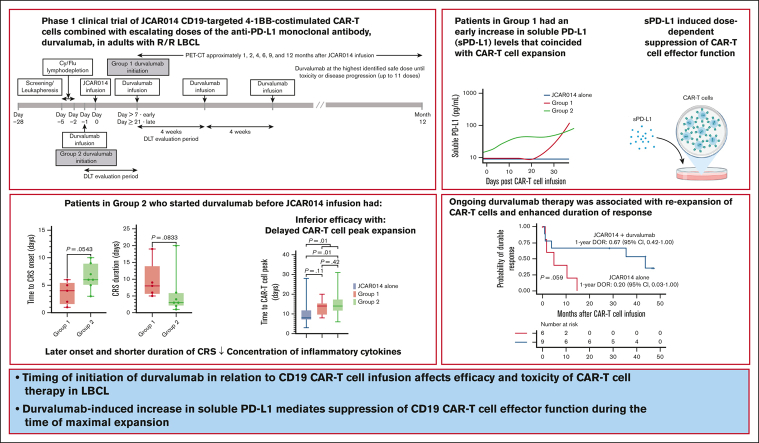
Key Points
-
•
Timing of initiation of durvalumab in relation to CD19 CAR T-cell infusion affects efficacy and toxicity of CAR T-cell therapy in LBCL.
-
•
Durvalumab-induced increase in sPD-L1 mediates suppression of CD19 CAR T-cell effector function during the time of maximal expansion.
Visual Abstract
Abstract
More than half of the patients treated with CD19-targeted chimeric antigen receptor (CAR) T-cell immunotherapy for large B-cell lymphoma (LBCL) do not achieve durable remission, which may be partly due to PD-1/PD-L1–associated CAR T-cell dysfunction. We report data from a phase 1 clinical trial (NCT02706405), in which adults with LBCL were treated with autologous CD19 CAR T cells (JCAR014) combined with escalating doses of the anti–PD-L1 monoclonal antibody, durvalumab, starting either before or after CAR T-cell infusion. The addition of durvalumab to JCAR014 was safe and not associated with increased autoimmune or immune effector cell–associated toxicities. Patients who started durvalumab before JCAR014 infusion had later onset and shorter duration of cytokine release syndrome and inferior efficacy, which was associated with slower accumulation of CAR T cells and lower concentrations of inflammatory cytokines in the blood. Initiation of durvalumab before JCAR014 infusion resulted in an early increase in soluble PD-L1 (sPD-L1) levels that coincided with the timing of maximal CAR T-cell accumulation in the blood. In vitro, sPD-L1 induced dose-dependent suppression of CAR T-cell effector function, which could contribute to inferior efficacy observed in patients who received durvalumab before JCAR014. Despite the lack of efficacy improvement and similar CAR T-cell kinetics early after infusion, ongoing durvalumab therapy after JCAR014 was associated with re-expansion of CAR T cells in the blood, late regression of CD19+ and CD19– tumors, and enhanced duration of response. Our results indicate that the timing of initiation of PD-L1 blockade is a key variable that affects outcomes after CD19 CAR T-cell immunotherapy for adults with LBCL.
Introduction
Lymphodepletion chemotherapy followed by infusion of CD19-targeted chimeric antigen receptor (CAR) T cells has achieved high response rates among patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL).1, 2, 3, 4 However, more than half of the treated patients do not achieve durable remissions,1, 2, 3, 4 which may partly be due to CAR T-cell dysfunction.5,6 High expression of the inhibitory receptor, programmed cell death-1 (PD-1), on tumor-infiltrating T cells7 and of its ligand, programmed cell death–ligand 1 (PD-L1), on tumor cells8 have been associated with inferior overall survival (OS) in patients with diffuse LBCL (DLBCL) treated in the chemoimmunotherapy era. Furthermore, high PD-L1 expression was observed in pretreatment tumor biopsy specimens from patients who failed to achieve durable responses to CD19 CAR T-cell immunotherapy.9 These data indicate that interruption of the PD-1/PD-L1 axis might improve the success of CAR T-cell therapy for LBCL.
Case reports and a small phase 1 clinical trial reported responses to anti–PD-1 monoclonal antibody therapy in a subset of patients with progression of LBCL after CD19 CAR T cells.10, 11, 12 However, the data from studies investigating CD19 CAR T-cell products combined with immune checkpoint blockade (ICB) immunotherapy have been disappointing.13, 14, 15 The reasons for the failure of ICB in combination with CD19 CAR T-cell therapies were not clear. Furthermore, these studies were not powered to address the differences in combination therapy regimens.
Durvalumab is an anti–PD-L1 monoclonal antibody approved for the treatment of non–small cell lung cancer, small cell lung cancer, biliary tract cancer, and hepatocellular carcinoma. Here, we report data from a phase 1 clinical trial of defined composition autologous CD19 CAR T cells administered in combination with durvalumab for adults with R/R LBCL. A PD-L1 inhibitor was favored over a PD-1 inhibitor because of the lower risk of severe adverse events (AEs), particularly immune-related AEs.16,17 We show that the timing of initiation of durvalumab in relation to CD19 CAR T-cell infusion was associated with differences in efficacy and toxicity of CAR T-cell therapy due to durvalumab-induced increase in soluble PD-L1 (sPD-L1), which mediated the suppression of CAR T-cell effector function.
Patients and methods
Study design and participants
We conducted an investigator-initiated, prospective, open-label, single-center, phase 1 dose-finding study (NCT02706405) of the safety and efficacy of combination therapy with defined composition autologous CD19 CAR T cells and escalating doses of durvalumab in adults with R/R LBCL. Eligible patients were aged ≥18 years and had positron emission tomography (PET)–positive R/R LBCL with either persistent disease after firstline chemoimmunotherapy containing an anti-CD20 monoclonal antibody and anthracycline, relapsed disease after firstline chemoimmunotherapy and ineligibility for autologous stem cell transplant (ASCT), or R/R disease after ≥2 lines of systemic therapy or ASCT (supplemental Methods). The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, with the approval of the Fred Hutchinson Cancer Center Institutional Review Board. All participants provided written informed consent.
Study treatment
Manufacturing of CD19 CAR T cells formulated at a 1:1 CD4+:CD8+ CAR T-cell ratio (JCAR014) has been previously described.18,19 Bridging antitumor therapy after leukapheresis was allowed during JCAR014 manufacturing, with reconfirmation of PET-positive disease required before lymphodepletion chemotherapy.
The first 2 treated patients received lymphodepletion with cyclophosphamide (Cy; 30 mg/kg) and fludarabine (Flu; 25 mg/m2 per day for 3 days) and 7 × 105 CAR T cells per kg. After establishing safety, subsequent patients received lymphodepletion with Cy 300 mg/m2 per day and Flu 30 mg/m2 per day for 3 days, followed by 2 × 106 CAR T cells per kg 2 to 5 days after the completion of lymphodepletion. Patients received durvalumab IV in 1 of 2 groups (supplemental Figure 1). Patients in group 1 initially received the first dose of durvalumab no earlier than 21 days after JCAR014. After analyses of safety, subsequent patients received durvalumab starting no earlier than 7 days after JCAR014. Patients in group 2 received the first dose of durvalumab 1 day before JCAR014 administration. The dose levels of the first durvalumab infusion in groups 1 and 2 are listed in supplemental Table 1. For both groups, durvalumab was administered at the highest identified safe dose approximately every 4 weeks for up to 10 cycles after JCAR014 infusion or until unacceptable toxicity or disease progression. Patients with progressive disease (PD) could continue durvalumab to the maximum cycle number if data indicated potential for subsequent benefit.
Toxicity and efficacy assessments
The primary end point was safety of JCAR014 in combination with durvalumab. A modified toxicity probability interval algorithm determined durvalumab dose escalation/deescalation.20 Patients were evaluable for dose-limiting toxicity (DLT; supplemental Methods) if they received the JCAR014 cell product conforming to dose and composition standards and full infusion of the first dose of durvalumab. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, with the exception of cytokine release syndrome (CRS), which was graded according to the Lee 2014 consensus criteria.21
Secondary end points included the proportion of patients who achieved complete response (CR), ORR, progression-free survival (PFS), and OS. Response was assessed centrally by PET and concurrent diagnostic-quality computed tomography (CT; PET/CT) according to the Lugano criteria22 at approximately 1, 2, 4, 6, 9, and 12 months after JCAR014 infusion. PFS was defined as the time from JCAR014 infusion to disease progression or death. OS was defined as the time from JCAR014 infusion to death.
CAR T-cell and serum cytokine quantification
CAR T-cell enumeration was assessed by flow cytometry and quantitative polymerase chain reaction (qPCR; lower limit of detection, 10 transgene copies per μg DNA), and serum cytokine concentrations were evaluated by Luminex assay (Luminex Corporation), as previously described.1,18,19
Statistical analyses
Median/range and count/percentage were calculated for continuous and categorical variables, respectively. Fisher exact test was used for comparisons of categorical variables, and Wilcoxon rank-sum/Mann-Whitney test was used for comparisons of continuous variables. Response rates were reported with a 95% confidence interval (CI) calculated using the Wilson/Brown method.23 The Kaplan-Meier method was used to estimate the duration of response (DOR), PFS, and OS, and log-rank test was used to compare the differences between the groups. The reverse Kaplan-Meier method was used to estimate the median follow-up time.24 Patients not experiencing an event were censored at the date of the last follow-up. One-way analysis of variance test was used for comparisons of the means between experimental conditions in the in vitro studies. In exploratory analyses, we compared patients with LBCL on study NCT02706405 with those who received the same lymphodepletion and JCAR014 dose (2 × 106 CAR T cells per kg) on a previously reported clinical trial (NCT01865617; supplemental Figure 2).1,19,25 Owing to low numbers, propensity score matching could not be performed. One-sample proportion test was used to compare response rates against the JCAR014 alone cohort. Statistical analyses were performed using Prism (version 9.3.1, GraphPad) and RStudio (version 2021.09.1+372, RStudion).
Results
Patient characteristics
Between 23 January 2017 and 2 July 2020, a total of 34 patients were screened, 32 underwent leukapheresis, and 29 were enrolled and received lymphodepletion and JCAR014 infusion (NCT02706405; supplemental Figure 3). Eleven patients were enrolled in group 1 and 18 in group 2. The baseline characteristics of patients in groups 1 and 2 were similar, with a trend toward higher lactate dehydrogenase and higher tumor cross-sectional area in group 1 (Table 1). Among all patients who received JCAR014 in the study, the median age was 62 years (range, 19-70 years). Thirteen patients (45%) had DLBCL not otherwise specified; 8 (28%) had DLBCL transformed from indolent histology, and 6 (21%) had high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (double-/triple-hit lymphoma). Patients had a median of 3 prior lines of therapy (range, 1-9), and 13 (45%) had primary refractory disease (failure to achieve CR to any line of therapy). Bridging therapy was administered to 10 patients (34%). Before lymphodepletion, 24 (83%) had stage III or IV disease, 18 (62%) had extranodal involvement, and 14 (48%) had elevated serum lactate dehydrogenase concentration. Among all patients, the median number of durvalumab cycles was 2 (range, 0-11), and the median dose administered was 750 mg (range, 7.5-750; supplemental Figure 4).
Table 1.
Patient characteristics
| Characteristic | Group 1 (n = 11) | Group 2 (n = 18) | P |
|---|---|---|---|
| JCAR014 dose level, n (%) | |||
| 1 (7.0 × 105/kg) | 2 (18) | 0 | |
| 2 (2.0 × 106/kg) | 9 (82) | 18 (100) | |
| Age | |||
| Median (IQR), y | 65 (59-67) | 58 (52-67) | .26 |
| ≥ 65 y, n (%) | 7 (64) | 6 (33) | .14 |
| Male sex, n (%) | 8 (73) | 11 (61) | .69 |
| ECOG performance score ≥ 1, n (%) | 6 (55) | 7 (39) | .47 |
| Disease histology, n (%) | |||
| DLBCL, NOS | 6 (55) | 7 (39) | .47 |
| DLBCL transformed from indolent histology | 4 (36) | 4 (22) | .43 |
| High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements | 1 (9) | 5 (28) | .36 |
| Other∗ | 0 | 2 (11) | .51 |
| MYC and BCL2 double-expressor† | |||
| Yes | 2 (20) | 2 (15) | 1 |
| No | 5 (50) | 6 (46) | 1 |
| Missing | 3 (30) | 5 (39) | 1 |
| Cell of origin (Hans algorithm), n (%) | |||
| Germinal center B-cell phenotype | 8 (73) | 9 (50) | .43 |
| Nongerminal-center B-cell phenotype | 3 (27) | 8 (44) | .43 |
| Missing | 0 | 1 (6) | 1 |
| Ann Arbor stage III or IV, n (%) | 10 (91) | 14 (78) | .62 |
| Extranodal disease, n (%) | 6 (55) | 12 (67) | .70 |
| LDH | |||
| Median (IQR), U/L | 227 (184-315) | 173 (149-343) | .29 |
| Elevated, n (%) | 7 (64) | 7 (39) | .26 |
| IPI, n (%)‡ | |||
| 0-2 | 5 (45) | 11 (61) | .47 |
| ≥ 3 | 6 (55) | 7 (39) | .47 |
| Tumor cross-sectional area, median (IQR), mm2§ | 4758 (2968-5475) | 3085 (984-6254) | .31 |
| Number of prior therapies, median (range) | 3 (2-7) | 2 (1-9) | .34 |
| Primary refractory disease, n (%) | 5 (45) | 8 (44) | 1 |
| Prior autologous hematopoietic stem cell transplantation, n (%) | 2 (18) | 3 (17) | 1 |
| Therapy between leukapheresis and lymphodepletion, n (%) | 4 (36) | 6 (33) | 1 |
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase; NOS, not otherwise specified.
P values per Wilcoxon rank-sum test or Fisher exact test (2-sided), as appropriate.
One patient with primary mediastinal B-cell lymphoma and 1 patient with Richter transformation.
Excluding double- or triple-hit lymphoma (n = 23).
Scores on the IPI include low risk (0 or 1 point), low-intermediate risk (2 points), high-intermediate risk (3 points), and high risk (4 or 5 points).
Sum of the product of the perpendicular diameters of up to 6 target measurable nodes and extranodal sites.
Safety of JCAR014 in combination with durvalumab
Among 29 patients who received lymphodepletion and JCAR014 infusion, 2 were not evaluable for DLT: 1 in group 1 did not receive durvalumab and 1 in group 2 received an out-of-specification JCAR014 product. Two of 27 patients (7%) had DLT. One patient in group 2 (first durvalumab dose, 225 mg) with DLBCL not otherwise specified, developed grade 4 CRS, transitioned to comfort care, and died 32 days after CAR T-cell infusion, and 1 patient in group 2 (first durvalumab dose, 750 mg) with high-grade B-cell lymphoma developed prolonged grade 3 neurotoxicity, which resolved by day 25 after CAR T-cell infusion, followed by PD and death 47 days after CAR T-cell infusion. Both patients received only the first dose of durvalumab 1 day before JCAR014 administration. One patient in group 1 died because of CRS and neurotoxicity 21 days after CAR T-cell infusion without receiving durvalumab. CTs on day 20 were consistent with PD, and autopsy showed CD19⁻ disease. No maximum-tolerated durvalumab dose was identified in either group.
All patients (n = 29) who received lymphodepletion and JCAR014 infusion were evaluated for AEs. The most common AEs of any grade attributed to study treatment (with or without durvalumab) were CRS (41%), neutropenia (21%), neurotoxicity (17%), and hypogammaglobulinemia (17%). supplemental Table 2 details treatment-emergent AEs by group and time of onset. Clinical signs and symptoms of autoimmune disease previously associated with ICB were not observed early after CAR T-cell infusion. Later, 1 patient developed grade 3 aspartate aminotransferase elevation on day 41 after JCAR014 infusion attributed to durvalumab. Isolated grade ≥3 neutropenia occurring in 5 patients (17%) at a median of 98 days (range, 79-195) after CAR T-cell infusion was attributed to durvalumab. All but 1 resolved after cessation of durvalumab; however, a contribution of lymphodepletion and JCAR014 to late neutropenia could not be discounted.
The combination of ICB with JCAR014 was not associated with increased immune effector cell–associated toxicities. The incidence of grade ≥1 and ≥3 CRS and neurotoxicity were consistent with prior experience and were similar between the 2 treatment groups (Table 2). However, compared with group 1, patients in group 2 had a later onset of CRS after CAR T-cell infusion (median, 6 vs 4 days; P = .05) and shorter duration of CRS (median, 3 vs 8 days; P = .08; Table 2).
Table 2.
Incidence and management of CRS and neurotoxicity
| Group 1 (n = 11) | Group 2 (n = 18) | P | |
|---|---|---|---|
| CRS | |||
| Any grade, n (%) | 5 (45) | 7 (39) | 1 |
| Grade ≥3, n (%) | 1 (9)∗ | 1 (6)† | 1 |
| Median time to onset, d (range) | 4 (1-6) | 6 (3-10) | .05 |
| Median duration, d (range) | 8 (5-19) | 3 (1-20) | .08 |
| Neurotoxicity | |||
| Any grade, n (%) | 3 (27) | 2 (11) | .34 |
| Grade ≥3, n (%) | 1 (9)∗ | 1 (6)‡ | 1 |
| Treatment for CRS and neurotoxicity | |||
| Tocilizumab, n (%) | 1 (9) | 1 (6) | 1 |
| Steroids, n (%) | 1 (9) | 2 (11) | 1 |
| Tocilizumab and steroids, n (%) | 1 (9) | 1 (6) | 1 |
CRS was graded according to the Lee criteria. Neurotoxicity was graded using the CTCAE v4.03. P values per Wilcoxon rank-sum test or Fisher exact test (2-sided), as appropriate.
Patient with grade 5 CRS and neurotoxicity who did not receive any durvalumab dose.
Patient from durvalumab 225 mg dose level with grade 4 CRS (DLT).
Patient from durvalumab 750 mg dose level with prolonged grade 3 neurotoxicity (DLT).
Efficacy of JCAR014 in combination with durvalumab
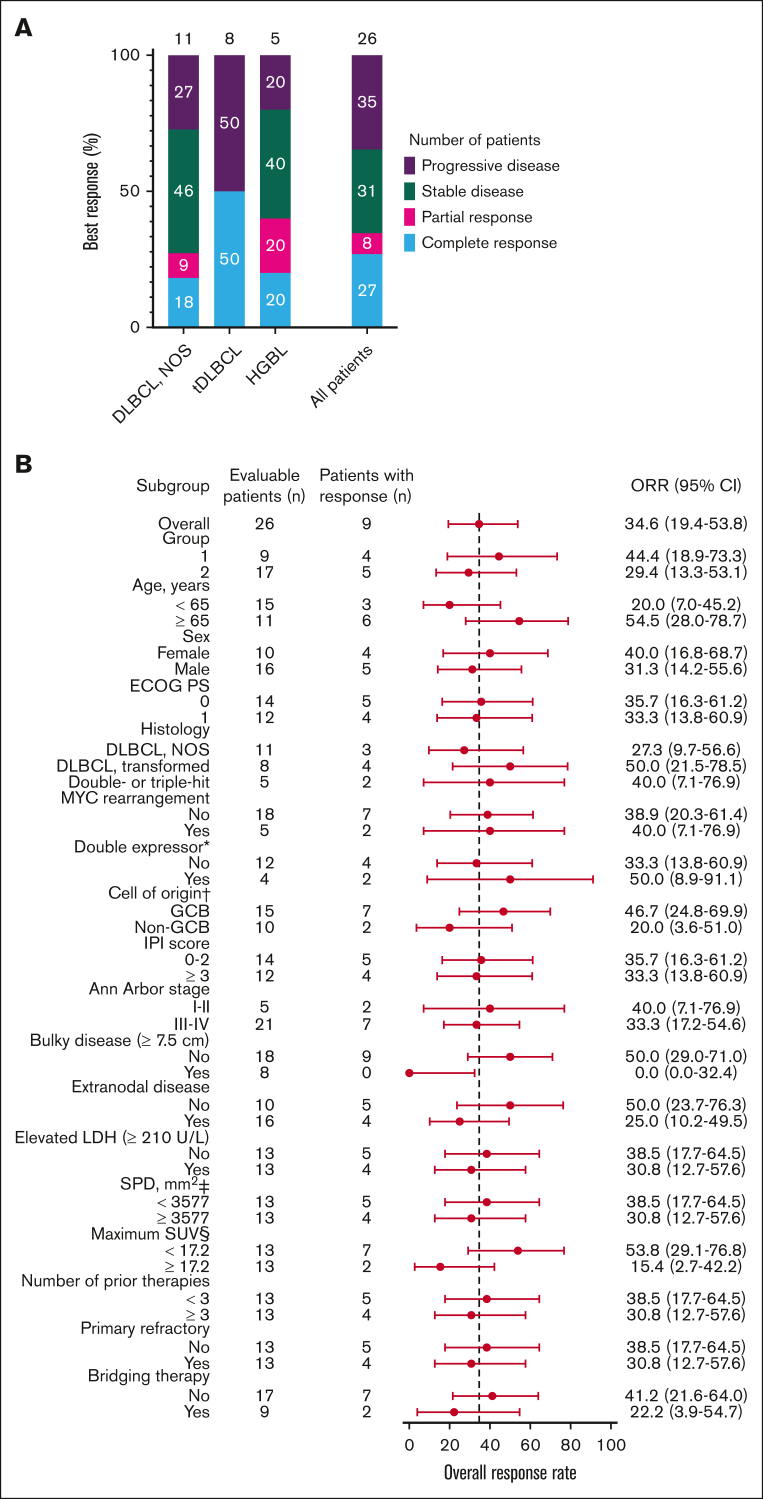
Twenty-six of the 29 patients were included in the efficacy-evaluable set. Two patients who received JCAR014 at 7 × 105 CAR T cells per kg and 1 who received an out-of-specification JCAR014 product (CD4+ CAR T cells only because of CD8+ fraction release failure) were excluded. The best ORR and CR rate were 38% (95% CI, 22%-57%) and 35% (95% CI, 19%-54%), respectively, including 2 late response conversions. The ORR and CR rate at 3 months were 35% (95% CI, 17%-56%) and 27% (95% CI, 14%-46%), respectively (Figure 1A). Patients who achieved CR after JCAR014 in combination with durvalumab had higher CAR T-cell peak counts and area under the curve from days 0 to 28 after CAR T-cell infusion (AUC0-28) in the blood than those who did not achieve CR (supplemental Figure 5A-B). Response rates were similar across key covariates, including age, performance status, histology subtype, prognostic parameters, tumor burden, number of prior therapies, and disease characteristics such as primary resistance to therapy and requirement of bridging therapy after leukapheresis (Figure 1B). No patient with bulky disease (maximum tumor diameter ≥ 7.5 cm)26 before lymphodepletion responded, and there was a trend toward lower response rates among patients with higher (greater than or equal to the median) maximum standardized uptake value on PET/CT before lymphodepletion. PFS and OS rates were superior among patients who achieved CR than among those who did not (supplemental Figure 6A-B).
Figure 1.
Best response rate at 3 months. (A) Best response at 3 months on 26 of the 29 patients included in the efficacy-evaluable set according to disease histology (DLBCL NOS; tDLBCL; and HGBL with MYC and BCL2 and/or BCL6 rearrangements) and in the whole cohort. (B) Subgroup analysis of the ORR for key baseline and clinical covariates. ∗Indicates double expressor excluding double- or triple-hit lymphoma (total of 23 patients). † indicates cell of origin by the Hans algorithm. ‡ indicates the sum of the product of the perpendicular diameters of up to 6 target measurable nodes and extranodal sites. § indicates the maximum standardized uptake value in target lesion. The 95% CI was calculated with the use of the Wilson/Brown method. ECOG PS, Eastern Cooperative Oncology Group performance score; HGBL, high-grade B-cell lymphoma; IPI, International Prognostic Index; LDH, lactate dehydrogenase; NOS, not otherwise specified; SPD, sum of the product of the perpendicular diameters of up to 6 target measurable nodes and extranodal sites; tDLBCL, DLBCL transformed from indolent histology.
The response rates in patients with LBCL treated with JCAR014 in combination with durvalumab in this study (NCT02706405) appeared lower than those of patients treated with JCAR014 alone in our previous phase 1/2 clinical trial (NCT01865617).1,19,25 We retrospectively compared the response rates of patients treated with JCAR014 in combination with durvalumab with those of patients with LBCL who received the same Cy/Flu lymphodepletion and JCAR014 dose (2 × 106 CAR T cells per kg) without durvalumab on our previous phase 1/2 clinical trial (NCT01865617; JCAR014 alone cohort). There was a trend toward lower ORR (P = .08) and CR rate (P = .09) in patients treated with JCAR014 in combination with durvalumab than those treated with JCAR014 alone (Table 3). Despite lower tumor burden, patients in group 2 (first durvalumab before JCAR014) appeared to have a lower CR rate than those treated in group 1 (first durvalumab after JCAR014; P = .16) and had lower ORR (P = .07) and CR rate (P = .03) than those treated with JCAR014 alone. No patient who received durvalumab 750 mg before JCAR014 achieved CR. There were no significant differences in ORR and CR rate between patients in group 1 and those who received JCAR014 alone. These data suggest that durvalumab initiation before JCAR014 was associated with inferior responses.
Table 3.
Response rates in JCAR014 in combination with durvalumab (NCT02706405) and JCAR014-alone cohorts (NCT01865617)
| ORR – n (%), (95% CI) | P | CR – n (%), (95% CI) | P | |
|---|---|---|---|---|
| JCAR014 + durvalumab | 9 of 26 (35), (19-54) | .08∗ | 7 of 26 (27), (14-46) | .09∗ |
| JCAR014 alone | 6 of 12 (50), (25-75) | 5 of 12 (42), (19-68) | ||
| Group 1 | 4 of 9 (44), (19-73) | .37† | 4 of 9 (44), (19-73) | .16† |
| Group 2 | 5 of 17 (29), (13-53) | 3 of 17 (18), (6-41) | ||
| Group 2 | 5 of 17 (29), (13-53) | .07∗ | 3 of 17 (18), (6 -41) | .03∗ |
| JCAR014 alone | 6 of 12 (50), (25-75) | 5 of 12 (42), (19-68) | ||
| Group 1 | 4 of 9 (44), (19-73) | .50∗ | 4 of 9 (44), (19-73) | .70∗ |
| JCAR014 alone | 6 of 12 (50), (25-75) | 5 of 12 (42), (19-68) |
P value per 1-sample proportion test (1-sided).
P value per Fisher exact test (1-sided).
We did not observe differences in PFS/OS between groups 1 and 2 (supplemental Figure 6C-D). At a median follow-up of 44.5 months (95% CI, 42.5 to not reached), the median 2-year estimates of PFS and OS rates were 22.2% (95% CI, 6.6%-75.4%) for patients in group 1 and 23.5% (95% CI, 10.0%-55.4%) for group 2 and 55.6% (95% CI, 31.0%-99.7%) in group 1 and 52.9% (95% CI, 33.8%-82.9%) in group 2, respectively.
CAR T-cell kinetics in patients treated with JCAR014 with and without durvalumab
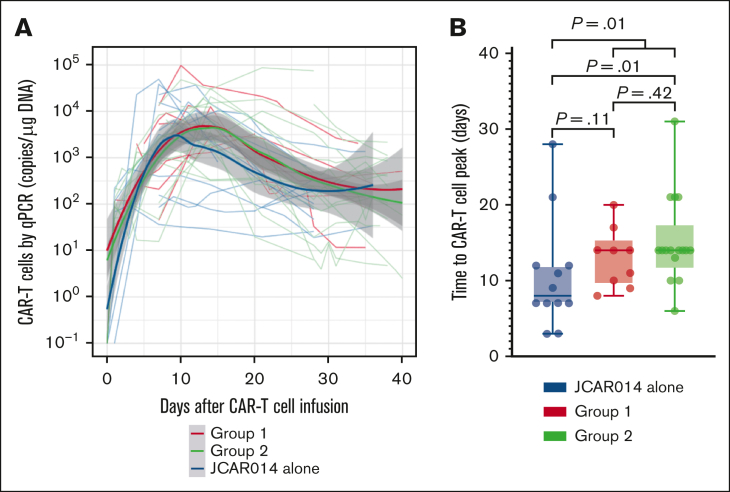
We had expected that administration of durvalumab before JCAR014 infusion would result in more robust CAR T-cell counts in the blood; therefore, we were surprised to see inferior efficacy, delayed onset, and shorter duration of CRS in group 2. We examined in vivo CAR T-cell kinetics in patients in groups 1 and 2 and retrospectively compared them with those of patients treated in the JCAR014 alone cohort (Figure 2A). The baseline characteristics of patients in these cohorts were similar, except for a higher median number of prior therapies in patients treated with JCAR014 alone (supplemental Table 3). We did not observe significant differences in the magnitude of CAR T-cell expansion, AUC0-28 after JCAR014 infusion, and day 28 CAR T-cell counts by qPCR and flow cytometry between the cohorts (supplemental Figure 7A-B). However, the time period to the maximum CAR T-cell counts in the blood was longer for patients treated with JCAR014 in combination with durvalumab than for patients treated with JCAR014 alone (median, 14 vs 8 days; P = .01; Figure 2B). This was most evident in patients treated in group 2 (Figure 2B) and was consistent with the later onset of CRS in this group; but it was also observed in group 1, likely because 5 of 9 patients (56%) in this group started durvalumab before the peak of CAR T-cell expansion.
Figure 2.
CAR T-cell kinetics in patients treated with JCAR014 with and without durvalumab. (A) CAR T-cell counts in the blood by qPCR in groups 1 and 2 (NCT02706405) and JCAR014 alone (NCT01865617) cohorts. Each thin line represents a single patient; bold lines represent the averaged data using local polynomial regression (LOESS) curve fitting approximation with the standard error in gray. (B) Time to CAR T-cell peak counts in the blood by qPCR in groups 1 and 2 (NCT02706405) and the JCAR014 alone (NCT01865617) cohorts. Mann-Whitney tests were used to compare differences between the groups.
We considered that ICB during CAR T-cell activation could promote activation-induced cell death and explain the delayed peak of CAR T-cell expansion in group 2. Consistent with increased activation, we observed higher CD69 expression and lower CD127 expression in CD8+ CAR T cells collected between days 7 and 14 after infusion from patients in group 2 (supplemental Figure 8A-B); however, there was no difference in activated caspase-3 expression between the groups (supplemental Figure 8C). The fractions of CD8+ CAR T cells expressing PD-1 or other inhibitory receptors (2B4, CD160, killer cell lectin-like receptor G1, lymphocyte-activation gene 3, T cell immunoreceptor with Ig and ITIM domains, and T cell immunoglobulin and mucin-domain containing-3) did not differ between patients treated in groups 1 and 2 (supplemental Figure 8D).
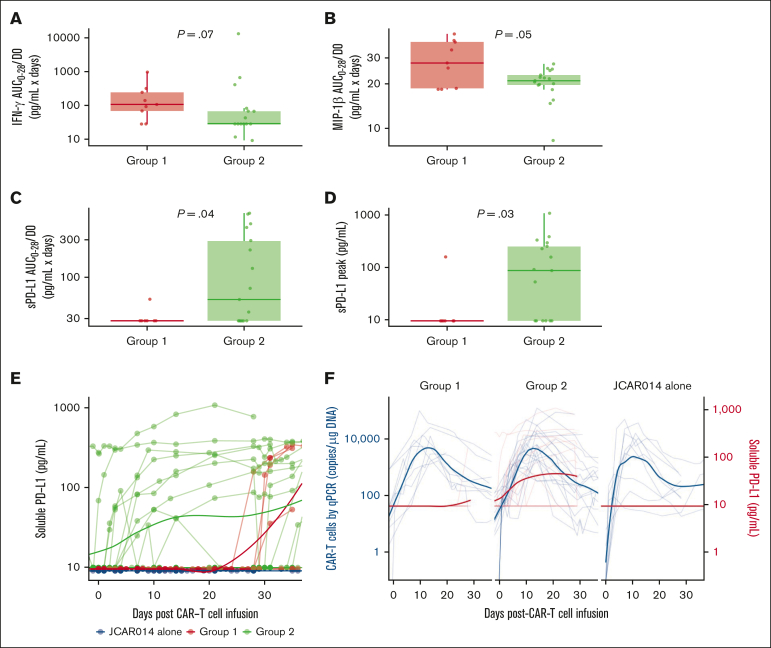
Given the slower accumulation of CAR T cells in the blood in group 2, we examined the serum concentrations of inflammatory cytokines associated with CRS as well as C-reactive protein and ferritin in each group, using concentration-time profiles adjusting for differences in day 0 concentrations (AUC0-28/D0). Patients in group 2 had lower soluble interferon gamma (IFN-γ; AUC0-28/D0: median, 28.0 pg/mL × days; interquartile range [IQR], 28.0-67.1 vs median, 106.0 pg/mL × days; IQR, 68.2-244.0; P = .07) and macrophage inflammatory protein-1β (AUC0-28/D0; median, 21.0 pg/mL × days; IQR, 19.6-23.0 vs median, 27.7 pg/mL × days; IQR, 18.6-38.5; P = .05) than patients in group 1 (Figure 3A-B). These data show that the lower efficacy and decreased duration of CRS in patients who received ICB with durvalumab starting before JCAR014 infusion was associated with slower accumulation of CAR T cells and lower concentrations of inflammatory cytokines in the blood.
Figure 3.
Serum biomarkers in patients treated with JCAR014 with and without durvalumab. AUC0-28/D0 after CAR T-cell infusion of serum IFN-γ (A), macrophage inflammatory protein-1β (MIP-1β) (B), and sPD-L1 (C) according to the treatment group. (D) Serum sPD-L1 peak concentration after CAR T-cell infusion according to treatment group. (E) Serum sPD-L1 in the JCAR014 in combination with durvalumab (groups 1 and 2; NCT02706405) and JCAR014 alone cohorts (NCT01865617). (F) Serum sPD-L1 and CAR T-cell counts in the blood by qPCR in the JCAR014 in combination with durvalumab (groups 1 and 2; NCT02706405) and JCAR014 alone cohorts (NCT01865617). (E-F) Each thin line represents a single patient, and each dot represents a sample; bold lines represent the averaged data using LOESS curve fitting approximation. Mann-Whitney tests were used to compare differences between groups.
High sPD-L1 coincides with CAR T-cell peak counts in patients receiving durvalumab before JCAR014
sPD-L1 increases early after the start of ICB therapy and has been reported to inhibit effector functions in CAR natural killer cells.27, 28, 29 We observed higher sPD-L1 peak concentration after JCAR014 infusion (median, 86.8 pg/mL; IQR, 9.5-253.1 vs median, 9.5; IQR, 9.5-9.5; P = .03) and AUC0-28/D0 (median, 52.3 pg/mL × days; IQR, 28.0-293.0 vs median, 28.0 pg/mL × days; IQR, 28.0-28.0; P = .04; Figure 3C-D) in patients in group 2 than in patients in group 1. Patients who received durvalumab starting the day before CAR T-cell infusion had an early increase in sPD-L1 levels that coincided with the period of CAR T-cell accumulation (Figure 3E-F). In contrast, sPD-L1 was not increased during CAR T-cell effector expansion in those who started durvalumab after CAR T-cell infusion (Figure 3E-F). No increase in sPD-L1 was detected in patients who did not receive durvalumab (JCAR014 alone).
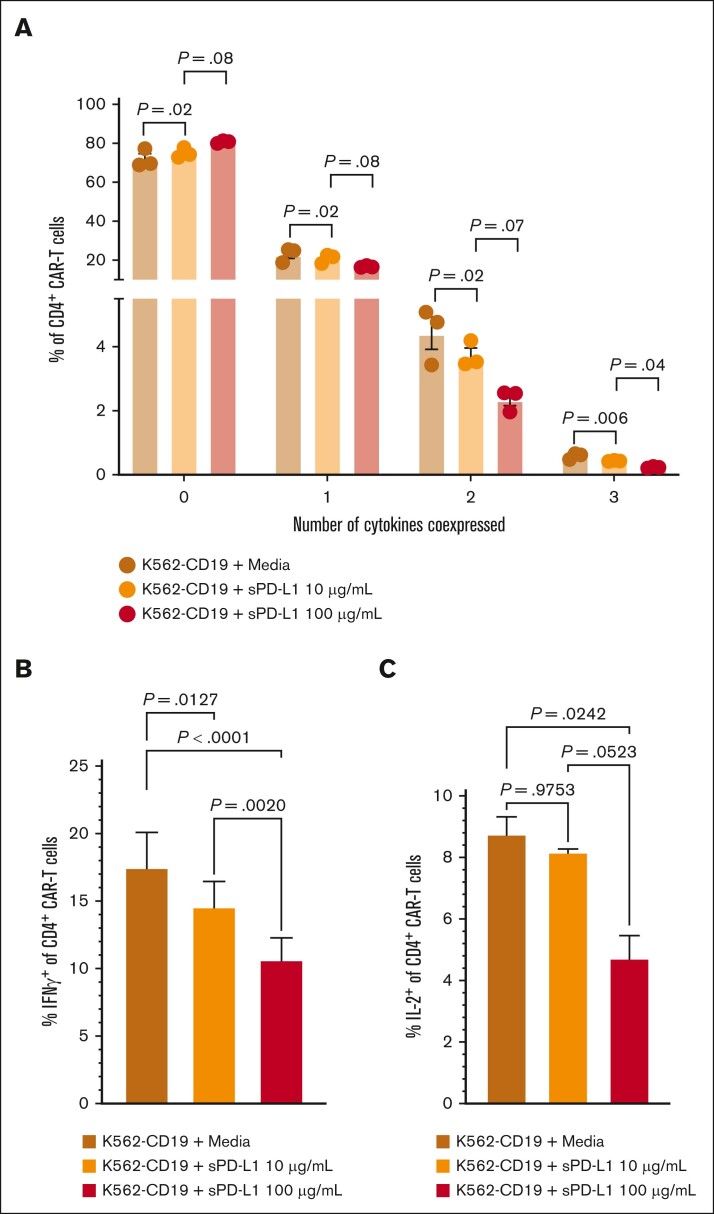
We considered that sPD-L1 detected during the CAR T-cell accumulation phase could inhibit CAR T-cell effector function, thereby contributing to later onset and shorter duration of CRS and inferior response rates in group 2. In in vitro studies, we found that the addition of sPD-L1 induced dose-dependent decreases in CD19-stimulated polyfunctionality of CD4+ CAR T-cell cytokine secretion (Figure 4A), particularly associated with decreased IFN-γ and interleukin-2 (Figure 4B-C). These results indicate that high sPD-L1 concentration coinciding with CAR T-cell accumulation may impair JCAR014 effector function and contribute to inferior efficacy in patients who received durvalumab before JCAR014 infusion.
Figure 4.
sPD-L1 inhibits CAR T-cell cytokine production in vitro in a dose-dependent manner. Human CD19 CAR T cells were generated from healthy donors (n = 3) and assayed on days 14 to 16 after the start of manufacturing. CD4+ CAR T cells were cocultured with CD19-expressing K562 cells at a 1:1 effector-to-target ratio and the indicated sPD-L1 concentrations or media alone for ∼24 hours. (A) The percentages of CD4+ CAR T cells expressing the indicated numbers of cytokines were measured by intracellular flow cytometry. IFN-γ (B) and IL-2 (C) individual production. Data are representative of at least 2 independent experiments. Figures show mean ± standard error of the mean. One-way analysis of variance tests with Tukey correction for multiple comparisons were used to compare differences between groups. IL-2, interleukin-2; ns, not significant.
CAR T-cell re-expansion events and late responses in patients receiving durvalumab and JCAR014
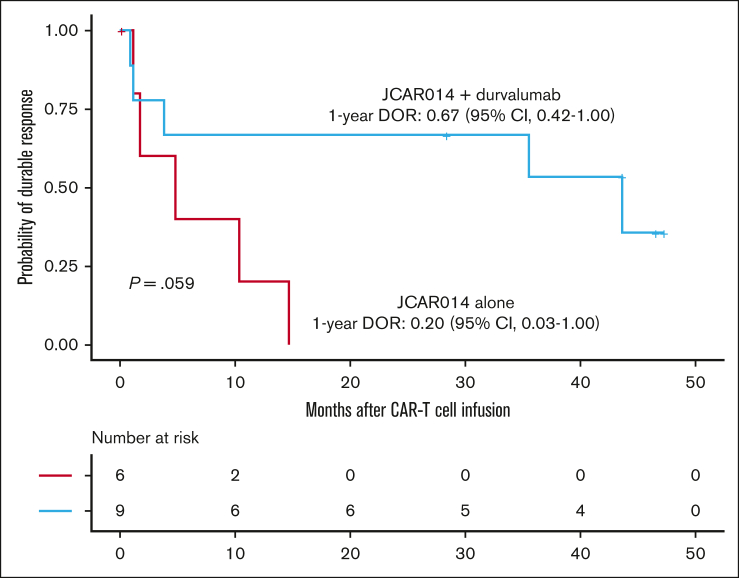
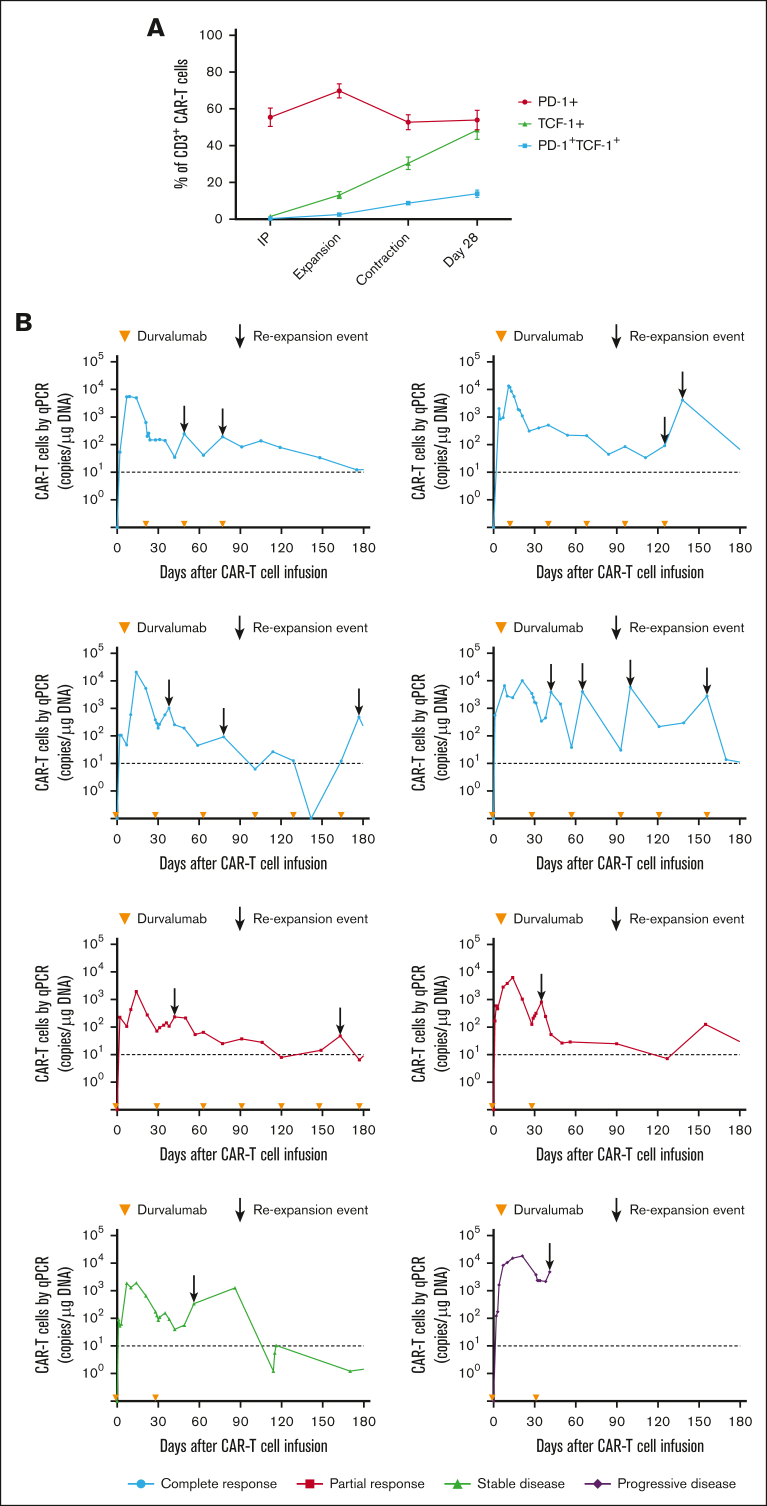
Despite the inferior response rates associated with a durvalumab-induced increase in sPD-L1 early after JCAR014 infusion in group 2, the DOR of patients who received JCAR014 and durvalumab was superior to that of those who received JCAR014 alone (1-year DOR estimate, 67%; 95% CI, 42-100 vs DOR, 20%; 95% CI, 3-100; Figure 5). We did not observe significant differences in the PFS/OS between these cohorts (supplemental Figure 9). We considered that, in spite of the initially inferior responses in a subset of patients, subsequent monthly durvalumab infusions might contribute to improved DOR after JCAR014. After JCAR014 infusion, we observed an increasing fraction of CAR T cells bearing a phenotype consistent with that of progenitor exhausted T cells that proliferate in response to ICB.30, 31, 32 Although the fraction of CD3+ CAR T cells that expressed PD-1 was stable, we found a progressive increase in both transcription factor-1 (TCF-1)+ and PD-1+TCF-1+ CAR T cells (Figure 6A), which prompted us to examine whether CAR T-cell re-expansion events were observed after durvalumab infusions. Eight of 26 patients (31%; 2 in group 1 and 6 in group 2) in the efficacy-evaluable set had re-expansion of CAR T cells in the blood beyond day 28 after JCAR014 infusion, defined as twofold or greater increase in transgene copy numbers from the previous level (Figure 6B; supplemental Figure 9). In the 8 patients with CAR T-cell re-expansion events, the ORR and CR rate at 3 months were 75% (95% CI, 41-96) and 50% (95% CI, 22-78), respectively. The median fold increase in CAR transgene copies per μg DNA was 5.9 (range, 2.2-78.5; n = 16) and occurred at a median of 15 days (range, 7-28 days) after the most recent durvalumab infusion. Re-expansion events were only observed in those who received durvalumab at the highest dose level of 750 mg.
Figure 5.
DOR in patients treated with JCAR014 with and without durvalumab. Kaplan-Meier estimates of the DOR according to treatment cohort. P value per log-rank test. The numbers of patients at risk at 10-month intervals are indicated.
Figure 6.
CAR T-cell progenitor exhausted phenotype and in vivo re-expansion in the blood with repeated dosing of durvalumab after JCAR014. (A) Aliquots of the infusion products (n = 26) and blood collected after CAR T-cell infusion (expansion, n = 26; contraction, n = 21; day 28, n = 12) were analyzed by flow cytometry. Percentage of PD-1+, TCF-1+, and PD-1+TCF-1+ CD3+ CAR T cells at each timepoint are shown. Figures show mean ± standard error of the mean. (B) CAR T-cell counts in the blood by qPCR in patients treated with JCAR014 in combination with durvalumab who experienced in vivo re-expansion of CAR T cells. Arrowheads on the x-axis indicate the time of durvalumab doses. Arrows show re-expansion events. Horizontal dashed lines show the qPCR assay limit of detection.
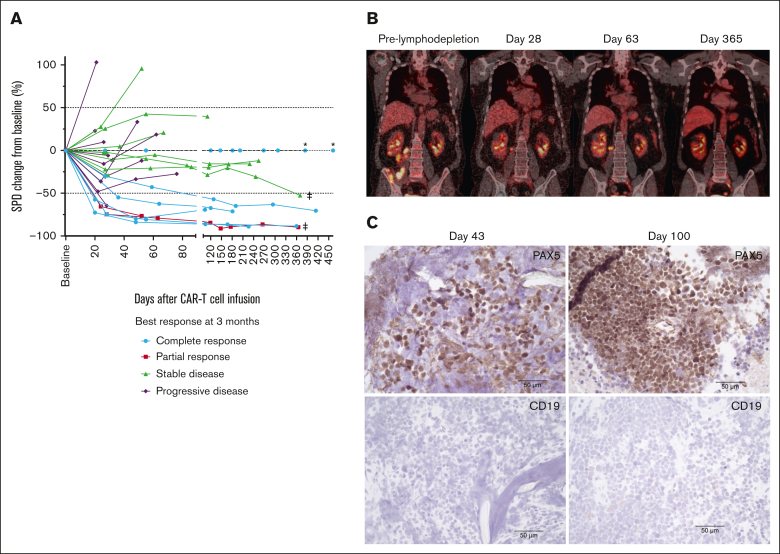
In a subset of patients who did not achieve CR and continued treatment with durvalumab, we observed tumor burden stabilization or ongoing regression (Figure 7A). Although conversions to CR can be observed within the first few months of CAR T-cell immunotherapy,1, 2, 3, 4 late responses are uncommon. We observed 2 patients with double-hit lymphoma who achieved CR 1 year after JCAR014 infusion with no additional antitumor therapy aside from monthly durvalumab. One patient received durvalumab 75 mg before JCAR014 infusion and 750 mg monthly and had stable disease on PET/CTs approximately 1, 2, 4, 6, and 9 months after JCAR014 infusion, achieving CR by PET/CT on day 370. CAR T cells were detected in the blood by qPCR above the limit of detection up to day 139 after CAR T-cell infusion. Another patient received durvalumab 22.5 mg before JCAR014 infusion and 750 mg monthly and achieved a partial response (PR) on day 28 with subsequent progression on day 63 (Figure 6B). Biopsy samples on days 43 and 100 after JCAR014 infusion showed CD19⁻ lymphoma (Figure 6C) that, remarkably, regressed to CR by day 365 on PET/CT (Figure 6B). CAR T cells were detected in the blood by qPCR at the time of achievement of CR. Both patients had ongoing grade 2 lymphopenia.
Figure 7.
Tumor burden stabilization/regression and late conversion to CR with repeated dosing of durvalumab after JCAR014. (A) Spider plot of the change in the sum of the product of the perpendicular diameters of ≤6 target measurable nodes and extranodal sites (SPD) over time compared with the baseline prelymphodepletion according to best response at 3 months. ∗ represents patients with extranodal disease and no measurable target lesion. ‡ represents patients who converted to CR at 1 year. PET/CT (B) and immunohistochemistry (C) images at different timepoints in a patient with late conversion to CR with continued durvalumab despite evidence of CD19– escape. Images were taken at original magnification ×40.
Together, these data indicate that although the administration of ICB with durvalumab before JCAR014 was associated with inferior efficacy 1 to 3 months after JCAR014, maintenance ICB was associated with better DOR. Regression of both CD19+ and CD19– double-hit lymphoma late after JCAR014 infusion during durvalumab maintenance suggests that late responses might be mediated by CAR T-cell re-expansion and/or CAR-independent mechanisms.
Discussion
In this phase 1 clinical trial, adults with R/R LBCL were treated with JCAR014 CD19-targeted 4-1BB–costimulated CAR T cells, escalating initial doses of durvalumab starting before or after CAR T-cell infusion, and ongoing durvalumab maintenance.
The combination of JCAR014 and durvalumab was safe and well-tolerated. We did not observe severe autoimmune AEs that have been described after durvalumab and other ICB therapies33, 34, 35 or increased incidence and severity of immune effector cell–associated toxicities. The most common AE attributed to durvalumab was late and isolated grade 3 to 4 neutropenia, consistent with the reports of pembrolizumab combined with CD19 CAR T cells.12,15 Prolonged cytopenias are observed after CAR T-cell therapy;36,37 neutrophil recovery often follows a biphasic temporal course.38 Therefore, a contribution of lymphodepletion and JCAR014 to late neutropenia cannot be discarded.
Patients in group 2 who received durvalumab starting before JCAR014 infusion had later onset and shorter duration of CRS, consistent with the observed delayed CAR T-cell peak expansion and lower concentration of inflammatory cytokines such as IFN-γ and macrophage inflammatory protein-1β. Response rates were also inferior in this group despite preserved CAR T-cell expansion/persistence in the first 28 days after JCAR014 infusion. These findings are consistent with reduced in vivo effector function of the CAR T cells when durvalumab is administered before JCAR014 infusion. Consistent with the effects of ICB therapy,27,28 we observed an earlier increase in sPD-L1 levels in patients who received durvalumab starting on the day before CAR T-cell infusion compared with those in group 1 who started after, resulting in elevated sPD-L1 concentrations during the CAR T-cell expansion phase in patients treated in group 2. Conceivably, commencing durvalumab very early after JCAR014 infusion (first week) might similarly result in increased sPD-L1 that overlaps the peak CAR T-cell expansion phase. sPD-L1 has been shown to maintain its capacity to bind PD-1 and inhibit T-cell function39,40 and CAR natural killer cell function in vitro.29 We hypothesized that sPD-L1 might inhibit CAR T-cell effector function and demonstrated that sPD-L1 resulted in a dose-dependent reduction in CAR T-cell cytokine production and polyfunctionality, providing a mechanism for reduced efficacy and CRS observed in group 2.
Outcomes of patients with LBCL after failure of CAR T-cell therapy are poor, with a median OS of less than 6 months.41,42 The tumor stabilization/regression observed in a subset of patients who continued treatment with durvalumab suggests a possible benefit of ICB therapy in this setting. Accordingly, we observed late CAR T-cell re-expansion in the blood after durvalumab infusion in 31% of treated patients, which might originate from PD-1+TCF-1+ CAR T cells, in line with previous studies that show TCF-1+ stem cell–like progenitor exhausted T cells give rise to proliferative and functional responses to ICB therapy,32,43,44 and that TCF-1+ progenitor exhausted CAR T cells are associated with long-lived CAR T-cell–mediated antitumor responses.45 The late emergence of the PD-1+TCF-1+ CAR T-cell subset might explain why increased CAR T-cell accumulation was not observed early after JCAR014 infusion in group 2. ICB-induced expansion of PD-1+TCF-1+ CAR T cells might explain late conversion to CR in those with CD19+ disease; however, the observation of a late conversion to CR in a patient with CD19– immune escape indicates CAR-independent benefits of ICB therapy, such as recruitment of endogenous tumor-specific T cells. Although the superior DOR in patients treated with CAR T cells in combination with durvalumab compared with those treated with CAR T cells alone is of note, additional studies will be needed to confirm the role of ICB in this finding.
Disruption of PD-1/PD-L1 signaling has been associated with improved efficacy in CD19 CAR T-cell therapy xenograft models.46,47 However, clinical studies of ICB and CD19 CAR T-cell combination therapy have demonstrated variable outcomes with limited insight into reasons why disruption of the PD-1/PD-L1 pathway has not been more effective.13, 14, 15,47 Our data show that ICB-induced sPD-L1 inhibition of CAR T-cell effector function is a mechanism of reduced in vivo efficacy that primarily occurs when ICB is administered before and potentially early after CAR T-cell infusion. This study provides a warning of caution and an opportunity to develop novel strategies to inhibit sPD-L1–mediated suppression of CAR T-cell effector function and improve the early response to ICB and CAR T-cell combination therapies while still enabling enhanced DOR through later ICB-mediated engagement of CAR-dependent and -independent antitumor mechanisms. Additional studies testing ICB for patients with PR at first restaging after CD19 CAR T-cell therapy aiming at increasing the CR conversion rate are warranted.
Conflict-of-interest disclosure: A.V.H. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, and Nektar Therapeutics, and honoraria from Bristol Myers Squibb and Novartis. E.L.K. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company. S.F. reports grants from Bristol Myers Squibb and other support in the form of pending equity from Link Immunotherapeutics outside of the submitted work; has issued patents for PCT/US2021/025255 and PCT/US2021/025248d; and a patent for PCT/US2021/025260 issued, licensed, and with royalties paid from Bristol Myers Squibb. J.G. has received research funding from Sobi, Juno Therapeutics, a Bristol Myers Squibb company, Celgene, and Angiocrine Bioscience, and has received honoraria/consulting fees from Sobi, Legend Biotech, Janssen, Kite Pharma, a Gilead company, and MorphoSys. C.C.S.Y. has received research funding from OBI Pharma, Lonza, Sensei, Signal One, and Pfizer, and serves on scientific advisory boards for Twinstrand Biosciences, AbbVie, Eli Lilly, and Loxo. R.D.C. has received research funding from Amgen, Incyte, Kite Pharma, a Gilead company, Pfizer, Servier, and Vanda Pharmaceuticals; has received honoraria/consulting fees from Amgen, Jazz, Kite/Gilead, and Pfizer; serves on a data and safety monitoring board for Pepromene Bio and on an independent response review committee for Autolus; and his spouse has been employed by and owned stock in Seagen. A.G.C. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company. D.J.G. has received research funding from, has served as an adviser for, and has received royalties from Juno Therapeutics, a Bristol Myers Squibb company; has served as an adviser and received research funding from Seattle Genetics; has served as an adviser to GlaxoSmithKline, Celgene, Janssen Biotech, and Legend Biotech; and has received research funding from SpringWorks Therapeutics, Sanofi, and Cellectar Biosciences. M.S. has received research funding from Mustang Bio, Bristol Myers Squibb, Pharmacyclics, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; has served as a consultant for AbbVie, Genentech, AstraZeneca, Pharmacyclics, Beigene, Bristol Myers Squibb, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC Therapeutics, Janssen, Fate Therapeutics, and MEI Pharma; and his spouse is an employee of Bristol Myers Squibb. B.G.T. has received research funding from Mustang Bio and Juno Therapeutics, a Bristol Myers Squibb company; serves on scientific advisory boards for Mustang Bio and Proteios Technology; and has the right to receive royalties from Fred Hutch as an inventor on licensed patents. S.R.R. is a cofounder and adviser to Lyell Immunopharma and has received research funding from and intellectual property licensed to Lyell Immunopharma; was a cofounder of Juno Therapeutics, a Bristol Myers Squibb company; is an inventor of patents licensed to Juno Therapeutics; and served as an adviser to Juno Therapeutics and Adaptive Biotechnologies. D.G.M. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, Celgene, and Kite Pharma, a Gilead company; has served on ad hoc advisory board meetings for Amgen, Bristol Myers Squibb, Genentech, Gilead, Incyte, Janssen, Legend Biotech, Mustang Bio, MorphoSys, Novartis, Pharmacyclics, and Umoja; has rights to receive royalties from Fred Hutch for patents licensed to Juno Therapeutics; and serves on scientific advisory board with stock options and compensations for A2 Biotherapeutics and Navan Technologies. C.J.T. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, NanoString Technologies, and Nektar Therapeutics; serves on scientific advisory boards for Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, and Cargo Therapeutics; serves on a data and safety monitoring board for Kyverna; has served on ad hoc advisory board meetings (last 12 months) for Legend Biotech, Nektar Therapeutics, and Syncopation Life Sciences; performs consulting for Century Therapeutics, Orna Therapetuics, and IGM Biosciences; has stock options in Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, Cargo Therapeutics, and ArsenalBio; and has the right to receive payments from Fred Hutch as an inventor on licensed patents. The remaining authors have declared no competing financial interests.
Acknowledgments
The authors thank the staff of the Fred Hutchinson Cancer Center (Fred Hutch) Cell Processing Facility, Fred Hutch Cell Therapy Laboratory, the Fred Hutch Integrated Immunotherapy Research Center, and the Fred Hutch Bezos Family Immunotherapy Clinic.
This work was supported by National Institutes of Health (NIH), National Cancer Institute grants R01 CA136551 and P30 CA15704, NIH National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK56465, NIH National Heart, Lung, and Blood Institute funded National Gene Vector Biorepository at Indiana University (contract no. 75N92019D00018), Life Science Discovery Fund, the Bezos family, Fred Hutch Immunotherapy Integrated Research Center, Juno Therapeutics, a Bristol Myers Squibb company, and AstraZeneca.
Authorship
Contribution: C.J.T. designed the clinical trial; A.V.H. conceived and designed research studies, and collected, analyzed, and interpreted data; A.V.H., E.L.K., N.G., B.S.P., D.R.K., A.T., and A.C. performed experiments; C.C.S.Y. reviewed pathology cases; A.V.H., J.M.V., and Q.W. performed statistical analyses; A.V.H. and C.J.T. wrote the manuscript; and all authors reviewed and edited the final version of the manuscript.
Footnotes
Individual participant data will not be shared. Original data are available on request from the corresponding author, Alexandre V. Hirayama (ahirayama@fredhutch.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Hirayama AV, Gauthier J, Hay KA, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–1887. doi: 10.1182/blood-2018-11-887067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 5.Deng Q, Han G, Puebla-Osorio N, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 2020;26(12):1878–1887. doi: 10.1038/s41591-020-1061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson Z, Hong C, Schauner R, et al. Sequential single-cell transcriptional and protein marker profiling reveals TIGIT as a marker of CD19 CAR-T cell dysfunction in patients with non-Hodgkin lymphoma. Cancer Discov. 2022;12(8):1886–1903. doi: 10.1158/2159-8290.CD-21-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu-Monette ZY, Xiao M, Au Q, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res. 2019;7(4):644–657. doi: 10.1158/2326-6066.CIR-18-0439. [DOI] [PubMed] [Google Scholar]
- 8.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137(19):2621–2633. doi: 10.1182/blood.2020007445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood. 2017;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill BT, Roberts ZJ, Xue A, Rossi JM, Smith MR. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2019;55(6):1184–1187. doi: 10.1038/s41409-019-0657-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong EA, Alanio C, Svoboda J, et al. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139(7):1026–1038. doi: 10.1182/blood.2021012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi T, Abramson JS, Lee HJ, et al. Safety of lisocabtagene maraleucel given with durvalumab in patients with relapsed/refractory aggressive B-cell non Hodgkin lymphoma: first results from the platform study. Hematol Oncol. 2019;37(S2):171–172. [Google Scholar]
- 14.Jacobson CA, Westin JR, Miklos DB, et al. Abstract CT055: phase 1/2 primary analysis of ZUMA-6: axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab (atezo) for the treatment of patients (pts) with refractory diffuse large B cell lymphoma (DLBCL) Cancer Res. 2020;80(16 suppl):CT055. [Google Scholar]
- 15.Jaeger U, Worel N, McGuirk JP, et al. Safety and efficacy of tisagenlecleucel plus pembrolizumab in patients with r/r DLBCL: results from the phase Ib PORTIA study. Blood Adv. 2023;7(11):2283–2286. doi: 10.1182/bloodadvances.2022007779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol. 2021;17(19):2545–2558. doi: 10.2217/fon-2020-1222. [DOI] [PubMed] [Google Scholar]
- 18.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7(6):653–663. doi: 10.1177/1740774510382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2):101–133. 33. [Google Scholar]
- 24.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 25.Hirayama AV, Gauthier J, Hay KA, et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood. 2019;134(7):636–640. doi: 10.1182/blood.2019000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 27.Oh SY, Kim S, Keam B, Kim TM, Kim DW, Heo DS. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-99311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Risch E, Halaban R, Zhen P, Bacchiocchi A, Risch HA. Dynamic changes of circulating soluble PD-1/PD-L1 and its association with patient survival in immune checkpoint blockade-treated melanoma. Int Immunopharmacol. 2023;118 doi: 10.1016/j.intimp.2023.110092. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Liu S, Adah D, et al. Soluble programmed death ligand-1-induced immunosuppressive effects on chimeric antigen receptor-natural killer cells targeting Glypican-3 in hepatocellular carcinoma. Immunology. 2023;169(2):204–218. doi: 10.1111/imm.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195–211.e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrelli F, Grizzi G, Ghidini M, et al. Immune-related adverse events and survival in solid tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother. 2020;43(1):1–7. doi: 10.1097/CJI.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 35.Balibegloo M, Nejadghaderi SA, Sadeghalvad M, et al. Adverse events associated with immune checkpoint inhibitors in patients with breast cancer: a systematic review and meta-analysis. Int Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107796. [DOI] [PubMed] [Google Scholar]
- 36.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. doi: 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143–155. doi: 10.1182/bloodadvances.2020002732. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rejeski K, Perez Perez A, Sesques P, et al. CAR-HEMATOTOX: A model for CAR T-cell related hematological toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassounah NB, Malladi VS, Huang Y, et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol Immunother. 2019;68(3):407–420. doi: 10.1007/s00262-018-2284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow VA, Gopal AK, Maloney DG, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94(8):E209–e213. doi: 10.1002/ajh.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Blasi R, Le Gouill S, Bachy E, et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: a DESCAR-T analysis. Blood. 2022;140(24):2584–2593. doi: 10.1182/blood.2022016945. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Tuong ZK, Dean I, et al. In vivo labeling reveals continuous trafficking of TCF-1+ T cells between tumor and lymphoid tissue. J Exp Med. 2022;219(6) doi: 10.1084/jem.20210749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai JA, Hellmann MD, Sauter JL, et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell. 2023;41(4):776–790.e7. doi: 10.1016/j.ccell.2023.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng W, Wei J, Zebley CC, et al. Regnase-1 suppresses TCF-1+ precursor exhausted T-cell formation to limit CAR-T-cell responses against ALL. Blood. 2021;138(2):122–135. doi: 10.1182/blood.2020009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Hu Y, Yang J, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609(7926):369–374. doi: 10.1038/s41586-022-05140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.