Abstract
Cardiac sarcoidosis (CS) can mimic any cardiomyopathy due to its ability to manifest with a variety of clinical presentations. The exact prevalence of CS remains unknown but has been reported ranging from 2.3% to as high as 29.9% among patients presenting with new onset cardiomyopathy and/or atrioventricular block. Early and accurate diagnosis of CS is often challenging due to the nature of disease progression and lack of diagnostic reference standard. The current diagnostic criteria for CS are lacking in sensitivity and specificity. Here, we review the contemporary role of advanced imaging modalities such as cardiac magnetic resonance imaging and positron emission tomography/computed tomography imaging in diagnosing and prognosticating patients with CS.
Keywords: Cardiac sarcoidosis, Cardiac magnetic resonance imaging, PET, Sarcoid, Sudden cardiac death
Key Findings.
-
▪
Cardiac sarcoidosis (CS) can mimic any cardiomyopathy, partly due to various clinical manifestations of the disease, depending on the location and extent of granuloma infiltration within the heart.
-
▪
Multiple diagnostic criteria have been proposed for diagnosing and treating CS early.
-
▪
Current evidence shows that cardiac magnetic resonance imaging and positron emission tomography/computed tomography scan have pivotal roles in the diagnosis, management, and prognostication of CS.
Introduction
Sarcoidosis is a systemic inflammatory disease that may affect any organ in the body. It is characterized by deposition of noncaseating granulomas in any organ leading to local inflammation and fibrosis. The exact etiology and pathogenesis of the disease remains unclear despite decades of extensive research.1 It was hypothesized that dysregulation in immunologic response to various environmental antigens in a genetically predisposed individual, and results in cytokine storm and granuloma formations in the affected organ.2 Cardiac sarcoidosis (CS) remains one of the leading causes of death among patients with sarcoidosis.2 The prevalence of CS, among patients presenting with new onset cardiomyopathy and/or atrioventricular block, is unknown but may be as high as 29.9%.3,4 The clinical manifestations of CS vary widely depending on the location of granulomas deposition in the heart. Often, high-grade atrioventricular block, ventricular arrhythmias, and heart failure may be the initial presenting signs of CS.5, 6, 7, 8 The present review focuses on the diagnostic approach to CS and the utility of advanced imaging studies in diagnosing and managing CS.
Diagnosis of CS
Initial approach
CS can mimic any cardiomyopathy in different stages, such as arrhythmogenic cardiomyopathy, dilated cardiomyopathy, acute coronary syndrome, giant cell myocarditis, and Chagas disease.9, 10, 11, 12 CS may affect different parts of the heart. The clinical manifestations of CS (Table 1) vary widely and usually depend on the location and extent of granuloma formation in the heart.7 Common initial signs of CS may be new left bundle branch block (Figure 1), high-grade atrioventricular block, atrial arrhythmias, heart failure with reduced left ventricular systolic function, or ventricular arrhythmias.7,13 Hence, the diagnosis of CS relies on a high index of suspicion of the disease across all age groups and incorporates relevant clues from the patient history and clinical data, imaging studies, and histopathological data.
Table 1.
Main clinical manifestations of cardiac sarcoidosis in a large cohort of 351 patients
| Clinical manifestations∗ | % |
|---|---|
| High-grade AVB | 43 |
| Heart failure | 15 |
| Sudden cardiac death | 14 |
| Sustained VT | 13 |
| Nonsustained VT | 6 |
| Syndrome mimicking AMI† | 3 |
| Atrial tachycardia | 1 |
| Others‡ | 4 |
AMI = acute myocardial infarction; AVB = atrioventricular block; VT = ventricular tachycardia.
From the study of Nordenswan and colleagues7 involving a nationwide registry of Myocardial Inflammatory Diseases in Finland with female predominance (71%) and a mean age of 51 years. The diagnosis of cardiac sarcoidosis was based on the 2014 Heart Rhythm Society and World Association of Sarcoidosis and Other Granulomatous Diseases diagnostic criteria for cardiac sarcoidosis.20,21
Chest pain and ischemic changes on the electrocardiogram with normal coronary angiogram.
1 or more of the following: unexplained syncope, elevated cardiac troponin, bundle branch block on the electrocardiogram, or typical angina, fatigue, or dyspnea.
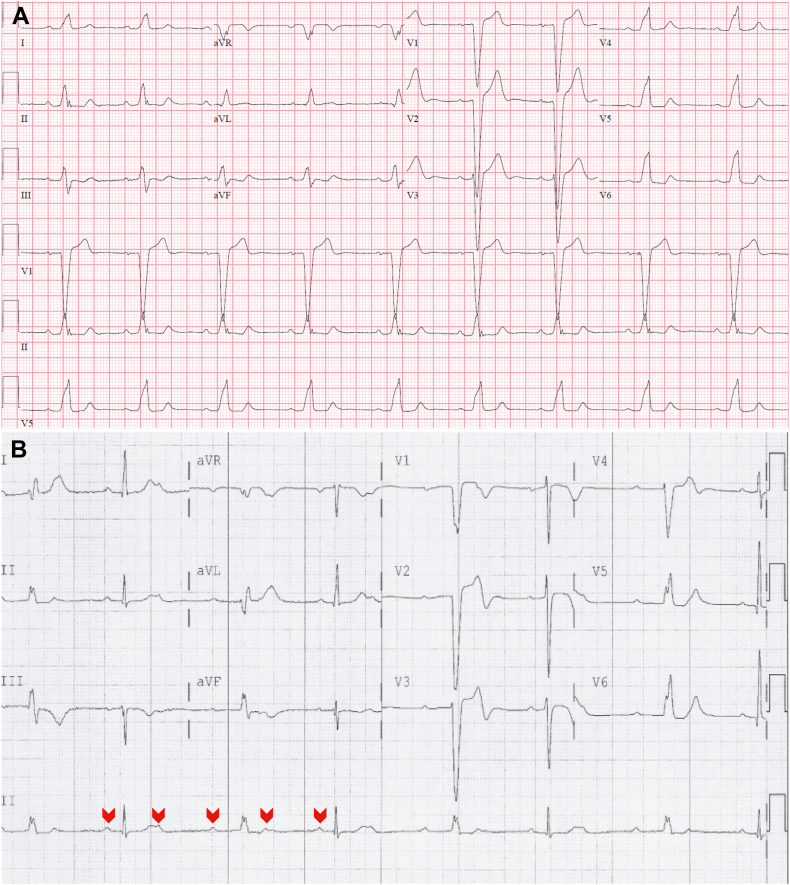
Figure 1.
A: A resting 12-lead electrocardiogram (ECG) showed sinus rhythm with new onset left bundle branch block and unremarkable coronary angiogram. B: Another resting 12-lead ECG showed 2:1 atrioventricular block with a normally conducted P-wave (first red arrowhead) to the ventricle, followed by a nonconducted P-wave, and then the following P-wave (third red arrowhead) conducted to the ventricle with a prolonged PR interval and left bundle branch block. This is a repetitive pattern. These nonspecific ECGs findings were the initial clinical manifestations of cardiac sarcoidosis in our patients with extracardiac biopsy-proven sarcoidosis and typical pattern for cardiac sarcoidosis on fluorodeoxyglucose positron emission tomography and cardiac magnetic resonance imaging studies.
The reported prevalence of CS among patients with biopsy-proven extracardiac sarcoidosis has been estimated at 5% to 10%.3,14 The prevalence of CS continued to increase over recent decades partly due to an increased recognition of the disease and wider use of advanced imaging tools for diagnosing CS. A majority of the patients with clinically manifest CS have conduction abnormalities. Patient with sarcoidosis should be screened for possible cardiac involvement with a 12-lead electrocardiogram (ECG) for conduction abnormalities (complete bundle branch block or atrioventricular block), atrial and/or ventricular arrhythmias, fragmented QRS complexes, Q waves or premature ventricular complexes.15 These are very nonspecific findings for CS.
In patients with suspected CS, 2-dimensional transthoracic echocardiography may be used to examine for left or right ventricular systolic function, wall motion abnormalities, ventricular wall thickening or thinning (involvement of basal anterior septal wall as shown in Figure 2), left ventricular aneurysm, pericardial effusion, and global left ventricular longitudinal strain. Other low-yield tests include Holter monitoring or treadmill exercise stress testing to detect arrhythmias or high-grade atrioventricular block and biomarkers such as angiotensin-converting enzyme, urinary calcium, natriuretic peptide, cardiac troponin, interleukin, or interferon.16, 17, 18 Nonetheless, all these tests lack sensitivity and specificity in detecting CS.
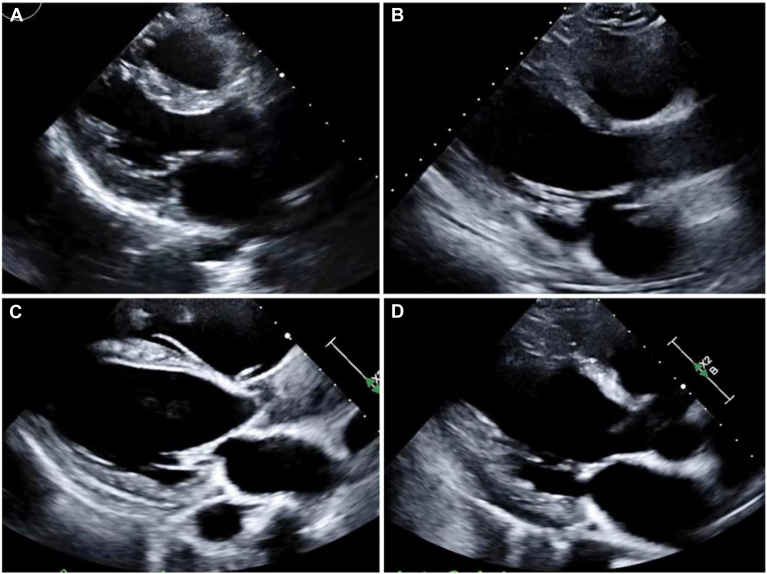
Figure 2.
Transthoracic echocardiogram (TTE) parasternal long-axis views showed (A) normal basal anteroseptal wall thickness and echogenicity in a healthy patient, (B) thinning of basal anteroseptal wall without fibrosis in a patient with endomyocardial biopsy proven cardiac sarcoidosis, (C) diffuse anteroseptal wall thinning and endocardial fibrosis, and (D) localized basal anteroseptal wall thinning and fibrosis. Involvement of basal anteroseptal wall is a highly suggestive of cardiac sarcoidosis.
Diagnostic criteria
Early recognition of CS is of utmost importance to improve clinical outcomes.19 However, early diagnosis of CS, especially isolated CS (without extracardiac manifestation) and subclinical CS (asymptomatic), remains difficult and poses some diagnostic challenges. Several clinical diagnostic criteria for CS (Table 2) have been established by the Heart Rhythm Society (HRS), World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG), and Japanese Circulation Society (JCS), to streamline the diagnosis of CS.20, 21, 22 Of note, both HRS and WASOG guidelines for the diagnosis of CS require positive histology of sarcoidosis (cardiac or extracardiac). These guidelines are certainly limited and have lower sensitivity in diagnosing isolated CS (without extracardiac manifestation).20,21 The revised 2016 JCS guidelines, on the other hand, do not require positive histology.22 Nonetheless, these guidelines were based on experts’ consensus, and they have not been systematically validated. Hence, a multidisciplinary approach with the incorporation of patient’s history, presenting symptoms, and clinical findings on ECG, transthoracic echocardiography, and advanced cardiac imaging tools for the diagnosis and management of CS as shown in Figure 3 has been suggested by the HRS, WASOG, and JCS.20, 21, 22
Table 2.
Summary of diagnostic criteria for CS
| Diagnostic criteria for CS | HRS∗ | JCS† | JCS (isolated CS)‡ | WASOG§ |
|---|---|---|---|---|
| Histological diagnoses | ||||
| EMB demonstrating presence of noncaseating granulomas with no other etiology identified | Definite CS | Definite CS | Definite isolated CS | Highly probable CS |
| Extracardiac biopsy demonstrating sarcoidosis with no other etiology identified | Probable CS | N/A | N/A | N/A |
| Clinical diagnoses | ||||
| Second-degree (Mobitz type II) or third-degree heart block | ✔ | ✔ (major) | ✔ (major) | ✔ (probable) |
| Unexplained HFrEF (LVEF <40%) | ✔ | ✔ (major) (LVEF <50%) |
✔ (major) (LVEF <50%) | ✔ (probable) |
| Heart block or cardiomyopathy responsive to immunosuppressive therapy | ✔ | N/A | N/A | ✔ (probable) |
| Unexplained VT >30 s (spontaneous or induced) | ✔ | ✔ (major)# | ✔ (major)# | ✔ (probable) |
| LGE on CMR (typical pattern for CS) | ✔ | ✔ (major) | ✔ (major) | ✔ (probable) |
| Patchy uptake on cardiac FDG-PET scan (typical pattern for CS) | ✔ | ✔ (major) | ✔ (major) | ✔ (probable) |
| Positive gallium uptake on scintigraphy (typical pattern for CS) | ✔ | ✔ (major) | ✔ (major) | ✔ (probable) |
| Echocardiogram: basal thinning of ventricular septum or abnormal ventricular wall anatomy (aneurysm, thinning of mid or basal septum, regional wall thickening) | N/A | ✔ (major) | ✔ (major) | N/A |
| Abnormal ECG findings (RBBB, LBBB, Q waves, axis deviation, frequent PVCs, NSVT) | N/A | ✔ (minor) | N/A | No consensus |
| Perfusion defects on SPECT | N/A | ✔ (minor) | N/A | ✔ (probable) |
| Endomyocardial biopsy: monocyte infiltration and moderate or severe myocardial interstitial fibrosis | N/A | ✔ (minor) | N/A | No consensus |
| T2 prolongation on CMR | N/A | N/A | N/A | ✔ (probable) |
| Reduced LVEF in the presence of other risk factors | N/A | N/A | N/A | ✔ (possible) |
| Atrial dysrhythmias | N/A | N/A | N/A | ✔ (possible) |
CMR = cardiac magnetic resonance; CS = cardiac sarcoidosis; EMB = endomyocardial biopsy; FDG-PET = 18F-fluorodeoxyglucose positron emission tomography; HFrEF = heart failure with reduced ejection fraction; HRS = Heart Rhythm Society; LBBB = left bundle branch block; JCS = Japanese Circulation Society; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; N/A = not applicable; NSVT = nonsustained ventricular tachycardia; RBBB = right bundle branch block; PVC = premature ventricular complex; SPECT = single-photon emission computed tomography; VT = ventricular tachycardia; WASOG = World Association of Sarcoidosis and Other Granulomatous Diseases.
Diagnosis of probable CS based on 2014 HRS diagnostic criteria: proof of extracardiac biopsy of sarcoidosis plus ≥1 of the included clinical diagnoses.20
Clinical diagnosis of CS without the need of histological proof based on 2016 JCS diagnostic criteria: (1) ≥2 of the major criteria are fulfilled; or (2) 1 of the major criteria plus ≥2 of the minor criteria are fulfilled.22
In patients with no clinical evidence of extracardiac CS on CT chest (no hilar/mediastinal lymphadenopathy) or other organs (eyes, skin, liver, nervous system) and no abnormal tracer uptake (FDG-PET scan or gallium scintigraphy) in any organs other than the heart, diagnosis of isolated CS can be made without the need of histological proof based on 2016 JCS diagnostic criteria if ≥3 of the major criteria are fulfilled.22
Probability of CS based on WASOG expert consensus statements: histological proof of granulomas in at least 1 organ plus 1 of the 3 categories: (1) highly probable (likelihood for CS causing this manifestation of at least 90%); (2) probable (likelihood for CS causing this manifestation of between 50% and 89%); (3) possible (likelihood for CS causing this manifestation of <50%).21
Including ventricular fibrillation.
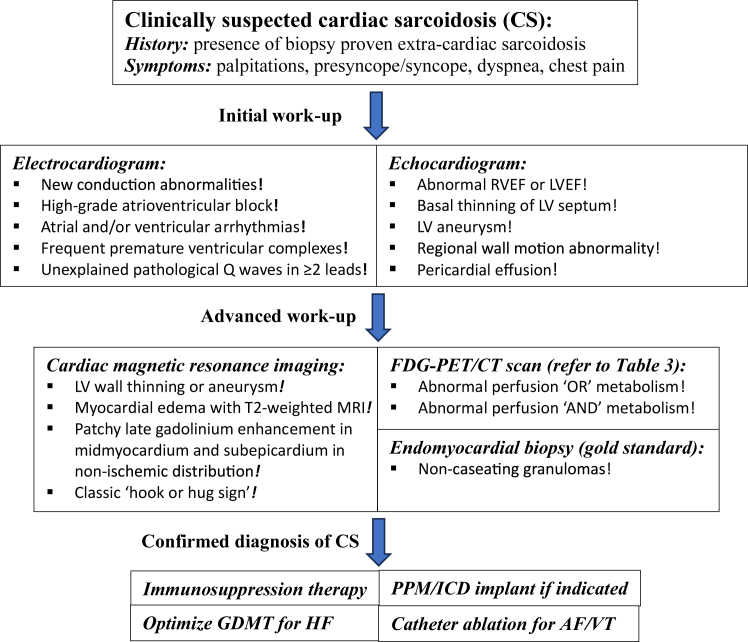
Figure 3.
General approach to the evaluation and management of cardiac sarcoidosis. AF = atrial fibrillation; CS = cardiac sarcoidosis; FDG-PET/CT = fluorodeoxyglucose positron emission tomography computed tomography; GDMT = guideline-directed medical therapy; HF = heart failure; ICD = implantable cardioverter-defibrillator; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; PPM = permanent pacemaker; RVEF = right ventricular ejection fraction; VT = ventricular tachycardia.
Utility of cardiac magnetic resonance
In patients with suspected CS, cardiac magnetic resonance (CMR) imaging offers a high-resolution assessment of cardiac anatomy and function, myocardial edema, and scarring. CMR imaging enables identification of systolic function of the ventricles with high accuracy and structural abnormalities of the ventricles such as wall thinning, aneurysm, myocardial edema with T2-weighted imaging, and late gadolinium enhancement (LGE) deposition for the diagnosis of CS. T2-weighted imaging can be used to detect myocardial edema, a regular feature of inflammation.23,24 However, this finding is not specific toward CS, as myocardial edema may be present in other conditions such as giant cell myocarditis, Lyme carditis, hypertrophic cardiomyopathy, or autoimmune-related acute myocarditis.23, 24, 25 Delayed postcontrast (15 minutes) imaging or LGE can be crucial for diagnosing CS.20, 21, 22 The presence of LGE provides meaningful diagnostic and prognostic information in patients with suspected CS.26, 27, 28 It is important to note that LGE highlights any process with increased extravascular space and therefore may represent not only fibrosis, but also inflammation. Thus, LGE is often visualized in both acute and chronic phases of CS. The typical pattern of LGE on CMR in patients with CS involves preferentially the midmyocardium and subepicardium in patchy, nonischemic distribution (Figure 4C and 4D).29 The LGE tends to involves the basal to mid septum of the left ventricle, which may extend into the right ventricle (Figure 4C and DD). LGE (Figure 4C) involving ventricular insertions across the left ventricular septum into the right ventricle has been described.30 However, this sign is nonspecific for CS as this can be seen in giant cell myocarditis.31 Our group has shown that the finding of basal inferoseptal triangular LGE pattern provides high specificity for the diagnosis of CS in a cohort of patients with nonischemic cardiomyopathy.32
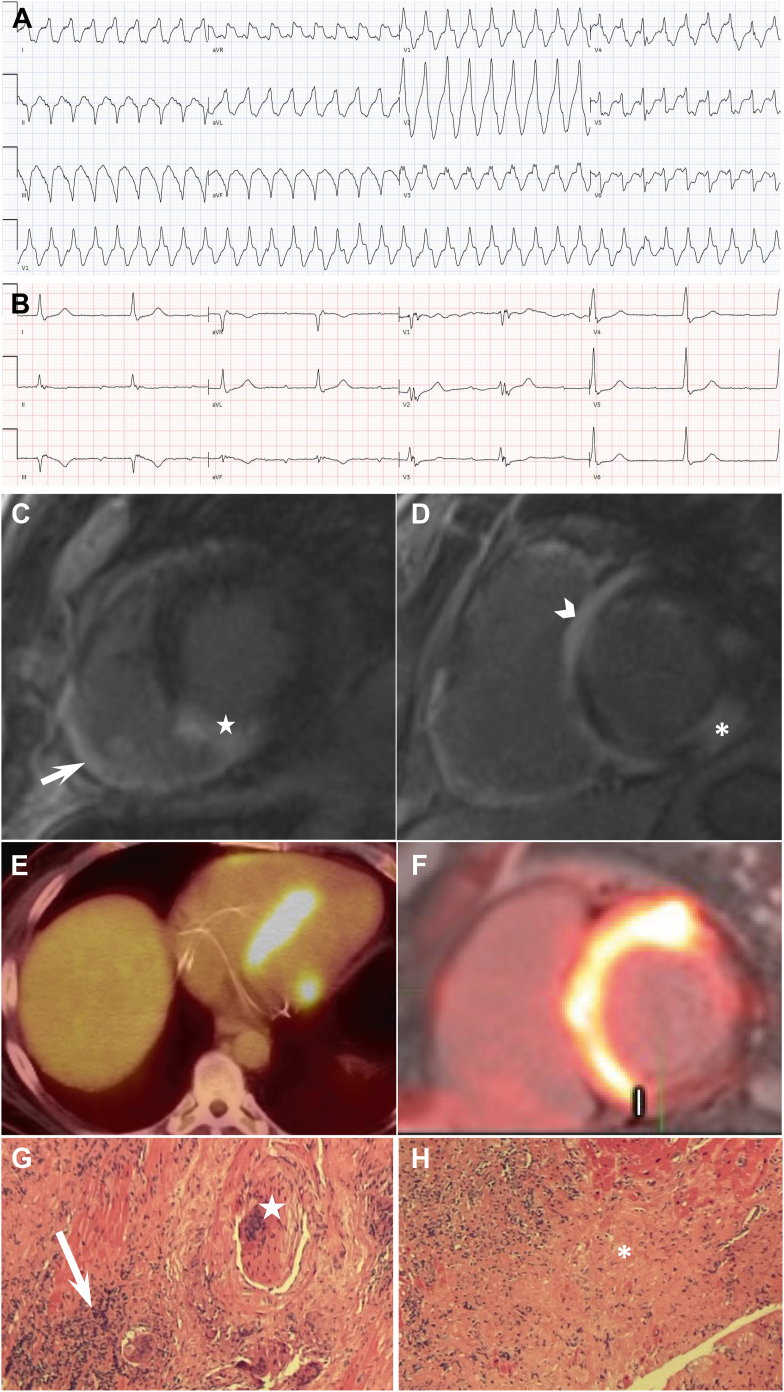
Figure 4.
Cardiac sarcoidosis and inflammation: case presentation. A 55-year-old man with history of inflammatory polyarthritis presented to our hospital for palpitation and presyncopal events. A: An initial 12-lead electrocardiogram upon arriving at emergency department showed monomorphic ventricular tachycardia at 214 beats/min. B: A repeated 12-lead electrocardiogram postcardioversion showed junctional rhythm and ectopic atrial rhythm with prolonged PR of 400 ms and right bundle branch block. C and D: Cardiac magnetic resonance showed diffuse late gadolinium enhancement in the right ventricular (RV) free wall (white arrow) and left ventricular (LV) basal to mid inferoseptal (white star), anteroseptal (white arrowhead), and inferolateral (asterisk) walls, and LV ejection fraction of 37% and RV ejection fraction of 38%. This patient has a classic hook or hug sign of late gadolinium enhancement involving ventricular insertions across the septum into the RV on cardiac magnetic resonance. E and F:18F-fluorodeoxyglucose positron emission tomography/computed tomography scan showed diffuse 18F-fluorodeoxyglucose uptake on the LV septum, basal anterior and inferior, and on RV free wall. G and H: Histological specimen from the RV myocardium of the patient showed diffuse lymphoplasmacytic infiltrate (white arrow) with Langhans multinucleated giant cell (white star) and non-necrotizing granulomas (asterisk).
CMR has high sensitivity of >90% and specificity between 77% and 85% for diagnosis of CS.4,26 The prognostic value of CMR imaging, specifically LGE, in patients with CS has been studied. In a meta-analysis of 10 studies with a total of 760 patients, Coleman and colleagues28 reported that patients with CS with LGE had 11-fold higher odds of arrhythmogenic events and all-cause mortality compared with those without LGE. Studies have also shown that a high LGE burden on CMR may be associated with lower chance of left ventricular function recovery and higher incidence of adverse outcomes (heart failure admission, life-threatening arrhythmias, cardiac-related death) in patients with CS post–immunosuppression therapy.4,33,34
Utility of fluorodeoxyglucose positron emission tomography
Cardiac fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is one of the best radionuclide imaging tools with higher spatial resolution and sensitivity than Gallium scintigraphy, Thallium, and Technicium-99m single-photon emission computed tomography to detect myocardial inflammatory activity.35,36 Accurate interpretation of the FDG-PET images are crucial in diagnosing CS. Blankstein and colleagues37 classified the results of the cardiac FDG-PET/CT into 3 major categories, as shown in Table 3.38 FDG-PET as a diagnostic tool for CS has several advantages. First, pre-existing cardiovascular implantable electronic devices do not cause significant artifact on PET images. Second, cardiac FDG-PET/CT studies can provide whole-body imaging; thus, any extracardiac involvement for sarcoidosis (such as lymph nodes, lungs, liver or spleen) may be incidentally detected as well. Third, as FDG uptake alone is a very nonspecific to CS, FDG-PET is performed along with a myocardial perfusion scan. A combination of FDG uptake and matched perfusion defects increased the sensitivity and specificity in diagnosing CS. In a recent meta-analysis of 6 studies examining the diagnostic accuracy of FDG-PET for CS, the pooled sensitivity and specificity of FDG-PET were 84% and 82%, respectively.26
Table 3.
Interpretation of cardiac FDG positron emission tomography/computed tomography perfusion and metabolism imaging
| Rest perfusion imaging | FDG (metabolic imaging) | Comments |
|---|---|---|
| Normal perfusion 'AND' metabolism (category 1) | ||
| Normal | No uptake | Normal study |
| Normal | Diffuse uptake | Abnormal metabolic imaging due to inadequate patient preparation; hence failure to suppress physiologic uptake of FDG by normal myocardium |
| Normal | Isolated lateral wall uptake | Normal variant study |
| Abnormal perfusion 'OR' metabolism (category 2) | ||
| Normal | Focal uptake | Could represent early stage of the disease |
| Abnormal perfusion | No uptake | Could represent scar from cardiac sarcoidosis or other etiologies |
| Abnormal perfusion 'AND' metabolism (category 3) | ||
| Abnormal perfusion | Focal uptake in area with abnormal perfusion (mismatch pattern) | Could represent active inflammation and/or scar in the same segment |
| Abnormal perfusion | Diffuse uptake with focal in area with abnormal perfusion | Could represent active inflammation and/or scar in the same segment with either diffuse inflammation or failure to suppress physiologic uptake of FDG by normal myocardium |
| Abnormal perfusion | Focal uptake in area with normal perfusion | Could represent both active inflammation and scar in different segments |
FDG = fluorodeoxyglucose.
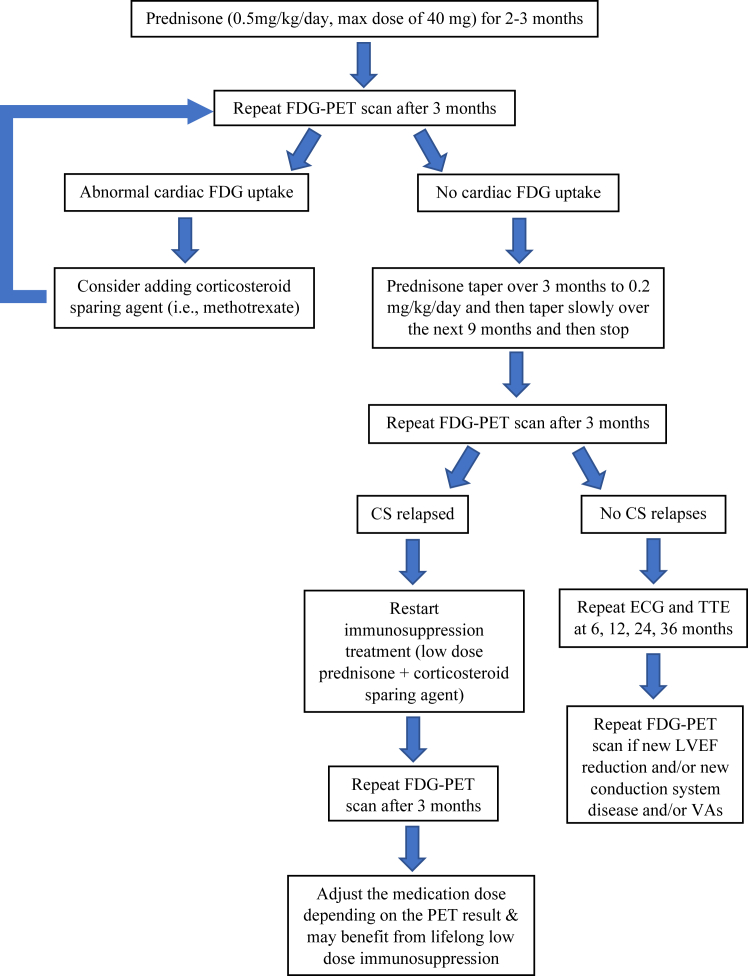
In addition, FDG-PET has important therapeutic and prognostic implications in managing patients with CS. The FDG-PET scan can be used to monitor treatment response and relapse after immunosuppression therapy. Immunosuppressive agents remain the cornerstone therapy for patients with active CS.39 The goal of the treatment is to suppress and halt any active inflammation and hence to minimize myocardial damage. There is currently no reliable biomarker to follow along patients’ clinical response to the immunosuppression therapy. Birnie and colleagues40 proposed obtaining a FDG-PET/CT study at baseline and 3 months after completing the immunosuppression treatment to determine their clinical response, as summarized in Figure 5. If there is no abnormal cardiac FDG uptake after 3 months of treatment, the clinician may further taper the steroid and stop after completing the 12-month course. The FDG-PET/CT study should be obtained 3 months after stopping steroid treatment to determine any CS relapse. If there is any abnormal cardiac FDG uptake on PET, a corticosteroid sparing agent is generally recommended.
Figure 5.
Treatment algorithm for patients with active cardiac sarcoidosis (CS). Adapted with permission from Birnie and colleagues.40 ECG = electrocardiography; FDG-PET = fluorodeoxyglucose positron emission tomography; LVEF = left ventricular ejection fraction; TTE = transthoracic echocardiography; VA = ventricular arrhythmia.
Subramanian and colleagues41 have demonstrated the use of the novel FDG myocardial uptake index (inflammatory burden based on FDG-PET scan) in 91 patients with CS. Patients with higher pretreatment myocardial uptake index of >30 are significantly associated with increased clinical (reduction in New York Heart Association functional class ≥I and freedom from ventricular arrhythmias and heart failure admissions) and echocardiographic (improvement in left ventricular ejection fraction [LVEF] >10%) responses to immunosuppression therapy.41 In addition, Muser and colleagues42 have investigated the prognostic role of serial FDG-PET scans in patients with CS presenting with ventricular arrhythmias. The authors reported reduction of myocardial inflammation quantified by standardized uptake value post–immunosuppression therapy correlated with improvement in LVEF and lower major adverse cardiac events.42 Emerging data support the role of FDG-PET scan in risk stratification of patients with CS. A retrospective analysis performed by Tuominen and colleagues43 reported that patients with suspected CS with a combined pathologic right ventricular uptake and high total cardiac metabolic activity have higher risk for future adverse cardiac events. Furthermore, a recent meta-analysis of 17 studies involving 1243 patients with CS reported patients with left or right ventricular FDG uptake were associated with major adverse cardiac events.44
Nonetheless, there are some unique challenges known to the use of FDG-PET imaging in the assessment of CS population. Prior to the FDG-PET imaging study, a patient is required to undertake dietary restriction of at least 2 high-fat (>35 g) and low-carbohydrate (<3 g) meals a day and fasting 4 to 12 hours.35,45 Alternatively, a prolonged fasting of 18 hours may be required to achieve the highest suppression of physiologic myocardial FDG uptake.35 Hence, FDG uptake in the myocardium, when present, truly represents ongoing inflammation. In addition, strict dietary preparation for FDG-PET imaging can present a major challenge for certain group of patients, especially insulin-dependent diabetic patients. Unfortunately, these tedious dietary preparations may lead to patient compliance issue and affect the result of the imaging study. It is also prudent to take note of an important limitation of FDG-PET imaging study: absence of FDG uptake cannot rule out the presence of CS.37 Diffuse FDG uptake may be seen in patient with inadequate preparation prior to the FDG-PET imaging study, due to failure to suppress physiological myocardial FDG uptake.37
Role of invasive electrophysiology study and ventricular tachycardia ablation
The diagnostic confirmation of CS is optimal with proof of CS histology but the sensitivity of conventional endomyocardial biopsy (EMB) remains relatively low (<25%),46,47 which has been attributed to the patchy and midmyocardial infiltration of the noncaseating granulomas in CS, which may not be sampled effectively by standard right ventricular myocardial biopsy techniques. Several articles have reported an additional role for invasive electrophysiology study (EPS) in combination with advanced imaging studies such as PET and CMR in diagnosing CS. Flautt and colleagues48 reported the use of PET and electroanatomic voltage mapping–guided EMB of the atrial septum for confirming the diagnosis of CS. Several other case reports and case series have reported higher diagnostic yield of CS at approximately 50% with the use of CMR and/or electroanatomic voltage mapping–guided EMB.49, 50, 51
Patients with CS may present with high-grade atrioventricular block. If a patient has an indication for pacemaker implantation, a patient shared decision-making discussion should take place to address the potential role of an implantable cardioverter-defibrillator (ICD) for primary prevention (class IIa indication) against sudden cardiac death (SCD).20,52 A nationwide registry in Finland reported the cumulative 5-year incidence of SCD among patients with clinically manifested CS with class I or IIa ICD indications by the HRS guideline was approximately 11% vs 5% in those without.53 Those without such an initial indication for ICD by the HRS guideline had a combined incidence of ventricular arrhythmias and emerging ICD indications >50% at 5 years of follow-up.53 Hence, CS patients who do not meet class I or IIa ICD indications may potentially benefit from invasive EPS for further risk stratification. Adhaduk and colleagues54 performed a recent meta-analysis of 8 studies with a total of 298 patients, finding that invasive EPS yielded a pooled sensitivity and specificity of 0.70 and 0.93, respectively, in predicting adverse events among CS patients with no prior ventricular tachycardia (VT). The authors also conducted a subgroup analysis on the utility of invasive EPS in predicting SCD among CS patients with no prior VT and with LVEF >35%. They reported that invasive EPS has a potential role of predicting adverse clinical events in CS patients with a pooled sensitivity and specificity of 0.63 and 0.97, respectively.
In patients with CS and VT, the management of VT remains challenging due to the complexity of the underlying substrate and the nature of the disease progression. Immunosuppressive therapy and antiarrhythmic drugs have been the main treatment strategies for these life-threatening ventricular arrhythmias.20 Although there is a paucity of data offering catheter ablation as first-line strategy in patients with CS and VT, observational studies support the role of catheter ablation in addition to medical treatment, especially in patients with incessant VT or VT storm.55,56 Muser and colleagues57 reported that catheter ablation provides long-term VT-free survival in 40% of patients with VT and CS. There was a significant reduction of ventricular arrhythmia burden in up to 90% of the cases. In a separate study, Muser and colleagues58 reported that in patients with CS and VT, the distribution of electroanatomical substrates correlates with the regions of LGE on CMR and FDG uptake on PET/CT. Hence, CMR and PET/CT have demonstrated potential roles in detecting electroanatomical substrates that could be targeted during substrate-based ablation approaches.58
Clinical perspective and future directions
At the current time, there are several established clinical diagnostic criteria for CS set by HRS, WASOG, and JCS. The current CS diagnostic criteria and guidelines are based mainly on expert consensus. However, discrepancies exist among the diagnostic criteria and guidelines, especially on early detection of isolated CS (without the involvement of other organs). Advanced imaging modalities such as CMR and PET/CT have promising and increasing clinical utility in the diagnosis, management, and prognostication of patients with CS. Incorporation of these advanced imaging modalities in caring for patients with CS may identify subgroups with higher risk of adverse events and improve their clinical outcomes and survival rates. The accuracy of CMR and PET/CT findings for CS remains debatable due to lack of an appropriate reference standard to diagnose and monitor treatment responses in patients with CS using these advanced imaging modalities. Additional studies are required to identify and close those gaps in providing the most comprehensive and contemporary care to patients with CS.
Conclusion
Early and accurate diagnosis of CS remains challenging, especially in patients with subclinical CS (clinically silent CS) or isolated CS (without extracardiac sarcoidosis). CMR and PET/CT have increasing roles in diagnosis, management, and prognostication of patients with CS. There is a need to encourage more prospective clinical studies to better understand the disease course and identify additional roles of CMR and PET/CT in the evaluation and management of patients with CS.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Saman Nazarian has served as the principal investigator for research funding from Biosense Webster to the University of Pennsylvania; and as a consultant for Biosense Webster. Jian Liang Tan and Gregory E. Supple have no relevant relationships to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Disclaimer
Given his role as Section Editor, Saman Nazarian had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to editors Nazem Akoum and Jeanne E. Poole.
References
- 1.Ueberham L., Hagendorff A., Klingel K., et al. Pathophysiological gaps, diagnostic challenges, and uncertainties in cardiac sarcoidosis. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drent M., Crouser E.D., Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018–1032. doi: 10.1056/NEJMra2101555. [DOI] [PubMed] [Google Scholar]
- 3.Baughman R.P., Teirstein A.S., Judson M.A., et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 4.Kouranos V., Tzelepis G.E., Rapti A., et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. J Am Coll Cardiol Img. 2017;10:1437–1447. doi: 10.1016/j.jcmg.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrom K., Lehtonen J., Nordenswan H.K., et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40:3121–3128. doi: 10.1093/eurheartj/ehz428. [DOI] [PubMed] [Google Scholar]
- 6.Fussner L.A., Karlstedt E., Hodge D.O., et al. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail. 2018;20:1713–1720. doi: 10.1002/ejhf.1319. [DOI] [PubMed] [Google Scholar]
- 7.Nordenswan H.K., Lehtonen J., Ekstrom K., et al. Manifestations and outcome of cardiac sarcoidosis and idiopathic giant cell myocarditis by 25-year nationwide cohorts. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaya Y., Kusano K.F., Nakamura K., Ito H. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. Am J Cardiol. 2015;115:505–509. doi: 10.1016/j.amjcard.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Sedaghat-Hamedani F., Kayvanpour E., Hamed S., et al. The chameleon of cardiology: cardiac sarcoidosis before and after heart transplantation. ESC Heart Fail. 2020;7:692–696. doi: 10.1002/ehf2.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton J.H., Tavora F., Farb A., Li L., Burke A.P. Unusual cardiovascular manifestations of sarcoidosis, a report of three cases: coronary artery aneurysm with myocardial infarction, symptomatic mitral valvular disease, and sudden death from ruptured splenic artery. Cardiovasc Pathol. 2010;19:e119–e123. doi: 10.1016/j.carpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Kandolin R., Ekstrom K., Simard T., et al. Spontaneous coronary artery dissection in cardiac sarcoidosis. Oxf Med Case Reports. 2019;2019:omz033. doi: 10.1093/omcr/omz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam C.S., Tolep K.A., Metke M.P., Glockner J., Cooper L.T., Jr. Coronary sarcoidosis presenting as acute coronary syndrome. Clin Cardiol. 2009;32:E68–E71. doi: 10.1002/clc.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusano K., Ishibashi K., Noda T., et al. Prognosis and outcomes of clinically diagnosed cardiac sarcoidosis without positive endomyocardial biopsy findings. JACC: Asia. 2021;1:385–395. doi: 10.1016/j.jacasi.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel M.R., Cawley P.J., Heitner J.F., et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohira H., Sato T., Manabe O., et al. Underdiagnosis of cardiac sarcoidosis by ECG and echocardiography in cases of extracardiac sarcoidosis. ERJ Open Res. 2022;8:00516–2021. doi: 10.1183/23120541.00516-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martusewicz-Boros M.M., Boros P.W., Wiatr E., Zych J., Piotrowska-Kownacka D., Roszkowski-Sliz K. Prevalence of cardiac sarcoidosis in white population: a case-control study: Proposal for a novel risk index based on commonly available tests. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiko T., Yoshihisa A., Kanno Y., et al. A Multiple biomarker approach in patients with cardiac sarcoidosis. Int Heart J. 2018;59:996–1001. doi: 10.1536/ihj.17-695. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T., Kanda T., Kubota S., Imai S., Murata K. Holter monitoring as a noninvasive indicator of cardiac involvement in sarcoidosis. Chest. 1994;106:1021–1024. doi: 10.1378/chest.106.4.1021. [DOI] [PubMed] [Google Scholar]
- 19.Padala S.K., Peaslee S., Sidhu M.S., Steckman D.A., Judson M.A. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–570. doi: 10.1016/j.ijcard.2016.10.101. [DOI] [PubMed] [Google Scholar]
- 20.Birnie D.H., Sauer W.H., Bogun F., et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Judson M.A., Costabel U., Drent M., et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 22.Terasaki F., Azuma A., Anzai T., et al. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis - digest version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 23.Eitel I., Friedrich M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson. 2011;13:13. doi: 10.1186/1532-429X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrich M.G., Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–839. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 25.Chen S., Huang L., Zhang Q., Wang J., Chen Y. T2-weighted cardiac magnetic resonance image and myocardial biomarker in hypertrophic cardiomyopathy. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aitken M., Chan M.V., Urzua Fresno C., et al. Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 2022;304:566–579. doi: 10.1148/radiol.213170. [DOI] [PubMed] [Google Scholar]
- 27.Hulten E., Agarwal V., Cahill M., et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman G.C., Shaw P.W., Balfour P.C., Jr., et al. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. J Am Coll Cardiol Img. 2017;10:411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blankstein R., Waller A.H. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.113.000867. [DOI] [PubMed] [Google Scholar]
- 30.Lehtonen J., Uusitalo V., Poyhonen P., Mayranpaa M.I., Kupari M. Cardiac sarcoidosis: phenotypes, diagnosis, treatment, and prognosis. Eur Heart J. 2023;44:1495–1510. doi: 10.1093/eurheartj/ehad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S., Chen X., Li J., et al. Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Fail. 2021;8:2320–2327. doi: 10.1002/ehf2.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo L., Han Y., Mui D., et al. Diagnostic specificity of basal inferoseptal triangular late gadolinium enhancement for identification of cardiac sarcoidosis. J Am Coll Cardiol Img. 2019;12:2574–2576. doi: 10.1016/j.jcmg.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Ise T., Hasegawa T., Morita Y., et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–1172. doi: 10.1136/heartjnl-2013-305187. [DOI] [PubMed] [Google Scholar]
- 34.Greulich S., Deluigi C.C., Gloekler S., et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. J Am Coll Cardiol Img. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Osborne M.T., Hulten E.A., Murthy V.L., et al. Patient preparation for cardiac fluorine–18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24:86–99. doi: 10.1007/s12350-016-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chareonthaitawee P., Beanlands R.S., Chen W., et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–1758. doi: 10.1007/s12350-017-0978-9. [DOI] [PubMed] [Google Scholar]
- 37.Blankstein R., Osborne M., Naya M., et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slart R., Glaudemans A., Lancellotti P., et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25:298–319. doi: 10.1007/s12350-017-1043-4. [DOI] [PubMed] [Google Scholar]
- 39.Baughman R.P., Valeyre D., Korsten P., et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58 doi: 10.1183/13993003.04079-2020. [DOI] [PubMed] [Google Scholar]
- 40.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian M., Swapna N., Ali A.Z., et al. Pre-treatment myocardial (18)FDG uptake predicts response to immunosuppression in patients with cardiac sarcoidosis. J Am Coll Cardiol Img. 2021;14:2008–2016. doi: 10.1016/j.jcmg.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Muser D., Santangeli P., Castro S.A., et al. Prognostic role of serial quantitative evaluation of (18)F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45:1394–1404. doi: 10.1007/s00259-018-4001-8. [DOI] [PubMed] [Google Scholar]
- 43.Tuominen H., Haarala A., Tikkakoski A., Kahonen M., Nikus K., Sipila K. FDG-PET in possible cardiac sarcoidosis: right ventricular uptake and high total cardiac metabolic activity predict cardiovascular events. J Nucl Cardiol. 2021;28:199–205. doi: 10.1007/s12350-019-01659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aitken M., Davidson M., Chan M.V., et al. Prognostic value of cardiac MRI and FDG PET in cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 2023;307 doi: 10.1148/radiol.222483. [DOI] [PubMed] [Google Scholar]
- 45.Bois J.P., Chareonthaitawee P. Continuing evolution in preparation protocols for (18)FDG PET assessment of inflammatory or malignant myocardial disease. J Nucl Cardiol. 2017;24:989–992. doi: 10.1007/s12350-016-0477-4. [DOI] [PubMed] [Google Scholar]
- 46.Casella M., Dello Russo A., Bergonti M., et al. Diagnostic yield of electroanatomic voltage mapping in guiding endomyocardial biopsies. Circulation. 2020;142:1249–1260. doi: 10.1161/CIRCULATIONAHA.120.046900. [DOI] [PubMed] [Google Scholar]
- 47.Cooper L.T., Baughman K.L., Feldman A.M., et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 48.Flautt T., Lador A., Da-Wariboko A., Schwartz M., Valderrabano M. PET-driven, voltage-guided atrial endomyocardial biopsy clinches the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol Case Rep. 2021;3:1764–1768. doi: 10.1016/j.jaccas.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haanschoten D.M., Adiyaman A., ‘t Hart N.A., Jager P.L., Elvan A. Value of 3D mapping-guided endomyocardial biopsy in cardiac sarcoidosis: case series and narrative review on the value of electro-anatomic mapping-guided endomyocardial biopsies. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13497. [DOI] [PubMed] [Google Scholar]
- 50.Trivieri M.G., Spagnolo P., Birnie D., et al. Challenges in cardiac and pulmonary sarcoidosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1878–1901. doi: 10.1016/j.jacc.2020.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang J.J., Hebl V.B., DeSimone C.V., et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. J Am Coll Cardiol HF. 2014;2:466–473. doi: 10.1016/j.jchf.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. doi: 10.1016/j.hrthm.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 53.Nordenswan H.K., Poyhonen P., Lehtonen J., et al. Incidence of sudden cardiac death and life-threatening arrhythmias in clinically manifest cardiac sarcoidosis with and without current indications for an implantable cardioverter defibrillator. Circulation. 2022;146:964–975. doi: 10.1161/CIRCULATIONAHA.121.058120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adhaduk M., Paudel B., Liu K., Ashwath M., Giudici M. The role of electrophysiology study in risk stratification of cardiac sarcoidosis patients: meta-analyses and systemic review. Int J Cardiol. 2022;349:55–61. doi: 10.1016/j.ijcard.2021.11.061. [DOI] [PubMed] [Google Scholar]
- 55.Siontis K.C., Santangeli P., Muser D., et al. Outcomes associated with catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. JAMA Cardiol. 2022;7:175–183. doi: 10.1001/jamacardio.2021.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan J.L., Jin C., Lee J.Z., Gaughan J., Iwai S., Russo A.M. Outcomes of catheter ablation for ventricular tachycardia in patients with sarcoidosis: insights from the National Inpatient Sample database (2002–2018) J Cardiovasc Electrophysiol. 2022;33:2585–2598. doi: 10.1111/jce.15708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muser D., Santangeli P., Pathak R.K., et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004333. [DOI] [PubMed] [Google Scholar]
- 58.Muser D., Santangeli P., Liang J.J., et al. Characterization of the electroanatomic substrate in cardiac sarcoidosis: correlation with imaging findings of scar and inflammation. J Am Coll Cardiol EP. 2018;4:291–303. doi: 10.1016/j.jacep.2017.09.175. [DOI] [PubMed] [Google Scholar]