher Scientific | Cat#377267 |
| SIGMA MM tube | Medical Wire & Equipment | Cat#MWMM |
| Parafilm | Bemis | Cat#PM-996 |
| Clinell disinfectant wipes | GAMA Healthcare Ltd. | Cat#CL44810 |
| Formalin | Sigma-Aldrich | Cat#HT5011 |
| 0° endoscope | Karl Storz | Cat#7230 AA |
| Light cable | Karl Storz | Cat#495 ND |
| Cold light fountain | Karl Storz | Cat#TL300 |
| Camera head | Karl Storz | Cat#TH 120 |
| Camera control unit | Karl Storz | Cat#TC 200 and TC 304 |
| Straight dissecting scissors | Karl Storz | Cat#790802 |
| Ferguson suction tube | Karl Storz | Cat#204810 |
| Hammer | Karl Storz | Cat#174200 |
| Chisel 6 mm | Karl Storz | Cat#484006 |
| Chisel 3 mm | Karl Storz | Cat#484003 |
| Ball probe | Karl Storz | Cat#629820 |
| Curved curette | Karl Storz | Cat#628714 |
| Elevator | Karl Storz | Cat#479000 |
| Sickle knife | Karl Storz | Cat#628001 |
| Heymann scissors | Karl Storz | Cat#449003 |
| 45° upturned Blakesley forceps | Karl Storz | Cat#456502 |
| Straight Blakesley forceps | Karl Storz | Cat#456001 |
| 45° upturned cutting forceps | Karl Storz | Cat#451502 B |
| Straight cutting forceps | Karl Storz | Cat#451002 B |
| Kerrison bone punch | Karl Storz | Cat#662122 |
Materials and equipment
Protective equipment
-
•
Deceased patients are potential sources of – sometimes undiagnosed – infections and the risk of transmission needs to be assessed and addressed with precautionary measures when appropriate.8 Therefore, follow general precautions. Before entering the room of the deceased patient, wear adequate water-proof garments and gloves; eye, nose, and mouth protection; and a hair bonnet.
-
•
Gloves, face mask, and bonnet are used not only for personal protection purposes but also to avoid contamination of the specimens with allogeneic material.
CRITICAL: Formalin is harmful and toxic by skin contact or inhalation. All procedures with formalin should be performed in a fume hood cabinet when possible. Formalin waste must be disposed carefully following local safety protocols.
-
•
As unfixed human tissue, blood, and other bodily fluids can be infectious, always wear protective equipment (lab coats, gloves, safety goggles, etc.) when handling samples.
Special precautions in case of infectious hazard
-
•
In case of infectious hazard, wear additional personal protective equipment (as indicated by applicable guidelines) and consider using double gloves (inner gloves preferably with long sleeves or attached to the apron with tape), and changing the outer gloves frequently. Make precautions when transporting material or samples, such as by triple-layer packaging.
-
•
In case of airborne infectious hazard (e.g., SARS-CoV-2), wear additional personal protective equipment and powered air-purifying respirator masks, or alternatively, a face shield and masks according to filtering face piece (FFP) standards (as indicated by applicable guidelines) during the surgical procedure (Figure 1D). Perform donning (putting on) and doffing (taking off) in separate rooms and according to applicable guidelines.9
-
•
In settings where aerosol formation should be avoided (such as airborne infectious hazard e.g., SARS-CoV-2), use cold instruments for the surgical procedure, rather than powered instruments.
Surgical and endoscopic equipment
-
•
Keep the equipment separate from equipment that will be used on living patients.
-
•
Take extra care with sharp objects, such as the sickle knife.
Alternative: More compact all-in-one devices are available, such as the “TELE Pack +” (Karl Storz, Cat#TP101), which combines illumination, image processing, and image display in one compact system.
Step-by-step method details
Postmortem bedside collection of non-tissue samples
Timing: ∼15 min
-
1.Blood sample.Note: Blood samples in a postmortem setting are often obtained via femoral vessel puncture.
Rationale for timing of sampling.
The composition of the blood starts to change after death, in part due to coagulation, and continues to change with increasing PMI.10 Blood is therefore best sampled first.
Rationale for site of sampling.
Due to the loss of blood pressure, peripheral venous blood collection after death is not feasible in smaller vessels such as the cephalic or arm vein, as is typically done in living patients. The femoral vein is large and easily accessible for blood sampling and, as it is relatively distant from the internal organs in the chest and abdomen, it is less influenced by the postmortem redistribution phenomenon.11 Alternatively, other large vessels such as subclavian or jugular blood vessels can be punctured.
Relevant anatomy.
The femoral artery and vein are the main vessels of the lower limb. They branch from the external iliac vessels and emerge underneath the mid-third of the inguinal ligament, medial to the femoral nerve.-
a.Femoral puncture:
-
i.Locate the midpoint between the anterior superior iliac spine and the pubic symphysis.
-
ii.Disinfect this midpoint with disinfectant (e.g., disposable alcohol pads, 70% isopropyl alcohol).
-
iii.Insert an 18G needle approximately 2 cm below this midpoint.
-
iv.Aspirate to check for blood.
-
i.
-
b.Collect whole-blood samples via a syringe (e.g., 10 mL syringe) while keeping the needle fixed.
-
c.Transfer the blood sample into one or more tubes specific for the assays, e.g., 5 mL Serum Separator Tubes for obtaining serum samples.Troubleshooting 4.Optional: Preferably, store the samples in a cool place (such as in a Styrofoam box with cool packs) during the remainder of the procedure.
-
a.
-
2.CSF sample.Note: To extract a CSF sample from the cisterna magna, perform a suboccipital cisternal puncture.
Rationale for timing of sampling.
Perform the cisternal puncture at the latest before endoscopic opening of the skull base. Preferably, do it prior to the endoscopic procedure because of the patient positioning that is required and the rigor mortis that occurs with increasing PMI.
Rationale for site of sampling.
The postmortem reduction of intrathecal pressure hinders the collection of CSF via a classical lumbar puncture.11 Sampling CSF via the nose, after gaining endonasal transcribriform access, poses the risk of contamination of the sample from nasal sources. Therefore, perform postmortem sampling of CSF preferably via a cisternal puncture.
Relevant anatomy.
The cerebellomedullary cistern, also called cisterna magna, is located between the medulla oblongata and the cerebellum in the posterior fossa.-
a.Position the patient in a supine anti-Trendelenburg position. Alternatively, the patient can be turned sideways. The operator is positioned behind the head of the patient.
-
b.Suboccipital puncture:
-
i.Flex the neck of the patient, with the chin touching the chest.
-
ii.Palpate the location for puncture by sliding with the thumb or index finger from the occiput in a caudal direction until the spinous process of the axis C2 vertebra is encountered.
-
iii.Disinfect the puncture location with disinfectant (e.g., disposable alcohol pads, 70% isopropyl alcohol).
-
iv.Insert an atraumatic spinal needle (90 mm, 22G) on the midline in the depression/dimple between the occiput and the spinous process.
-
i.
-
c.Direct the needle upwards toward the midpoint of an imaginary line joining the left and right external auditory meatus, until the needle is felt to pierce the atlanto-occipital ligament, after which loss of resistance can be felt. The needle tip is now positioned within the cisterna magna.
-
i.Inspect the needle hub to confirm that the liquid is clear and colorless, before continuing to sample.
-
ii.Connect the syringe (e.g., a syringe of 5 or 10 mL) and continue to sample CSF.
-
i.
-
d.Aliquot the CSF sample directly into tubes that will be used for storage or analysis, e.g., 1.8 mL cryotubes.Optional: Preferably, store the samples in a cool place (such as in a Styrofoam box with cool packs) during the remainder of the procedure.
-
a.
-
3.
Nasopharyngeal swab.
Rationale for timing of sampling.
Nasopharyngeal swabbing is best performed prior to tissue sampling to avoid contamination with blood or CSF. Perform the swabbing under endoscopic vision and after suction cleaning of nasal secretions. In the case of blind swapping, as is routinely done on living patients, prior suction cleaning will remove secretions.
Rationale for site of sampling.
Nasopharyngeal swabs often have higher sensitivity for respiratory virus detection or result in a higher viral load compared to nasal aspirates or oropharyngeal swabs.12
Relevant anatomy.
The nasopharynx is the region of the throat located behind the nasal cavity.-
a.Insert the swab into the nostril and continue to insert it further in the lower nasal meatus parallel to the nasal floor onto the posterior nasopharyngeal wall.
-
b.Gently rub and roll the swab against the nasopharyngeal mucosa, leaving it in place for several seconds to absorb secretions.
-
c.Remove the swab while rotating it and insert the swab tip into the allocated tube, e.g., Sigma MM tube.Optional: Preferably, store the samples in a cool place (such as in a Styrofoam box with cool packs) during the remainder of the procedure.
-
a.
Bedside endoscopic endonasal procedure for tissue samples
Timing: ∼30 min
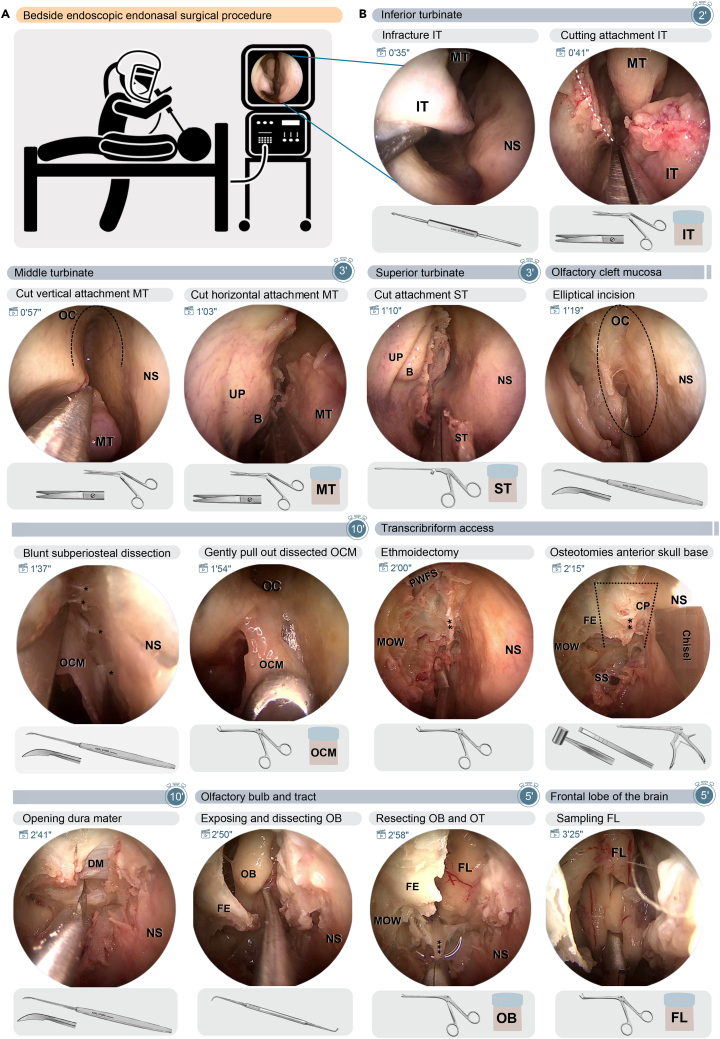
Figure 2 depicts the key steps of the endoscopic endonasal tissue sampling. The time in min and sec next to the clapperboards in the left-upper corner of each snapshot refers to the corresponding time in Video S1,1 which can also be viewed at and downloaded from https://data.mendeley.com/datasets/363xfhrnv5. The steps listed below can be performed unilaterally or bilaterally; the timing is given for one side.
-
4.Endoscopic inspection and preparatory steps.
-
a.Perform an initial endoscopic inspection to examine the sinonasal anatomy and the surgically relevant landmarks, to identify pathology or prior surgery (Figure 3), and to obtain an understanding of the space that will be accessible for surgical instrumentation. Pay attention to the following anatomical structures:
-
i.Nasal septum, and its individual variations in shape.
-
ii.Lateral nasal wall with the nasal turbinates (inferior, middle, superior) and their meatus.
-
iii.Axilla of middle turbinate and the ostiomeatal unit.
-
iv.Olfactory cleft.
-
v.Anterior wall of the sphenoid sinus and the sphenoethmoidal recess.
-
i.
-
b.Remove factors that could hinder either the endoscopic vision or the surgical instrumentation by performing the following preparatory steps:
-
i.Obstructing structural abnormalities of the septum, such as a septal spur or deviation (Figure 3A), can be fractured and removed via a mucosal incision.
-
ii.Large nasal polyps that are obstructing the view of the operator should be removed (Figure 3C).
-
iii.In case of abundant secretions or crusts (Figures 3E–3H), prior (suction) cleaning may be required. Remove detritus with grasping forceps or aspirate using suction cannulas. If necessary, the nasal cavity can be flushed with 0.9% sodium chloride solution.
-
i.
-
a.
Figure 2.
Bedside endoscopic endonasal surgical procedure in steps
(A) Pictographic display of the postmortem bedside endoscopic endonasal procedure.
(B) Step-by-step depiction of key-frames of the endoscopic endonasal procedure, shown for the right nasal cavity. The time in min and sec next to the clapperboards in the left-upper corner of each snapshot refers to the corresponding time in Video S1,1 which can also be viewed at and downloaded from https://data.mendeley.com/datasets/363xfhrnv5.. Under each snapshot, the required surgical and collection material are displayed. The duration for each group of steps is indicated in minutes in the stopwatch. Abbreviations: B, ethmoid bulla; CP, cribriform plate; DM, dura mater; FE, fovea ethmoidalis; FL, frontal lobe of the brain; IT, inferior turbinate; MOW, medial orbital wall; MT, middle turbinate; NS, nasal septum; OB, olfactory bulb; OC, olfactory cleft; OCM, olfactory cleft mucosa; OT, olfactory tract; PWFS, posterior wall of the frontal sinus. White dashed line: cutting line of the inferior turbinate attachment. Black dashed line: area of the olfactory cleft. Black dotted line: indicating the osteotomies for the transcribriform access. ∗, fila olfactoria; ∗∗, attachment middle turbinate; ∗∗∗, olfactory tract.
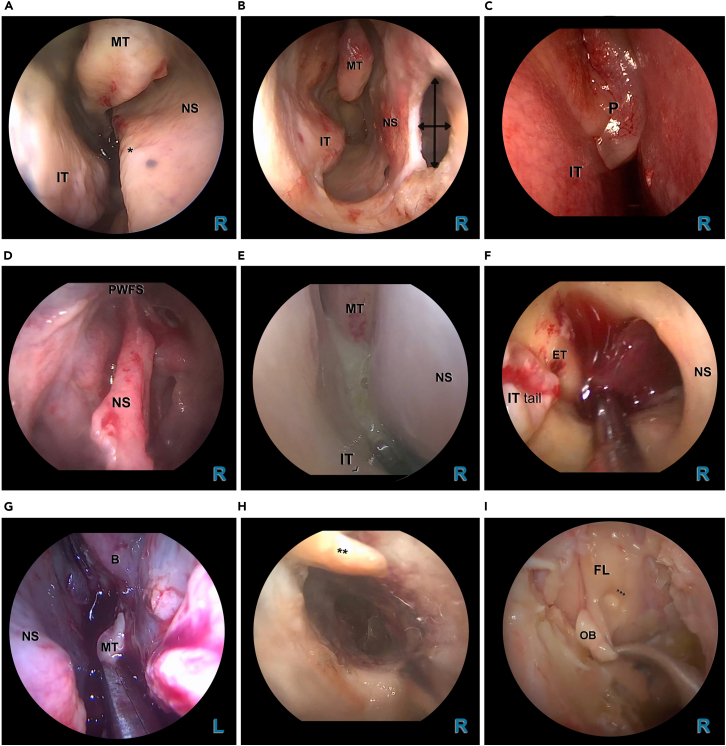
Figure 3.
Examples and endoscopic images of common endonasal and intracranial aberrations
(A) 0° endoscopic view via the right nostril showing a septal spur (∗) extending from antero-inferior to postero-superior, complicating endoscopic and surgical instrumentation.
(B) 0° endoscopic view via the right nostril showing an anterior septal perforation.
(C) 0° endoscopic view via the right nostril showing polyposis nasi extending beyond the middle meatus (grade 3, according to the Meltzer grading system).13
(D) 0° median endoscopic view (with the endoscope inserted via the right nostril in the septal defect), from a case with a history of previous rhinologic surgery with a frontal sinusotomy (DRAF III procedure).
(E) 0° endoscopic view via the right nostril showing purulent secretions emanating laterally of the middle turbinate.
(F) 0° endoscopic view via the right nostril showing a blood clot being suction cleaned in the nasopharynx.
(G) 0° endoscopic view via the left nostril showing excessive blood upon resection of the middle turbinate, due to anticoagulative therapy.
(H) 0° endoscopic view via the right nostril showing dry and atrophic mucosal surface and crusting (∗∗), due to longtime transnasal oxygen therapy.
(I) 0° endoscopic view via the right nostril showing edematous brain tissue in a case of increased intracranial pressure. The brain tissue shows to be fragile and easily damaged (∗∗∗) during atraumatic dissection of the equally fragile olfactory bulb. Abbreviations: B, ethmoid bulla; ET, Eustachian tube; FL, frontal lobe of the brain; IT, inferior turbinate; L, left; MT, middle turbinate; NS, nasal septum; OB, olfactory bulb; PWFS, posterior wall of the frontal sinus; R, right. ∗, septal spur. ∗∗, secretion/crust. ∗∗∗, damage to brain tissue during dissection of olfactory bulb.
-
5.Inferior turbinate (respiratory mucosa).Note: Relevant surgical anatomy of the inferior turbinate: The inferior turbinate, also called concha inferior, is an independent bone lined with respiratory mucosa and is the lowest of the three nasal turbinates. It articulates with the maxilla and extends horizontally toward posterior along the lateral nasal wall over a length of ∼5 cm.
-
a.In-fracture of the inferior turbinate.
-
i.Insert an elevator far enough within the inferior meatus.
-
ii.Move the entire inferior turbinate in a superior and medial direction. To facilitate cutting, fracture the bone of the inferior turbinate by dislocating it medially.
-
i.
-
b.Cutting the attachment.
-
i.Place Heymann nasal scissors with one blade situated above and one blade below the shoulder of the inferior turbinate.
-
ii.Position the endoscope below the inferior turbinate to avoid inadvertent damage to the lateral nasal wall.
-
iii.Cut the attachment of the inferior turbinate across the entire length in an antero-posterior direction.
 CRITICAL: When cutting, stay close to the lateral nasal wall while maintaining the scissors vertically to avoid cutting into the medial wall of the maxillary sinus.
CRITICAL: When cutting, stay close to the lateral nasal wall while maintaining the scissors vertically to avoid cutting into the medial wall of the maxillary sinus.
-
i.
-
c.Transfer the resected inferior turbinate to the fixative solution.
-
i.Pull the resected turbinate out using a Blakesley nasal forceps.
-
ii.Transfer the sample into a container with 10% neutral buffered formalin.
-
i.
-
a.
-
6.Middle turbinate (respiratory mucosa).Note: Relevant surgical anatomy of the middle turbinate: The middle turbinate, also called concha media, inserts antero-superiorly into the region of the agger nasi, in a structure known as the axilla, and extends vertically from the roof of the nasal cavity. Its attachment zone then deviates laterally and inferiorly to end postero-caudally in a horizontal segment at the lateral nasal wall. Anatomical variations in superior attachments are possible.
-
a.Cut the vertical attachment of the middle turbinate. Start from the axilla of the middle turbinate, cut the superior vertical attachment using Heymann nasal scissors or straight cutting forceps.
-
b.Cut the horizontal attachment of the middle turbinate.
-
i.Continue the cut along the course of the attachment zone until the horizontal attachment, staying close to the lateral nasal wall.
-
ii.Incise the posterior attachment of the middle turbinate using a cutting forceps or Heymann scissors until the middle turbinate is free.
 CRITICAL: Lower the dissected middle turbinate after each cut, maintaining a view of the cutting line, to avoid cutting through the superior turbinate.
CRITICAL: Lower the dissected middle turbinate after each cut, maintaining a view of the cutting line, to avoid cutting through the superior turbinate.
-
i.
-
c.Transfer the resected middle turbinate to the fixative solution.
-
i.Extract the resected middle turbinate using a Blakesley nasal forceps.
-
ii.Transfer the sample into a container with 10% neutral buffered formalin solution.
-
i.
-
a.
-
7.Superior turbinate (respiratory mucosa).Note: Relevant surgical anatomy of the superior turbinate: The superior turbinate, also called the concha superior, and the middle turbinate are both parts of the ethmoid bone and extend from a common surface area, the lamina conchalis. The tail of the superior turbinate projects toward the anterior wall of the sphenoid, often pointing at the sphenoethmoidal recess, which is located infero-medially of the postero-inferior insertion of the superior turbinate.
-
a.Cut the postero-inferior insertion of the superior turbinate. Start cutting the postero-inferior insertion of the superior turbinate with a 45° upturned cutting forceps.
-
b.Cut the superior attachment of the superior turbinate. Resect the superior insertion of the superior turbinate to the lamina conchalis using a straight cutting forceps or Heymann endoscopic nasal scissors.
-
c.Transfer the resected superior turbinate to the fixative solution.
-
i.Extract the resected superior turbinate using a Blakesley nasal forceps.
-
ii.Transfer the sample into a container with 10% neutral buffered formalin solution.
-
i.
-
a.
-
8.Olfactory cleft mucosa.Note: Relevant surgical anatomy of the olfactory cleft: The olfactory cleft is a narrow, inverted U-shaped slit in the roof of the nasal cavity and is located between the conchal lamina on the lateral side and the nasal septum on the medial side. The olfactory cavity is closed off superiorly by the cribriform plate of the ethmoid bone, posteriorly by the anterior wall of the sphenoid sinus, and anteriorly by the nasal bone. The mucosa of the olfactory cleft is a mixture of respiratory mucosa and olfactory mucosa — but these two types of tissues cannot be distinguished endoscopically.
-
a.Elliptical incision (part 1) – Anterior inverted-U-shaped incision.By using a Sickle knife, make an incision, starting from the lateral side of the olfactory cleft, over the roof, and going down medially, toward the nasal septum.
-
i.Start the incision laterally in the antero-superior remnant of the vertical attachment of the middle turbinate.
 CRITICAL: At this point, carefully identify the subperiosteal dissection plane, separating the bone and the submucosal layer.
CRITICAL: At this point, carefully identify the subperiosteal dissection plane, separating the bone and the submucosal layer. -
ii.Continue the dissection antero-superiorly and gradually turn the sharp edge of the sickle knife upwards and medially while crossing the roof of the olfactory cleft, all the while maintaining the tip of the knife in the subperiosteal plane.
-
iii.Incise further down on the medial side over the septum until the level corresponding with anterior remnant of the middle turbinate attachment.
-
i.
-
b.Elliptical incision (part 2) – Posterior horizontal incision.
-
i.Identify the anterior sphenoid wall and the natural sphenoid ostium, found at the level of postero-inferior edge of the superior turbinate, superior to the choana.
-
ii.Make a horizontal incision on the anterior sphenoid wall, just above the level of the sphenoid ostium, in continuity with the cut edge of the superior turbinate.
-
i.
-
c.Elliptical incision (part 3) – Connect the incisions.
-
i.On the lateral side, connect the incision on the anterior sphenoid wall to the attachment of the resected superior turbinate and middle turbinate (lamina conchalis).
-
ii.Connect the incision medially over to the nasal septum at the same height.
-
i.
-
d.Blunt subperiosteal dissection.
-
i.Dissect gently with the blunt side of the sickle knife, raising the olfactory cleft mucosa from the cribriform plate. The first olfactory fiber (filum olfactorium) can usually be expected at the level of the axilla of the middle turbinate.Note: Upon dissection and removal of olfactory cleft mucosa, do not apply to much pressure on the often very thin cribriform plate, to avoid penetrating it accidentally.
-
ii.Continue the subperiosteal dissection in the roof of the olfactory cleft until reaching the posterior limit on the sphenoid face. Transect the fila olfactoria with the sharp edge of the sickle knife.
-
iii.If not detached already, dissect the lateral and medial sides until the level of the incisions.
-
i.
-
e.Transfer the resected olfactory cleft tissue to the fixative solution.
-
i.Remove the olfactory cleft tissue sample atraumatically using a Blakesley nasal forceps.
-
ii.Transfer the sample into a container with 10% neutral buffered formalin solution.Optional: For en-bloc sampling of a specimen comprising the olfactory cleft mucosa and olfactory bulb, steps 8d–10 are reduced as follows:Instead of step 8d: Perform a minimal subperiosteal dissection to expose the bone for osteotomy.Instead of steps 8e – 10: Do not remove the olfactory cleft mucosa covering the anterior skull base and the cribriform plate (steps 8e and 9b are thus not performed). Instead, immediately perform osteotomies, incise the dura mater or cut it on all four sides, and include the resected part in the en-bloc specimen. Then remove the olfactory cleft mucosa and olfactory bulb en bloc and fix them together in formalin.
-
i.
-
a.
-
9.Transcribriform access.Note: Relevant surgical anatomy for the transcribriform approach: The anterior skull base (anterior cranial fossa) is endoscopically accessible over its full antero-posterior extent (from the posterior table of the frontal sinus to the planum sphenoidale) and lateral extent (from the median nasal septum until the bilateral medial orbital walls bilaterally). Between the frontal bone and the sphenoid bone, the ethmoidal part of the skull base consists of the fovea ethmoidalis (at the lateral side) and the cribriform plates (at the medial side). The cribriform plate is a paper-thin bony plate, with 15–20 small foramina traversed by the fila olfactoria.Note: Terminology: strictly speaking, the term “transcribriform approach” is anatomically inaccurate here, as the approach not only involves the cribriform plate but also the ethmoidal roof (fovea ethmoidalis).Note: The goal of step 9 is to create access to the intracranial space via a bone window in the anterior skull base. Skeletonize the anterior skull base completely over its entire length, from the frontal sinus anteriorly until the sphenoid sinus posteriorly. Therefore, open or disassemble the sinonasal compartments according to the needs of exposure. After complete skeletonization of the skull base, remove the bony ethmoid roof and cribriform plate, thereby creating a window to the intracranial space.
-
a.Pansinus procedure. Similar to a pansinus surgical procedure (“full-house FESS", functional endoscopic sinus surgery), it is composed of the following main sequential steps:
-
i.Uncinectomy and medial antrostomy.Note: Uncinectomy to identify the ethmoidal bulla and expose the natural maxillary ostium. Medial antrostomy to expose the orbital floor and posterior wall of the maxillary sinus (anatomical landmarks).
-
ii.Disassemble the ethmoidal box (anterior and posterior ethmoidectomy) by opening of the ethmoidal bulla and resection of the anterior and posterior ethmoidal cells, until the lamina papyracea and the ethmoidal roof are completely exposed.
-
iii.Identify the sphenoid sinus ostium and make a wide sphenoidotomy.
-
iv.Landmark the frontal sinus by identification and widening of the frontal recess.
-
i.
-
b.Expose the anterior skull base completely. Remove any remnants of the attachment of the middle turbinate, bony septations, and mucosal lining.
 CRITICAL: Mind the landmarks. At this point, the anterior skull base is skeletonized over its entire length, from the frontal sinus anteriorly until the sphenoid sinus posteriorly, and from the septum medially until the lamina papyracea laterally. The position of the anterior and posterior ethmoidal arteries can help in guiding the orientation.
CRITICAL: Mind the landmarks. At this point, the anterior skull base is skeletonized over its entire length, from the frontal sinus anteriorly until the sphenoid sinus posteriorly, and from the septum medially until the lamina papyracea laterally. The position of the anterior and posterior ethmoidal arteries can help in guiding the orientation. -
c.Osteotomies. Perform four osteotomies with hammer and chisel.Troubleshooting 20.Note: This step can be performed with either cold instruments alone (hammer and chisel), or with the help of powered instruments (high-speed drill with rinsing system, such as the Medtronic Anterior Skull Base Diamond Bur, 15°). In settings where aerosol formation should be avoided (such as risk of infection with SARS-CoV-2), cold instruments are the preferred choice.
-
i.Perform the anterior osteotomy at the level of the frontal recess / the anterior ethmoidal artery.
-
ii.Perform the lateral osteotomy medial to the medial orbital wall (lamina papyracea) in the ethmoid roof (fovea ethmoidalis).
-
iii.Perform the medial osteotomy directly along the nasal septum.
-
iv.Perform the posterior osteotomy at the level of the planum sphenoidale.
-
i.
-
d.Bone removal.
-
i.Starting from the osteotomies, fracture the skull base outwards (toward the nasal cavity) using a ball probe or a curved curette.
-
ii.Use an upturned Blakesley forceps to clean up the loose bone pieces.
-
iii.Use a Kerrison bone punch for widening of the window on thicker bone fragments.
 CRITICAL: Clean the instruments prior to incising the dura mater and sampling the olfactory bulbs and brain to avoid cross-contamination from intranasally collected tissues.
CRITICAL: Clean the instruments prior to incising the dura mater and sampling the olfactory bulbs and brain to avoid cross-contamination from intranasally collected tissues.
-
i.
-
e.Opening and resection of the dura mater.
-
i.Incise the dura mater longitudinally with a sickle knife.
 CRITICAL: To avoid damage to the olfactory bulb, cut as laterally as possible in the transcribriform window, and turn the sickle knife facing the edge. When cut, the dura mater will retract partly, and the olfactory bulb should become visible medially, next to the crista galli.
CRITICAL: To avoid damage to the olfactory bulb, cut as laterally as possible in the transcribriform window, and turn the sickle knife facing the edge. When cut, the dura mater will retract partly, and the olfactory bulb should become visible medially, next to the crista galli. -
ii.The dura mater will retract and open. Remove any pieces of the dura mater with a Blakesley forceps, for better exposure.Optional: when of interest, samples of dura mater can be collected at this point. Resect a piece of dura mater using a Blakesley forceps, either immediately after incision of the dura mater, or after resection of the olfactory bulb (to avoid accidently damaging the olfactory bulb upon resection of dura mater).Optional: At this point, a CSF sample can also be collected with a long (spinal) needle transnasally, either before incising the dura mater (but with the risk of damaging the olfactory bulb) or after (but with the risk of less successful sampling due to overflow into the nose).
-
i.
-
a.
-
10.Olfactory bulb.Note: Relevant surgical anatomy of the olfactory bulb: The olfactory bulbs are located at the bottom of the forebrain in the olfactory sulcus formed medially by the gyrus rectus and laterally by the gyri orbitales and are contained within the bony groove of the cribriform plate on either side of the crista galli. The olfactory tracts run from each olfactory bulb posteriorly and slightly laterally, to eventually split around the anterior perforated substance into a lateral and medial olfactory stria.
-
a.Dissect the olfactory bulb.
-
i.Identify the olfactory bulb and olfactory tract.Note: After removal of the bone and dura mater, a ventral view of the olfactory bulb is obtained and, more posteriorly, of the olfactory tract. The olfactory bulb is covered with leptomeninges.Note: The olfactory bulb can be located more posteriorly in some postmortem cases or be more difficult to identify in cases with pre-existing intracranial overpressure.
-
ii.Dissect the olfactory bulb from its surroundings (arachnoidea and blood vessels) in the plane of the subarachnoid space, by placing the small ball of the ball probe posterior and superior of the olfactory bulb and gently pulling it to the front.
 CRITICAL: By using this dissection technique, the olfactory bulb can be separated from the forebrain in an atraumatic way and can be luxated into the nasal cavity.
CRITICAL: By using this dissection technique, the olfactory bulb can be separated from the forebrain in an atraumatic way and can be luxated into the nasal cavity.
-
i.
-
b.Resect the olfactory bulb.
-
i.Use a Blakesley forceps to grab the olfactory tract as posteriorly as possible and gently pull it in an anterior direction and remove the olfactory bulb, with an adjacent part of the olfactory tract, from the nasal cavity.Note: The olfactory tract can be resected even more posteriorly by slight traction in anterior direction, pulling it in like a rope.Note: The olfactory bulb and adjacent olfactory tract are fragile. Therefore, a cutting instrument is not necessary for resection.
-
ii.Transfer the sample into a container with 10% neutral buffered formalin solution.
-
i.
-
a.
-
11.Frontal lobe of the brain (gyrus rectus or gyri orbitales).Note: Relevant surgical anatomy of the gyrus rectus and gyri orbitales: The transethmoid-transcribriform window provides direct access to the basal surface of the frontal lobe, more specifically to the gyrus rectus and gyri orbitales.
-
a.Sampling the ventromedial frontal lobe.
-
i.Obtain samples of the gyrus rectus or gyri orbitales by a wedge-shaped or cone-shaped incision (using sickle knife for instance), or more easily by punching it out using an upturned Blakesley forceps.
 CRITICAL: The resection must be sufficiently deep so that it contains both gray and white matter.
CRITICAL: The resection must be sufficiently deep so that it contains both gray and white matter. -
ii.Immediately after resection, transfer the sample into a container with 10% neutral buffered formalin solution.
-
i.
-
a.
Clean-up procedure
Timing: 4 h
-
12.
Note the time of the end of the procedure. Stop the endoscopic video recording as desired.
-
13.Cleaning and disinfection.
-
a.Clean the body and the room.
-
i.Stuff both sides of the nasal cavity with cotton wool to prevent fluid leakage.
-
ii.Clean the nose and face, making sure not to leave external traces.
-
iii.Dispose all wasted materials in designated garbage bins.
-
i.
-
b.Clean the used material.
-
i.Change the outer pair of gloves and disinfect hands.
 CRITICAL: After this point, do not make direct contact with the deceased or any sample.
CRITICAL: After this point, do not make direct contact with the deceased or any sample. -
ii.Clean the outer side of the sample tubes and containers. Wrap parafilm around the lid.
-
iii.Clean and disinfect the mobile endoscopy unit with Clinell Disinfectant Wipes.
-
iv.Bag endoscopic and surgical material for industrial sterilization.
-
i.
-
a.
-
14.Label and bag the samples.
-
a.Change the outer pair of gloves and disinfect hands.
-
b.Mark or label the samples, as appropriate.
-
c.Double bag or triple bag the samples, as appropriate.
-
a.
-
15.Exit the room.
-
a.Take off disposable garments.
-
b.Disinfect the mobile endoscopy unit again, with 70% ethanol in a clean area, and cover it with a washable synthetic cover during transport and in storage.
-
a.
-
16.Industrial disinfection of the endoscopic equipment and surgical instruments.
-
a.Machine cleaning and thermal disinfection.
-
b.Steam sterilization for surgical instruments and hydrogen peroxide sterilization for the endoscopic equipment.
-
a.
Initial sample processing and storage of non-tissue (fluid) samples
Timing: 25 min
-
17.
CSF samples and nasopharyngeal swabs can be stored at ‒80°C without further processing.
-
18.Processing of blood samples.Note: Whole-blood samples are not suitable for long-term storage. Most hematologic parameters in whole blood samples are not stable in the frozen state.14
-
a.Centrifuge (3724 × g) whole-blood samples to obtain serum or plasma samples.
 CRITICAL: Centrifugation should be done within 6 h after sampling.
CRITICAL: Centrifugation should be done within 6 h after sampling. -
b.Aliquot the serum or plasma samples (e.g., in 1.8 mL cryotubes) and store at ‒80°C.
 CRITICAL: To avoid unnecessary pipetting, tube transfers, or thawing/freezing cycles, store the samples in small aliquots (e.g., 1.8 mL cryotubes) and, when possible, directly into the tube that will be used for analysis.
CRITICAL: To avoid unnecessary pipetting, tube transfers, or thawing/freezing cycles, store the samples in small aliquots (e.g., 1.8 mL cryotubes) and, when possible, directly into the tube that will be used for analysis.
-
a.
Initial sample processing and storage of tissue samples
Timing: 10–16 days
-
19.
Formalin fixation. Keep the samples in formalin for a period of >24 h, to sufficiently fix the tissues and inactivate infectious agents, if applicable.
Note: Fixation time and biosafety level depend on tissue type, pathogen type, and local guidelines. If the tissues will be processed in less than 48–72 h, fix at room temperature (18°C–26°C). For longer fixation times, keep the sample at 4°C.
-
20.Serial sucrose immersions for dehydration / cryoprotection.
-
a.Transfer all tissue samples from formalin to 15% sucrose in PBS for a period of 3 d at 4°C on a see-saw rocker.
 CRITICAL: The volume of sucrose must be at least 5 times the volume of the tissue.
CRITICAL: The volume of sucrose must be at least 5 times the volume of the tissue. -
b.Transfer the samples into 25% sucrose in PBS for 3 d at 4°C on a see-saw rocker.
-
c.Transfer the samples into 30% sucrose in PBS for 3–7 d (or until the tissue sinks) at 4°C on a see-saw rocker.
 CRITICAL: Intracranial samples, especially brain samples, benefit from longer 30% sucrose immersion (5–7 d). Sucrose removes water from within the tissue, preventing freezing artifacts.Troubleshooting 26.Note: Tissue is still very delicate soon after death, which makes it vulnerable for damage when dissecting the mucosa from the bone. Tissues can be dissected before or after cryoprotection steps. Fixation and cryoprotection consolidate the tissue, which makes it easier to handle and dissect the tissue from the bone. If the sampled structures are large (like nasal turbinates), consider dissecting them in order to facilitate sucrose penetration. Consider performing the dissection immediately prior to embedding the sample in optimal cutting temperature (O.C.T.) compound, while orienting the sample.
CRITICAL: Intracranial samples, especially brain samples, benefit from longer 30% sucrose immersion (5–7 d). Sucrose removes water from within the tissue, preventing freezing artifacts.Troubleshooting 26.Note: Tissue is still very delicate soon after death, which makes it vulnerable for damage when dissecting the mucosa from the bone. Tissues can be dissected before or after cryoprotection steps. Fixation and cryoprotection consolidate the tissue, which makes it easier to handle and dissect the tissue from the bone. If the sampled structures are large (like nasal turbinates), consider dissecting them in order to facilitate sucrose penetration. Consider performing the dissection immediately prior to embedding the sample in optimal cutting temperature (O.C.T.) compound, while orienting the sample.
-
a.
-
21.Subperiosteal dissection of turbinates. After fixation and cryoprotection, dissect out the bone from the inferior turbinate, middle turbinate, and superior turbinate under a stereomicroscope, using a fine tissue forceps and a sharp scalpel for sharp dissection in the subperiosteal plane.Note: The mucosa of the middle turbinate and superior turbinate is usually much thinner than that of the inferior turbinate.
-
22.
Embedding. Embed the tissues either in Paraffin (routine formalin-fixed and paraffin-embedded, FFPE), or Tissue-Tek O.C.T. compound on dry ice.
Optional: Take snapshots of each tissue sample upon embedding to document its orientation (Figure 4).
-
23.Sectioning. Depending on the chosen type of embedding:
-
a.Cut cryosections using a cryostat and collect on microscope slides, e.g., SuperFrost Plus Gold Slides (Thermo Fisher Scientific, Cat# K5800AMNZ72) or SuperFrost Plus Micro slides (VWR, Cat# 48311–703).
-
b.Cut paraffin-embedded blocks using a vibratome/microtome and collect on slides.
-
a.
Note: We cut cryosections of 6–8 μm for all tissue samples and in selected cases 5 μm paraffin-embedded sections of olfactory bulb and the frontal lobe of the brain. Slides were air-dried and stored at ‒80°C (cryosections) or 4°C (paraffin-embedded sections).
-
24.
Staining. In our studies,1,2 the cryosectioned samples were processed with hematoxylin and eosin staining, or with fluorescence RNAscope combined with fluorescence immunohistochemistry.
Note: RNAscope is a highly specific technique using commercially available probes for a target. Run positive and negative controls on the tissue samples.
Note: The results of immunohistochemistry depend on the antibody.
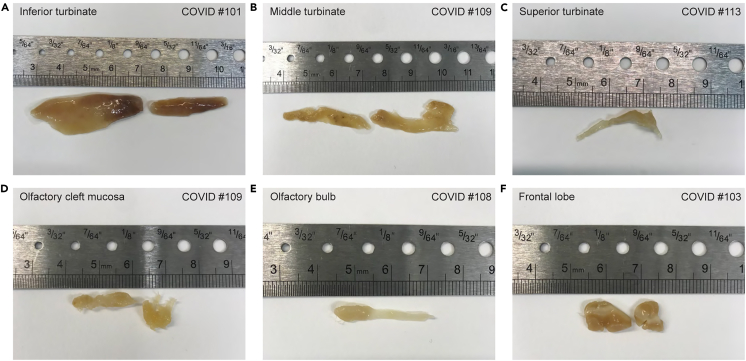
Figure 4.
Tissue samples before embedding
Photos taken with a mobile phone show representative tissue samples after fixation and cryoprotection and prior to embedding in O.C.T. A ruler is included as size reference.
(A–C) Nasal turbinates: inferior turbinate in (A), middle turbinate in (B), and superior turbinate in (C). After fixation and cryoprotection, samples were separated into medial and lateral mucosal surfaces by subperiosteal dissection and removal of bone fragments. The mucosal surface areas range from ∼1 to 6 cm2.
(D) Olfactory cleft mucosa.
(E) Olfactory bulb and olfactory tract, ∼2.5 cm in length.
(F) Two tissue samples from the frontal lobe of the brain, each ∼1 cm3 in size, containing white and gray matter.
Expected outcomes
We developed, streamlined, and validated the protocol over a period of two years to visualize the patterns of SARS-CoV-2 infection in the olfactory system and adjacent tissues.1,2 The rapid bedside surgical procedure enabled us to obtain pristine-quality tissue samples of the respiratory mucosa and olfactory cleft mucosa, whole olfactory bulbs, and the frontal lobe of the brain, without leaving externally visible incisions. The mobile endoscopy unit supported a high level of flexibility, in terms of both timing (24/7) and location (ward, intensive care unit, operation room), and can be extended to other settings with an infectious hazard.
We included a total of 138 cases from May 2020 to May 2022, of two types: 115 COVID-19 cases and 23 (noninfected) control cases. The COVID-19 cases died from or with COVID-19 and were infected either with the ancestral SARS-CoV-2 strain or variants of concern Alpha, Delta, Omicron BA.1, or Omicron BA.2. During this two-year period, we progressively refined the protocol and expanded it with the collection of a blood sample, a CSF sample, and a nasopharyngeal swab. The PMI at the start of endoscopic tissue sampling was <3 h in >90% of the inclusions (median: 89 min; IQR: 60–118 min). The duration of the procedure of endoscopic tissue sampling was 1–1.5 h (median: 71 min; IQR: 49–102 min). The death-to-sampling time using this protocol is thus much shorter compared to autopsy studies, resulting in superior tissue preservation.
As is inherent to most postmortem studies, the cases were typically aged individuals (median age: 78, IQR: 70–85) with multiple comorbidities. We observed no detrimental impact on tissue quality related to age or specific comorbidities. The quality of the intracranial samples (olfactory bulb and frontal lobe) was compromised in a few cases with increased intracranial pressure, and such cases were not included in later phases of the project.
Tissue samples and microscopy
Figure 4 shows samples of various resected tissues before embedding, illustrating the size and structure of samples that can be obtained using this protocol. The high yields of tissue samples obtained are suitable for various applications. In the ANOSMIC-19 project, the samples were processed with RNAscope combined with immunohistochemistry, or with conventional hematoxylin and eosin staining. Attesting to the quality of the protocol, the collected tissue samples were suitable for histological staining and confocal imaging in 100% of the 138 cases.
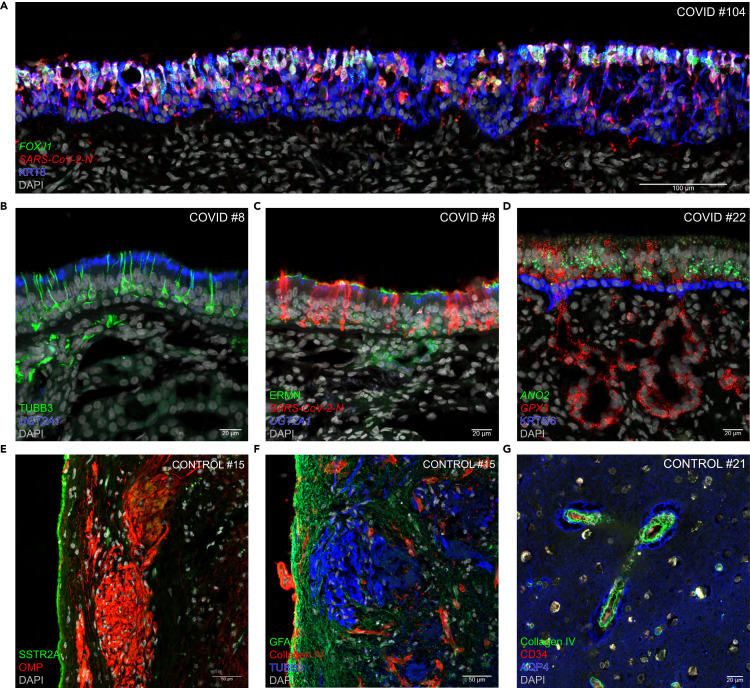
Related to the short PMIs, tissue samples retained their biomolecular integrity, allowing for advanced analyses of tissue morphology, gene expression, and protein expression. The pristine protein and nucleic acid preservation resulted in strong and unambiguous signals for RNAscope and immunohistochemistry, allowing for excellent visualization of RNA and protein expression and cell type identification. Figure 5 shows representative examples of confocal images, combining RNAscope with immunohistochemistry of sections of the respiratory mucosa and olfactory mucosa, olfactory bulb, and frontal lobe of the brain. A section through the respiratory mucosa of a COVID-19 case shows SARS-CoV-2 N puncta in the great majority of ciliated cells harboring FOXJ1 puncta and KRT8-immunoreactive signal (Figure 5A). In the olfactory epithelium of another COVID-19 case, TUBB3-immunoreactive signal marks olfactory sensory neurons (Figure 5B), densely packed SARS-CoV-2-N puncta are harbored by sustentacular cells (Figure 5C), and basal cells contain KRT5/6-immunoreactive signal (Figure 5D). In the olfactory bulb of a control case, an intact layer of SSTRA-2-immunoreactive pia mater snugly covers the external surface of the olfactory bulb (Figure 5E), and OMP-immunoreactive or TUBB3-immunoreactive axons of olfactory sensory neurons project to glomeruli within the GFAP-immunoreactive parenchyma of the olfactory bulb (Figures 5E and 5F). In the olfactory bulb and the frontal lobe of two control cases, the blood vessel walls can be visualized with collagen-IV immunoreactive signal (Figures 5F and 5G). In the frontal lobe of a control case, the glia limitans perivascularis can be visualized with AQP4-immunoreactive signal (Figure 5G).
Figure 5.
Examples of multiplex confocal imaging
Confocal images of sections stained fluorescently with RNAscope and immunohistochemistry of the respiratory mucosa (A), olfactory mucosa (B–D), olfactory bulb (E and F), and frontal lobe (G) from COVID-19 and control cases. Markers are at bottom left, either in italics (for genes, RNAscope) or roman (for proteins, immunohistochemistry). DAPI served as nuclear stain.
(A) A long stretch of infected respiratory mucosa of COVID #104. FOXJ1 is a marker for ciliated cells and KRT8 is a marker for epithelial cells. Densely packed SARS-CoV-2-N puncta occur within cells harboring FOXJ1 puncta and KRT8-immunoreactive signal.
(B and C) Olfactory mucosa of COVID #8. Olfactory sensory neurons contain TUBB3-immunoreactive signal. Sustentacular cells in the olfactory epithelium and Bowman’s gland cells in the underlying lamina propria harbor UGT2A1 puncta, and ERMN-immunoreactive signal caps sustentacular cells. (B) In this uninfected patch, TUBB3-immunoreactive olfactory sensory neurons are closely appositioned to sustentacular cells harboring UGT2A1 puncta. (C) In this infected patch, densely packed SARS-CoV-2-N puncta delineate sustentacular cells, which are devoid of UGT2A1 puncta or ERMN-immunoreactive signal as a result of the infection with SARS-CoV-2.
(D) Olfactory mucosa of COVID #22, with no signs of viral presence at the time of death. Olfactory sensory neurons harbor ANO2 puncta. GPX3 puncta are detected in sustentacular cells in the olfactory epithelium and in the Bowman’s gland cells in the lamina propria. A layer of KRT5/6-immunoreactive basal cells demarcates the border between the olfactory epithelium and the lamina propria.
(E and F) Olfactory bulb of control #15. (E) OMP-immunoreactive olfactory sensory neuron axons project to glomeruli of the olfactory bulb. A layer of SSTRA2-immunoreactive pia mater snugly covers the surface of the olfactory bulb. (F) TUBB3-immunoreactive olfactory sensory neuron axons project to glomeruli within the GFAP-immunoreactive parenchyma of the olfactory bulb. Collagen-IV-immunoreactive signal labels the blood vessel walls.
(G) Frontal lobe of control #21. Collagen-IV-immunoreactive signal labels the blood vessel walls and AQP4-immunoreactive signal labels the glia limitans perivascularis, formed by the endfeet of astrocytes; the unstained area in between is the Virchow-Robin Space.
Protocol modifications and variations
Modifications were undertaken in attempts to improve the tissue collection and processing protocol for our research purposes, as follows.
For the respiratory mucosa and the olfactory cleft mucosa samples, we observed various levels of epithelial detachment in the first cases. We evaluated the effect of tissue dissection techniques on tissue quality. Initially, the turbinates were dissected with blunt instruments bedside soon after sampling and transferred into the fixative solution without the bone. We suspected that tissue integrity was disturbed and that epithelial detachment occurred due to tissue manipulation and traction during the dissection. We then dissected the mucosa from the turbinate bone after fixation and obtained better results.
For brain tissue samples, cryoprotection with sucrose is a critical step in tissue preparation for long term storage at -80°C. Initially, we performed sucrose cryoprotection with shorter durations: 15% sucrose for 1 day, 25% sucrose 1 day, then 30% sucrose for 2 d at 4°C. The images showed holes in the tissues after cryosectioning especially in the brain and olfactory bulb — so-called “Swiss cheese artifacts”. Troubleshooting was performed by prolonging the sucrose step. Sucrose solutions above 10% are hypertonic and cause water to flow out of cells, hence maintaining tissue integrity and preventing freezing artifact by removing water. For O.C.T. embedding, the duration of immersion in sucrose solutions was prolonged at each step; 3 d for 15% sucrose, up to 4 d for 25% sucrose and 7 d for 30% sucrose. The images showed improvement with absence of holes. An alternative to freezing artifacts would be paraffin embedding.
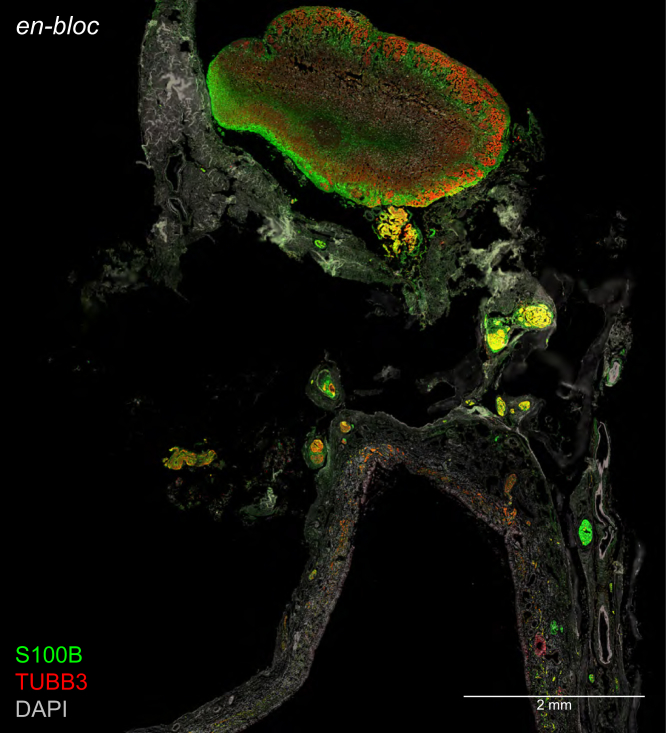
To preserve the topographical relation of the olfactory cleft mucosa and olfactory bulb, we extracted an en-bloc specimen in a case with a thin skull base, where fractioning of the skull base between the two tissues can be controlled better. Figure 6 shows immunohistochemistry of a section of an en-bloc specimen of a COVID-19 case, illustrating the topographical relation of the olfactory cleft mucosa and the olfactory bulb within a wide field of view.
Figure 6.
Variation of the protocol
Fluorescent confocal image of a section stained with immunohistochemistry for S100B (a marker for olfactory ensheathing cells) and TUBB3 (a marker for olfactory sensory neurons). DAPI served as nuclear stain. Section is of an en-bloc specimen extracted from COVID #105 comprising olfactory cleft mucosa (below) and olfactory bulb (above), illustrating their topographical relation. Olfactory sensory neurons in the olfactory epithelium, their axons, and their axons terminating in glomeruli of the olfactory bulb contain TUBB3-immunoreactive signal. Axon bundles enwrapped by ensheathing cells contain S100B-immunoreactive signal and colocalize with TUBB3-immunoreactive signal.
Rationale to develop the protocol
The literature on the human olfactory mucosa and olfactory bulb is largely based on postmortem samples. After death, tissues start to undergo changes, the rate of which is affected by several factors such as the ambient temperature, tissue pH, and agonal factors. A determinant of tissue quality that can be measured and experimentally controlled is the PMI, defined as the period of time between the death of the patient and the beginning of the tissue sampling. With increasing PMI, biomolecular integrity, gene expression levels, and ultrastructural quality are affected.10,15,16,17 For a typical autopsy, as performed by pathologists and assistants in a dedicated autopsy or dissection room, PMIs tend to be long (24 h or more) and vary considerably between patients, affecting the interpretation of histological or biochemical findings.18
Early in the COVID-19 pandemic, olfactory dysfunction emerged as a prominent first and often sole symptom of infection with SARS-CoV-2 and speculations were raised about the scenario of viral neuroinvasion via the olfactory route from the nasal into cranial cavity. To procure tissue samples of the olfactory mucosa, olfactory bulb, and adjacent brain regions that were suitable for advanced microscopy, we reasoned that the sampling ought to be performed postmortem and in a way that minimized the risk of nucleic acid degradation and tissue autolysis associated with prolonged PMIs. We recognized the need for an alternative method to procure rapidly, at the bed of the deceased patient and with consistently short PMIs, tissue samples from patients who died from or with COVID-19 during the acute phase of SARS-CoV-2 infection and who may still be contagious. We developed a protocol in the context of the multicentric project with acronym ANOSMIC-19 (Analyzing Olfactory dySfunction Mechanisms In COVID-19).1,2
The postmortem bedside surgical procedure is based on an endoscopic endonasal transcribriform approach that we adapted from skull base surgery.19 We designed a dedicated mobile endoscopy unit to facilitate timely transport of the equipment to the bed of the deceased patient within the infectious environment of the COVID-19 pandemic, keeping in mind that the deceased patient may still be contagious and transmit SARS-CoV-2 to the operators. This logistic flexibility enabled rapid bedside sampling in the hospital settings of a ward, an intensive care unit, and — in the case of organ donors serving as control patients — an operating room. As the time of death of a COVID-19 patient is unpredictable, we implemented a 24/7 workflow that was initiated by an on-site health care worker who phoned a member of a team of ear, nose and throat (ENT) surgeons shortly after the death of a patient who was hospitalized in a ward or intensive care unit.
Compared to the skull base surgery performed on living patients, the postmortem procedure does not include the following steps: coagulation and transection of the anterior and posterior ethmoidal arteries, removal of the superior parts of the septum, frontal drill out (Draf III), resection of the crista galli, and transection of the falx cerebri. There is much less bleeding in deceased patients and obviously no risk of inflicting complications, making cauterization and careful dissection unnecessary. The postmortem procedure should be performed by a team of ENT surgeons or neurosurgeons (including their trainees). Prior experience in endoscopic endonasal surgery, particularly in the transnasal endoscopic resection of malignancies of the anterior skull base, will lead to an easier implementation of the procedure. Transcribriform access with cold instruments requires two operators: a lead surgeon and an assistant.
Applications of the protocol
The protocol can be applied for procuring postmortem tissue samples to investigate other types of olfactory dysfunction such as related to aging or neurological disease or of post-traumatic nature, and for other research questions at the nose-brain interface in humans such as neuroinvasion or neurotropism of other infectious agents or transport of intranasally delivered medication to the brain.
The protocol can also be applied if one is interested only in procuring whole olfactory bulbs or tissue samples of the frontal lobe of the brain. For this restricted purpose, the middle and superior turbinates still need to be resected in order to gain access to the cribriform plate.
Comparison with other methods
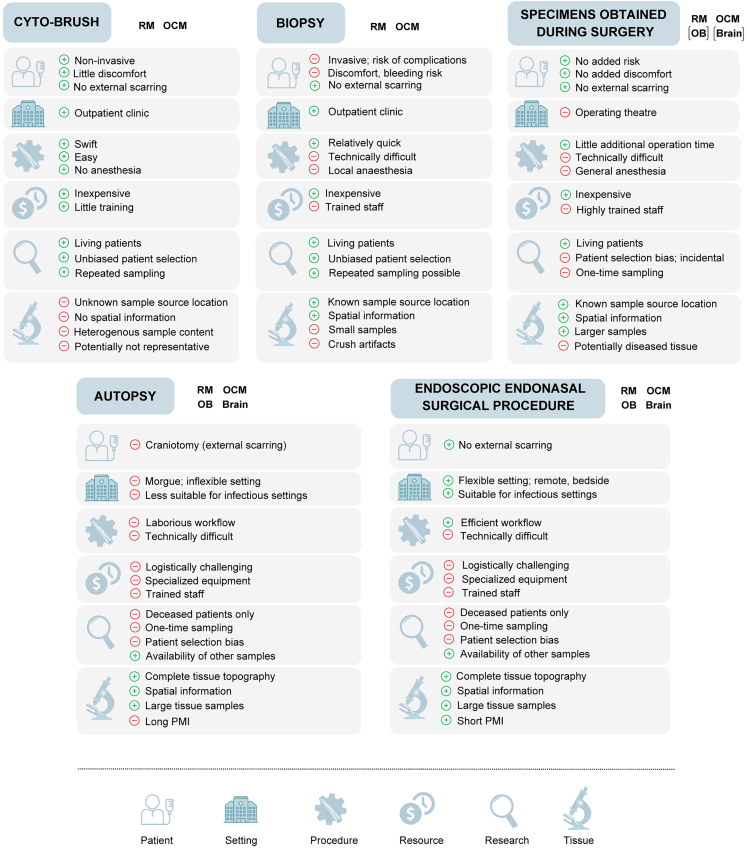
The protocol describes the sampling of the respiratory mucosa and olfactory cleft mucosa, of whole olfactory bulbs, and of white and gray matter from the ventromedial part of the frontal lobe of the brain, the gyrus rectus and gyri orbitales. Several methods have been described to sample these tissues in living or deceased patients, including cytobrushing, biopsies, surgical resection specimens, or postmortem collection during autopsies (Figure 7).
Figure 7.
Comparison of tissue collection methods
Overview of the advantages and disadvantages of different sampling methods for collecting respiratory mucosa, olfactory cleft mucosa, olfactory bulb, and brain tissue. Abbreviations: OB, olfactory bulb; OCM, olfactory cleft mucosa; PMI, postmortem interval; RM, respiratory mucosa.
Cytobrushing is a non-invasive method for collecting cell samples of the respiratory mucosa and the olfactory cleft mucosa from living patients.20,21,22,23 Superficially located epithelial cells are gently exfoliated over a wide surface area. Cytobrushing is relatively straightforward, can be performed quickly in an outpatient clinic, has few or no procedural complications, and can be safely repeated over time, making it suitable for longitudinal studies. The use of cytobrushing for research purposes is limited by several factors, including the uncertainty about the tissue origin of the collected sample, the lack of spatial information in terms of tissue context, and doubts about the representativeness of exfoliated cells for the corresponding tissue.
Endoscopically guided biopsies overcome some of the limitations of cytobrushing by sampling a small piece of olfactory cleft mucosa. Such biopsies can be taken relatively easily under local anesthesia in an outpatient clinic, safely and without overt damage to the sense of smell.24,25,26,27 But instrumentation in this anatomically narrow region is difficult, can cause substantial discomfort to the patient, and carries the risk of traumatic injury to the thin cribriform plate, potentially causing CSF leakage. A safer alternative is to take olfactory cleft mucosa biopsies under general anesthesia in an operation room, usually in patients undergoing surgery for other reasons.5,28,29 The controlled environment and patient sedation can improve sampling conditions, but there is the remaining difficulty of obtaining olfactory mucosa containing olfactory sensory neurons.5,28
In selected surgical procedures, carried out for removal of a tumor, pieces of healthy olfactory cleft mucosa and/or respiratory mucosa,30,31 olfactory bulb,6,7 and brain tissue17,32 are resected and used for research purposes, as these pieces that would otherwise be discarded as medical waste. This approach is reserved to a highly selected group of patients. The topography and dimensions of the resected tissue samples vary according to the pathology or surgical procedure and may harbor pathological alterations.
Given the myriad challenges to procure tissue samples from living patients, an alternative approach is postmortem sampling in the setting of an autopsy in a dedicated room.4,33,34,35,36 The main advantage is that adequate amounts of tissue and whole olfactory bulbs can be sampled and that their topographical origin is known, extending as much as to an en-bloc extraction of a specimen containing the olfactory cleft and both olfactory bulbs. To access the olfactory bulb and other regions of the brain during an autopsy, a craniotomy is performed in order to gain access the intracranial space. This open surgical technique is an invasive and mutilating procedure and, when carried out on a patient with an airborne infectious disease, the formation of aerosols carries a substantial risk of infecting the operator collecting the samples.9,37 Additionally, autopsy specimens tend to have long PMIs because of the logistics of transporting the body to a dedicated room and the availability of pathologists and their staff.
Limitations
The need for specialized equipment and trained staff limits the applicability to hospitals with an ENT or neurosurgical department. When sampling patients who may still be contagious, there is a variable and unknown risk of infection for the operators. Suitable personal protective equipment must be available at all time and operators must be trained in donning and doffing techniques.9 A limitation of the brain sampling is that the transcribriform access limits the sampling to the ventromedial part of the frontal lobe: typically, the gyrus rectus and the gyri orbitales are sampled.
Troubleshooting
Problem 1
Prolonged PMI, due to.
-
•
Delay in transport of equipment to bedside.
-
•
Delay due to collection of other samples (blood, CSF, nasopharyngeal swab). Related to “before you begin” item 3.
Potential solution
-
•
Store the mobile endoscopy unit in a central place.
-
•
Work with a team of two for a rapid set-up.
Problem 2
Defective surgical instruments, due to susceptibility of surgical instruments to wear and tear; for instance, subperiosteal dissection renders the sickle knife blunt.
Related to “before you begin” item 7.
Potential solution
-
•
Keep a full set of spare instruments.
-
•
Sharpen the sickle knife.
Problem 3
Difficult femoral puncture, due to.
-
•
Absence of pulsations in deceased patients, which make femoral vessels challenging to identify.
-
•
Femoral fat may impede topographical landmarks and the estimation of the depth of the puncture. Related to step 1a.
Potential solution
-
•
Palpate the anterior superior iliac spine with the thumb and the pubic bone with the middle finger; the index finger will be along the direction of the femoral vessels.
-
•
Aspirate while retrieving the needle.
-
•
Bring sufficient material.
Problem 4
Blood clotting in syringe, due to activation of the intrinsic coagulation pathway through contact with surfaces such as plastic.
Related to step 1c.
Potential solution
Transfer the blood into the tube within 30 s after sampling.
Problem 5
Difficult suboccipital puncture trajectory, due to muscle rigidity / rigor mortis with longer PMI, which can complicate puncture with an atraumatic needle. Related to step 2b.
Potential solution
Use a conventional pencil-point spinal needle.
Problem 6
Traumatic CSF puncture, due to accidental piercing of a blood vessel along the trajectory of the puncture. Related to step 2c.
Potential solution
-
•
Stop when observing blood in the needle hub.
-
•
Restart with new material.
-
•
Use an atraumatic needle.
Problem 7
Previous endoscopic surgery (Figure 3D) in cases with sinonasal inflammatory disease. Related to step 4.
Potential solution
Focus on other landmarks to avoid disorientation.
Problem 8
Nasal polyps (Figure 3C) in chronic rhinosinusitis.
Related to step 4.
Potential solution
Start with polypectomy to get maximal exposure of landmarks.
Problem 9
Abundant secretions (Figure 3E), for instance due to recent sinonasal infection. Related to step 4.
Potential solution
Perform suction cleaning prior to sampling.
Problem 10
Septal deviation or spur (Figure 3A), due to e.g., previous nasal trauma, congenital variation. Related to step 4.
Potential solution
Remove/fracture septal deviations prior to sampling.
Problem 11
Septal perforation (Figure 3B), due to e.g., acquired conditions (such as digital manipulation, trauma). Related to step 4.
Potential solution
Focus on other landmarks to avoid disorientation.
Problem 12
Nasal bleeding (Figure 3G), due to therapeutic anticoagulative therapy such as after ECMO therapy. Related to step 4.
Potential solution
Perform suction cleaning.
Problem 13
Nasal dryness and (blood) crusts (Figure 3H), due to oxygen therapy, or previous manipulation. Related to step 4.
Potential solution
-
•
Perform suction cleaning.
-
•
Flush/rinse with saline when necessary.
Problem 14
Pressure necrosis of nasal mucosa (Figure 3A), due to feeding tube, or nasal packing. Related to step 4.
Potential solution
Decide after processing whether samples can be used for interpretation.
Problem 15
Damaged olfactory bulb and brain (Figure 3I), due to increased intracranial pressure (intracranial hemorrhage, tumor). Related to step 4.
Potential solution
-
•
Dissect with extra care.
-
•
Decide after processing whether the samples should be used.
Problem 16
Narrow/limited workspace (Figures 3A–3C), due to patient-related structural pathology (septal spur, septal deviation, polyposis). Related to step 4b.
Potential solution
Remove or correct obstructing structures accordingly (see above).
Problem 17
Suboptimal visualization (Figures 3E–3G), due to blood, CSF, or nasal secretions. Related to step 4b.
Potential solution
Perform suction cleaning.
Problem 18
Dissecting in the incorrect plane of the olfactory cleft mucosa, due to misidentifying the correct subperiosteal plane for olfactory cleft mucosa dissection. Related to step 8a.
Potential solution
Keep the tip of the sickle knife in contact with the bone while incising and dissecting.
Problem 19
Tears and crush artifacts of the olfactory cleft mucosa, due to squeezing too hard with the Blakesley forceps upon removal. Related to step 8e.
Potential solution
-
•
Dissect and detach the olfactory cleft mucosa completely and transect all fila olfactoria.
-
•
Handle the tissue with care and grasp it lightly.
Problem 20
Olfactory bulb damage during osteotomies, due to applying too much force on the chisel, penetrating bone, and olfactory bulb at once. Related to step 9c.
Potential solution
-
•
Perform osteotomies more carefully on the contralateral side.
-
•
Identify position of olfactory bulb anteriorly before performing posterior osteotomy.
Problem 21
Olfactory bulb damage during dura mater incision, due to incautiously incising the dura mater. Related to step 9e.
Potential solution
Identify the position of the olfactory bulb endoscopically through a small lateral incision in the dura mater.
Problem 22
Excessive CSF flow, due to intracranial overpressure. Related to step 9e.
Potential solution
-
•
Leave the tip of the suction in place.
-
•
Flex the head slightly (20°).
Problem 23
Edematous olfactory bulb/brain tissue (Figure 4I) due to increased intracranial pressure (such as after craniocerebral hemorrhage). Related to steps 10 and 11.
Potential solution
Focus on atraumatic dissection.
Problem 24
Poor quality of antibody staining, due to overfixation or underfixation. Related to step 19.
Potential solution
Adjust fixation time according to tissue type and antibody.
Problem 25
Irregular sinking times of turbinates during sucrose immersion, due to bone within the tissue or a concha bullosa (air pocket) can influence sinking times. Related to step 20.
Potential solution
Ensure sufficient sucrose immersion by using sucrose steps of 3 days.
Problem 26
Freezing artifacts, due to water trapped inside the tissue forming crystals. Related to step 20c.
Potential solution
Apply longer 30% sucrose immersion periods (5–7 days) for olfactory bulb and brain samples.
Problem 27
Challenging turbinate dissection, due to concha bullosa (pneumatized anterior middle turbinate). Related to step 21.
Potential solution
Practice to dissect with less pressure.
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the lead contact, Laura Van Gerven (laura.vangerven@uzleuven.be).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Laura Van Gerven (laura.vangerven@uzleuven.be).
Materials availability
This study did not generate new unique materials or reagents.
Data and code availability
-
•
This study did not generate any unique data sets or code.
-
•
The multicentric ANOSMIC-19 project,1,2 under which the protocol was developed, is registered on clinicaltrials.gov (NCT04445597). See Khan et al. (2021)1 and Khan et al. (2022)2 for data and microscopy images generated using this protocol. Video S11 can be viewed at and downloaded from https://data.mendeley.com/datasets/363xfhrnv5.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank the nurses, doctors, and paramedical staff of the participating centers for their support and cooperation in the ANOSMIC-19 project; Florence Bourgeois, Michiel Claerhout, Floor Couvreur, Samuel Klein, Charlotte Lietaer, Laure Van Breda, and Gill Verstappen for their efforts during the project and for their input and feedback on the protocol; and Florian Lindenblatt, Wouter Meert, Erick Rea Escalante, and Gert Timmermans for technical and material support. P.M. acknowledges the generous financial support of the Max Planck Society. L.V.G. was supported by Senior Clinical Investigator Fellowship 18B2222N of the Research Foundation Flanders (FWO).
Author contributions
L.V.G. and P.M. conceived and supervised the ANOSMIC-19 project and together with W.B., M.K., and M.C. developed the protocol, from bed to bench. W.V.D.B. and C.V. contributed to the development of the protocol for postmortem collection of blood and CSF samples. W.B., P.V.B., K.S., M.J., M.C., and L.V.G. were responsible for the collection of the tissue and non-tissue samples. M.K. took the macroscopic photos and generated the confocal images. M.C. wrote the original draft of the manuscript. P.M., C.V., and L.V.G. revised the manuscript. All authors edited the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Khan M., Yoo S.J., Clijsters M., Backaert W., Vanstapel A., Speleman K., Lietaer C., Choi S., Hether T.D., Marcelis L., et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–5949.e15. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M., Clijsters M., Choi S., Backaert W., Claerhout M., Couvreur F., Van Breda L., Bourgeois F., Speleman K., Klein S., et al. Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron. 2022;110:3919–3935.e6. doi: 10.1016/j.neuron.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinna F.d.R., Ctenas B., Weber R., Saldiva P.H., Voegels R.L. Olfactory neuroepithelium in the superior and middle turbinates: which is the optimal biopsy site? Int. Arch. Otorhinolaryngol. 2013;17:131–138. doi: 10.7162/S1809-97772013000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook E.H., Wu E., Curry W.T., Lin D.T., Schwob J.E. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanos T., Saibene A.M., Pipolo C., Battaglia P., Felisati G., Rubio A. Isolation of putative stem cells present in human adult olfactory mucosa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maresh A., Rodriguez Gil D., Whitman M.C., Greer C.A. Principles of glomerular organization in the human olfactory bulb – implications for odor processing. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lötsch J., Schaeffeler E., Mittelbronn M., Winter S., Gudziol V., Schwarzacher S.W., Hummel T., Doehring A., Schwab M., Ultsch A. Functional genomics suggest neurogenesis in the adult human olfactory bulb. Brain Struct. Funct. 2014;219:1991–2000. doi: 10.1007/s00429-013-0618-3. [DOI] [PubMed] [Google Scholar]
- 8.Nine J.S. In: Autopsy Performance & Reporting. Collins K.A., editor. CAP Press; 2017. High-risk autopsy cases; pp. 91–97. [Google Scholar]
- 9.Van Gerven L., Hellings P.W., Cox T., Fokkens W., Hopkins C., Hox V., Jorissen M., Schuermans A., Sinonquel P., Speleman K., et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID-19 outbreak. Rhinology. 2020;58:289–294. doi: 10.4193/Rhin20.119. [DOI] [PubMed] [Google Scholar]
- 10.Antiga L.G., Sibbens L., Abakkouy Y., Decorte R., Van Den Bogaert W., Van de Voorde W., Bekaert B. Cell survival and DNA damage repair are promoted in the human blood thanatotranscriptome shortly after death. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinis-Oliveira R.J., Vieira D.N., Magalhães T. Guidelines for collection of biological samples for clinical and forensic toxicological analysis. Forensic Sci. Res. 2016;1:42–51. doi: 10.1080/20961790.2016.1271098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn M.F., Kelly M., Dooley J.S.G. Nasopharyngeal swabs vs. nasal aspirates for respiratory virus detection: A systematic review. Pathogens. 2021;10 doi: 10.3390/pathogens10111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gevaert P., De Craemer J., Bachert C., Blauwblomme M., Chaker A., Cingi C., Hellings P.W., Hopkins C., Hox V., Fokkens W.J., et al. European Academy of Allergy and Clinical Immunology position paper on endoscopic scoring of nasal polyposis. Allergy. 2023;78:912–922. doi: 10.1111/all.15650. [DOI] [PubMed] [Google Scholar]
- 14.Tang O., Selvin E., Arends V., Saenger A. Short-term stability of hematologic parameters in frozen whole blood. J. Appl. Lab. Med. 2019;4:410–414. doi: 10.1373/jalm.2018.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birdsill A.C., Walker D.G., Lue L., Sue L.I., Beach T.G. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 2011;12:311–318. doi: 10.1007/s10561-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glausier J.R., Konanur A., Lewis D.A. Factors affecting ultrastructural quality in the prefrontal cortex of the postmortem human brain. J. Histochem. Cytochem. 2019;67:185–202. doi: 10.1369/0022155418819481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dachet F., Brown J.B., Valyi-Nagy T., Narayan K.D., Serafini A., Boley N., Gingeras T.R., Celniker S.E., Mohapatra G., Loeb J.A. Selective time-dependent changes in activity and cell-specific gene expression in human postmortem brain. Sci. Rep. 2021;11:6078. doi: 10.1038/s41598-021-85801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrich F., Mertz K.D., Glatzel M., Beer M., Krasemann S. Using autopsies to dissect COVID-19 pathogenesis. Nat. Microbiol. 2023;8:1986–1994. doi: 10.1038/s41564-023-01488-7. [DOI] [PubMed] [Google Scholar]
- 19.Kassam A., Snyderman C.H., Mintz A., Gardner P., Carrau R.L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg. Focus. 2005;19:E3. [PubMed] [Google Scholar]
- 20.Zanusso G., Bongianni M., Caughey B., Ferrari S., Hughson A.G., Groveman B.R., Fiorini M., Pocchiari M., Monaco S., Caughey B., Zanusso G. A test for Creutzfeldt–Jakob disease using nasal brushings. N. Engl. J. Med. 2014;371:1842–1843. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brozzetti L., Sacchetto L., Cecchini M.P., Avesani A., Perra D., Bongianni M., Portioli C., Scupoli M., Ghetti B., Monaco S., et al. Neurodegeneration-associated proteins in human olfactory neurons collected by nasal brushing. Front. Neurosci. 2020;14:145. doi: 10.3389/fnins.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefani A., Iranzo A., Holzknecht E., Perra D., Bongianni M., Gaig C., Heim B., Serradell M., Sacchetto L., Garrido A., et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain. 2021;144:1118–1126. doi: 10.1093/brain/awab005. [DOI] [PubMed] [Google Scholar]
- 24.Jafek B.W., Murrow B., Michaels R., Restrepo D., Linschoten M. Biopsies of human olfactory epithelium. Chem. Senses. 2002;27:623–628. doi: 10.1093/chemse/27.7.623. [DOI] [PubMed] [Google Scholar]
- 25.Witt M., Bormann K., Gudziol V., Pehlke K., Barth K., Minovi A., Hähner A., Reichmann H., Hummel T. Biopsies of olfactory epithelium in patients with Parkinson’s disease. Mov. Disord. 2009;24:906–914. doi: 10.1002/mds.22464. [DOI] [PubMed] [Google Scholar]
- 26.Holbrook E.H., Rebeiz L., Schwob J.E. Office-based olfactory mucosa biopsies. Int. Forum Allergy Rhinol. 2016;6:646–653. doi: 10.1002/alr.21711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pipolo C., Bottai D., Fuccillo E., Aronica E., Bruschi F., Bulfamante A.M., Castellani L., Canevini M.P., Chiumello D., Ferrari S., et al. Evidence of SARS-CoV-2 in nasal brushings and olfactory mucosa biopsies of COVID-19 patients. PLoS One. 2022;17 doi: 10.1371/journal.pone.0266740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winstead W., Marshall C.T., Lu C.L., Klueber K.M., Roisen F.J. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am. J. Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- 29.Finlay J.B., Brann D.H., Abi Hachem R., Jang D.W., Oliva A.D., Ko T., Gupta R., Wellford S.A., Moseman E.A., Jang S.S., et al. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.add0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraiva L.R., Riveros-McKay F., Mezzavilla M., Abou-Moussa E.H., Arayata C.J., Makhlouf M., Trimmer C., Ibarra-Soria X., Khan M., Van Gerven L., et al. A transcriptomic atlas of mammalian olfactory mucosae reveals an evolutionary influence on food odor detection in humans. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omura K., Han B., Nishijima H., Aoki S., Ebihara T., Kondo K., Otori N., Kojima H., Yamasoba T., Kikuta S. Heterogeneous distribution of mature olfactory sensory neurons in human olfactory epithelium. Int. Forum Allergy Rhinol. 2022;12:266–277. doi: 10.1002/alr.22885. [DOI] [PubMed] [Google Scholar]
- 32.Loomba S., Straehle J., Gangadharan V., Heike N., Khalifa A., Motta A., Ju N., Sievers M., Gempt J., Meyer H.S., Helmstaedter M. Connectomic comparison of mouse and human cortex. Science. 2022;377 doi: 10.1126/science.abo0924. [DOI] [PubMed] [Google Scholar]
- 33.Godoy M.D.C.L., Fornazieri M.A., Doty R.L., Pinna F.d.R., Farfel J.M., Santos G.B.D., Molina M., Ferretti-Rebustini R.E.L., Leite R.E.P., Suemoto C.K., et al. Is olfactory epithelium biopsy useful for confirming Alzheimer's disease? Ann. Otol. Rhinol. Laryngol. 2019;128:184–192. doi: 10.1177/0003489418814865. [DOI] [PubMed] [Google Scholar]
- 34.Klingenstein M., Klingenstein S., Neckel P.H., Mack A.F., Wagner A.P., Kleger A., Liebau S., Milazzo A. Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 2020;209:155–164. doi: 10.1159/000513040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 36.Ho C.Y., Salimian M., Hegert J., O'Brien J., Choi S.G., Ames H., Morris M., Papadimitriou J.C., Mininni J., Niehaus P., et al. Postmortem assessment of olfactory tissue degeneration and microvasculopathy in patients with COVID-19. JAMA Neurol. 2022;79:544–553. doi: 10.1001/jamaneurol.2022.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly A.J., Finkbeiner W.E., Ursell P.C., Davis R.L., editors. Autopsy Pathology: A Manual and Atlas. Elsevier; 2016. Basic postmortem evaluation; pp. 33–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
This study did not generate any unique data sets or code.
-
•
The multicentric ANOSMIC-19 project,1,2 under which the protocol was developed, is registered on clinicaltrials.gov (NCT04445597). See Khan et al. (2021)1 and Khan et al. (2022)2 for data and microscopy images generated using this protocol. Video S11 can be viewed at and downloaded from https://data.mendeley.com/datasets/363xfhrnv5.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.