Abstract
Immunization of mice with DNA vaccines encoding the full-length form and C and N termini of Plasmodium yoelii merozoite surface protein 1 provided partial protection against sporozoite challenge and resulted in boosting of antibody titers after challenge. In C57BL/6 mice, two DNA vaccines provided protection comparable to that of recombinant protein consisting of the C terminus in Freund’s adjuvant.
Merozoite surface protein 1 (MSP-1) has been the focus of intense efforts to develop malaria blood-stage vaccines (6). A large body of evidence from in vitro studies with Plasmodium falciparum and from challenge studies in murine and primate models indicate that MSP-1 can be a target of protective immune responses, suggesting that a vaccine against MSP-1 could protect humans against malaria (2, 3, 5, 10, 13, 14, 16, 20). Here we report that immunization of mice with DNA vaccines encoding Plasmodium yoelii MSP-1 (PyMSP-1) provides protective immunity against sporozoite challenge, that the protection is comparable to that achieved by a recombinant-protein–adjuvant formulation of the same antigen, and that immunized mice exhibit boosting of antibody responses after infection.
Construction of PyMSP-1 DNA vaccines.
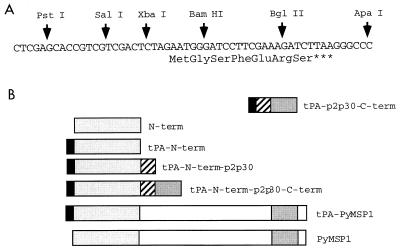
The VR1012 vector (11) was modified by insertion of a new polylinker (Fig. 1) to accept minigene cassettes and also to permit addition of the signal sequence of human tissue plasminogen activator (tPA) from VR1020 (17). Another vector, VR1012 tPA p2p30, which contains the p2 and p30 T-helper epitopes of tetanus toxin, was constructed (18, 23). Full-length and partial PyMSP-1 gene fragments were amplified from P. yoelii genomic DNA (strain 17XNL; nonlethal) and cloned into the minigene vectors (Fig. 1). The fragments corresponding to the N and C termini encoded amino acids 1 to 466 and 1659 to 1757 of PyMSP-1 (15), respectively. The constructs were verified by sequence analysis. Plasmid DNAs were prepared with CsCl gradients (7). To verify that the constructs were able to express antigen, UM449 cells were transfected with plasmid DNA, and 24 h later an indirect fluorescent-antibody test (IFAT) with monoclonal antibody 302 (1) or a polyclonal serum against a recombinant protein consisting of the PyMSP-1 C terminus (3, 24) was used to detect antigen expression.
FIG. 1.
DNA vaccine constructs used in this study. (A) Sequence of the minigene polylinker used to replace the original polylinker in the VR1012 vector. (B) Schematic representation of PyMSP-1 DNA vaccine constructs encoding the C terminus, the N terminus, and full-length PyMSP-1. These inserts were cloned into the minigene vectors derived from VR1012, as described in the text. The human tPA signal sequence (black boxes) and p2p30 T-helper epitopes (hatched boxes) are indicated.
Immunization and challenge regimen.
The experiments reported here were conducted in accordance with reference 13a. Female 6- to 8-week-old BALB/c and C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine) were injected intramuscularly with 50 μl of plasmid DNA (1 mg/ml) in saline in each tibialis anterior muscle with a 0.3-ml insulin syringe and 29½ gauge needle (Becton Dickinson no. 329431). Plasmid mixtures contained 100 μg of each plasmid. Positive control C57BL/6 mice received subcutaneous injections of one dose of 50 μg of recombinant protein consisting of the MSP-1 C terminus (produced in Escherichia coli) in complete Freund’s adjuvant, followed by two doses of MSP-19 in incomplete Freund’s adjuvant (22). Mice were challenged 2 weeks after the third immunization by intravenous injection of 50 P. yoelii sporozoites (17XNL). Geometric mean parasitemias were calculated and graphed. The repeated-measures analysis of variance (ANOVA) was used to determine whether groups differed from one another. Differences in group means on each day were calculated by one-way ANOVA and by the nonparametric Kruskal-Wallis test. ANOVA and Kruskal-Wallis outcomes were equivalent. Multiple-comparison post hoc analyses subsequent to ANOVA were done with Tukey’s honestly significant difference test in order to identify the groups that differed. Sera were collected 4 days before challenge. Antibody titers in pooled sera were measured by IFAT against air-dried P. yoelii-infected erythrocytes.
Immunogenicity and protection against sporozoites in BALB/c mice.
All of the PyMSP-1 DNA vaccines were immunogenic in BALB/c mice (Table 1). Interestingly, the antibody response to constructs encoding full-length PyMSP-1 and the PyMSP-1 N terminus was higher without the tPA signal peptide. Fusion of the PyMSP-1 N terminus to the C terminus resulted in a twofold increase in antibody titer over that obtained with C terminus alone (1:640 and 1:320, respectively). The highest antibody titer (1:1,280) was in mice immunized with the mixtures of the tPA–p2p30–N-term and tPA–p2p30–C-term constructs. Thus, the mixture of N- and C-terminus-encoding plasmids induced titers (1:1,280) of antibody to the whole parasite fourfold greater than was achieved by immunization with the C-terminus sequence alone (1:320). Addition of N-terminus-encoding sequence to the C-terminus-encoding construct, either as a gene fusion on a single plasmid or as two plasmids mixed together, improved antibody responses after intramuscular immunization.
TABLE 1.
Antibody titers and day 5 parasitemias in BALB/c mice after immunization with PyMSP-1 DNA vaccines and challenge with sporozoites
| Immunogen(s) | Titera
|
Parasitemia (%)b | |
|---|---|---|---|
| Prechallenge | Postchallenge | ||
| tPA–p2p30–C-term | 1:320 | 1:5,120 | 0.46 |
| tPA–N-term–p2p30–C-term | 1:640 | 1:5,120 | 0.69 |
| N-term | 1:640 | 1:1,280 | 0.46 |
| tPA–N-term | 1:320 | 1:5,120 | 0.33 |
| tPA–p2p30–N-term | 1:640 | 1:2,560 | 0.42 |
| PyMSP-1 | 1:320 | 1:2,560 | 0.16 |
| tPA–PyMSP-1 | 1:80 | 1:640 | 0.16 |
| tPA–p2p30–N-term + tPA–p2p30–C-term | 1:1,280 | 1:1,280 | 0.075 |
| tPA–PyMSP-1 + tPA–p2p30–C-term | 1:320 | 1:160 | 0.087 |
| Vector control | Neg | 1:80 | 0.43 |
Antibody titers in pooled sera were determined by IFAT on air-dried P. yoelii-infected erythrocytes 4 days prechallenge and 15 days postchallenge. Neg, negative.
Values are geometric mean parasitemias on day 5 postchallenge.
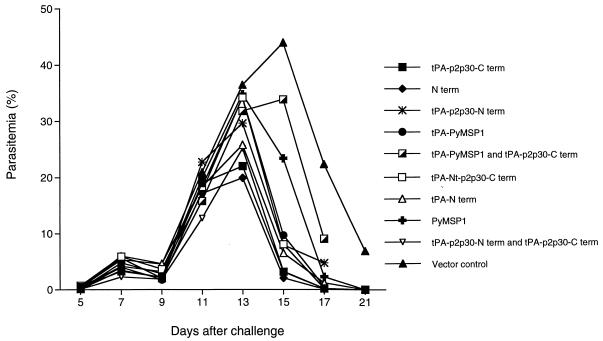
Upon challenge with sporozoites all control mice became infected; peak parasitemia (geometric mean) in the controls was 44% on day 15. In contrast, all groups immunized with the MSP-1 DNA vaccines exhibited lower peak parasitemias; in most groups, peak parasitemia occurred 1 day earlier than in controls and the parasitemia was resolved more rapidly. Peak parasitemias in mice immunized with the tPA–p2p30–C-term and N-term vaccines were 22 and 20%, respectively, ∼50% lower than those in controls, and occurred on day 13. Furthermore, one of eight mice in the groups immunized with the PyMSP-1 and tPA–PyMSP-1 constructs was completely protected, and one of eight mice in the group immunized with the mixture of C-term and tPA–PyMSP-1 plasmids had a very low level of parasitemia on day 5 postinfection and was aparasitemic thereafter. Statistically significant differences (P < 0.05; Tukey’s honestly significant difference test) in parasitemia levels between experimental groups and the vector controls were also noted on days 15, 17, and 21 in mice immunized with tPA–p2p30–C-term, tPA–N-term–p2p30–C-term, N-term, tPA–N-term, tPA–PyMSP-1, and tPA–p2p30–C-term plus tPA–p2p30–N-term. Levels of parasitemia in mice immunized with the tPA–N-term–p2p30 and PyMSP-1 DNA vaccines were significantly lower than those in controls on days 17 and 21. In addition, mice immunized with either of the two plasmids encoding the full-length PyMSP-1 sequence exhibited lower levels of parasitemia on day 5 than other groups, suggesting an effect on liver-stage parasites (Table 1). These data indicate that the mice immunized with PyMSP-1 DNA vaccines controlled and cleared their parasitemias more rapidly than the controls.
Antibody titers were measured 15 days after sporozoite challenge (Table 1). Most groups immunized with PyMSP-1 DNA vaccines exhibited 2- to 16-fold increases in antibody titers, up to a maximum of 1:5,120 in mice immunized with the tPA–p2p30–C-term, the tPA–N-term, or the tPA–N-term–p2p30–C-term fusion vaccine. However, mice that received the two plasmid mixtures had either no increase in titer or a small decrease. Control mice had no detectable antibodies prechallenge and titers of only 1:80 15 days postchallenge. These data indicate that the animals were primed by immunization with the PyMSP-1 DNA vaccines and that subsequent exposure to blood-stage parasites in the course of the infection boosted the antibody response.
Immunogenicity and protection against sporozoite challenge in C57BL/6 mice.
Previous work indicated that C57BL/6 mice show the highest level of protection against challenge with infected erythrocytes after immunization with recombinant proteins consisting of the PyMSP-1 C terminus in adjuvants (22). We therefore repeated the experiments with C57BL/6 mice and, to compare the DNA and recombinant-protein vaccines, immunized one group with recombinant MSP-19 protein in Freund’s adjuvant.
All the PyMSP-1 DNA vaccines tested were immunogenic in C57BL/6 mice (Table 2). The highest antibody titer (1:2,560) was induced in mice that received the mixture of tPA–p2p30–C-term and tPA–PyMSP-1 plasmids or the mixture of tPA–p2p30–C-term and tPA–p2p30–N-term plasmids. Mice immunized with the tPA–p2p30–C-term plasmid had antibody titers of 1:1,280. Mice immunized with three doses of the MSP-19 recombinant protein had IFAT titers of 1:5,120. Thus, in C57BL/6 mice, the best PyMSP-1 DNA vaccines induced antibody titers twofold lower than those induced by the recombinant-protein vaccine, and the comparable construct induced antibody titers fourfold lower than did the recombinant protein in Freund’s adjuvant.
TABLE 2.
Antibody titers and day 5 parasitemias in C57BL/6 mice after immunization with PyMSP-1 DNA vaccines and challenge with sporozoites
| Immunogen(s) | Titera | Parasitemia (%)b |
|---|---|---|
| tPA–p2p30–C-term | 1:1,280 | 0.49 |
| PyMSP-1 | 1:40 | 0.10 |
| tPA–p2p30–C-term + PyMSP-1 | 1:1,280 | 0.59 |
| tPA–p2p30–C-term + tPA–PyMSP-1 | 1:2,560 | 0.36 |
| tPA–p2p30–C-term + tPA–p2p30–N-term | 1:2,560 | 0.46 |
| Vector control | Neg | 0.55 |
| MSP-19 recombinant protein | 1:5,120 | 0.12 |
Antibody titers in pooled sera were determined by IFAT on air-dried P. yoelii-infected erythrocytes 4 days prechallenge. Neg, negative.
Values are geometric mean parasitemias on day 5 postchallenge.
Upon sporozoite challenge, all control mice were parasitemic on day 5, but five of eight mice immunized with recombinant protein, one of eight mice immunized with tPA–p2p30–C-term, one of eight mice immunized with tPA–p2p30–C-term plus PyMSP-1, one of eight mice immunized with tPA–p2p30–C-term plus tPA–PyMSP-1, one of eight mice immunized with tPA–p2p30–C-term plus tPA–p2p30–N-term, and two of eight mice immunized with PyMSP-1 DNA did not have detectable parasitemias. The lowest levels of parasitemia on day 5 were in the groups that received the full-length PyMSP-1 DNA vaccine or the recombinant protein in adjuvant (Table 2). As in the previous experiment, all of the groups immunized with DNA vaccines had lower peak parasitemias and resolved their parasitemias more rapidly than controls. All control mice became infected and had a peak geometric mean parasitemia of 33% on day 15. One of eight mice that had received the mixtures of tPA–p2p30–C-term plus tPA–p2p30–N-term and of tPA–p2p30–C-term plus tPA–PyMSP-1 were completely protected. In the MSP-19 group, two of eight mice were completely protected. With regard to geometric mean peak parasitemia, the DNA vaccine group with the best results, which received the mixture of tPA–p2p30–C-term and tPA–p2p30–N-term vaccines, had a peak parasitemia of 3%, a reduction of 88% compared to mice that received vector DNA alone. The group that received the tPA–p2p30–C-term DNA vaccine had the second best results, with a peak parasitemia of 5.86%. Among the mice that received recombinant MSP-19 protein the peak parasitemia was 6.25%. In this experiment the mixture of tPA–p2p30–C-term and tPA–p2p30–N-term DNA vaccines was as good as or better than the recombinant MSP-19 protein in reducing peak parasitemia after challenge with sporozoites (P ≤ 0.5), and the tPA–p2p30–C-term plasmid was comparable to the recombinant protein in this regard. When administered to BALB/c mice the mixture of tPA–p2p30–C-term and tPA–p2p30–N-term also reduced peak parasitemia significantly (Fig. 2).
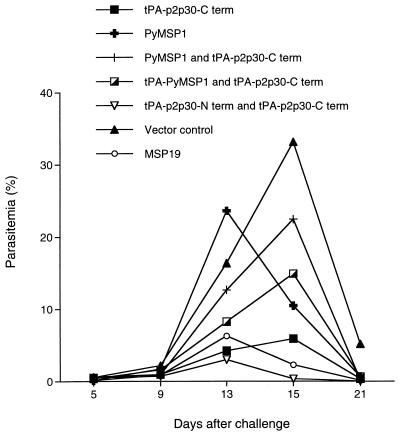
FIG. 2.
Geometric mean parasitemias after intravenous challenge with P. yoelii 17XNL sporozoites. BALB/c mice were immunized with DNA vaccines containing the C-terminus, N-terminus, and full-length PyMSP-1 genes in the VR1012 vector.
The most promising finding in this study was that the PyMSP-1 DNA vaccines protected immunized mice against sporozoite challenge. This was shown by a reduction in peak parasitemia in both BALB/c and C57BL/6 mice immunized with PyMSP-1 DNA vaccines compared to vector controls. In the case of the C57BL/6 mice, the protection observed in mice immunized with the mixture of tPA–p2p30–N-term and tPA–p2p30–C-term DNA vaccines, or the tPA–p2p30–C-term DNA vaccine alone, was equivalent to or better than that obtained in mice immunized with MSP-19 recombinant protein plus adjuvant (Fig. 3).
FIG. 3.
Geometric mean parasitemias in C57BL/6 mice after challenge with P. yoelii 17XNL sporozoites. Mice were immunized with DNA vaccines containing the C-terminus, N-terminus, and full-length PyMSP-1 genes in the VR1012 vector or with MSP-19 recombinant protein.
The mechanism of protection against sporozoite challenge in these experiments has not been defined. We believe that extracellular merozoites or intraerythrocytic parasites were the major targets of the protective immune responses, because in mice immunized with pre-erythrocytic-stage vaccines that do not prevent blood-stage infection, the course of blood-stage parasitemia after sporozoite challenge is similar to that seen in control animals (24). Since parasitemias in mice immunized with the two most protective DNA vaccine regimens were not significantly different than in negative control C57BL/6 mice on day 5 (Table 2 and Fig. 3) but were markedly different thereafter, we believe that the major immune effects were on the erythrocytic stages of the parasite life cycle. However, we cannot exclude the possibility that a protective effect against infected hepatocytes reduced the number of merozoites released from the liver.
We also demonstrated that DNA vaccines encoding the PyMSP-1 N terminus induced protection against sporozoite challenge in BALB/c mice, which indicates that the PyMSP-1 N terminus should be considered as a target for protective immunity. This confirms recent work by Tian et al. (21), who observed protection against blood-stage challenge in mice immunized with a recombinant protein consisting of the N terminus of PyMSP-1 (amino acids 20 to 792), and work in primate models with protein vaccines consisting of N-terminal fragments of P. falciparum MSP-1 (8, 9, 12, 19).
The full-length PyMSP-1 DNA vaccines also elicited protection. This was particularly clear in BALB/c mice, in which mice immunized with the tPA–PyMSP-1 construct exhibited statistically significant reductions in parasitemia on days 15 through 21. The mechanism of protection in mice immunized with the N-term and full-length PyMSP-1 DNA vaccines was not clear, but antibodies reactive with infected erythrocytes were detected in immunized animals, so protection may have been antibody mediated. The day 5 parasitemia data in BALB/c mice immunized with the full-length PyMSP-1 DNA may also support an anti-liver-stage effect, as parasitemia was significantly reduced on day 5 (Table 1). Studies are in progress to characterize the protective immune responses, so new DNA vaccines can be designed to more efficiently induce the appropriate response(s). These studies should also examine the isotype and specificity of the antibody response, factors which have also been shown to influence protection (4).
Mice immunized with PyMSP-1 DNA vaccines were also able to boost antibody responses after challenge. Boosting of the antibody response after exposure to blood-stage parasites would be a very desirable feature for a malaria vaccine, because it would allow individuals who are constantly exposed to parasites to boost protective responses upon infection. We propose that after priming with DNA vaccines, infection in the field will lead to boosting of primed immune responses and limitation of the parasite burden.
We have shown that DNA vaccines encoding PyMSP-1 are immunogenic in mice, that they can induce protective immunity to sporozoites comparable to that obtained with recombinant protein, that immunization with the PyMSP-1 N terminus induces protection, and that there is boosting of the antibody response upon a challenge infection. These results suggest that MSP-1 should be investigated as one potential component of multivalent DNA vaccines currently being developed for P. falciparum malaria. To this end, we are investigating the immunogenicity and protective efficacy of DNA vaccines encoding N- and C-terminal fragments of P. falciparum MSP-1 in animal models.
Acknowledgments
We thank Sanjai Kumar for providing recombinant MSP-1 and the staff of the Malaria Program who provided the sporozoites used in this work.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and the Naval Medical Research and Development Command Work Units, 61102A.S13.00101.BFX1431, 612787A.870.00101.EFX.1432, and 623002A.810.00101.HFX.1433. S.I.B. was supported by a Research Training Fellowship for Medical Students awarded by the Howard Hughes Medical Institute.
REFERENCES
- 1.Burns J M, Majarian W R, Young J F, Daly T M, Long C A. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J Immunol. 1989;143:2670–2676. [PubMed] [Google Scholar]
- 2.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee N C, Barr P J, Yokota B T, Hut G S. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly T M, Long C A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 4.Daly T M, Long C A. Influence of adjuvants on protection induced by a recombinant fusion protein against malarial infection. Infect Immun. 1996;64:2602–2608. doi: 10.1128/iai.64.7.2602-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diggs C L, Ballou W R, Miller L H. The major merozoite surface protein as a malaria vaccine target. Parasitol Today. 1993;9:300–302. doi: 10.1016/0169-4758(93)90130-8. [DOI] [PubMed] [Google Scholar]
- 7.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enders B, Hundt E, Knapp B. Protection of Aotus monkeys after immunization with recombinant antigens of Plasmodium falciparum. Mem Inst Oswaldo Cruz. 1992;3:413–422. doi: 10.1590/s0074-02761992000700070. [DOI] [PubMed] [Google Scholar]
- 9.Etlinger H M, Caspers P, Matile H, Schoenfeld H J, Stueber D, Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991;59:3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman R R, Holder A A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983;54:609–616. [PMC free article] [PubMed] [Google Scholar]
- 11.Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing L, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M. An improved plasmid expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996;7:1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- 12.Herrera M A, Rosero F, Herrera S, Caspers P, Rotmann D, Sinigaglia F, Certa U. Protection against malaria in Aotus monkeys immunized with a recombinant blood-stage antigen fused to a universal T-cell epitope: correlation of serum gamma interferon levels with protection. Infect Immun. 1992;60:154–158. doi: 10.1128/iai.60.1.154-158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 13a.Institute of Laboratory Animals Resources. Guide for the care and use of laboratory animals. Publication no. (NIH) 86-23. Washington, D.C: National Research Council, Department of Health and Human Services; 1985. [Google Scholar]
- 14.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue G K, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis A P. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol Biochem Parasitol. 1989;36:271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- 16.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 17.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 18.Panina Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 19.Patarroyo M E, Romero P, Torres M L, Clavijo P, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui W A, Tam L Q, Kramer K J, Hui G S, Case S E, Yamaga K M, Chang S P, Chan E B, Kan S C. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian J H, Kumar S, Kaslow D C, Miller L H. Comparison of protection induced by immunization with recombinant proteins with different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian J H, Miller L H, Kaslow D C, Ahlers J, Good M F, Alling D W, Berzofsky J A, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 23.Valmori D, Pessi A, Bianchi E, Corradin G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J Immunol. 1992;149:717–721. [PubMed] [Google Scholar]
- 24.Wang R, Charoenvit Y, Daly T M, Long C A, Corradin G, Hoffman S L. Protective efficacy against malaria of a combination sporozoite and erythrocytic stage malaria vaccine. Immunol Lett. 1996;53:83–93. doi: 10.1016/s0165-2478(96)02610-7. [DOI] [PubMed] [Google Scholar]