Abstract
Bartonella henselae, the causative agent of cat scratch disease, establishes long-term bacteremia in cats, in which it attaches to and invades feline erythrocytes (RBC). Feline RBC invasion was assessed in vitro, based on gentamicin selection for intracellular bacteria or by laser confocal microscopy and digital sectioning. Invasion rates ranged from 2 to 20% of the inoculum, corresponding to infection of less than 1% of the RBC. Invasion was a slow process, requiring >8 h before significant numbers of intracellular bacteria were detected. Pretreatment of the bacteria with trypsin, or of the RBC with trypsin or neuraminidase, had no effect, but pronase pretreatment of RBC resulted in a slight increase in invasion frequency. The ability to model B. henselae invasion of feline RBC in vitro should permit identification of bacterial surface components involved in this process and elucidate the significance of RBC invasion to transmission and infection in cats.
Bartonella henselae causes cat scratch disease (CSD), bacillary angiomatosis, visceral peliosis, and endocarditis in humans (1, 5, 14, 22, 23). The domestic cat is a confirmed reservoir for B. henselae (15); >90% of CSD cases are associated with cat contact, typically with superficial trauma in the form of a bite or scratch. While B. henselae is not readily detectable by PCR in the saliva or nail clippings of infected cats (8), cat fleas (Ctenocephalides felis) can harbor B. henselae in the gut, shed the organism in their feces (12), and transmit infections to specific-pathogen-free kittens (7). These findings raise the possibility that B. henselae infections are acquired by scratching contaminated flea feces into the skin at sites of flea bites, in much the same way as Bartonella quintana, the agent of trench fever, is transmitted in the feces of the human body louse.
The clinical course of experimental B. henselae infection in cats includes persistent bacteremia accompanied by a specific antibody response (9, 17). Bacteremic cats are generally asymptomatic (25) but can experience histopathological lesions in multiple organs (10). The prevalence of B. henselae bacteremia or Bartonella-specific antibody in cat populations is striking (4, 6, 11, 13, 18, 24) and may represent a significant public health threat, particularly for individuals with frequent cat contact. For example, in a study of 205 feral, clinic, pet, and impounded cats in northern California, 5% were associated with human cases of CSD or bacillary angiomatosis. However, 40% were positive for blood culture of B. henselae, with prevalences ranging from 4 to 47% in the pet group, 53% in impounded cats, and 70% in the feral cat group, and antibodies to B. henselae were detected in 81% of the cats tested (6). A similar pattern was observed in a study of Australian cat populations, in which 22 of 77 cats tested (35%) were culture positive for B. henselae (4); 40% of these were feral and 16% were domestic cats.
Bartonella spp. are believed to require hemin for growth (20), and they exhibit an affinity for erythrocytes (RBC) that is best characterized with Bartonella bacilliformis, the agent of Carrión’s disease and verruga peruana (19). B. bacilliformis is transmitted to humans by nocturnal sand flies in parts of South America, and upon reaching the blood it invades and ultimately lyses most circulating RBC, leading to severe anemia. B. henselae likewise exhibits an affinity for RBC, although the extent of RBC invasion varies with the host. In contrast to the bacteremia that is common in cats, blood cultures are rarely positive for humans, in which the organism appears to localize primarily in regional lymph nodes (9, 10, 17, 18, 25). Kordick and Breitschwerdt (16) described the intraerythrocytic location of B. henselae during persistent bacteremia in cats. Electron micrographs revealed bacteria within 2.9 to 6.2% of the RBC examined, but no bacteria were found to be free of RBC or associated with the RBC surface. The significance of RBC invasion to long-term infections in cats, acquisition by cat fleas, and transmission to humans is unknown. Detailed investigation of the RBC invasion process, including the potential isolation of noninvading mutants, will require a suitable model for RBC invasion in vitro; this was the focus of the present study.
B. henselae invasion of feline RBC was analyzed in vitro as described for B. bacilliformis invasion of human RBC (3), with modifications. B. henselae Houston-1 was supplied by R. Regnery (Centers for Disease Control and Prevention, Atlanta, Ga.) and tested by PCR (21) and indirect fluorescent antibody binding (9). Bartonella cultures were grown on plates of heart infusion agar supplemented with 5% defibrinated rabbit blood (HIBA) in 4% CO2 for 6 to 7 days at 35°C. Sterile feline blood, preserved and anticoagulated with EDTA, was obtained from Harlan Bioproducts (Indianapolis, Ind.). One-milliliter aliquots were each supplemented with 0.14 ml of acid-citrate-dextrose anticoagulant solution (Sanofi Animal Health, Inc., Overland Park, Kans.) to help maintain RBC integrity. Sterility was confirmed by inoculating HIBA plates and incubating them for up to 7 days at 37°C.
Feline RBC in EDTA and acid-citrate-dextrose were washed three times in Hanks balanced salt solution without bicarbonate and resuspended at approximately 1.7 × 108 to 2 × 108/ml, based on direct hemocytometer counts. For each invasion assay, one HIBA plate containing a lawn of B. henselae cells (6 to 7 days of growth) was overlaid with 1 ml of Bartonella liquid growth medium (RPMI 1640 without bicarbonate, supplemented with 1% l-glutamine, HEPES buffer, sodium pyruvate, nonessential-amino-acid solution, and 2 mg of hemin/ml [prepared in 0.01 N NaOH]; Sigma Chemical Co., St. Louis, Mo.) (30). Bacterial cells were suspended in the liquid growth medium, 2- or 20-μl aliquots were added to sterile 1.5-ml microcentrifuge tubes, and the total volume in each tube was adjusted to 100 μl with Bartonella liquid growth medium. A 100-μl aliquot from each HIBA plate was also serially diluted and plated to determine the viable-cell count (typically approximately 6.0 × 109 B. henselae CFU/ml). Feline RBC in Hanks balanced salt solution were added to tubes containing the B. henselae suspension and incubated at 35°C for various lengths of time, ranging from 1 to 24 h. In some experiments, the samples were centrifuged at 900 × g for 4 min to increase bacterial association with RBC (3), but this step did not appear to affect the results. Feline RBC were separated from unassociated bacteria by washing with phosphate-buffered saline (PBS), since Percoll gradient centrifugation (3) resulted in substantial lysis of these RBC.
Evaluation of RBC invasion by confocal microscopy.
Infected RBC were analyzed by laser scanning confocal microscopy to discriminate between intracellular and epicellular B. henselae cells. RBC invasion samples prepared as described above were diluted 1:10 or 1:100 in PBS, and 10-μl volumes were added to individual wells of an eight-well glass slide. The slides were air dried, heat fixed, stained for 20 min with a 1:100 dilution of acridine orange (0.5 mg/ml), washed in distilled water, and again air dried. A 1:100 dilution of fluorescein isothiocyanate (1 mg/ml) was used as a secondary stain for 20 min, after which the slides were rinsed and air dried. Coverslips were sealed with Citifluor mounting fluid, and slides were viewed at the University of Georgia Center for Ultrastructural Research, using a Bio-Rad (Hercules, Calif.) MRC 600 laser scanning confocal microscope with an excitation wavelength of 488 nm and an emission wavelength of 617 nm. Z scans were performed to section RBC images digitally in depth in increments of 0.5 μm. Feline RBC are rounded (as opposed to biconcave) and, with a diameter of 3.9 to 5.5 μm, can be completely traversed in 8 to 11 digital sections taken in 0.5-μm increments.
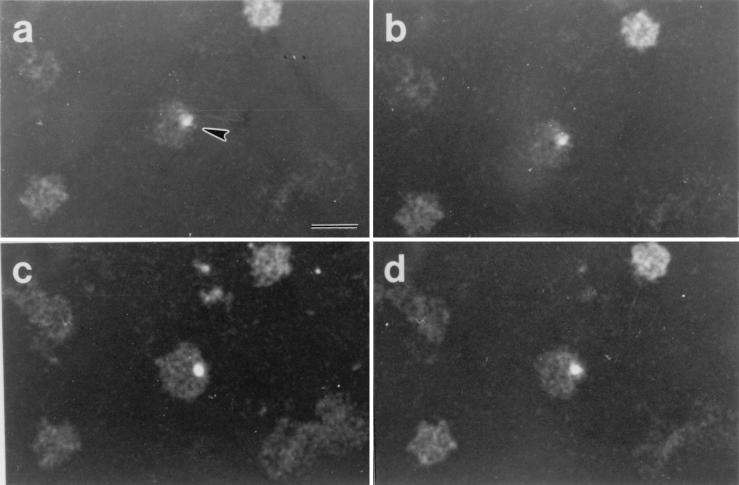
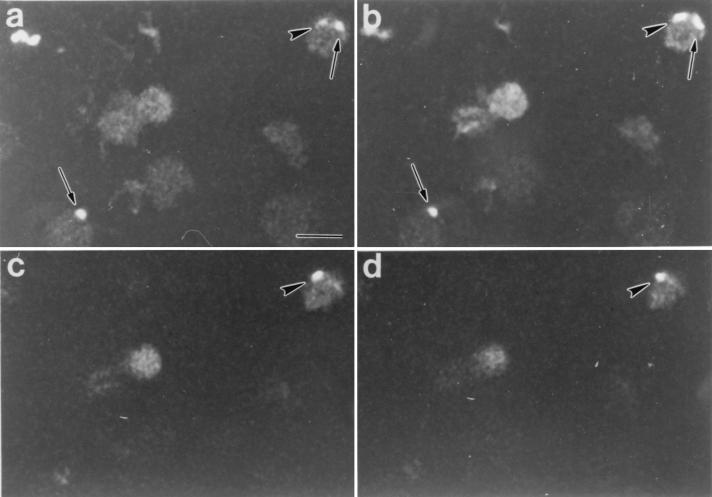
Typical results following digital sectioning of infected RBC are shown in Fig. 1. Figure 1a to d are digital sections 1, 5, 8, and 11 (from top to bottom, respectively) of feline RBC following B. henselae invasion in vitro. Bacterial cells stained with acridine orange were easily distinguished by their intense fluorescence. The bacterial cell in this series was weakly fluorescent in the uppermost sections (Fig. 1a and b), but the fluorescence increased in intensity in section 8 (Fig. 1c). Significantly, the bacterial cell in Fig. 1c was clearly seen interior to the edge of the RBC and, based on this observation and the depth of the section, probably was intracellular. The fluorescence intensity of the indicated cell diminished as digital sectioning approached the lower surface of the RBC (Fig. 1d). A similar pattern was observed in the serial sections shown in Fig. 2. The images in this series were from sections 2, 6, 8, and 10 (Fig. 2a to d, respectively) from top to bottom. Likely epicellular bacteria, which were common in the RBC smears, are evident, suggesting that RBC association does not lead to rapid invasion. A B. henselae cell exhibiting a fluorescence pattern similar to that observed in Fig. 1 and consistent with an intracellular location is also evident. Both bacteria in the upper right corner of Fig. 2a appear to be within the margin of the RBC. Through optical scanning, however, it was evident that they were localized in different planes. The bacterial cell indicated by the arrow in Fig. 2b was barely seen in Fig. 2c and d, suggesting a location on or near the top of the RBC. In contrast, the fluorescence pattern of the bacterial cell indicated by the arrowhead suggested a location in a lower plane that probably corresponds to the RBC interior. Thus, analysis by confocal microscopy suggests the presence of both intracellular and epicellular B. henselae organisms associated with feline RBC but also underscores the difficulty in distinguishing between each by this technique.
FIG. 1.
Digital sections of feline RBC after infection with B. henselae, as viewed by laser scanning confocal microscopy. Shown are sections 1 (a), 5 (b), 8 (c), and 11 (d); sections were taken in 0.5-μm increments from top to bottom. Note that the fluorescence intensity of the B. henselae cell (arrowhead) was low in panels a and b and increased in panel c. The B. henselae cell can be seen clearly within the RBC membrane and was probably intracellular. In panel d, the fluorescence intensity of the Bartonella cell once again diminished. Bar, 5 μm; magnification, ×1,000.
FIG. 2.
Digital sections of feline RBC after infection with B. henselae, as viewed by laser scanning confocal microscopy. Sections were taken as described for Fig. 1; shown are sections 2 (a), 6 (b), 8 (c), and 10 (d). The arrowhead indicates a likely intracellular B. henselae cell, while the arrows indicate probable epicellular B. henselae cells. Note that the fluorescence intensity of the organism indicated by the arrowhead in panel a increased in panel b and then faded in panels c and d. Bar, 5 μm; magnification, ×1,000.
Assessment of RBC invasion by gentamicin selection.
Intracellular B. henselae cells were quantitated on the basis of protection from gentamicin. Invasion mixtures prepared and incubated from 1 to 24 h as described above were treated with gentamicin sulfate (250 μg/ml) for 3 h at 35°C to kill extracellular B. henselae cells. RBC were centrifuged at 900 × g for 4 min, washed three times in PBS to remove residual antibiotic, and serially diluted in PBS. Aliquots (0.1 ml) were each combined with 0.3 ml of sterile water to lyse the RBC, and 100-μl volumes of each dilution were individually inoculated onto HIBA plates that were subsequently incubated at 35°C in 4% CO2 for 6 to 7 days. Bartonella viable-cell counts consistently reflected substantial RBC invasion (Table 1). No CFU were recovered when RBC infection was carried out at 4°C, reflecting a temperature-dependent process. Invasion was also dose dependent, but variability in CFU recovery between experiments was high. CFU counts after gentamicin selection (reported as percentages of the initial inoculum) ranged from 2 to 17%, averaging 7% for the 24-h incubation. In some experiments, total CFU before and after the 24-h incubation, prior to the gentamicin treatment, were compared. Little change in viable numbers was observed during this incubation, consistent with the slow growth of these bacteria in culture (data not shown). No CFU were recovered when RBC were omitted, and no spontaneous gentamicin resistance was observed at the gentamicin concentration used.
TABLE 1.
Evaluation of B. henselae invasion of feline RBC, based on gentamicin selection
| Expt | Inoculum (CFU)a | Recovered CFU/sampleb | % of inoculum recovered |
|---|---|---|---|
| 1 | 6.4 × 103 | 5.7 × 102 | 8.9 |
| 6.4 × 104 | 5.0 × 103 | 7.8 | |
| 6.4 × 105 | 1.0 × 104 | 1.6 | |
| 6.4 × 104c | 0 | 0 | |
| 2 | 5.0 × 103 | 6.0 × 102 | 12 |
| 5.0 × 104 | 4.0 × 103 | 8 | |
| 5.0 × 105 | 1.0 × 104 | 2 | |
| 5.0 × 104c | 0 | 0 | |
| 3 | 3.4 × 104 | 5.8 × 103 | 17 |
| 3.4 × 105 | 1.1 × 104 | 3.2 | |
| 3.4 × 106 | 1.0 × 105 | 2.9 | |
| 3.4 × 105c | 0 | 0 | |
| 4 | 1.8 × 104 | 1.0 × 103 | 5.6 |
| 1.8 × 105 | 1.0 × 104 | 5.6 | |
| 1.8 × 106 | 1.0 × 105 | 5.6 |
The RBC count was 1.4 × 108 to 2.5 × 108 per sample.
Determined after a 24-h incubation of B. henselae with RBC.
Infection was performed at 4°C.
Invasion of feline RBC by B. henselae, based on gentamicin selection, was a time-dependent process (Table 2). Significant numbers of intracellular bacteria were not observed until the 24-h time point postinfection. This rate was much lower than that reported for invasion of human RBC in vitro by B. bacilliformis (3), for which adherence follows a 15- to 30-min lag and invasion progresses rapidly, peaking by 6 h (19). Analysis of infected RBC by confocal microscopy suggested that B. henselae attachment to RBC likewise occurred relatively rapidly, with internalization being the rate-limiting step. Flagellar function is thought to be important in B. bacilliformis invasion (27), but B. henselae lacks flagella and is thought to employ a twiddle motility driven by type IV fimbriae (19). This difference in motility may account for the lower level of RBC invasion by B. henselae.
TABLE 2.
Time course of B. henselae invasion of feline RBC, based on gentamicin selection
| Expt | Time point (h) | Inoculum (CFU)a | Recovered CFU/sample | % of inoculum recovered |
|---|---|---|---|---|
| 1 | 1 | 1.2 × 106 | 0 | 0 |
| 1 | 1.2 × 107 | 0 | 0 | |
| 4 | 1.2 × 106 | 0 | 0 | |
| 4 | 1.2 × 107 | 8.5 × 10b | <1 | |
| 8 | 1.2 × 106 | 5.0 × 102b | <1 | |
| 8 | 1.2 × 107 | 1.0 × 103b | <1 | |
| 24 | 1.2 × 106 | 2.8 × 105 | 23 | |
| 24 | 1.2 × 107 | 2.4 × 106 | 20 | |
| 2 | 1 | 1.0 × 105 | 0 | 0 |
| 1 | 1.0 × 106 | 0 | 0 | |
| 4 | 1.0 × 105 | 0 | 0 | |
| 4 | 1.0 × 106 | 8.5 × 102b | <1 | |
| 8 | 1.0 × 105 | 2.5 × 102b | <1 | |
| 8 | 1.0 × 106 | 1.5 × 103b | <1 | |
| 24 | 1.0 × 105 | 2.0 × 104 | 20 | |
| 24 | 1.0 × 106 | 1.0 × 105 | 10 |
The RBC count was 1.9 × 108 to 2.5 × 108 per sample.
Value reflects less than 30 CFU per plate.
A direct comparison of gentamicin treatment and laser confocal microscopy for the evaluation of feline RBC invasion by B. henselae in vitro was made (Table 3). Samples from the same invasion mixture were processed by the two techniques in parallel. Bartonella viable-cell counts following gentamicin selection were comparable to results obtained previously and totaled between 1 and 10% of the initial B. henselae inoculum, corresponding to invasion of <1% of the RBC in the invasion mixture. However, by confocal microscopy, between 1 and 12% of the feline RBC appeared to contain intracellular bacteria. The infection rate determined by confocal microscopy is probably less reliable due to the difficulty in distinguishing intracellular and epicellular bacteria and the inability to discriminate between viable and nonviable bacteria by this technique. The latter problem would likely be accentuated by the use of plate-grown bacteria for the invasion assay. Furthermore, gentamicin selection requires that the bacteria be completely separated from the extracellular environment to avoid elimination. Kordick and Breitschwerdt (16) described an apparent pore at the RBC surface that may reflect the route of entry into the cell interior. If these pores persisted for some time, they might permit accessibility of the internalized bacteria to the gentamicin in the extracellular space. Only when the pore was sealed would the bacteria be expected to survive gentamicin selection.
TABLE 3.
Comparison of gentamicin selection and confocal microscopy for assessing in vitro feline RBC invasion by B. henselae
| Expt | Inoculuma | CFU recovered as determined by gentamicin selection | % of RBC infected as determined by:
|
|
|---|---|---|---|---|
| Gentamicin selection | Confocal microscopyb | |||
| 1 | 6.4 × 103 | 5.7 × 102 | <1 | 10 |
| 6.4 × 104 | 5.0 × 103 | <1 | 15 | |
| 2 | 5.0 × 103 | 6.0 × 102 | <1 | 1 |
| 5.0 × 104 | 4.0 × 103 | <1 | 12 | |
The RBC count was 1.5 × 108 to 2.0 × 108 per sample.
Only intracellular organisms were counted.
A preliminary comparison between RBC invasion frequencies in vitro and in vivo in bacteremic cats was achieved by using gentamicin selection. Blood samples from cats experimentally infected with B. henselae by subcutaneous and intradermal inoculation were evaluated for viable-cell counts with and without gentamicin selection. The infection, sampling, and treatment of cats in experimental infections were conducted as described previously (9). Cats were inoculated intradermally and subcutaneously with 3.5 × 104 CFU of a low-passage-number B. henselae stock culture grown on HIBA plates and stored at −70°C prior to use. For enumeration of intracellular B. henselae cells, gentamicin sulfate was added to a concentration of 250 μg/ml to whole blood from a bacteremic cat and the blood was then incubated for 3 h at 35°C. The blood was subsequently centrifuged at 900 × g for 4 min, and the blood cell pellet was washed three times with PBS. RBC were lysed with sterile distilled water, and the lysate was serially diluted and inoculated onto HIBA plates. The total B. henselae CFU in the blood was enumerated by lysing the RBC (without gentamicin treatment) with sterile distilled water and then diluting the lysate serially and inoculating HIBA plates. In a cat at peak bacteremia, for example, the total CFU count was 1.8 × 105/ml. After gentamicin selection, a value of 2.2 × 104 CFU was obtained, representing 12% of the total CFU in the blood and within the range of invasion rates observed in vitro. These results are very preliminary, and expanded studies are planned for more-thorough documentation of invasion rates in vivo based on gentamicin selection.
Approximately 5% of the RBC examined from cats naturally infected with B. henselae were reported to contain bacteria, and no epicellular bacteria were observed by electron microscopy (16). In the present study, the percentage of RBC containing intracellular bacteria on the basis of gentamicin selection was much lower (≪1%) both in experimentally infected cats (see preceding paragraph) and with invasion in vitro. This discrepancy might be due to any one of several factors, including differences between natural and experimental infections, viable versus total cell counts, or the B. henselae strains studied in each case (2).
Characterization of B. henselae-feline RBC interaction.
To analyze biochemically the interaction between B. henselae and feline RBC, we assessed the effects of protease or neuraminidase pretreatment of the RBC, or protease pretreatment of the bacteria, on RBC invasion. Treatments were carried out as described previously for B. bacilliformis (3), with minor changes. Briefly, trypsin (15, 25, or 50 μg) was added to 1.5-ml microcentrifuge tubes containing feline RBC or B. henselae cells in Bartonella liquid growth medium, and the tubes were incubated at 37°C for 10 min. Antitrypsin was added in a twofold molar excess, and the tubes were placed on ice. Trypsinized RBC suspensions were evaluated visually for hemolysis, while trypsinized B. henselae cells were diluted and inoculated on HIBA plates to confirm bacterial viability. In some experiments, both the bacteria and the RBC were treated with trypsin (50 μg). Alternatively, RBC were pretreated with type XIV bacterial protease from Streptomyces griseus (pronase, 1 mg/ml in PBS; Sigma Chemical Co.) or neuraminidase (C. perfringens neuraminidase type VIII, 1 U/ml; Sigma Chemical Co.) for 30 min at 37°C. RBC were washed twice with PBS after each treatment and then used in invasion assays as described above. Trypsin pretreatment of feline RBC, B. henselae cells, or both had no detectable effect on invasion, but a 23 to 50% increase in invasion was observed with pronase-pretreated RBC (data not shown). Pretreatment of feline RBC with neuraminidase had no effect on invasion, indicating that sialic acid moieties on the RBC surface are probably not required. Significantly, the same pattern of resistance to enzymatic pretreatment of bacteria or RBC was reported for B. bacilliformis (3, 19, 29), suggesting that similar surface structures may be functional in the interplay between bacteria and RBC in each case.
Our evaluation of bacteremic cats during natural and experimental infections suggests the presence of intracellular, epicellular, and extracellular bacteria in the blood, with RBC invasion perhaps being limited to a small percentage of the bacterial population. Significantly, the rate of RBC invasion in vitro described here was similarly low. This raises the question of the role that RBC invasion may play in the natural history of B. henselae, and at least four potential benefits seem likely: (i) RBC invasion might confer a nutritional advantage (e.g., hemin acquisition); (ii) immune evasion might be enhanced by intraerythrocytic localization; (iii) spread to other anatomical sites (e.g., the liver or spleen) might be enhanced by RBC invasion; and/or (iv) survival in the cat flea, perhaps during the initial stages of digestion of a blood meal, may be enhanced for intracellular bacteria. It is difficult to reconcile a nutritional advantage with low levels of invasion. B. henselae cells are sensitive to antibody-independent, complement-mediated cytolysis (26); hence, RBC invasion might afford a means to escape this defense mechanism. However, this fails to account for the high numbers of extracellular bacteria seen in experimentally infected cats. There is insufficient information regarding the spread of B. henselae to major organs in infected cats to address a possible role for RBC invasion in this context. However, Vaughan and Azad (28) reported that ingested RBC are rapidly hemolyzed within human body lice (Pediculus humanus) and cat fleas (C. felis), vectors for B. quintana and B. henselae, respectively. Intraerythrocytic B. henselae might withstand initial digestion better than their extracellular counterparts and thereby survive to replicate in the digestive tract of their arthropod vector.
ACKNOWLEDGMENTS
This study was supported by a grant from the University of Georgia Program in Biological Resources and Biotechnology.
We thank Mark Farmer and Cathy Kelloes from the University of Georgia Center for Ultrastructural Research for their assistance with confocal microscopy and Macon Miles, Michelle McDermott, and Erez Sternberg for their technical assistance.
REFERENCES
- 1.Anderson B, Sims K, Regnery R, Robinson L, Schmidt M J, Goral S, Hager C, Edwards K. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol. 1994;32:942–948. doi: 10.1128/jcm.32.4.942-948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterman H J, Peek J A, Loutit J S, Falkow S, Tompkins L S. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun. 1995;63:4553–4556. doi: 10.1128/iai.63.11.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson L A, Kar S, McLaughlin G, Ihler G M. Entry of Bartonella bacilliformis into erythrocytes. Infect Immun. 1986;54:347–353. doi: 10.1128/iai.54.2.347-353.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branley J, Wolfson C, Waters P, Gottlieb T, Bradbury R. Prevalence of Bartonella henselae bacteremia, the causative agent of cat scratch disease, in an Australian cat population. Pathology. 1996;28:262–265. doi: 10.1080/00313029600169124. [DOI] [PubMed] [Google Scholar]
- 5.Breathnach A S, Hoare J M, Eykyn S J. Culture-negative endocarditis: contribution of Bartonella infections. Heart. 1997;77:474–476. doi: 10.1136/hrt.77.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomel B B, Abbott R C, Kasten R W, Floyd-Hawkins K A, Kass P H, Glaser C A, Pederson N C, Koehler J E. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995;33:2445–2450. doi: 10.1128/jcm.33.9.2445-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomel B B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield A N, Abbott R C, Pederson N C, Koehler J E. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demers D M, Bass J W, Vincent J M, Person D A, Noyes D K, Staege C M, Samlaska C P, Lockwood N H, Regnery R L, Anderson B E. Cat-scratch disease in Hawaii: etiology and seroepidemiology. J Pediatr. 1995;127:23–26. doi: 10.1016/s0022-3476(95)70251-2. [DOI] [PubMed] [Google Scholar]
- 9.Greene C E, McDermott M, Jameson P H, Atkins C L, Marks A M. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682–1685. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guptill L, Slater L, Wu C C, Lin T L, Glickman L T, Welch D F, Hogenesch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 11.Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J A, Radulovic S, Jaworski D C, Azad A F. Acquisition of the cat scratch disease agent Bartonella henselae by cat fleas (Siphonatpera: Pulicidae) J Med Entomol. 1996;33:490–495. doi: 10.1093/jmedent/33.3.490. [DOI] [PubMed] [Google Scholar]
- 13.Jameson P, Greene C, Regnery R, Dryden M, Marks A, Brown J, Cooper J, Glaus B, Greene R. Prevalence of Bartonella henselae antibodies in pet cats throughout regions of North America. J Infect Dis. 1995;172:1145–1149. doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 14.Koehler J E. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 15.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection: a new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 16.Kordick D L, Breitschwerdt E B. Intraerythrocytic presence of Bartonella henselae. J Clin Microbiol. 1995;33:1655–1656. doi: 10.1128/jcm.33.6.1655-1656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kordick D L, Breitschwerdt E B. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res. 1997;58:492–497. [PubMed] [Google Scholar]
- 18.Kordick D L, Wilson K H, Sexton D J, Hadfield T L, Berkhoff H A, Breitschwerdt E B. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol. 1995;33:3245–3251. doi: 10.1128/jcm.33.12.3245-3251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minnick M F. Cell entry and the pathogenesis of Bartonella infections. Trends Microbiol. 1996;9:343–347. doi: 10.1016/0966-842x(96)10055-x. [DOI] [PubMed] [Google Scholar]
- 20.Myers W F, Cutler L D, Wisseman C L., Jr Role of erythrocytes and serum in the nutrition of Rickettsia quintana. J Bacteriol. 1969;97:663–666. doi: 10.1128/jb.97.2.663-666.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norman A F, Regnery R, Jameson P, Greene C, Krause D C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regnery R, Tappero J. Unraveling mysteries associated with cat-scratch disease, bacillary angiomatosis, and related syndromes. Emerg Infect Dis. 1995;1:16–21. doi: 10.3201/eid0101.950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regnery R L, Martin M, Olson J. Naturally occurring Rochalimeae henselae infection in domestic cat. Lancet. 1992;340:557–558. doi: 10.1016/0140-6736(92)91760-6. [DOI] [PubMed] [Google Scholar]
- 25.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 26.Rodriguez-Barradas M C, Bandres J C, Hamill R J, Trial J, Clarridge III J E, Baughn R E, Rossen R D. In vitro evaluation of the role of humoral immunity against Bartonella henselae. Infect Immun. 1995;63:2367–2370. doi: 10.1128/iai.63.6.2367-2370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherer D C, DeBuron-Connors I, Minnick M F. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun. 1993;61:4962–4971. doi: 10.1128/iai.61.12.4962-4971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan J A, Azad A F. Patterns of erythrocyte digestion by bloodsucking insects: constraints on vector competence. J Med Entomol. 1993;30:214–216. doi: 10.1093/jmedent/30.1.214. [DOI] [PubMed] [Google Scholar]
- 29.Walker T S, Winkler H H. Bartonella bacilliformis: colonial types and erythrocyte adherence. Infect Immun. 1981;31:480–486. doi: 10.1128/iai.31.1.480-486.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong M T, Thornton D C, Kennedy R C, Dolan M J. A chemically defined liquid medium that supports primary isolation of Rochalimaea (Bartonella) henselae from blood and tissue specimens. J Clin Microbiol. 1995;33:742–744. doi: 10.1128/jcm.33.3.742-744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]