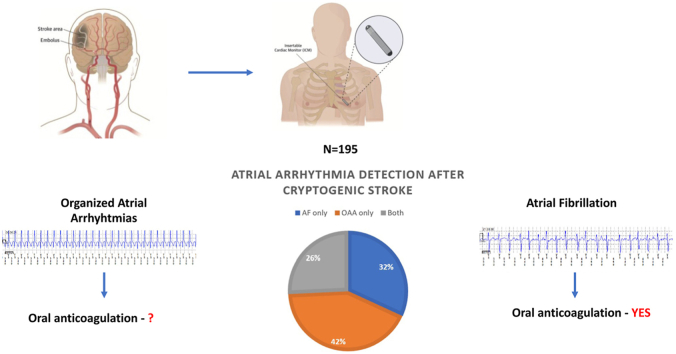
Abstract
Background
Long-term rhythm monitoring to detect atrial fibrillation (AF) following a cryptogenic stroke (CS) is well established. However, the burden of organized atrial arrhythmias in this population is not well defined.
Objective
The purpose of this study was to assess the incidence and risk factors for organized atrial arrhythmias in patients with CS.
Methods
We evaluated all patients with CS who received an insertable cardiac monitor (ICM) between October 2014 and April 2020. All ICM transmissions categorized as AF, tachycardia, or bradycardia were reviewed. We evaluated the time to detection of organized AF and the combination of either organized atrial arrhythmia or AF.
Results
A total of 195 CS patients with ICMs were included (51% men; mean age 66 ± 12 years; mean CHA2DS2-VASC score 4.6). Over mean follow-up of 18.9 ± 11.2 months, organized atrial arrhythmias lasting ≥30 seconds were detected in 45 patients (23%), of whom 62% did not have AF. Seventeen patients had both organized atrial arrhythmia and AF, and another 21 patients had AF only. Compared to those with normal left atrial size, patients with left atrial enlargement had a higher adjusted risk for development of atrial arrhythmias (mild left atrial enlargement: hazard ratio 1.99; 95% confidence interval 1.06–3.75; moderate/severe left atrial enlargement: hazard ratio 3.06; 95% confidence interval 1.58–5.92).
Conclusion
Organized atrial arrhythmias lasting ≥30 seconds are detected in nearly one-fourth of CS patients. Two-thirds of these patients did not have AF. Further studies are required to evaluate the impact of organized atrial arrhythmias on recurrent stroke risk.
Keywords: Cryptogenic stroke, Atrial fibrillation, Atrial flutter, Organized atrial arrhythmias, Insertable cardiac monitor
Graphical abstract
Key Findings.
-
▪
Approximately one-fourth of cryptogenic stroke patients will have evidence of organized atrial arrhythmias on follow-up monitoring.
-
▪
The majority of patients with organized atrial arrhythmias do not have evidence of concurrent atrial fibrillation.
-
▪
Left atrial size at the time of stroke is independently associated with the development of atrial arrhythmias in the poststroke population.
Introduction
Atrial flutter is associated with a similar rate of ischemic stroke as atrial fibrillation (AF).1 As a result, primary and secondary stroke prevention guidelines recommend similar long-term, oral anticoagulation for atrial flutter as is recommended for AF.1,2 However, poststroke monitoring studies have focused primarily on AF detection. These studies have used automatic device algorithms that recognize AF based primarily on irregular ventricular rates and a lack of organized, atrial electrical activity on electrograms. Furthermore, these studies have consistently demonstrated increased rates of AF detection with intermittent or continuous long-term rhythm monitoring in patients who have experienced an ischemic stroke.3, 4, 5, 6 However, limited studies have evaluated the detection and risk factors associated with organized atrial arrhythmias such as atrial flutter or atrial tachycardia in this vulnerable population.
We sought to evaluate the incidence of organized atrial arrhythmias in a real-world cohort of patients with cryptogenic stroke (CS) who had an insertable cardiac monitor (ICM). We also evaluated the incidence of atrial arrhythmias defined as the first detection of either an organized atrial arrhythmia or AF. In additional analyses, we evaluated the risk factors associated with the presence of these atrial arrhythmias that often are deemed to be clinically significant and warrant anticoagulant therapy for prevention of thromboembolic complications.
Methods
Study design
All patients with CS who underwent ICM implantation between 2014 and 2020 at 1 of the 3 Philadelphia-based Penn Medicine hospitals including the Hospital of the University of Pennsylvania, Presbyterian Medical Center, or Pennsylvania Hospital were included. All patients were evaluated and managed by the stroke–neurology service and were determined to have experienced a CS after extensive evaluation, including vascular imaging (100% of patients) and echocardiography (transthoracic in 98% and/or transesophageal in 50%) before ICM insertion. Patients were discharged with both neurology and arrhythmia monitoring care within the Penn Medicine system. Patients who had a previous diagnosis of an atrial arrhythmia or who had an ICM for a known etiology of ischemic stroke were excluded from this analysis.
Covariate data
We evaluated the demographic and clinical characteristics of patients at the time of the index stroke event that led to insertion of the cardiac monitor. In particular, we evaluated age, sex, and race. In addition, we recorded medical comorbidities, including any history of hypertension, hyperlipidemia, diabetes, coronary heart disease, heart failure, and chronic kidney disease. We recorded any history of stroke or transient ischemic attack and the use of antiplatelets and oral anticoagulants at the time of ICM placement. We also recorded left atrial (LA) volume by echocardiography (mild LA dilation 34–42 mL/m2, moderate LA dilation 42–48 mL/m2, severe LA dilation >48 mL/m2).7
Cardiac rhythm monitoring
All patients were implanted with the LINQ device (Medtronic, Inc., Minneapolis, MN). This ICM can record rhythm abnormalities for 3–4 years. Devices were programmed to detect any AF episode ≥2 minutes. The 2-minute timeframe is the minimum duration required to trigger an automatic AF recording in the ICM’s arrhythmia log. In addition, all devices were programmed to detect tachycardia (>150 bpm). All patients were enrolled in remote monitoring (MyCareLink, Medtronic), which allowed for routine arrhythmia surveillance. All remote transmissions were reviewed by clinical teams that included the implanting cardiac electrophysiologist. Remote transmissions were reviewed on a monthly basis.
Separate adjudication for all ICM transmissions was performed by our research team. In particular, we reviewed all transmissions that were categorized as AF, tachycardia, and bradycardia. Episodes with an irregular R-R interval and no obvious P waves were classified as true AF episodes. Tracings that were categorized as AF by the device but had clear evidence of sinus activity were classified as false AF episodes. All tachycardia episodes that were regular were reviewed with careful attention to heart rate scatter plots and detection of P waves. All tracings were consistently analyzed using the zoom function on CareLink to better visualize potential P waves.
The tracings of all tachycardia episodes were reviewed and adjudicated as an organized atrial arrhythmia if they met one of the following criteria: heart rate during the episode >220 – patient’s age; evidence of organized atrial activity on electrograms (sawtooth appearance) with ventricular rates in the tachycardia zone (>150 bpm) suggestive of atrial flutter/atrial tachycardia with variable atrioventricular conduction; or abrupt transition from sinus rhythm to an arrhythmia in the tachycardia zone on scatterplot analysis of the arrhythmic event. These detection criteria are congruent with the mechanistic basis of various arrhythmias and similar to those in other randomized trials assessing organized atrial arrhyhtmias.6,8 Only organized arrhythmia episodes that lasted ≥30 seconds were considered clinically significant based on previous studies and were included in our analysis. The electrocardiograms of all episodes were reviewed initially by the faculty electrophysiologist involved in the clinical care of the patient. As part of the adjudication process for this analysis, all electrocardiograms were reviewed independently by 2 investigators (NKP, UB). All episodes and electrocardiograms were then reviewed as a team including a faculty electrophysiologist (RD). Episodes without available electrocardiograms were excluded from the analysis.
Study outcomes
The primary outcome of our analysis was time from implantation of the ICM to the detection of organized atrial arrhythmia. We also evaluated the time to atrial arrhythmias defined as the first occurrence of either an organized atrial arrhythmia or AF. The follow-up censoring date was December 31, 2020.
Statistical analysis
Categorical variables are given as frequency (proportion). Continuous variables are given as mean ± SD. A series of time to event analyses were performed using Kaplan-Meier survival curves. Specifically, we evaluated the time to detection of either an organized arrhythmia, AF, or the first occurrence of either type of atrial arrhythmia. We then evaluated risk factors for incident atrial arrhythmias. Cox proportional hazards regression analysis was performed to identify independent risk factors/markers of primary and secondary outcomes. Variables that were significant at P <.20 in the univariate model were included in the multivariate model to identify independent predictors. P <.05 was considered significant. The proportional hazards assumption was not violated in any of these analyses. All statistical analyses were completed using SAS Version 9.4 (SAS Institute Inc., Cary, NC). The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board at the University of Pennsylvania. Finally, because this was a retrospective analysis of stored insertable cardiac monitor data, a waiver of informed consent was approved by the Institutional Review Board.
Results
Our multicenter cohort consisted of 195 patients with CS. Mean age was 66 ± 12 years, nearly half were women, and approximately one-fifth were Black (Table 1). Mean CHA2DS2-VASc score at the time of ICM insertion was 4.6 ± 1.5. More than one-fourth had a history of stroke before the index stroke event that led to insertion of the cardiac monitor. The majority of patients had a history of hypertension or hyperlipidemia. Few patients had a history of heart failure. LA size was normal in the majority of patients, mildly dilated in 20%, and moderately/severely dilated in 11%. Twenty-two patients were placed on oral anticoagulation therapy by the neurology service at the time of the ICM. These patients did not have any previously documented atrial arrhythmias. Antiplatelet therapy was prescribed at discharge in 92% of patients.
Table 1.
Demographic and clinical characteristics of the study cohort (N = 195)
| Age (y) | 66 ± 12 |
| Male | 100 (51) |
| Black | 41 (22) |
| Comorbid conditions | |
| Hypertension | 143 (73) |
| Hyperlipidemia | 140 (72) |
| Diabetes mellitus | 59 (30) |
| Coronary heart disease | 43 (22) |
| Heart failure | 7 (4) |
| Chronic kidney disease | 50 (27) |
| Previous stroke/TIA | 54 (28) |
| CHA2DS2-VASc score | 4.6 ± 1.5 |
| Left atrial size on echocardiography | |
| Normal | 127 (68) |
| Mildly dilated | 38 (20) |
| Moderately dilated | 11 (6) |
| Severely dilated | 10 (5) |
| Medications | |
| Aspirin | 105 (54) |
| Clopidogrel | 14 (7) |
| Aspirin + clopidogrel | 58 (30) |
| Aspirin + dipyridamole | 2 (1) |
| Anticoagulants before ILR | 22 (12) |
Values are given as mean ± SD or n (%).
ILR = implantable loop recorder; TIA = transient ischemic attack.
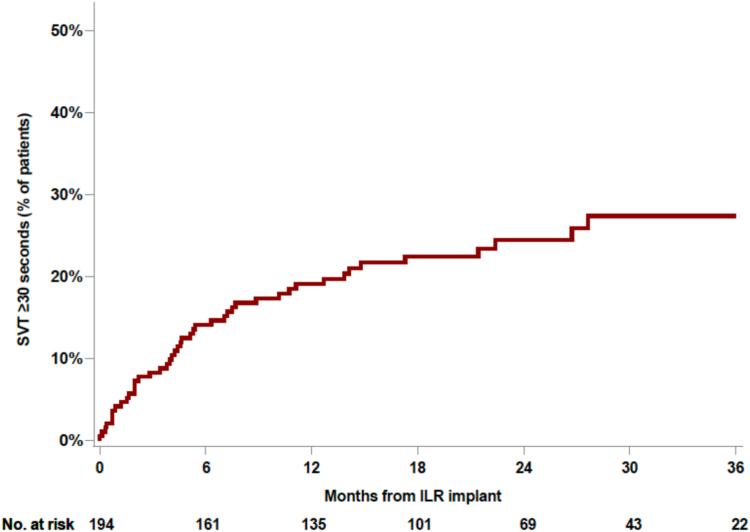
Over a mean follow-up period of 18.9 ± 11.2 months, 45 patients had an organized atrial arrhythmia lasting ≥30 seconds (Figure 1). Representative examples of ICM tracings and scatterplots that demonstrate organized atrial arrhythmias are shown in Figure 2. Of these cases, the majority (n = 28 [62%]) did not have any evidence of AF at any point in the follow-up monitoring period. The remaining 17 patients had both organized atrial arrhythmias and AF. An additional 21 patients in our cohort had AF only (Supplemental Figure 1). Nearly all cases of organized atrial arrhythmia and AF were identified within the first year after implantable loop recorder (ILR) insertion (mean duration 7.0 ± 7.1 months for detection of organized atrial arrhythmia and 8.4 ± 8.7 months for AF). Among the patients who did not have an atrial arrhythmia, mean duration of monitoring was 21.5 ± 9.4 months. All 17 patients who had both AF and organized atrial arrhythmias were started on oral anticoagulation. Among the 28 patients who had organized atrial arrhythmias only, 3 were on long-term anticoagulation before ILR implantation at the discretion of the stroke neurologist, and 5 patients were started after review of ILR findings.
Figure 1.
Cumulative incidence of organized atrial arrhythmias. Kaplan-Meier curve depicts the time to first detection of organized atrial arrhythmias lasting ≥30 seconds. ILR = implantable loop recorder; SVT = supraventricular tachycardia.
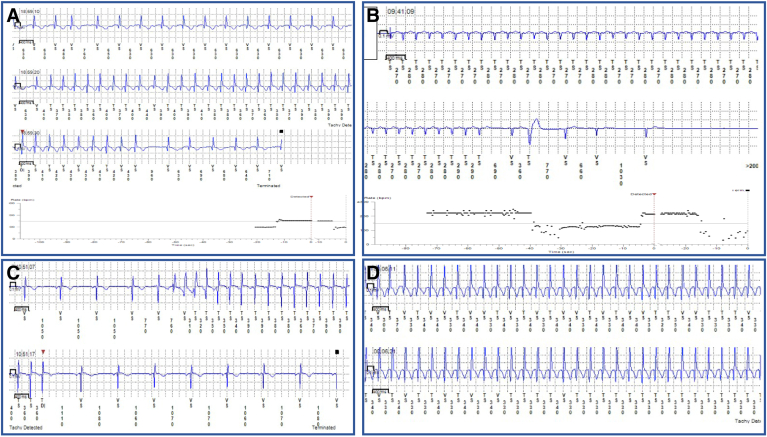
Figure 2.
Examples of organized atrial arrhythmias. A: Sudden onset and offset of regular narrow complex tachycardia. B: Episode of organized atrial arrhythmia showing abrupt offset and a peak ventricular rate greater than maximal age-predicted heart rate. Note the sinus pause at the termination of an episode suggestive of sinus node dysfunction. C: Initiation of this organized atrial arrhythmias starts with a wider QRS secondary to aberrant conduction. Clear p waves are seen during the tachycardia. D: Regular narrow complex tachycardia at a rate of 188 bpm in an 82-year-old patient with peak heart rate exceeding maximal age-predicted heart rate.
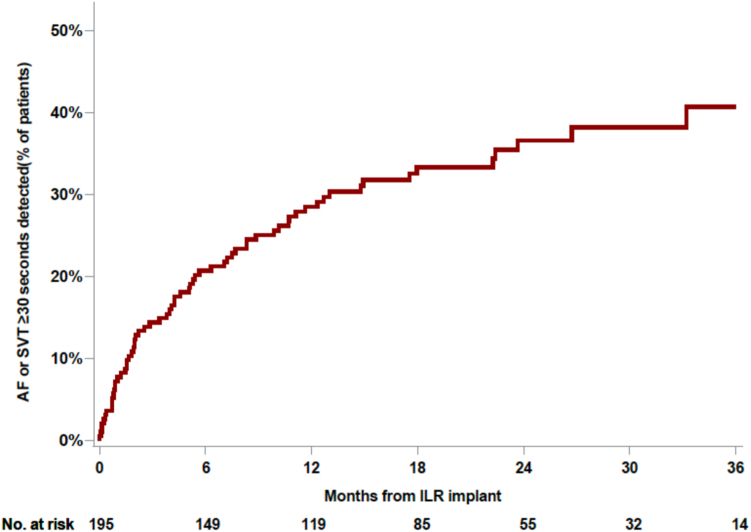
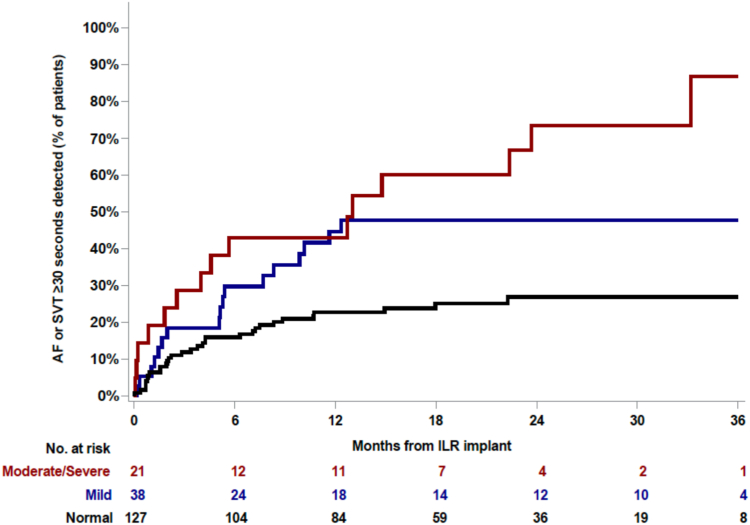
A total of 66 poststroke patients had evidence of an atrial arrhythmia on long-term ICM evaluation (Figure 3). In staged multivariable modeling, only LA size was an independent marker of risk for the development of atrial arrhythmias (Table 2). Compared to those with normal LA size, patients with moderate-to-severe LA dilation had a >3-fold increased, adjusted risk of incident atrial arrhythmias that was noted early and was sustained throughout the follow-up period (Figure 4). Similar associations with LA size were observed when evaluating organized atrial arrhythmias and AF as independent arrhythmic endpoints (Supplemental Tables 1 and 2). Female sex was associated with less organized atrial arrhythmias. A history of heart failure was associated with a > 3-fold risk of incident AF in unadjusted analysis; however, this estimate was rendered nonsignificant after controlling for other risk factors. Age, race, and clinical risk factors such as hypertension, hyperlipidemia, diabetes, coronary heart disease, and chronic kidney disease were not associated with organized atrial arrhythmias or AF.
Figure 3.
Cumulative incidence of atrial arrhythmias. Kaplan-Meier curve showing time to first detection of any atrial arrhythmia (atrial fibrillation [AF] or organized atrial arrhythmias lasting ≥30 seconds) in a postcryptogenic stroke population using long-term continuous cardiac monitoring. Patients with both AF and organized atrial arrhythmias were censored at the first occurrence of the endpoint. Abbreviations as in Figure 1.
Table 2.
Risk factors of incident atrial arrhythmias
| Unadjusted HR | P value | Adjusted HR | P value | |
|---|---|---|---|---|
| Age | 1.02 (1.00–1.04) | .055 | 1.02 (0.99–1.05) | .15 |
| Female sex | 0.68 (0.42–1.11) | .13 | 0.64 (0.37–1.09) | .10 |
| Black | 1.17 (0.64–2.13) | .62 | 1.13 (0.61–2.08) | .70 |
| Heart failure | 3.43 (1.38–8.57) | .01 | 1.99 (0.77–5.18) | .16 |
| Mild left atrial dilation | 2.04 (1.12–3.68) | .02 | 1.99 (1.06–3.75) | .03 |
| Moderate/severe left atrial dilation | 3.59 (1.94–6.66) | <.001 | 3.06 (1.58–5.92) | <.001 |
Hypertension, hyperlipidemia, diabetes, coronary heart disease, heart failure, chronic kidney disease, previous stroke/transient ischemic attack, and left atrial size were each evaluated as risk factors for the specified arrhythmia in unadjusted analysis. Only those variables that were associated with the arrhythmia endpoint at P <.20 were further assessed in multivariable analysis. Age, sex, and race were forced into the multivariable analysis.
HR = hazard ratio.
Figure 4.
Cumulative incidence of atrial arrhythmias according to left atrial size. Left atrial size was categorized according to American Society of Echocardiography criteria as normal (black line), mildly dilated (blue line), or moderately/severely dilated (red line). Abbreviations as in Figures 1 and 3.
Discussion
Among patients with CS undergoing ICM insertion, an organized atrial arrhythmia such as atrial flutter or atrial tachycardia was detected in nearly one-fourth of patients. The majority of these poststroke patients did not have evidence of AF during the follow-up period. Our findings also suggest that among a series of demographics and clinical risk factors, only LA size assessed at the time of stroke was independently associated with the development of atrial arrhythmias in this poststroke population.
Our study is one of the first to systematically evaluate the incidence of organized atrial arrhythmias in this population. Despite clinical guidelines recommending anticoagulation for organized atrial arrhythmias such as atrial flutter,1 previous ICM monitoring studies in poststroke populations did not report the incidence of non-AF arrhythmias.3,5,6 Differentiating between arrhythmia types using ICM tracings can be challenging because P-wave deflection and amplitude vary, often because of the patient’s position or habitus. Furthermore, the underlying mechanism including either a reentrant flutter vs microreentrant or focal atrial tachycardia requires electrophysiological study. However, the possibility of atrial flutter or a rapid atrial tachycardia leading to electromechanical dyssynchrony would likely justify oral anticoagulation in this vulnerable, poststroke population according to current guidelines. Although the mechanisms of cardioembolic stroke in patients with AF and atrial flutter are strongly associated with turbulent blood flow and subsequent thrombus formation in the LA appendage, the pathophysiology of atrial tachycardia leading to a cardioembolic event is unclear. Part of the risk for thromboembolic complications may be related to a fast atrial rate. For example, an atrial tachycardia at a rate of 250 bpm may have similar implications for hemostasis and appendage thrombosis as an atrial flutter. The occurrence of atrial tachycardia also may be a sign of underlying atrial myopathy, which can be a risk factor for stroke. Detailed specifications that define the thresholds for initiating anticoagulation require an evaluation of both the rate and burden of organized atrial arrhythmias that increase the risk of stroke and thromboembolic events. Until then, the detection of these episodes should trigger a shared decision approach between provider and patient on anticoagulation and antiarrhythmic management.
The assessment of AF in our real-world, multicenter analysis helps to generalize our overall findings. AF detection rate in the first year of monitoring was 14%, which is similar to that observed in the CRYSTAL AF (Cryptogenic Stroke and Underlying Atrial Fibrillation) trial3 and other prolonged monitoring studies of patients who had an ischemic stroke.5,6 These consistent rates of AF detection observed across studies also suggest that the incidence of organized atrial arrhythmias from our population would be similar to that of other poststroke populations. The proportion of individuals in our analysis who had evidence of atrial arrhythmias (34%) is similar to that found in ASSERT-II (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial II), which assessed subclinical AF using an ICM in the elderly with comorbidities including a history of stroke in half.9 Furthermore, previous studies have demonstrated correlations between atrial cardiopathy or LA size and function and AF.10, 11, 12, 13, 14 We extend these observations between LA size and a broader atrial arrhythmic endpoint that includes other atrial arrhythmias. The link between ischemic stroke, LA size, AF, and now organized atrial arrhythmias continues to support the idea that structural and electrical activity of the atrium are correlated in this high-risk population. Whether these organized atrial arrhythmias are independent risk factors for recurrent stroke or are markers of the underlying substrate related to age and cardiovascular comorbidities remains unclear.
Study limitations
The analysis in this study was performed in a tertiary care population, and the results may not be generalizable to a broader population of stroke patients. Assessment of AF and organized atrial arrhythmia burden was not possible because of the retrospective study design and the lack of availability of electrograms representing all arrhythmic episodes. As a result, adjudication and an assessment of overall burden were not possible. In addition, there is a possibility of missing some episodes of rate controlled atrial flutter with ICM monitoring. The cycle length regularity of atrial flutter prevents the device from triggering an AF/tachycardia alarm when the ventricular response is controlled and is below the programmed tachycardia zone. In addition, we evaluated measures obtained in routine clinical practice. As such, we did not have a complete assessment of potential risk markers such as cardiac biological markers, cardiovascular medications, and LA strain measures for incident atrial arrhythmias. Finally, we used different time cutoffs for detection of AF (2 minutes) and organized atrial arrhythmias (30 seconds). This difference was primarily a function of ICM programming for which the lowest time cutoff for AF detection is 2 minutes but allows a 30-second cutoff for “tachycardia” detection. Future studies in which the ICM reliably stores all arrhythmia episodes will be important to understanding the overall duration and burden of organized atrial arrhythmias.
Conclusion
We report a high incidence of organized atrial arrhythmias in patients with CS undergoing ICM monitoring. LA size is the only independent risk marker for the development of atrial arrhythmias. Further studies are required to evaluate the significance of organized atrial arrhythmias on the risk of recurrent stroke.
Acknowledgments
Funding Sources
Partial support for this project was provided by the Winkelman Family Fund in Cardiovascular Innovation.
Disclosures
The authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Because this was a retrospective analysis of stored insertable cardiac monitor data, a waiver of informed consent was approved by the Institutional Review Board.
Ethics Statement
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board at the University of Pennsylvania.
Disclaimer
Given his role as Section Editor, Saman Nazarian had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Editors Dennis Lau and Jeanne E. Poole.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.11.016.
Appendix. Supplementary Data
References
- 1.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 2.Kleindorfer D.O., Towfighi A., Chaturvedi S., et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 3.Sanna T., Diener H.C., Passman R.S., et al. CRYSTAL AF Investigators Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone D.J., Spring M., Dorian P., et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein R.A., Kamel H., Granger C.B., et al. Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA. 2021;325:2169–2177. doi: 10.1001/jama.2021.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck B.H., Hill M.D., Quinn F.R., et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA. 2021;325:2160–2168. doi: 10.1001/jama.2021.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C., Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin. 2011;3:23–45. doi: 10.1016/j.ccep.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healey J.S., Alings M., Ha A., et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. doi: 10.1161/CIRCULATIONAHA.117.028845. [DOI] [PubMed] [Google Scholar]
- 10.Edwards J.D., Healey J.S., Fang J., Yip K., Gladstone D.J. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghi S., Boehme A.K., Hazan R., et al. Atrial Cardiopathy and cryptogenic stroke: a cross-sectional pilot study. J Stroke Cerebrovasc Dis. 2016;25:110–114. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sajeev J.K., Kalman J.M., Dewey H., Cooke J.C., Teh A.W. The atrium and embolic stroke: myopathy not atrial fibrillation as the requisite determinant? JACC Clin Electrophysiol. 2020;6:251–261. doi: 10.1016/j.jacep.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Ricci B., Chang A.D., Hemendinger M., et al. A simple score that predicts paroxysmal atrial fibrillation on outpatient cardiac monitoring after embolic stroke of unknown source. J Stroke Cerebrovasc Dis. 2018;27:1692–1696. doi: 10.1016/j.jstrokecerebrovasdis.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Thijs V.N., Brachmann J., Morillo C.A., et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86:261–269. doi: 10.1212/WNL.0000000000002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.