Abstract
Background
Catheter ablation of premature ventricular complexes (PVCs) and ventricular tachycardia (VT) from the left ventricular summit (LVS) may require advanced ablation techniques. Bipolar ablation from the coronary veins and adjacent endocardial structures can be effective for refractory LVS arrhythmias.
Objective
The aim of this study was to investigate the outcomes of bipolar ablation performed between the coronary venous system and adjacent endocardial left ventricular outflow tract (LVOT) or right ventricular outflow tract (RVOT).
Methods
This multicenter study included consecutive patients with LVS PVC/VT who underwent bipolar ablation between the anterior interventricular vein (AIV) or great cardiac vein (GCV) and the endocardial LVOT/RVOT after failed unipolar ablation. Ablation was started with powers of 10–20 W and uptitrated to achieve an impedance drop of at least 10%. Angiography was performed in all cases to confirm a safe distance (>5 mm) of the catheter from the major coronary arteries.
Results
Between 2013 and 2023, bipolar radiofrequency ablation between the AIV/GCV and the adjacent LVOT/RVOT was attempted in 20 patients (4 female; age 57 ± 16 years). Unipolar ablation from sites of early activation (AIV/GCV, LVOT, aortic cusps, RVOT) failed to effectively suppress the PVC/VT in all subjects. Bipolar ablation was delivered with a maximum power of 30 ± 8 W and total duration of 238 ± 217 s and led to acute PVC/VT elimination in all patients. No procedural-related complications occurred. Over a follow-up period of 30 ± 24 months, the freedom from arrhythmia recurrence was 85% (1 recurrence in the VT group and 2 in the PVC group). PVC burden was reduced from 22% ± 10% to 4% ± 8% (P <.001).
Conclusion
In cases of LVS PVC/VT refractory to unipolar ablation, bipolar ablation between the coronary venous system and adjacent endocardial LVOT/RVOT is safe and effective if careful titration of power and intraprocedural angiography are performed to ensure a safe distance from the coronary arteries.
Keywords: Ablation techniques, Catheter ablation, Intramural, Mapping, Radiofrequency, Ventricular arrhythmia
Key Findings.
-
▪
In this multicenter study, bipolar ablation between the coronary venous system and the adjacent left ventricular outflow tract/right ventricular outflow tract was attempted in 20 patients with left ventricular summit premature ventricular complexes (PVCs) or ventricular tachycardia (VT) that had been refractory to unipolar ablation.
-
▪
Bipolar ablation was delivered with a maximum power of 30 ± 8 W and total duration of 238 ± 217 seconds. Baseline impedance of the circuit was on average 217 ± 77 Ω, and mean impedance drop during bipolar radiofrequency applications was 21 ± 9 Ω.
-
▪
Acute PVC/VT elimination was achieved in all patients with no procedure-related complications.
-
▪
Over a follow-up period of 30 ± 24 months, the freedom from ventricular arrhythmia recurrence was 85%.
Introduction
Radiofrequency (RF) catheter ablation is an effective and well-established therapy for idiopathic and scar-related ventricular arrhythmias (VAs).1 In absence of structural heart disease, outcomes of catheter ablation are very good, with reported success rates between 80% and 90%.2,3 However, the outcomes are less favorable when the arrhythmia origin is intramural or epicardial.2,4,5
Premature ventricular complexes (PVCs) and ventricular tachycardia (VT) from the epicardial aspect of the left ventricular (LV) ostium, also known as the left ventricular summit (LVS), are particularly challenging to ablate due to proximity to the coronary arteries, the presence of epicardial fat, and the often deep intramural location of the arrhythmogenic substrate,6, 7, 8 which limit accessibility from the endocardial or epicardial surfaces. LVS arrhythmias typically are ablated from the coronary veins (great cardiac vein [GCV] or anterior interventricular vein [AIV]) or from adjacent endocardial or endovascular structures, including the left coronary cusp, the commissure between the left coronary cusp and right coronary cusp, the subaortic left ventricular outflow tract (LVOT), the septal right ventricular outflow tract (RVOT), or the left pulmonic cusp.9 Often, ablation from >1 site is required to achieve arrhythmia suppression.10
When standard ablation fails, advanced ablation approaches can be attempted alone or in combination to create deeper or transmural lesions.11,12 Bipolar RF ablation involves the application of alternating current between 2 ablation catheters positioned on opposite sites of the arrhythmogenic substrate, one connected to the active port of the RF generator (active catheter [AC]) and the second connected to the indifferent port instead of the skin dispersive “ground” patch (return catheter [RC]). Consequently, RF current flows between the distal tip of both catheters, resulting in higher current density to the myocardial tissue and increased lesion transmurality.13,14
Bipolar ablation between the coronary venous system and adjacent endocardial structures can be effective in treating refractory LVS arrhythmias,15,16 but evidence for the efficacy and safety of this approach is limited. In this multicenter study, we report the feasibility, safety, and outcomes of bipolar ablation applied between the coronary veins and adjacent endocardial LVOT/RVOT in patients with LVS arrhythmias refractory to standard ablation.
Methods
Patients
This multicenter retrospective series included patients undergoing bipolar ablation of LVS arrhythmias in 8 international centers between 2013 and 2023. Inclusion criteria were (1) age ≥18 years; (2) PVC/VT with earliest local activation in the GCV or AIV; and (3) failed unipolar ablation from endocardium and earliest vein site(s).
Patients were excluded if (1) mapping or ablation catheters could not be advanced to the GCV/AIV; or (2) ablation within the GCV/AIV was deemed unsafe due to proximity to an epicardial coronary artery (<5 mm as determined by intraprocedural angiography). Collection of data was approved by institutional review boards of the participating centers, and the research was conducted according to the Helsinki Declaration guidelines on human research. All patients provided written informed consent before the ablation procedure. Baseline clinical characteristics including age, sex, clinical presentation, and 12-lead electrocardiogram (ECG) of the PVC/VT were recorded. Four patients were included in a previous report.15
Mapping
Patients presented to the cardiac electrophysiology laboratory in the fasting state. Conscious sedation was used in most cases (75%) to prevent arrhythmia suppression. Antiarrhythmic medications were discontinued for 5 half-lives before the study. All procedures were guided by electroanatomic mapping using the EnSite (Abbott, St. Paul, MN) or CARTO (Biosense Webster, Diamond Bar, CA) system. Intracardiac echocardiography was used at the discretion of the operator. Both the RVOT and LVOT, including the aortic sinuses of Valsalva, were mapped in all cases. For LVOT mapping, a retrograde aortic approach was used, and heparin was administered to maintain an activated clotting time >250–300 seconds. Multipolar catheters (PentaRay, Biosense Webster; or Advisor HD Grid, Abbott) were used for mapping in 35% of cases. In 20% of cases, mapping of the septal perforator veins was performed using a multielectrode catheter (EPstar, Baylis Medical, Toronto, Canada).
For PVCs or focal VT, activation mapping was performed, recording the earliest bipolar activation time compared with surface QRS. In case of infrequent ectopy, induction was attempted with isoproterenol infusion (2–20 μg/min) or salbutamol boluses (0.125 mg) and ventricular or atrial burst pacing. Pacemapping was also used to complement activation mapping, but ablation based solely on pacemapping was not performed. For reentrant VTs, the circuit was characterized using activation and entrainment mapping. Sites with concealed QRS fusion and return cycle within 30 ms of the VT cycle length with matching stimulus-QRS and electrogram-QRS intervals or where VT terminated during pacing without global capture were considered critical.
Bipolar ablation
An open-irrigated ablation catheter (ThermoCool, Biosense Webster; or Coolflex or TactiCath, Abbott) or solid-tip 8-mm catheter (Therapy Dual-8, Abbott) was advanced into the GCV/AIV to the site of earliest PVC/VT activation. A second irrigated catheter or a nonirrigated 4- or 8-mm catheter (AlCath, Biotronik, Berlin, Germany) was positioned into the LVOT or RVOT at the site of earliest endocardial activation or the site anatomically closest to the earliest epicardial electrode. In most cases the AC was positioned in the GCV/AIV; in other cases the endocardial catheter served as AC; and in a minority of cases the polarity was sequentially alternated. The RC was connected to the indifferent port of the RF generator (EP Shuttle, Stockert, Freiburg, Germany) using a custom cable, T-type connector (IBI GenConnect, Abbott) or a prototype adapter (formerly IndiCath Switchbox, Corsystem, Rzeszow, Poland). In 1 case, the second ablation catheter could not be advanced to the site of earliest activation in the GCV, and a multipolar mapping catheter (EPstar, Baylis) was used instead as return electrode.17
RF ablation was performed by starting with power of 10–20 W and titrating up gradually to achieve an impedance drop of at least 10% from baseline values. Temperature limit was set at 42°C. The primary target for ablation was the site of earliest bipolar activation, ideally exhibiting a QS unipolar electrogram and good pacemap (morphology matching score >95% by PASOTM [CARTO] or Score Map [EnSite]). In all cases, coronary angiography was performed before ablation to confirm a safe distance (>5 mm) of the catheter tip from epicardial coronary arteries.
ECG analysis
ECG analysis was performed offline using the Prucka CardioLab (GE, Houston, TX) or EP-Tracer (Cardiotek, Maastricht, The Netherlands) recording system at a sweep speed between 100 and 300 mm/s using digital calipers. QRS morphology was evaluated with respect to bundle branch block pattern, QRS duration, maximum deflection index, axis, and precordial transition.
Outcomes and follow-up
Acute procedural success was defined as follows—(1) for PVCs: complete elimination of spontaneous or inducible PVCs after a waiting time of 30 minutes despite isoproterenol or salbutamol administration; and (2) for VT: noninducibility of any sustained monomorphic VT at the end of the procedure. Serious complications were defined as those that caused prolonged hospitalization or resulted in significant morbidity or mortality. These included pericardial effusion with or without tamponade, transient or permanent cerebrovascular event, complete heart block, acute coronary syndrome, major bleeding requiring transfusion, or vascular injury that required surgical intervention.
Postprocedure, patients remained in the hospital overnight under continuous ECG monitoring and were discharged the next day if no complications occurred. Follow-up included inpatient telemetry, outpatient rhythm monitoring, and follow-up clinic visits. In PVC patients, a 24-/48-hour Holter or 2-week event monitor was obtained to assess residual PVC burden between 1 and 3 months after discharge. Follow-up visits were performed at intervals as deemed necessary by the treating physician or as clinically indicated.
Statistical analysis
Results are summarized as mean ± SD for continuous variables and as frequency (percentage) for categorical variables. The paired t test was used to compare PVC burden before and after catheter ablation. Analyses were performed using Stata Version 14.2 (Stata Corporation, College Station, TX).
Results
Baseline characteristics
Bipolar RF ablation between the AIV/GCV and the adjacent LVOT/RVOT was attempted in 20 patients (4 females; age 57 ± 16 years; 1–4 patients included per center). Baseline characteristics are listed in Table 1. Fourteen patients (70%) had a structurally normal heart, 5 patients (25%) had nonischemic cardiomyopathy, including cardiac sarcoidosis in 1 case, and 1 patient (5%) had ischemic cardiomyopathy. The indication for ablation was VT in 2 cases and PVCs in the remaining 18 patients. Nine patients had failed unipolar ablation in a previous separate procedure, with the remaining converting to bipolar setup due to failed unipolar ablation within the index procedure. In 3 of the 9 patients with previous failed procedures, bipolar ablation was conducted up front, without additional attempts at unipolar ablation.
Table 1.
Clinical characteristics of the study population (n = 20)
| Age (y) | 57 ± 16 |
| Male | 16 (80) |
| BMI (kg/m2) | 29 ± 6 |
| Ventricular arrhythmia | |
| PVC | 18 (90) |
| VT | 2 (10) |
| Etiology | |
| Idiopathic | 14 (70) |
| Nonischemic | 5 (25) |
| Ischemic | 1 (5) |
| PVC burden (%) | 24 ± 11 |
| LV ejection fraction by 2-dimensional echocardiography (%) | 51 ± 17 |
| Failed antiarrhythmic drugs | |
| Beta-blocker | 15 (75) |
| Class III | 6 (30) |
| Class I | 3 (15) |
Values are given as mean ± SD or n (%).
BMI = body mass index; LV = left ventricle; PVC = premature ventricular contraction; VT = ventricular tachycardia.
ECG characteristics
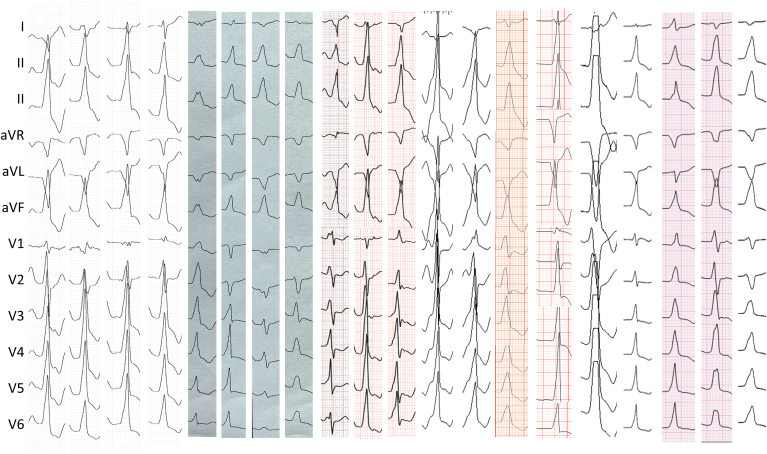
PVC/VT morphology was right bundle branch block in 13 cases (65%) and left bundle branch block in 7 cases (35%) (Figure 1). In those with left bundle branch block, the transition was V2 in 2 patients, V3 in 4 patients, and V4 in 1 patient. The frontal plane axis was right inferior in 12 (60%) and left inferior in 8 (40%). Average QRS duration was 155 ± 13 ms, and the maximum deflection index was 0.46 ± 0.08.
Figure 1.
Twelve-lead morphology of the premature ventricular complexes/ventricular tachycardias.
Procedural data
Electroanatomic mapping was performed with CARTO (Biosense Webster) in 7 patients (35%) and EnSite (Abbott) in 13 patients (65%) (Table 2). Previous unipolar ablation from sites of early activation adjacent to the LVS (GCV/AIV, RVOT, LVOT, aortic cusps) failed to effectively suppress the PVC/VT in all subjects. Mapping of the septal veins using multielectrode catheters was performed in 4 cases (20%), and intracardiac echocardiography was used in 13 cases (65%).
Table 2.
Procedural characteristics (n = 20)
| Catheter location | |
| GCV/AIV and LVOT | 17 (85) |
| GCV/AIV and RVOT | 2 (10) |
| Septal perforator vein and LVOT | 1 (5) |
| Polarity of RF application | |
| Vein to endocardium | 11 (55) |
| Endocardium to vein | 7 (35) |
| Alternating | 2 (10) |
| Active catheter | |
| 3.5-mm irrigated | 20 (100) |
| Return catheter | |
| 3.5-mm irrigated | 12 (60) |
| 8-mm nonirrigated | 4 (20) |
| 4-mm nonirrigated | 3 (15) |
| 2F EPstar catheter | 1 (5) |
| Mapping system | |
| EnSite | 13 (65) |
| CARTO | 7 (35) |
| Circuit impedance (Ω) | 217 ± 77 |
| Impedance drop (Ω) | 21 ± 9 (range 8–35) |
| Power (W) | 30 ± 8 |
| No. of bipolar RF applications | 5 ± 3 |
| Bipolar RF total time (s) | 238 ± 217 |
| Use of half-normal saline | 9 (45) |
Values are given as n (%) or mean ± SD.
AIV = anterior intraventricular vein; GCV = great cardiac vein; LVOT = left ventricular outflow tract; RF = radiofrequency; RVOT = right ventricular outflow tract.
Catheter location during bipolar ablation was as follows: GCV/AIV and LVOT in 17 patients (85%) (Figures 2 and 3); GCV/AIV and RVOT in 2 (10%); and septal perforator vein and LVOT in 1 (5%) (Figure 4). The AC was in the coronary vein in 11 cases (55%); the endocardium in 7 cases (35%); and in 2 cases (10%) the polarity was reversed so RF was applied sequentially from both the vein and the endocardium. Whereas the AC was irrigated in all cases, the RC varied: irrigated catheter in 60%, non–irrigated 8-mm catheter in 20%, nonirrigated 4-mm catheter in 15%, and multipolar mapping catheter in 5% (Figure 5). Mean distance between the catheters was 12.4 ± 4.2 mm. Earliest bipolar activation recorded at the GCV or AIV preceded the QRS onset on surface ECG by 31.5 ± 12.8 ms.
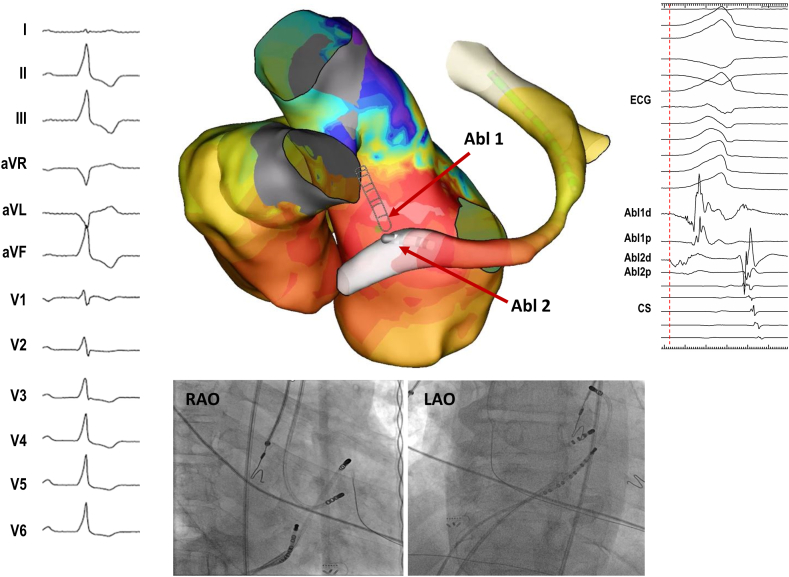
Figure 2.
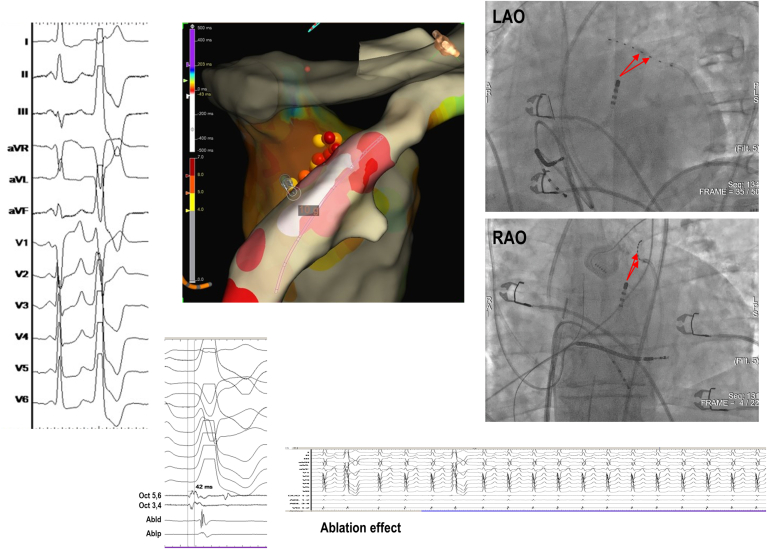
Left ventricular summit premature ventricular complex (PVC) eliminated by bipolar radiofrequency ablation from the left ventricular outflow tract (LVOT) and coronary venous system after failed standard ablation using EnSite (Abbott, St. Paul, MN). Earliest activation was recorded in the great cardiac vein (GCV)/anterior interventricular vein (AIV) junction (–40 ms), whereas the earliest endocardial site was the subaortic LVOT, below the left coronary cusp (–20 ms). Ablation from the GCV/AIV junction was limited by impedance and temperature rise, and ablation from the earliest LVOT site resulted in only transient suppression. One single bipolar radiofrequency application (20 W, 60 s) from LVOT (Abl1; active catheter) to GCV/AIV (Abl2; return catheter) resulted in durable PVC suppression. CS = coronary sinus; ECG = electrocardiogram; LAO = left anterior oblique; RAO = right anterior oblique.
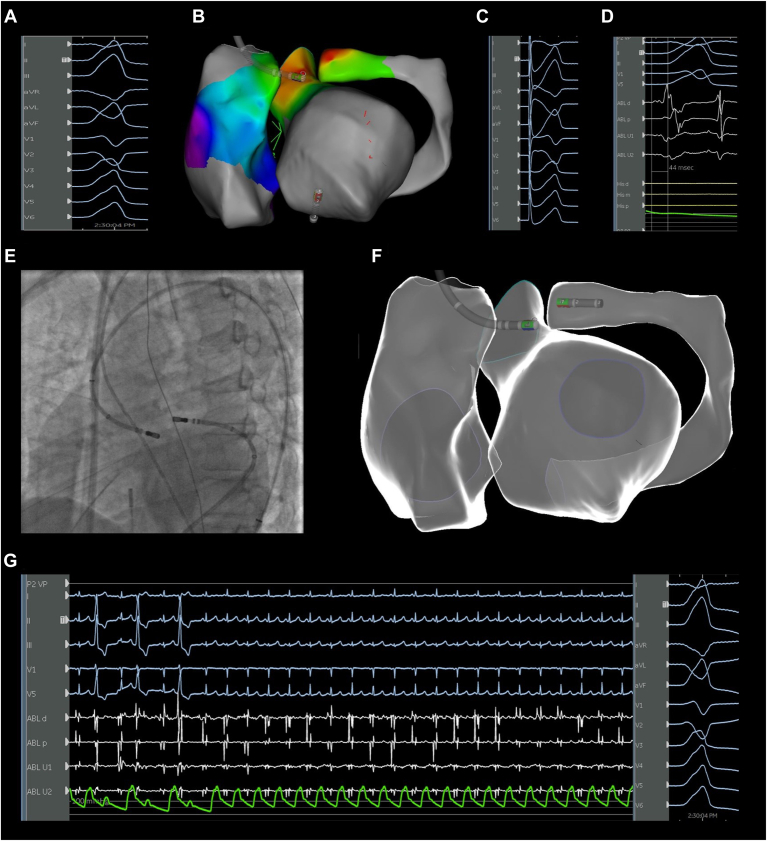
Figure 3.
LVS PVC eliminated with bipolar ablation between the GCV and left coronary cusp after failed standard ablation using CARTO (Biosense Webster, Diamond Bar, CA). A: Clinical PVC morphology. B: Activation map with an ablation catheter at the point of earliest activation. C: Pacemapping from the point of earliest activation with a 98.2% match in the PASOTM module. D: Earliest activation time (44 ms) with the sensing catheter located in the left coronary cusp. E: Fluoroscopic view of the location of the ablation catheters during bipolar ablation. F: Electroanatomic map showing the location of the ablation catheters during bipolar ablation. G: Suppression of PVCs during bipolar ablation delivery Abbreviations as in Figure 2.
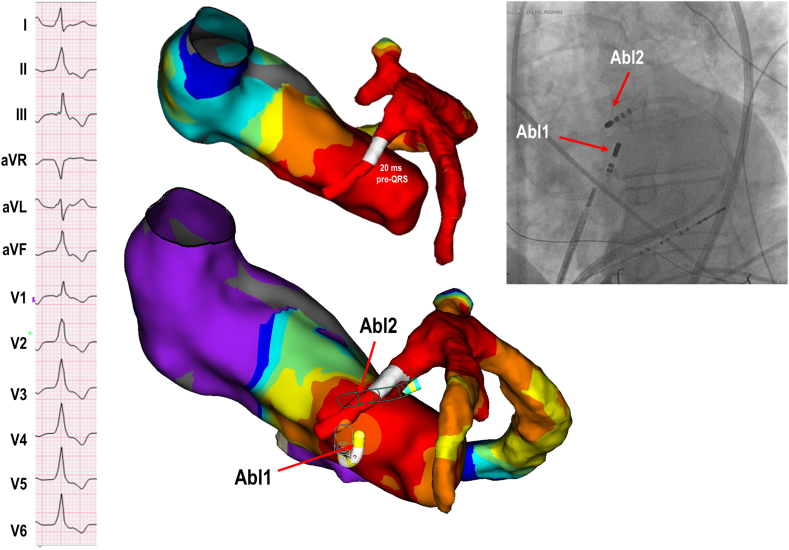
Figure 4.
Bipolar ablation between LVOT and septal perforator vein. Patient with cardiac sarcoidosis presenting with incessant bigeminy and symptomatic with dyspnea. Activation mapping showed earliest activation at the first septal perforator vein (–20 ms pre-QRS). Unipolar ablation from the LVOT opposite the intramural catheter and from the earliest site within the perforator vein resulted in only transient PVC suppression. Bipolar ablation from LVOT to the septal vein achieved PVC elimination (distance between active catheter and return catheter measured at 13 mm). Abbreviations as in Figure 2.
Figure 5.
Multipolar ablation using an irrigated ablation catheter and multielectrode mapping catheter (EPstar). Earliest local ventricular activation was recorded in the GCV (42 ms pre-QRS), but the ablation catheter could not be advanced to this site, and endocardial ablation from the LVOT was unsuccessful. Therefore, ablation was performed between the LVOT and electrodes 5 and 6 of the EPstar catheter, which were connected to the ground port of the generator via jumper cables. This resulted in successful and durable PVC suppression. Abbreviations as in Figure 2.
Bipolar ablation was delivered with maximum power of 30 ± 8 W and total duration of 238 ± 217 seconds (average number of bipolar RF applications 5 ± 3). Baseline impedance of the circuit was on average 217 ± 77 Ω (193 ± 45 Ω with 8-mm RC vs 233 ± 84 Ω with 3.5- or 4-mm RC; P = .38), and mean impedance drop during bipolar RF applications was 21 ± 9 Ω. Concomitant catheter irrigation with half-normal saline was used in 9 cases (45%), all which had previously failed unipolar RF ablation with half-normal saline. In those cases in which unipolar ablation was performed during the index procedure (n = 17), total unipolar RF time before bipolar ablation was 380 ± 276 seconds.
Outcomes
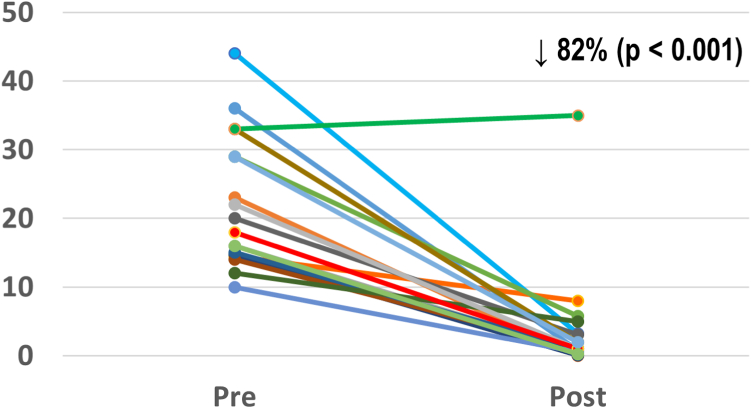
Acute PVC/VT elimination was achieved in all 20 patients (Table 3). No procedural-related complications occurred. Postablation, PVC burden was reduced from 22.2% ± 9.6% to 3.9% ± 8.1% (P <.001) (Figure 6). Over a follow-up period of 30 ± 24 months, the freedom from all VA recurrence was 85% (1 recurrence in the VT group and 2 in the PVC group). One patient had recurrent VT treated with an implantable cardioverter-defibrillator shock 28 months after the index ablation, which was the patient’s third ablation and the first in which bipolar ablation was attempted. This patient underwent a fourth procedure, in which VT was mapped again to the LVS and ablated using sequential and bipolar ablation between the GCV and the LVOT below the left coronary cusp. No recurrent arrhythmia events had been documented since then, after 12 months from the procedure. Another patient in the PVC group developed recurrent symptomatic PVCs and runs of nonsustained VT 50 months postablation. These were also mapped to the LVS and successfully ablated with bipolar ablation, this time using an 8-mm-tip catheter in the GCV. Finally, a second patient in the PVC group had recurrence of PVCs between 3 and 6 months post-procedure and had not undergone a repeat ablation at the time of this study.
Table 3.
Ablation outcomes
| Acute success | 20 (100) |
| Long-term success | 17 (85) |
| Complications | 0 (0) |
| Duration of follow-up (mo) | 30 ± 24 |
| Residual PVC burden (%) | 3.9 ± 8.1 |
| Repeat ablation | 2 (10) |
Values are given as n (%) or mean ± SD.
PVC = premature ventricular contraction.
Figure 6.
Premature ventricular contraction burden reduction after bipolar ablation (n = 18).
Discussion
In this multicenter clinical experience, we report the efficacy and safety of bipolar RF delivered between the coronary venous system and the endocardial LVOT/RVOT for treatment of LVS arrhythmias refractory to standard ablation. The study included all patients in whom this ablation approach was attempted after failed unipolar RF ablation during the same and/or a previous procedure, achieving acute and long-term success in 100% and 85% of cases, respectively. This outcome is remarkable in this highly challenging population, typically characterized by high rates of ablation failure and arrhythmia recurrence.18,19 In this series, no serious adverse events such as steam pops, myocardial perforation, pericardial effusion, or coronary injury were reported.
Earliest activation in the GCV or AIV after comprehensive mapping is the hallmark of LVS arrhythmias. However, when the septal branches of the AIV are also mapped using small wires or multielectrode catheters, many of these arrhythmias are found to originate not from the epicardium but from the intramural aspect of the basal septum,20 which explains in part the higher rates of ablation failure. Other elements that suggest an intramural origin include earliest endo/epicardial activation <20 ms pre-QRS, similar activation timing in different cardiac chambers (within 10 ms), and suboptimal pacemapping at the sites of earliest activation.
Bipolar ablation, used in the early days of catheter ablation to treat atrioventricular accessory pathways, more recently has been explored as a therapeutic option for refractory intramural arrhythmias.13 Compared to sequential unipolar or simultaneous unipolar ablation, lesions created with bipolar ablation are deeper, narrower, and more likely transmural.14 Although simultaneous unipolar and bipolar ablation may produce similar lesion volumes, the geometry of the lesions is different, with bipolar ablation having a denser necrotic core, reflective of greater core lesion temperatures. Although sequential unipolar ablation has limited ability to achieve transmural lesions in tissues >15 mm, bipolar ablation was able to achieve transmurality in tissues as deep as 25 mm.13 In an ex vivo bipolar ablation model, total lesion depth was affected by time of ablation but not by force (20g, 30g, or 40g), power (30 or 40 W), tissue thickness, or catheter orientation.21
The complex anatomy of the LVS provides several vantage points to perform bipolar ablation: (1) RVOT and LVOT to target the septal region of the LVS; (2) left pulmonary cusp and LVOT for the more apical aspect of the LVS or so-called inaccessible region; and (3) coronary veins (GCV/AIV) and LVOT/RVOT endocardium, with the GCV giving access to the more lateral region of the summit and the AIV to its more septal region. The last approach can be considered when earliest activation is recorded in the GCV or AIV, but durable arrhythmia suppression is not achieved with unipolar ablation from the endocardium and veins, suggesting an intramural site of origin. Additionally, bipolar ablation may have a role when earliest activation is in the GCV/AIV, but ablation inside the vein is limited by impedance and/or temperature rise, and endocardial ablation is unsuccessful. As with other special ablation techniques, we believe bipolar ablation should not be considered up front in most procedures but used instead as a bailout strategy after reasonable attempts using standard unipolar ablation have failed. Other simple strategies to increase lesion transmurality can be attempted first, including extended RF applications,22 catheter irrigation with low-ionic solutions,23 or modulation of baseline impedance by repositioning or adding a dispersive patch.24
Of interest, no steam pops or other complications were reported in this series despite the use of powers up to 40 W in some cases and half-normal saline irrigation in 45% of patients. In our opinion, a key element for safe bipolar ablation is careful power titration, starting at 10–20 W and gradually increasing energy (in steps of 5 W) to achieve an impedance drop >10%. As expected, impedance drops during bipolar RF application are larger compared to unipolar ablation (mean impedance drop 21 ± 9 Ω in this series), consistent with simultaneous lesion formation in both catheter–tissue interfaces. Exaggerated impedance drops are associated with an increased risk of steam pops,25 and ablation should be stopped if impedance drop exceeds 20%, especially if a rapid increase in tissue density is seen on intracardiac echocardiography. Adequate catheter selection is another important aspect to consider. An open irrigated catheter is the preferred choice for AC in most cases, especially when applying RF energy inside a coronary vein, whereas either an irrigated catheter or an 8-mm catheter is a reasonable option for return electrodes. Data from experimental studies indicate that the largest and deepest lesions are produced by using irrigated active and ground catheters oriented perpendicular to the tissue.26 Conversely, using a nonirrigated catheter as the active electrode is associated with an increased risk of steam pops, especially when there is a size mismatch between the active and return electrodes.26 In our series, baseline impedances values in bipolar configuration often were >200 Ω (lower when an 8-mm catheter was used as RC) and as high as 400 Ω in some cases. This is expected considering that the circuit involves 2 catheter–tissue interfaces, and the surface area of the RC is significantly smaller than a ground patch. In addition, the impedance inside a coronary vein often is high due to the connective tissue composition of the vein wall and the smaller blood volume surrounding the ablation catheter. To overcome these high impedance values, the upper limit impedance often needs to be adjusted or turned off during ablation. Finally, another safety measure is evaluation of the coronary anatomy with an intraprocedural angiogram, which is mandatory in every case of bipolar ablation involving the coronary venous system, and the 5-mm rule applies whether the catheter positioned in the vein is the active or return electrode.
In one of our cases, multipolar ablation was performed by incorporating 2 electrodes of a mapping catheter in the circuit, a strategy that has been recently reported.17 This novel approach could be considered only in exceptional cases when the ablation catheter cannot be advanced to the area of interest, but caution should be exercised considering this catheter is not intended for ablation. In this case, the dispersive patch still should be included in the circuit as a means to lower the impedance and minimize the risk of steam pops.
Study limitations
This was a retrospective study and included patients from 8 different centers (each center contributed 1–4 cases). Therefore, ablation settings and tools were not homogeneous and varied across the different centers. The ablation strategy was not standardized, half-normal saline was not used in all cases, and the decision when to switch from unipolar to bipolar ablation was at the discretion of each operator. Thus, it is difficult to assert that some of these arrhythmias may not have been eliminated, for example, by using half-normal saline irrigation or extended duration RF lesions. Finally, the septal coronary veins were not routinely mapped in all cases in order to distinguish true epicardial VAs from those that originated in intramural foci.
Conclusion
In cases of LVS PVC/VT refractory to unipolar ablation, bipolar ablation performed between GCV/AIV and opposite endocardial LVOT/RVOT is effective and safe if careful titration of power and intraprocedural angiography are performed to ensure a safe distance from the coronary arteries.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Piotr Futyma reports a patent application for a bipolar ablation device. All other authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided written informed consent prior to the ablation procedure.
Ethics Statement
Collection of data was approved by Institutional Review Boards of the participating centers and the research was conducted according to the Helsinki Declaration guidelines on human research.
References
- 1.Cronin E.M., Bogun F.M., Maury P., et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019;21:1143–1144. doi: 10.1093/europace/euz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latchamsetty R., Yokokawa M., Morady F., et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol. 2015;1:116–123. doi: 10.1016/j.jacep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Ling Z., Liu Z., Su L., et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract prospective randomized study. Circ Arrhythm Electrophysiol. 2014;7:237–243. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- 4.Ghannam M., Liang J., Sharaf-Dabbagh G., et al. Mapping and ablation of intramural ventricular arrhythmias: a stepwise approach focused on the site of origin. JACC Clin Electrophysiol. 2020;6:1339–1348. doi: 10.1016/j.jacep.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Hanson M., Futyma P., Bode W., et al. Catheter ablation of intramural outflow tract premature ventricular complexes: a multicentre study. Europace. 2023;25 doi: 10.1093/europace/euad100. May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada T., McElderry H.T., Doppalapudi H., et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol. 2010;3:616–623. doi: 10.1161/CIRCEP.110.939744. [DOI] [PubMed] [Google Scholar]
- 7.Santangeli P., Marchlinski F.E., Zado E.S., et al. Percutaneous epicardial ablation of ventricular arrhythmias arising from the left ventricular summit. Circ Arrhythm Electrophysiol. 2015;8:337–343. doi: 10.1161/CIRCEP.114.002377. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T., Doppalapudi H., Litovsky S.H., McElderry H.T., Kay G.N. Challenging radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from the left ventricular summit near the left main coronary artery. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004202. [DOI] [PubMed] [Google Scholar]
- 9.Enriquez A., Malavassi F., Saenz L.C., et al. How to map and ablate left ventricular summit arrhythmias. Heart Rhythm. 2017;14:141–148. doi: 10.1016/j.hrthm.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Di Biase L., Romero J., Zado E.S., et al. Variant of ventricular outflow tract ventricular arrhythmias requiring ablation from multiple sites: Intramural origin. Heart Rhythm. 2019;16:724–732. doi: 10.1016/j.hrthm.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Neira V., Santangeli P., Futyma P., et al. Ablation strategies for intramural ventricular arrhythmias. Heart Rhythm. 2020;17:1176–1184. doi: 10.1016/j.hrthm.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Hanson M., Futyma P., Bode W., et al. Catheter ablation of intramural outflow tract premature ventricular complexes: a multicentre study. Europace. 2023;25 doi: 10.1093/europace/euad100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koruth J.S., Dukkipati S., Miller M.A., Neuzil P., D’Avila A., Reddy V.Y. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9:1932–1941. doi: 10.1016/j.hrthm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen D.T., Zheng L., Zipse M.M., et al. Bipolar radiofrequency ablation creates different lesion characteristics compared to simultaneous unipolar ablation. J Cardiovasc Electrophysiol. 2019;30:2960–2967. doi: 10.1111/jce.14213. [DOI] [PubMed] [Google Scholar]
- 15.Futyma P., Sander J., Ciąpała K., et al. Bipolar radiofrequency ablation delivered from coronary veins and adjacent endocardium for treatment of refractory left ventricular summit arrhythmias. J Interv Card Electrophysiol. 2020;58:307–313. doi: 10.1007/s10840-019-00609-9. [DOI] [PubMed] [Google Scholar]
- 16.Tokioka S., Fukamizu S., Kawamura I., Kitamura T., Hojo R. Bipolar radiofrequency catheter ablation between the left ventricular endocardium and great cardiac vein for refractory ventricular premature complexes originating from the left ventricular summit. J Arrhythm. 2020;36:363–366. doi: 10.1002/joa3.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes G.C., Nguyen T., Creed E., et al. Multipolar ablation using mapping electrodes: a novel approach to intramural arrhythmia substrates. JACC Clin Electrophysiol. 2023;9:680–685. doi: 10.1016/j.jacep.2022.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Chung F.P., Lin C.Y., Shirai Y., et al. Outcomes of catheter ablation of ventricular arrhythmia originating from the left ventricular summit: a multicenter study. Heart Rhythm. 2020;17:1077–1083. doi: 10.1016/j.hrthm.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Te-Rosano A.L.D., Chung F.P., Lin Y.J., Chen S.A. Outcomes of catheter ablation of left ventricular summit arrhythmias. Card Electrophysiol Clin. 2023;15:85–92. doi: 10.1016/j.ccep.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Guandalini G.S., Santangeli P., Schaller R., et al. Intramyocardial mapping of ventricular premature depolarizations via septal venous perforators: differentiating the superior intraseptal region from left ventricular summit origins. Heart Rhythm. 2022;19:1475–1483. doi: 10.1016/j.hrthm.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 21.John M., Rook A., Post A., Mersman A., Allen W., Schramm C., Razavi M. Bipolar ablation's unique paradigm: Duration and power as respectively distinct primary determinants of transmurality and steam pop formation. Heart Rhythm O2. 2020;1:290–296. doi: 10.1016/j.hroo.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg L., Daubert T., Lin A., et al. Utility of prolonged duration endocardial ablation for ventricular arrhythmias originating from the left ventricular summit. JACC Clin Electrophysiol. 2022;8:465–476. doi: 10.1016/j.jacep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen D.T., Gerstenfeld E.P., Tzou W.S., et al. Radiofrequency ablation using an open irrigated electrode cooled with half-normal saline. JACC Clin Electrophysiol. 2017;3:1103–1110. doi: 10.1016/j.jacep.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Shapira-Daniels A., Barkagan M., Rottmann M., et al. Modulating the baseline impedance: an adjunctive technique for maximizing radiofrequency lesion dimensions in deep and intramural ventricular substrate. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John M., Rook A., Post A., et al. Bipolar ablation’s unique paradigm: duration and power as respectively distinct primary determinants of transmurality and steam pop formation. Heart Rhythm O2. 2020;1:290–296. doi: 10.1016/j.hroo.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen D.T., Tzou W.S., Brunnquell M., et al. Clinical and biophysical evaluation of variable bipolar configurations during radiofrequency ablation for treatment of ventricular arrhythmias. Heart Rhythm. 2016;13:2161–2171. doi: 10.1016/j.hrthm.2016.07.011. [DOI] [PubMed] [Google Scholar]