Abstract
Chronic myelomonocytic leukemia (CMML) is a hematologic malignancy affecting the bone marrow and resulting in peripheral blood monocytosis. Kidney and urinary tract involvement is common and can present dramatically with life-threatening consequences. Kidney involvement can be the result of direct or indirect mechanisms, including prerenal azotemia, glomerular disease, tubulointerstitial involvement, and renovascular disorders. Urinary tract involvement, electrolyte and acid-base disorders, as well as nephrotoxicity from treatment of the disorder can also occur. Given this multifactorial pathogenesis involving several mechanisms concomitantly, nephrologists must exercise heightened awareness and maintain a low threshold for kidney biopsy. There is a pressing need for future research endeavors to elucidate and target the manifestations of CMML that involve the kidneys with the ultimate goal of augmenting overall prognosis and therapeutic outcomes.
Index Words: Chronic myelomonocytic leukemia, glomerulopathy, kidney injury, lysozyme-induced nephropathy, onconephrology, tumor lysis syndrome, vasculitis
Chronic myelomonocytic leukemia (CMML) is a unique hematologic malignancy characterized by a combination of features from both myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS) (Fig 1).1,2 Its reported incidence is 2.5 cases per 100,000 individuals, and it predominantly affects older adults, with a median age of onset ranging from 71 to 75 years.3 Although the exact pathophysiology of CMML remains incompletely understood, various genetic and epigenetic abnormalities contribute to the development of the disease.4,5
Figure 1.
Major overlap syndromes between myeloproliferative neoplasm (MPN) and myelodysplastic syndrome (MDS). Although MDS and MPN have distinct defining features, certain syndromes combine both myeloproliferative characteristics, such as an elevated white blood cell count and splenomegaly, with myelodysplastic traits, including anemia, thrombocytopenia, and an excess of blasts. The 3 major overlap syndromes are chronic myelomonocytic leukemia, atypical chronic myeloid leukemia, and MDS or MPN with ringed sideroblasts and thrombocytosis.
The diagnosis of CMML is established based on the 2016 World Health Organization criteria, which include specific clinical and hematologic parameters (Box 1).6 The disease is characterized by both myeloproliferative features, such as elevated white blood cell (WBC) count and splenomegaly, and myelodysplastic features, including anemia, thrombocytopenia, and excess blasts. More importantly, the key hallmark of CMML is significant monocytosis, which plays a crucial role in causing end-organ damage.7
Box 1. 2016 WHO diagnostic criteria for CMML.
| 2016 WHO Diagnostic Criteria For CMML |
|---|
|
|
|
|
|
|
Abbreviations: CMML, chronic myelomonocytic leukemia; WHO, World Health Organization.
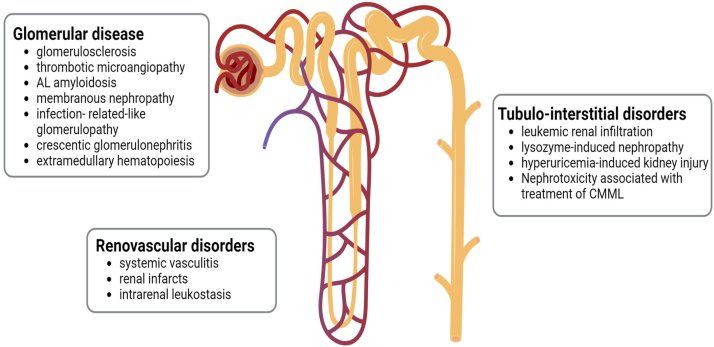
Among the various end-organ damage events observed in CMML, kidney injury is the most frequent, affecting up to 35% of patients.8 The occurrence of kidney injury is more common in proliferative CMML and directly correlates with peak WBC counts and absolute monocyte counts. Notably, the damage can manifest across various kidney compartments, including glomeruli, tubulointerstitium, and vessels, while also affecting different segments of the urinary tract (Fig 2). Adding to the complexity, multiple mechanisms of injury can concomitantly coexist.
Figure 2.
Renal involvement in chronic myelomonocytic leukemia (CMML). CMML can affect all the different renal compartments, including the glomeruli, tubulointerstitial space, and even blood vessels. Each compartment can be affected through multiple mechanisms of injury.
Kidney related complications not only confer susceptibility to kidney failure in patients with CMML but also exert a deleterious effect on progression-free survival.9 This has prompted experts to propose creatinine as a potential prognostic marker in CMML.10 Consequently, it becomes incumbent on both hematologists and nephrologists to exercise keen vigilance in the early identification of these complications, thereby enabling timely implementation of appropriate therapeutic measures.
In this work, we comprehensively review the potential mechanisms through which CMML can affect the kidney and the urinary tract. We also delve into the intricate pathophysiology of injury and explore diverse treatment modalities that have been used to address each distinct condition.
Prerenal Acute Kidney Injury
Prerenal acute kidney injury (AKI) can occur in patients with CMML. Frequent contributors to intravascular volume depletion encompass instances of emesis or diarrhea, diminished oral intake, escalated insensible losses stemming from fever and tachypnea, and the utilization of pharmaceutical agents, such as diuretics. Supportive treatment with intravenous fluids is usually sufficient to restore kidney function.
Glomerular Disease
Several types of CMML-related glomerulopathies have been described in the literature. These conditions typically present clinically with worsening proteinuria, microscopic hematuria, AKI, and even nephrotic syndrome. The exact mechanism by which CMML causes nephrotic syndrome remains unclear, but one hypothesis suggests that it may be due to the upregulation of tumor necrosis factor-alpha (TNF-α) mRNA expression from circulating monocytes, leading to increased permeability of glomerular capillaries.11,12 Others have suggested that abnormalities in antigen presentation because of dysfunctional T- and B-cell interactions may be responsible for autoimmunity and subsequent glomerular manifestations.13 As a result, glomerulonephritis may either be part of a constellation of symptoms in acute systemic vasculitis or may present as an isolated autoimmune manifestation.14 One other theory explaining glomerular involvement in CMML is related to high levels of immune complexes because of monocyte proliferation and B-cell activation.15 Finally, Saitoh et al16 found that patients with nephrotic syndrome had higher monocyte counts than those without it, suggesting that kidney infiltration by monocytes is playing a role (Fig 3).
Figure 3.
Mechanisms of glomerular injury in chronic myelomonocytic leukemia. Monocytes can cause direct injury by infiltrating the glomerulus or indirect injury through accumulation of immune complexes along the glomerular basement membrane or upregulation of TNF-α, leading to increased glomerular capillary permeability. Autoimmunity from abnormal T- and B-cell interactions can also potentiate glomerular damage.
The most common form of glomerular involvement reported with MPNs in general, and more specifically in CMML, is glomerulosclerosis, both global or segmental, which appears to exceed age-related norms. This pattern of glomerular injury was described in multiple reports, including a large series that examined kidney postmortem findings in 57 patients with MPNs.17, 18, 19, 20, 21 The pathophysiology behind the accelerated glomerulosclerosis remains uncertain, but it has been postulated that growth factors, such as fibroblast growth factor, platelet-derived growth factor, and transforming growth factor-β (TGF-β), which play a central role in MPN-related myelofibrosis, may be implicated.22 These growth factors are potent triggers of mesangial cell proliferation, mesangial and subendothelial matrix production, and subsequent endothelial and podocyte damage that eventually lead to glomerular scarring.19,23,24 As a result, it is possible that new TGF-β inhibitors, such as luspatercept, recently approved for the treatment of anemia in MPNs, may also have the potential to reverse glomerulosclerosis and improve kidney function.25,26
Furthermore, chronic endothelial cell injury has been proposed as an essential mechanism in the pathogenesis of CMML-related glomerulopathies. Apart from the influence of cytokines and growth factors, endothelial injury can result from an increased propensity for intracapillary erythrocyte sludging and platelet aggregation. This phenomenon could potentially explain the features of chronic thrombotic microangiopathy (TMA) observed in many cases.18,19
Other glomerulopathies associated with CMML include amyloid light chain amyloidosis, membranous nephropathy, infection-related-like glomerulopathy, crescentic glomerulonephritis, and even extramedullary (intracapillary) hematopoiesis.14,19,27, 28, 29 In a recent case series by Gipe et al,20 out of 14 patients with CMML and kidney involvement, 2 patients presented with nephrotic syndrome and had minimal change disease (MCD), 1 patient had incidental IgA nephropathy, 1 patient had resolving postinfectious glomerulonephritis, and 1 patient had unspecified MPN-related glomerulopathy. “Pseudocrescentic” glomerulonephritis was also described in association with CMML by Asano et al30 in a patient with extensive extracapillary mononuclear cell proliferation resembling cellular crescents. Further immunostaining however revealed that these cells were positive for myeloperoxidase, CD68, and lysozyme, indicating that they were in fact leukemic cells infiltrating the glomerulus in a pseudocrescentic fashion.
CMML-related glomerulopathies often coexist with monocytic tubulointerstitial infiltration or with lysozyme-induced nephropathy (LyN), and the combination of these leads to rapid kidney failure. The treatment of the glomerular disease is mostly focused on managing the underlying CMML, typically with hydroxyurea or chemotherapy. Immunosuppression may also play a role. Steroids have been tried without success, but mycophenolate mofetil at a dose of 1.5 g/d successfully lowered proteinuria from 27 g/d to 1 g/d in a case of membranous nephropathy.18,28 Cyclophosphamide and azathioprine were useful in controlling the disease in 1 report.14 Unfortunately, the prognosis of patients with glomerular involvement is guarded, and they often experience a progressive deterioration in kidney function despite therapy, eventually reaching kidney failure.29 Table 1 summarizes the cases of CMML-related glomerulopathies reported in the literature.31
Table 1.
Cases of CMML-Related Glomerulopathy Reported in the Literature
| Reference (Year) | No. of Patients With CMML | Clinical Presentation |
Glomerular Pathology on Biopsy | Concomitant Tubulointerstitial Findings | Therapies Instituted at Kidney Diagnosis | Change in Kidney Function After Therapy |
|---|---|---|---|---|---|---|
| Schwarze et al31 (1975) | N = 18 (postmortem kidney examination of autopsy cases of CMML) | N/A | Leukemic infiltration of glomeruli | Leukemic infiltration of cortex and medulla (N = 17), tubular atrophy, secondary uric acid diathesis, nephrocalcinosis, interstitial edema, tubular hyaline droplet change | N/A | N/A |
| Morschhauser et al27 (1995) | N = 45 | NS (N = 3), AKI (N = 1) |
AL amyloidosis (N = 1), extracapillary glomerulonephritis (N = 1) | - | VP16 and hydroxyurea | Improved in 2 patients, stable in 1 patient, and worsened in 1 patient |
| Enriquez et al28 (2008) | N = 1 | NS | Membranous glomerulopathy | - | Initially hydroxyurea or prednisone and cyclosporine then MMF | No response to hydroxyurea or prednisone or cyclosporine; MMF brought proteinuria down from 27 g/d to 1 g/d |
| Büttner-Herold et al19 (2021) | N = 4 | Hematuria (N = 2), Proteinuria (N = 4) (no cases of NS) | Endothelial damage (N = 4), mesangiolysis (N = 2), double contours (N = 4), podocytopathy >75% (N = 1), TMA (N = 1), IR-like GN (N = 1) | |||
| Belliere et al29 (2021) | N = 8 | FSGS (N = 2), extramedullary hematopoiesis (N = 3), monocyte infiltration (N = 1) | Decitabine, azacitidine, ruxolitinib | Improved in 1 patient, worsened in 2 patients, continued dialysis dependence in 1 patient | ||
| Asano et al30 (2021) | N = 1 | Subnephrotic proteinuria (2 g/g), microscopic hematuria with red cell casts, AKI | Extracapillary infiltration of CMML (diagnosed by immunostaining) | Tubulointerstitial nephritis with positive staining for MPO and CD68, positive staining for lysozyme in proximal tubule | Not discussed | Not discussed |
| Person et al21 (2021) | N = 18 (postmortem kidney findings) | N/A | Diffuse glomerulosclerosis (N = 2, both of which were post allo-HCT) | Extramedullary hematopoiesis, leukemic infiltration | N/A | N/A |
| Sun et al12 (2022) | N = 1 | AKI and NS. Proteinuria 15/d | MCD (complete foot process effacement of podocytes and no immune complex deposition) | Proximal tubular injury, focal mononuclear infiltration in the interstitium, positive lysozyme staining, and increased lysosomal granules in proximal tubular | ||
| Gipe et al20 (2023) | N = 14 | AKI, nephrotic syndrome with CKD, nonnephrotic range proteinuria with CKD | Focal global glomerulosclerosis (N = 3), FSGS (N = 2), glomerular ischemia (N = 1), acute post infectious glomerulonephritis (N = 1), minimal change disease (N = 1), myeloproliferative related neoplasm (MPN) related glomerulopathy (N = 1), IgA nephropathy (N = 1) |
Acute tubular injury, interstitial nephritis, arteriosclerosis, moderate to severe tubular atrophy and interstitial fibrosis, lysozyme-induced nephropathy, tubulointerstitial CMML infiltration | Supportive care, decitabine, hydroxyurea, and prednisone taper | Decrease in creatinine between 0.5 and 1.2 mg/dL (N = 2), 3 patients died |
Abbreviations: AKI, acute kidney injury; AL, amyloid light chain; AZA, azacytidine; CKD, chronic kidney disease; CMML, chronic myelomonocytic leukemia; DAC, decitabine; EMH, extramedullary hematopoiesis; HCT, hematopoietic cell transplant; IR-like GN, infection-related-like glomerulopathy; FSGS, focal segmental glomerulosclerosis; HU, hydroxyurea; MCD, minimal change disease; MMF, mycophenolate mofetil; N/A, not available; NS, nephrotic syndrome; PIGN, post infectious glomerulonephritis; TMA, thrombotic microangiopathy; VP16, etoposide.
Tubulointerstitial Disorders
The 3 main forms of tubulointerstitial disorders associated with CMML include lysozyme-induced nephropathy, hyperuricemia-induced kidney injury, and leukemic kidney infiltration.
Lysozyme-Induced Nephropathy
Until recently, the full clinicopathologic spectrum of lysozyme-induced nephropathy in CMML remained elusive. Lysozyme, also known as muramidase, is a small bactericidal enzyme produced mainly by monocytes and macrophages. Under physiologic conditions, lysozyme is freely filtered by the glomerulus because of its small size and cationic charge. It is then almost completely reabsorbed in the proximal tubule by endocytosis and later undergoes proteolysis and destruction in the endolysosomes.32 As a result of the kidney’s excellent reabsorptive capacity, the amount of lysozyme excreted in the urine is, in general, negligible under normal circumstances. However, in CMML, there is excessive production of lysozyme by monocytes, surpassing the proximal tubule’s absorptive ability and leading to the presence of excessive lysozyme in the urine. The presence of lysozyme can therefore be suspected when comparing urine albumin and urine total protein concentration, as lysozymuria is typically a nonalbumin proteinuria and manifests as a “cationic peak” on urine protein electrophoresis.33,34 Although it is widely accepted that high concentrations of lysozyme can damage the proximal tubular cells, ultimately leading to acute tubular necrosis and tubular atrophy with interstitial fibrosis (IFTA), the pathophysiology of tubular injury is not entirely clear.35, 36, 37 Some authors postulate that the tubular toxic effect is exerted through impaired endolysosomal function resulting in “amorphous aggregation” or “nonamyloid aggregation” of lysozyme. In a case series of patients with CMML, including 8 patients with CMML, IFTA was the main pathologic findings on kidney biopsy, present in 75% of cases.29
Kudose et al38 recently published the largest case series comprising 37 patients with LyN, among whom 15 were diagnosed with CMML. The kidney manifestations commonly observed with lysozymuria included AKI, chronic kidney disease (CKD), and subnephrotic range proteinuria, with a median estimated glomerular filtration rate (eGFR) of 21.7 mL/min per 1.73 m2 and median proteinuria of 1.7 g at the time of biopsy. Some patients also exhibited microscopic hematuria and partial Fanconi syndrome, consistent with previous reports.39 The hallmark finding on pathology was the presence of abundant periodic acid–Schiff (PAS)-positive hypereosinophilic intracytoplasmic inclusions, exhibiting a characteristic staining pattern with lysozyme immunohistochemical staining, in agreement with earlier case reports.36,40, 41, 42 Notably, these proximal tubular inclusions tested negative on silver methenamine Jones (JMS) stain, appeared fuchsinophilic with trichrome staining, and exhibited a pale pink hue with Congo red staining. Further characterization through electron microscopy revealed that these intracytoplasmic inclusions were membrane-bound vacuoles containing homogenous or granular electron-dense material, which likely represent autophagolysosomes.20 These degenerating cellular organellar debris were diffusely distributed in proximal tubular epithelia. Figure 4 demonstrates classical pathology findings in LyN.
Figure 4.
Lysozyme-induced nephropathy. (A) There is no significant glomerular abnormality, whereas the proximal tubules show enlargement because of accumulated protein droplets (periodic acid–Schiff stain, 200×). (B) Epithelial cells of the proximal tubules are engorged by protein reabsorption droplets (periodic acid–Schiff stain, 400×) (C) Round eosinophilic and refractile protein reabsorption droplets fill out the entire cell, with epithelial cell nuclei pushed to the basolateral aspect of the cell (hematoxylin and eosin stain, 400×). (D) Immunohistochemistry staining for lysozyme reveals strong reaction in the intratubular protein reabsorption granules (100×). Courtesy of Dr Vanesa Bijol and Dr Yihe Yang, Nephropathologists at Northwell Health.
When tubular dysfunction because of LyN is suspected, measurement of serum and urine lysozyme is often helpful in establishing the diagnosis. Serum lysozyme is available in most referral centers, and takes approximately 5 days to result. In fact, serum lysozyme measurements were shown to have a strong correlation with both absolute monocyte count and serum creatinine level.43 Consequently, some authors have advocated that an elevated serum lysozyme level in the context of evident proximal tubular impairment is sufficient to establish a diagnosis of LyN and may obviate the need for a kidney biopsy. This approach is particularly relevant for CMML patients who often have thrombocytopenia, rendering the risk of bleeding from a kidney biopsy unacceptable.44 However, one must be aware that LyN can still occur, although rarely, in patients with hematologically stable CMML with normal serum lysozyme levels, thus highlighting the importance of having a high index of suspicion for this condition when patients with CMML develop AKI as well as performing a kidney biopsy when the diagnosis remains unclear.45
Because there is no available pharmacologic therapy to specifically decrease lysozyme levels, the treatment of LyN is largely aimed at aggressive reduction of monocyte counts and usually consists of intensive cytoreduction with a combination of leukapheresis, chemotherapy, and hydroxyurea. Outcomes reported in the literature have been variable. Some patients experienced improvement in kidney function with therapy, whereas others had stabilization of kidney function.40,41,44,46,47 Unfortunately, therapy was ineffective in a number of patients, leading to continued dialysis dependence.36
Hyperuricemia-Induced Kidney Injury
Elevated uric acid levels in CMML can adversely affect kidney function in many ways. One of the potential consequences is the development of acute urate nephropathy, which occurs when serum uric acid levels sharply increase to >15 mg/dL.48 This phenomenon is mostly observed in cases of CMML transformation to acute myeloid leukemia (AML), accompanying a sharp increase in monocyte count.49 In these cases, AKI can be observed alongside hyperuricemia, but without major electrolyte abnormalities. On the other hand, tumor lysis syndrome (TLS) can also occur and is characterized by massive uric acid release into the systemic circulation by cancerous cells. According to the Cairo-Bishop classification, TLS is defined as the presence of 2 or more of the classic metabolic abnormalities (hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia) within 3 days before or 7 days after instituting chemotherapy in the setting of adequate hydration and use of a hypouricemic agent.50 Nonetheless, TLS has also been described after initiation of minimally cytotoxic therapy with hydroxyurea alone.51 Spontaneous TLS can also occur in the setting of secondary transformation of CMML to AML.52 The pathophysiology of kidney injury secondary to uric acid is complex. Traditionally, it was attributed to precipitation of uric acid within the distal tubules and collecting ducts.53,54 However, more recent research has proposed endothelial injury because of extracellular histone release as an important pathologic mechanism.55 The treatment of choice for urate nephropathy and TLS consists of acutely lowering uric acid levels with allopurinol, febuxostat, or rasburicase as well as promoting the elimination of uric acid crystals by increasing urine output with intravenous fluids and loop diuretics.56
Leukemic Kidney Infiltration
Kidney infiltration by monocytes can lead to a spectrum of consequential outcomes. In a study by Lieutaud et al57 the infiltration of CMML was observed to manifest as a hemorrhagic kidney and adrenal tumor. Other investigations have shown that leukemic cells can lead to direct and extensive invasion of the kidney interstitial space, resulting in kidney failure.58, 59, 60, 61 In these cases, kidney biopsy commonly shows histologically unremarkable glomeruli alongside acute tubulointerstitial nephritis characterized by an abundance of monocytic and reactive lymphoid cells with light microscopy. Although immunofluorescence microscopy (IF) generally yields negative results, immunohistochemical staining for CD14 frequently exposes the presence of numerous mature monocytes. Aggressive therapeutic intervention for CMML control is paramount, aimed at the restoration of kidney function.
Renovascular Disorders
Renovascular disorders in CMML include kidney injury related to vasculitis, kidney infarcts, and intrarenal leukostasis.
Kidney Injury Related to Systemic Vasculitis
Systemic vasculitis is the most frequent autoimmune disease described with CMML, affecting up to 32% of patients.62,63 Various types of vasculitis affecting the kidney have been described, such as polyarteritis nodosa (PAN), cryoglobulinemia, and granulomatosis with polyangiitis (GPA).15,29,64, 65, 66, 67 In the case series assessed by Hamidou et al,64 8 patients with CMML met the American College of Rheumatology criteria for PAN, out of whom 7 were antineutrophil cytoplasmic antibody (ANCA)-negative. All patients had kidney involvement manifested by proteinuria and hematuria, and 3 patients had kidney angiograms that showed diffuse intraparenchymatous microaneurysms. Interestingly, several authors have reported that these arterial aneurysms in PAN-like vasculitis associated with CMML can rupture and lead to spontaneous massive perirenal hemorrhage.15,64,66,68,69 Embolization or unilateral radical nephrectomy are usually required to control the hemorrhage.
Kidney Infarcts
Thromboembolic events, specifically kidney infarcts, are uncommon occurrences in CMML but have been rarely described. Yen et al70 described the case of an older female with CMML blast crisis who experienced a sudden occurrence of extensive bilateral infarction of the kidneys, leading to oliguric kidney failure and eventually mortality. Similarly, Chadachan et al71 described a similar case of a patient with CMML who had a primary aortic thrombus resulting in multiple visceral embolization, including infarcts of the kidneys. In both cases, the infarcts were attributed to a hypercoagulable state secondary to CMML.
Intrarenal Leukostasis
Hyperleukocytosis and leukostasis syndrome can be a severe complication leading to end-organ damage in patients with CMML who experience a rapid increase in WBC counts.72,73 The pathophysiologic mechanism involves plugging of the capillary vasculature with immobile monocytes, which adhere to vascular endothelium.74 Leukostasis has been associated with AKI and new-onset CKD.8 Its prognosis remains poor despite treatment with cytoreductive therapies and leukapheresis.75
Urinary tract involvement
Upper Urinary Tract Involvement
Every part of the urinary tract may be affected by CMML (Fig 5). When CMML involves the upper urinary tract, particularly the pelvic kidney and ureter, it can lead to uncontrollable hematuria, which often does not resolve with conservative management. In such challenging situations, nephrectomy becomes necessary to effectively control bleeding, as demonstrated in studies by Hyams et al76 and Bane et al.77 Pathological examination of involved kidney specimens in these cases showed infiltration of the suburothelial connective tissue of the pelvic kidney and ureter by primitive myeloid cells. Another noteworthy and unusual finding in these reports was the presence of foci of extramedullary hematopoiesis within the kidney parenchyma. Additionally, another mechanism of upper urinary tract obstruction associated with CMML involves the formation of massive uric acid nephrolithiasis, resulting in bilateral hydronephrosis and progressive kidney failure, specifically in the context of TLS.28,78 Ureteral stenting is therefore needed to relieve the obstruction and restore kidney function. Finally, retroperitoneal fibrosis encasing the kidneys has been also reported with CMML.62,79,80
Figure 5.
Urinary tract involvement in chronic myelomonocytic leukemia (CMML). CMML can lead to obstruction in either the upper or lower urinary tract. Upper urinary tract involvement can result from the infiltration of the pelvic kidney and ureter, uric acid nephrolithiasis, or retroperitoneal fibrosis. Lower urinary tract involvement is typically due to bladder outlet obstruction or prostate infiltration.
Lower Urinary Tract Involvement
Bladder outlet obstruction causing postobstructive AKI has also been described in patients with CMML. In an interesting case report by Hope-Gill et al,81 transurethral resection of the prostate was performed on a patient with CMML and urinary retention. Histologic examination of the prostate revealed dense infiltration by myelomonocytes, which was unexpected given the patient’s low peripheral blood monocyte count.
Nephrotoxicity Associated With Select Therapies in CMML
Hypomethylating Agents
Hypomethylating agents (HMA), such as azacitidine or decitabine, are often prescribed to CMML patients who require therapy because of disease progression or complications. HMAs can be given both to allogeneic stem cell transplant eligible and ineligible patients.82 High-dose azacitidine (200 mg/m2/d) has been shown to be toxic to both proximal and distal renal tubules, affecting up to 70% of patients. This toxicity can lead to consequences such as renal tubular acidosis and kidney wasting of phosphate, calcium, magnesium, potassium, sodium, glucose, and amino acids, ultimately resulting in kidney failure.83,84 Salt wasting may cause orthostatic hypotension, and polyuria can occur, usually starting about 4 days after the treatment’s initiation and occasionally persisting despite drug withdrawal.85 However, kidney injury and complications in current standard practice with 50-75 mg/m2/d azacitidine are rare. Decitabine has been rarely reported to induce kidney thrombotic microangiopathy with glomerular crescent formation and tubular necrosis, but further studies are needed to confirm this association.86 According to a study conducted by Otiker et al,87 patients who were treated with AZA had more severe AKI compared with those who received decitabine. Therapy for patients experiencing nephrotoxicity from AZA or decitabine is mostly supportive with drug discontinuation and judicious electrolyte replacement.88
Ruxolitinib
There have been case reports and studies suggesting potential nephrotoxicity associated with ruxolitinib, a selective inhibitor of Janus kinase 1 and 2 (JAK1 and JAK2) used off-label in the treatment of symptomatic CMML. However, it is important to consider that these reports are relatively limited compared with the widespread use of ruxolitinib. Despite several claims of ruxolitinib-induced nephrotoxicity in the US Food and Drug Administration Adverse Events Reporting System (FAERS) database, Strohbehn et al89 demonstrated that unexplained AKI was infrequent with ruxolitinib, and other factors could have contributed to the decline in kidney function.90
Acid-Base and Electrolyte Disorders Associated With CMML and its Therapy
Various acid-base and electrolyte disorders have been associated with CMML or its treatment. First, hyponatremia is common in hematologic malignancies and can be due to effective arterial volume contraction (from poor oral intake, emesis, diarrhea, etc.) or syndrome of inappropriate antidiuretic hormone secretion.91 TLS, whether spontaneous or induced by therapy, can lead to the well-known combination of hyperkalemia, hyperphosphatemia, and hypocalcemia in addition to hyperuricemia.92 The highest risk is during the initiation of chemotherapy.93 TLS is considered an oncologic emergency as its consequences include cardiac arrhythmia, seizures, and even death.94 The treatment of choice for TLS, as discussed above, consists of hydration and administration of urate-lowering agents.56,95 In addition to TLS, LyN can also lead to severe metabolic derangements resulting from tubular toxicity and subsequent renal tubular acidosis. Increased kaliuresis and urinary excretion of magnesium and phosphorus occurs, resulting in profound hypokalemia, hypomagnesemia and hypophosphatemia.40,96, 97, 98, 99 The severe electrolyte abnormalities noted in LyN frequently necessitate intravenous (IV) electrolyte repletion.
Gardner et al100 described a case of type B lactic acidosis associated with CMML. The proposed mechanism is a shift from oxidative metabolism to glycolysis by leukemic cells even at normal oxygen concentrations. Continuous veno-venous hemofiltration failed to clear lactic acidemia; however, urgent initiation of chemotherapy lowered lactate levels successfully within 8 hours.
CMML therapies also cause electrolyte abnormalities. For example, sorafenib-induced profound hypocalcemia has been reported by Gupta et al.101 Hypocalcemia was attributed to diarrhea and vitamin D malabsorption from exocrine pancreatic dysfunction induced by sorafenib. Normalizing serum calcium levels was particularly challenging and required IV calcium drip and high doses of IV calcitriol. Finally, azacytidine can induce hyponatremia, hypophosphatemia and hypokalemia. Potassium depletion can persist after stopping the drug and require prolonged parenteral supplementation.102
Conclusion
Kidney involvement in CMML is a frequent occurrence that is characterized by a multifactorial pathogenesis involving several mechanisms concomitantly. Given the complexity of these mechanisms, nephrologists must exercise heightened awareness and maintain a low threshold for kidney biopsy. The significance of kidney biopsy lies in its pivotal role in identifying the specific etiology of kidney dysfunction, thereby enabling targeted treatment interventions. Although the primary focus of management centers around addressing the underlying malignancy, it is imperative to recognize that kidney response may not always follow. Hence, there is a pressing need for future research endeavors to elucidate and target the kidney manifestations of CMML specifically, with the ultimate goal of augmenting overall prognosis and therapeutic outcomes.
Article Information
Authors’ Full Names and Academic Degrees
Rose Mary Attieh, MD, Farhana Begum, MD, David Chitty, DO, Hassan Izzedine, MD, PhD, and Kenar D Jhaveri, MD.
Support
None.
Financial Disclosure
Rose Mary Attieh is the Galdi fellow in glomerular diseases and onconephrology at Northwell with grant support from Greg and Linda Galdi. Kenar D. Jhaveri reports consultancy agreements with PMV Pharmaceuticals, Calliditas, ChemoCentryx, GlaxoSmithKline, Secretome, and George Clinicals and honoraria from the American Society of Nephrology; is a paid contributor to UpToDate.com and Micromedex; and is the onconephrology section editor for Nephrology Dialysis Transplantation. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received September 2, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form September 21, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Valent P., Orazi A., Savona M.R., et al. Proposed diagnostic criteria for classical chronic myelomonocytic leukemia (CMML), CMML variants and pre-CMML conditions. Haematologica. 2019;104(10):1935–1949. doi: 10.3324/haematol.2019.222059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber D.A., Orazi A., Hasserjian R.P., et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patnaik M.M., Tefferi A. Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management. Am J Hematol. 2018;93(6):824–840. doi: 10.1002/ajh.25104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elena C., Gallì A., Such E., et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016;128(10):1408–1417. doi: 10.1182/blood-2016-05-714030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomo L., Garcia O., Arnan M., et al. Targeted deep sequencing improves outcome stratification in chronic myelomonocytic leukemia with low risk cytogenetic features. Oncotarget. 2016;7(35):57021–57035. doi: 10.18632/oncotarget.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arber D.A., Orazi A., Hasserjian R., et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 7.Patnaik M.M., Tefferi A. Chronic myelomonocytic leukemia: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(3):352–372. doi: 10.1002/ajh.26455. [DOI] [PubMed] [Google Scholar]
- 8.Hunter A.M., Al Ali N., Mai A., et al. Leukocytosis is associated with end organ damage and mortality in chronic myelomonocytic leukemia and can be mitigated by cytoreductive therapy. Leuk Res. 2021;109 doi: 10.1016/j.leukres.2021.106640. [DOI] [PubMed] [Google Scholar]
- 9.Strati P., Abdelrahim M., Selamet U., et al. Ruxolitinib therapy is associated with improved renal function in patients with primary myelofibrosis. Ann Hematol. 2019;98(7):1611–1616. doi: 10.1007/s00277-019-03708-9. [DOI] [PubMed] [Google Scholar]
- 10.Heschl J., Geissler K. Significance of reduced renal function in patients with chronic myelomonocytic leukemia. Wien Med Wochenschr. 2023;173(1-2):3–8. doi: 10.1007/s10354-022-00977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustos C., González E., Muley R., Alonso J.L., Egido J. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur J Clin Invest. 1994;24(12):799–805. doi: 10.1111/j.1365-2362.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 12.Sun C.Y., Lin C.C., Liao I.C., Hsu Y.T., Chang Y.T. Acute kidney injury in a man with chronic myelomonocytic leukemia. J Nephrol. 2022;35(4):1303–1304. doi: 10.1007/s40620-021-01157-0. [DOI] [PubMed] [Google Scholar]
- 13.Saif M.W., Hopkins J.L., Gore S.D. Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2002;43(11):2083–2092. doi: 10.1080/1042819021000016186. [DOI] [PubMed] [Google Scholar]
- 14.Enright H., Jacob H.S., Vercellotti G., Howe R., Belzer M., Miller W. Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol. 1995;91(2):403–408. doi: 10.1111/j.1365-2141.1995.tb05310.x. [DOI] [PubMed] [Google Scholar]
- 15.Aslangul-Castier E., Papo T., Amoura Z., et al. Systemic vasculitis with bilateral perirenal haemorrhage in chronic myelomonocytic leukaemia. Ann Rheum Dis. 2000;59(5):390–393. doi: 10.1136/ard.59.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saitoh T., Murakami H., Uchiumi H., et al. Myelodysplastic syndromes with nephrotic syndrome. Am J Hematol. 1999;60(3):200–204. doi: 10.1002/(sici)1096-8652(199903)60:3<200::aid-ajh6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Au W.Y., Chan K.W., Lui S.L., Lam C.C., Kwong Y.L. Focal segmental glomerulosclerosis and mesangial sclerosis associated with myeloproliferative disorders. Am J Kidney Dis. 1999;34(5):889–893. doi: 10.1016/S0272-6386(99)70047-8. [DOI] [PubMed] [Google Scholar]
- 18.Said S.M., Leung N., Sethi S., et al. Myeloproliferative neoplasms cause glomerulopathy. Kidney Int. 2011;80(7):753–759. doi: 10.1038/ki.2011.147. [DOI] [PubMed] [Google Scholar]
- 19.Büttner-Herold M., Sticht C., Wiech T., Porubsky S. Renal disease associated with myeloproliferative neoplasms and myelodysplastic syndrome/myeloproliferative neoplasms. Histopathology. 2021;78(5):738–748. doi: 10.1111/his.14282. [DOI] [PubMed] [Google Scholar]
- 20.Gipe N., Leung N., Lasho T., et al. Spectrum of renal pathological findings in patients with chronic myelomonocytic leukemia and kidney injury. Am J Hematol. 2023;98(6):E148–E153. doi: 10.1002/ajh.26902. [DOI] [PubMed] [Google Scholar]
- 21.Person F., Meyer S.C., Hopfer H., Menter T. Renal post-mortem findings in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Virchows Arch. 2021;479(5):1013–1020. doi: 10.1007/s00428-021-03129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardy A., Tiple A., Rabant M., et al. The myeloproliferative neoplasms-related glomerulopathy. Rev Med Interne. 2014;35(4):222–230. doi: 10.1016/j.revmed.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Floege J., Topley N., Resch K. Regulation of mesangial cell proliferation. Am J Kidney Dis. 1991;17(6):673–676. doi: 10.1016/s0272-6386(12)80349-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R.J., Raines E.W., Floege J., et al. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenaux P., Kiladjian J.J., Platzbecker U. Luspatercept for the treatment of anemia in myelodysplastic syndromes and primary myelofibrosis. Blood. 2019;133(8):790–794. doi: 10.1182/blood-2018-11-876888. [DOI] [PubMed] [Google Scholar]
- 26.Fenaux P., Platzbecker U., Mufti G.J., et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med. 2020;382(2):140–151. doi: 10.1056/NEJMoa1908892. [DOI] [PubMed] [Google Scholar]
- 27.Morschhauser F., Wattel E., Pagniez D., et al. Glomerular injury in chronic myelomonocytic leukemia. Leuk Lymphoma. 1995;18(5-6):479–483. doi: 10.3109/10428199509059648. [DOI] [PubMed] [Google Scholar]
- 28.Enriquez R., Sirvent A.E., Marin F., Perez M., Alpera M.R., Amorós F. Severe renal complications in chronic myelomonocytic leukemia. J Nephrol. 2008;21(4):609–614. [PubMed] [Google Scholar]
- 29.Belliere J., Colombat M., Kounde C., et al. Kidney involvement in patients with chronic myelomonocytic leukemia or BCR-ABL-negative myeloproliferative neoplasms. Kidney Int Rep. 2021;6(3):737–745. doi: 10.1016/j.ekir.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano M., Hase H., Naruse Y., et al. A rare cause of acute kidney injury with chronic myelomonocytic leukemia. CEN Case Rep. 2021;10(3):320–325. doi: 10.1007/s13730-020-00567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarze E.W. Pathoanatomical features of the kidney in myelomonocytic and chronic lymphocytic leukemia. Virchows Arch A Pathol Anat Histol. 1975;368(3):243–251. doi: 10.1007/BF00432526. [DOI] [PubMed] [Google Scholar]
- 32.Christensen E.I., Maunsbach A.B. Intralysosomal digestion of lysozyme in renal proximal tubule cells. Kidney Int. 1974;6(6):396–407. doi: 10.1038/ki.1974.125. [DOI] [PubMed] [Google Scholar]
- 33.Osserman E.F., Lawlor D.P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruzanski W., Platts M.E. Serum and urinary proteins, lysozyme (muramidase), and renal dysfunction in mono- and myelomonocytic leukemia. J Clin Invest. 1970;49(9):1694–1708. doi: 10.1172/JCI106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillen J.M., Raemaekers J.M., Steenbergen E.J., Wetzels J.F.M., Verhave J.C. Progressive kidney failure in chronic myelomonocytic leukaemia: don’t forget lysozyme damage. Neth J Med. 2018;76(9):407–410. [PubMed] [Google Scholar]
- 36.Santoriello D., Andal L.M., Cox R., D’Agati V.D., Markowitz G.S. Lysozyme-induced nephropathy. Kidney Int Rep. 2017;2(1):84–88. doi: 10.1016/j.ekir.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klockars M., Azar H.A., Hermida R., et al. The relationship of lysozyme to the nephropathy in chloroleukemic rats and the effects of lysozyme loading on normal rat kidneys. Cancer Res. 1974;34(1):47–60. [PubMed] [Google Scholar]
- 38.Kudose S., Cossey L.N., Canetta P.A., et al. Clinicopathologic spectrum of lysozyme-associated nephropathy. Kidney Int Rep. 2023;8(8):1585–1595. doi: 10.1016/j.ekir.2023.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamadou I., Buob D., Rabant M., et al. The case: acute kidney injury associated with chronic myelomonocytic leukemia. Kidney Int. 2021;99(2):495–496. doi: 10.1016/j.kint.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Robinet-Zimmermann G., Rioux-Leclercq N., Frouget T., Le Naoures C. Lysozyme-induced nephropathy: a rare manifestation of chronic myelomonocytic leukaemia. Ann Pathol. 2020;40(6):478–482. doi: 10.1016/j.annpat.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Maraj A., MacEneaney O., Doyle B., Quinn J. Lysozyme-induced nephropathy: a rare manifestation of chronic myelomonocytic leukaemia. Br J Haematol. 2020;189(3):393. doi: 10.1111/bjh.16455. [DOI] [PubMed] [Google Scholar]
- 42.Patel T.V., Rennke H.G., Sloan J.M., DeAngelo D.J., Charytan D.M. A forgotten cause of kidney injury in chronic myelomonocytic leukemia. Am J Kidney Dis. 2009;54(1):159–164. doi: 10.1053/j.ajkd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel A.B., Miles R.R., Deininger M.W. Lysozyme nephropathy in chronic myelomonocytic leukemia. Clin Case Rep. 2019;7(6):1263–1264. doi: 10.1002/ccr3.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulo N., Baptista P., Nogueira F., Pereira C., Cerqueira A., Rocha A. Lysozyme-induced nephropathy: a diagnosis not to forget. Cureus. 2023;15(1) doi: 10.7759/cureus.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh T.L., Carpenter L., Ly E. Lysozyme nephropathy in haematologically stable chronic myelomonocytic leukaemia. Nephrology. 2018;23(4):377. doi: 10.1111/nep.13056. [DOI] [PubMed] [Google Scholar]
- 46.Borges T., Rêgo I., Badas J., et al. Chronic myelomonocytic leukaemia: a presentation with rare extramedullary involvement. Port J Nephrol Hypert. 2016;30(2) [Google Scholar]
- 47.Donati A., Luciano R., Shirali A. Lysozyme nephropathy: a rare and reversible cause of acute kidney injury in chronic myelomonocytic leukemia. J Onco-Nephrol. 2021;5(2):120–121. [Google Scholar]
- 48.Fathallah-Shaykh S.A., Cramer M.T. Uric acid and the kidney. Pediatr Nephrol. 2014;29(6):999–1008. doi: 10.1007/s00467-013-2549-x. [DOI] [PubMed] [Google Scholar]
- 49.DeBoer R., Garrahy I., Rettew A., Libera R. Transformation of CMML to AML presenting with acute kidney injury. J Community Hosp Intern Med Perspect. 2020;10(4):353–357. doi: 10.1080/20009666.2020.1774271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cairo M.S., Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 51.Otrock Z.K., Taher A.T., Mahfouz R.A.R., Makarem J.A., Shamseddine A.I. Acute tumor lysis syndrome secondary to hydroxycarbamide in chronic myelomonocytic leukemia. Am J Hematol. 2006;81(3):220–221. doi: 10.1002/ajh.20497. [DOI] [PubMed] [Google Scholar]
- 52.Langridge A., Musgrave K., Upadhye Y. Spontaneous tumour lysis syndrome secondary to the transformation of chronic myelomonocytic leukaemia into acute myeloid leukaemia. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conger J.D., Falk S.A., Guggenheim S.J., Burke T.J. A micropuncture study of the early phase of acute urate nephropathy. J Clin Invest. 1976;58(3):681–689. doi: 10.1172/JCI108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanwar Y.S., Manaligod J.R. Leukemic urate nephropathy. Arch Pathol. 1975;99(9):467–472. [PubMed] [Google Scholar]
- 55.Arnaud M., Loiselle M., Vaganay C., et al. Tumor lysis syndrome and AKI: beyond crystal mechanisms. J Am Soc Nephrol. 2022;33(6):1154–1171. doi: 10.1681/ASN.2021070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coiffier B., Altman A., Pui C.H., Younes A., Cairo M.S. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 57.Lieutaud T., Mejean A., Prayssac P., et al. Renal and adrenal gland localization of chronic myelomonocytic leukemia presenting as a kidney tumor. Leuk Lymphoma. 1999;34(3-4):405–408. doi: 10.3109/10428199909050967. [DOI] [PubMed] [Google Scholar]
- 58.Robinson G.T., Sundaram K.R., Dilly S.A., Bevan D.H., Andrews P.A. Renal failure in a patient with chronic myelomonocytic leukaemia. Nephrol Dial Transplant. 1997;12(7):1500–1502. doi: 10.1093/ndt/12.7.1500. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi K., Yokote T., Tsuji M., Takubo T., Inoue T., Hanafusa T. Renal infiltration associated with chronic myelomonocytic leukaemia. Br J Haematol. 2009;147(4):414. doi: 10.1111/j.1365-2141.2009.07785.x. [DOI] [PubMed] [Google Scholar]
- 60.Spapen J., Fostier K., De Raeve H., Janssens P., Spapen H. An unexpected complication of chronic myelomonocytic leukemia: severe renal failure due to malignant tubulo-interstitial cell infiltration. Int J Nephrol Renovasc Dis. 2016;9:1–4. doi: 10.2147/IJNRD.S98528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robins J., Roncolato F., Katz I. Case report: uric acid nephropathy and myelomonocytic neoplastic renal infiltration in a patient with chronic myelomonocytic leukaemia and chronic kidney disease. Nephrology. 2010:1576. [Google Scholar]
- 62.Grignano E., Mekinian A., Braun T., et al. Autoimmune and inflammatory diseases associated with chronic myelomonocytic leukemia: a series of 26 cases and literature review. Leuk Res. 2016;47:136–141. doi: 10.1016/j.leukres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Mekinian A., Grignano E., Braun T., et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology. 2016;55(2):291–300. doi: 10.1093/rheumatology/kev294. [DOI] [PubMed] [Google Scholar]
- 64.Hamidou M.A., Boumalassa A., Larroche C., El Kouri D., Blétry O., Grolleau J.Y. Systemic medium-sized vessel vasculitis associated with chronic myelomonocytic leukemia. Semin Arthritis Rheum. 2001;31(2):119–126. doi: 10.1053/sarh.2001.27717. [DOI] [PubMed] [Google Scholar]
- 65.Fain O., Guillevin L., Kaplan G., et al. Vasculitis and neoplasms. 14 cases. Ann Med Interne (Paris) 1991;142(7):486–504. [PubMed] [Google Scholar]
- 66.Stergiou I.E., Christoforou P., Sypsa G., et al. Extraordinary extrahaematological manifestations of chronic myelomonocytic leukaemia. Lancet. 2020;396(10254):853. doi: 10.1016/S0140-6736(20)31905-X. [DOI] [PubMed] [Google Scholar]
- 67.Smail A., Ducroix J.P., Sahir R., et al. Wegener’s disease and myelomonocytic leukemia: a fortuitous association? Rev Med Interne. 1989;10(5):463–465. doi: 10.1016/s0248-8663(89)80055-4. [DOI] [PubMed] [Google Scholar]
- 68.Brickner L.A., Scannell K.A. Medium-vessel PAN-type vasculitis associated with myelodysplastic syndrome and presenting as bilateral perinephric hematomas. Clin Exp Rheumatol. 1997;15(2):221–222. [PubMed] [Google Scholar]
- 69.Georgiou C., Krokidis M., Elworthy N., Dimopoulos S. Spontaneous bilateral renal aneurysm rupture secondary to polyarteritis nodosa in a patient with chronic myelomonocytic leukaemia: a case report study. Int J Surg Case Rep. 2016;26:61–64. doi: 10.1016/j.ijscr.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yen T.H., Chang C.T., Ng K.K., Wu M.S. Bilateral renal infarction in chronic myelomonocytic leukemia on blast crisis. Ren Fail. 2003;25(6):1029–1035. doi: 10.1081/jdi-120026038. [DOI] [PubMed] [Google Scholar]
- 71.Primary aortic thrombus with multiple visceral embolisation: a presenting manifestation of chronic myelomonocytic leukemia. ISTH Congress Abstracts. https://abstracts.isth.org/abstract/primary-aortic-thrombus-with-multiple-visceral-embolisation-a-presenting-manifestation-of-chronic-myelomonocytic-leukemia/
- 72.Azoulay E. Springer Science & Business Media; 2011. Pulmonary Involvement in Patients with Hematological Malignancies. [Google Scholar]
- 73.McCormick J., III, Henderson S.O. Leukemic hyperleukocytosis-induced unstable angina and congestive heart failure. Am J Emerg Med. 1999;17(2):217–219. doi: 10.1016/s0735-6757(99)90073-6. [DOI] [PubMed] [Google Scholar]
- 74.Stucki A., Rivier A.S., Gikic M., Monai N., Schapira M., Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97(7):2121–2129. doi: 10.1182/blood.v97.7.2121. [DOI] [PubMed] [Google Scholar]
- 75.Stemmler J., Wittmann G.W., Hacker U., Heinemann V. Leukapheresis in chronic myelomonocytic leukemia with leukostasis syndrome: elevated serum lactate levels as an early sign of microcirculation failure. Leuk Lymphoma. 2002;43(7):1427–1430. doi: 10.1080/1042819022386671. [DOI] [PubMed] [Google Scholar]
- 76.Hyams E.S., Gupta R., Melamed J., Taneja S.S., Shah O. Renal involvement by chronic myelomonocytic leukemia requiring nephroureterectomy. Rev Urol. 2009;11(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- 77.Bane A.L., Enright H., Sweeney E.C. Chronic myelomonocytic leukemia revealed by uncontrollable hematuria. Arch Pathol Lab Med. 2001;125(5):657–659. doi: 10.5858/2001-125-0657-CMLRBU. [DOI] [PubMed] [Google Scholar]
- 78.Trachsler J., Gaspert A., Previsdomini M., Wüthrich R.P., Fehr T. Massive uric acid nephrolithiasis with progressive renal failure due to spontaneous tumour lysis syndrome. NDT Plus. 2008;1(5):307–309. doi: 10.1093/ndtplus/sfn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Embase. https://www.embase.com/records?subaction=viewrecord&rid=3&page=1&id=L612112379
- 80.Ricard L., Abisror N., Droin N., et al. Retroperitoneal fibrosis as extramedullary hematopoiesis of a chronic myelomonocytic leukemia. Leuk Lymphoma. 2018;59(10):2503–2505. doi: 10.1080/10428194.2018.1427857. [DOI] [PubMed] [Google Scholar]
- 81.Hope-Gill B., Goepel J.R., Collin R.C. Obstructive uropathy associated with myelomonocytic infiltration of the prostate. J Clin Pathol. 1998;51(4):340–342. doi: 10.1136/jcp.51.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patnaik M.M., Tefferi A. Chronic myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95(1):97–115. doi: 10.1002/ajh.25684. [DOI] [PubMed] [Google Scholar]
- 83.Kintzel P.E. Anticancer drug-induced kidney disorders. Drug Saf. 2001;24(1):19–38. doi: 10.2165/00002018-200124010-00003. [DOI] [PubMed] [Google Scholar]
- 84.Peterson B.A., Collins A.J., Vogelzang N.J., Bloomfield C.D. 5-Azacytidine and renal tubular dysfunction. Blood. 1981;57(1):182–185. [PubMed] [Google Scholar]
- 85.Liu S., Tan J. Epigenetic Regulation in Overcoming Chemoresistance. Elsevier; 2021. DNA methyltransferase inhibitors (DNMTis) as sensitizing agents to overcome chemoresistance; pp. 9–23. [Google Scholar]
- 86.Qin A.B., Tan Y., Su T. Decitabine-induced kidney thrombotic microangiopathy with glomerular crescents formation and tubular necrosis: a case report. Medicine. 2020;99(43) doi: 10.1097/MD.0000000000022901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Otiker H., Rivero G., Sidebottom A., Hwang G. Is renal function equally impacted by hypomethylating agents during acute myelogenous leukemia induction therapy? Blood. 2022;140(suppl 1):3297–3298. [Google Scholar]
- 88.Sahni V., Choudhury D., Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol. 2009;5(8):450–462. doi: 10.1038/nrneph.2009.97. [DOI] [PubMed] [Google Scholar]
- 89.Strohbehn S., Seethapathy H., Rusibamayila N., et al. Acute kidney injury after ruxolitinib: common complication, uncommon cause. Am J Hematol. 2020;95(7):E181–E183. doi: 10.1002/ajh.25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qlik sense. US Food and Drug Administration https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis
- 91.Luciano R.L., Brewster U.C. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;21(1):27–35. doi: 10.1053/j.ackd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Lupușoru G., Ailincăi I., Frățilă G., et al. Tumor lysis syndrome: an endless challenge in onco-nephrology. Biomedicines. 2022;10(5) doi: 10.3390/biomedicines10051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howard S.C., Jones D.P., Pui C.H. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abu-Alfa A.K., Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55(5 suppl 3):S1–S13. doi: 10.1053/j.ajkd.2009.10.056. ;quiz S14-S19. [DOI] [PubMed] [Google Scholar]
- 95.Belay Y., Yirdaw K., Enawgaw B. Tumor lysis syndrome in patients with hematological malignancies. J Oncol. 2017;2017 doi: 10.1155/2017/9684909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pérez-Pinzón J., Olin R.L., Banerjee R. Severe electrolyte derangements from lysozymuria in acute myeloid leukemia. EJHaem. 2022;3(3):1018–1020. doi: 10.1002/jha2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans J.J., Bozdech M.J. Hypokalemia in nonblastic chronic myelogenous leukemia. Arch Intern Med. 1981;141(6):786–787. [PubMed] [Google Scholar]
- 98.Perazella M.A., Eisen R.N., Frederick W.G., Brown E. Renal failure and severe hypokalemia associated with acute myelomonocytic leukemia. Am J Kidney Dis. 1993;22(3):462–467. doi: 10.1016/s0272-6386(12)70154-3. [DOI] [PubMed] [Google Scholar]
- 99.Muggia F.M., Heinemann H.O., Farhangi M., Osserman E.F. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am J Med. 1969;47(3):351–366. doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 100.Gardner A.J., Griffiths J. A case of type B lactic acidosis as a complication of chronic myelomonocytic leukaemia: a case report and review of the literature. J Med Case Rep. 2015;9(1):16. doi: 10.1186/1752-1947-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Embase. https://www.embase.com/records?subaction=viewrecord&rid=1&page=1&id=L633699867
- 102.Verzicco I., Regolisti G., Quaini F., et al. Electrolyte disorders induced by antineoplastic drugs. Front Oncol. 2020;10:779. doi: 10.3389/fonc.2020.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]