Abstract
Caspase-3 is a crucial component of the apoptotic machinery in many cell types. Here, we report the timescale of caspase-3 activation in single living cells undergoing apoptosis. This was achieved by measuring the extent of fluorescence resonance energy transfer within a recombinant substrate containing cyan fluorescent protein (CFP) linked by a short peptide possessing the caspase-3 cleavage sequence, DEVD, to yellow fluorescent protein (YFP; i.e. CFP–DEVD–YFP). We demonstrate that, once initiated, the activation of caspase-3 is a very rapid process, taking 5 min or less to reach completion. Furthermore, this process occurs almost simultaneously with a depolarization of the mitochondrial membrane potential. These events occur just prior to the characteristic morphological changes associated with apoptosis. Our results clearly demonstrate that, once initiated, the commitment of cells to apoptosis is a remarkably rapid event when visualized at the single cell level.
INTRODUCTION
Apoptosis, or programmed cell death, is responsible for many normal developmental processes. For example, it is crucial during embryonic development, in shaping the adult organism, in surveillance of the cell cycle, and in some forms of chemically induced cell death. Inappropriate apoptosis has been implicated in many human diseases such as neurodegeneration and neoplasia, and also in acute organ failure.
The activation of caspase-3 is a central event in the process of apoptosis (Thornberry and Lazebnik, 1998; Budihardjo et al., 1999; Wolf and Green, 1999). This cysteine protease, which is proteolytically activated by cleavage of pro-caspase-3 by caspase-8, cleaves several intracellular proteins. One such target protein is the DNA repair enzyme poly(ADP-ribose) polymerase (PARP), which is cleaved by caspase-3 after the tetrapeptide motif Asp-Glu-Val-Asp (DEVD; Lazebnik et al., 1994; Thornberry et al., 1997).
Mitochondria are also crucial components of the apoptotic response. Depolarization of the mitochondrial inner membrane potential and the release of cytochrome c accompany the activation of the caspase-3 via a mechanism involving the apoptosome (a protein complex comprising Apaf-1 and procapase-9; Li et al., 1997; Zou et al., 1999). Transient opening of the mitochondrial permeability transition pore (MPTP) has been proposed to be important in the release of cytochrome c. Currently however, the precise temporal and spatial interrelationships between these processes are poorly characterized (Kroemer and Reed, 2000). Indeed, it should be noted that in many cases cytochrome c release has been observed without detectable mitochondrial depolarization (reviewed in Halestrap et al., 2000).
Green fluorescent protein (GFP) is a well established fluorescent reporter allowing a variety of intracellular events that occur in living cells to be visualized non-invasively (Tsien, 1998). The development of mutants of GFP with distinct spectral properties, has allowed in vivo measurements of protein–protein interaction to be performed by measuring fluorescence resonance energy transfer (FRET). For example, the emission spectrum of cyan fluorescent protein (CFP) overlaps significantly with the excitation spectrum of yellow fluorescent protein (YFP); placing CFP in close proximity to YFP (i.e. <5 nm) allows FRET between the two fluorescent moieties to occur. FRET between CFP fused to calmodulin, and YFP attached to an M13 peptide (a calmodulin binding peptide; Cameleons), has been reported to be increased by raising intracellular Ca2+; this induces an alteration in the spatial arrangement of the CFP and YFP moieties leading to the change in FRET (Miyawaki et al., 1997). Similarly, FRET between two spectrally distinct GFP mutants, fused covalently with a peptide linker containing a protease cleavage sequence, was used to monitor the degree of cleavage by trypsin and factor Xa in vitro (Heim and Tsien, 1996; Mitra et al., 1996).
We have adapted the latter type of approach to measure the activity of caspase-3 in single living cells by transfecting COS-7 cells with a construct encoding a fusion protein in which CFP is linked to YFP by a peptide containing the sequence DEVD, which is cleaved by caspase-3 (CFP–DEVD–YFP; Figure 1A) (Xu et al., 1998; Mahajan et al., 1999).
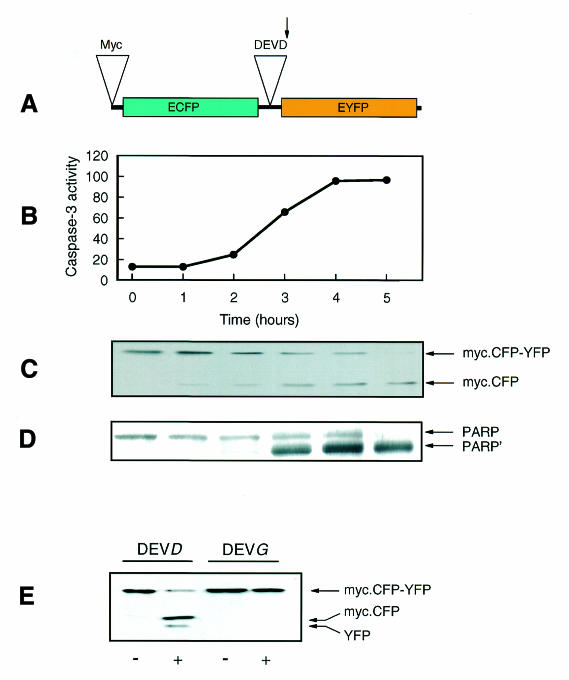
Fig. 1. Activation of caspase-3 and the cleavage of the CFP–DEVD–YFP fusion protein and PARP occur in parallel in response to staurosporine. (A) A schematic diagram of the CFP–DEVD–YFP fusion protein containing an 18 amino acid linker possessing the caspase-3 specific cleavage sequence, DEVD, and an N-terminal Myc-epitope tag. The arrow indicates the site of cleavage by caspase-3. (B–D) COS-7 cells transfected with the CFP–DEVD–YFP fusion protein were incubated with 1 µM staurosporine for the times indicated, and the activity of caspase-3 was measured in subsequently prepared lysates (pmol AMC released per min per µg protein; B) and compared with the extent of cleavage of CFP–DEVD–YFP (C) and PARP (D) by western blotting with anti-Myc and anti-PARP antibodies, respectively. The migration positions of intact PARP and cleaved PARP (PARṔ) are indicated. (E) COS-7 cells transfected with either CFP–DEVD–YFP (DEVD) or CFP–DEVG–YFP (DEVG) were incubated in the absence (–) or presence (+) of 1 µM staurosporine for 4 h, as indicated. Lysates were subjected to western blotting with an anti-GFP antibody.
RESULTS AND DISCUSSION
COS-7 cells were transfected with the CFP–DEVD–YFP construct. Staurosporine-induced apoptosis of these cells [as confirmed by TdT-mediated dUTP nick-end labelling (TUNEL) assay; data not shown] was accompanied by an activation of caspase-3, which slowly proceeded to completion by 4–5 h after an initial 1–2 h lag phase (Figure 1B). The activation of caspase-3 was accompanied by an almost parallel increase in cleavage of the introduced CFP–DEVD–YFP fusion protein (Figure 1C), as determined by western blotting of cell lysates with 9E10 antibody. This recognizes the Myc epitope present at the N-terminus of CFP (Figure 1A). The timescale of CFP–DEVD–YFP cleavage was almost identical to that of the appearance of the 85 kDa product (PARP’) of caspase-3-dependent cleavage of the 116 kDa PARP holoenzyme, as determined in parallel (Figure 1D). The cleavage of the CFP–DEVD–YFP fusion protein was completely blocked when the critical aspartic acid at the fourth residue of the cleavage motif was substituted with a glycine (CFP–DEVG–YFP; Figure 1E). It should be noted that transfection of cells with the CFP–DEVD–YFP construct has no apparent effect on basal caspase-3 activity, or its rate of activation by staurosporine (data not shown).
These experiments demonstrate that the extent of CFP–DEVD–YFP cleavage correlates well with both the activity of caspase-3 and the degree of cleavage of PARP. At the cell population level, these phenomena appear to occur quite slowly. However, the experiments tell us little about the timescale of caspase-3 activation and PARP cleavage at the single cell level.
In Figure 2, cells transfected with the CFP–DEVD–YFP fusion protein were imaged by confocal microscopy over the 1.5–3 h time period in which, according to Figure 1, the rate of increase of caspase-3 activation and cleavage of the CFP–DEVD–YFP fusion protein were the greatest. Simultaneously, we measured the inner mitochondrial membrane potential by loading the cells with tetramethylrhodamine ethyl ester (TMREE), which partitions across the mitochondrial inner membrane according to its electrical potential; a depolarization leads to decreased TMREE fluorescence in mitochondria (see Figure 2A and the quantitative analysis of representative cells in Figure 2B). It should be noted that the presence of TMREE had no apparent effect on the kinetics of caspase-3 activation as measured by confocal microscopy (data not shown).
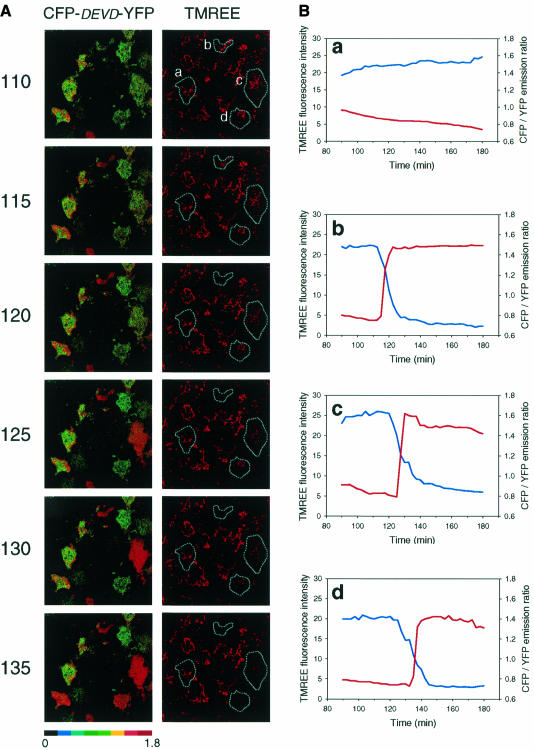
Fig. 2. The activation of caspase-3 and depolarization of the mitochondrial inner membrane are very rapid and occur in parallel. COS-7 cells transfected with CFP–DEVD–YFP were pre-loaded with the TMREE dye prior to incubation for 90 min in the presence of 1 µM staurosporine in a tissue culture incubator. The cells were then placed on the stage of the confocal microscope in modified KRP buffer supplemented with 1 µM staurosporine at 37°C. (A) A CFP/YFP emission ratio image (470–485 nm/520–540 nm) was collected at 2.5 min intervals and a selected region of the time course beginning at 110 min is shown on the left. The fluorescence intensity of TMREE was measured simultaneously across a 580–665 nm window. (B) The CFP/YFP ratio (red lines) for four representative cells in the field of view (as indicated by a–d), was plotted alongside the TMREE fluorescence intensity (blue lines) and is shown on the right hand side of the figure over the entire 1.5 h duration of imaging. Plot a shows a cell that did not respond during the experiment. Plots b–d show three responsive cells. A full animation of this experiment is available as supplementary data to this paper at EMBO Reports Online.
Cleavage of the CFP–DEVD–YFP fusion protein is indicated by an increase in the CFP/YFP emission ratio, which reflects a decrease in FRET. Very little cleavage of CFP–DEVD–YFP occurred during the first 2 h after the addition of staurosporine; this is demonstrated by quantitative analysis of the non-responsive cell ‘a’ (see Figure 2B, panel a). However, it can be seen clearly from the images in Figure 2A and the quantitative analysis shown in Figure 2B (panels b–d) that once CFP–DEVD–YFP cleavage initiates in an individual cell it moves to completion within 5 min or less. Furthermore, individual cells initiate their response at quite different times; clearly when this is averaged out over the cell population the apparently ‘slow’ increases in caspase-3 activity, and CFP–DEVD–YFP and PARP cleavage are observed (Figure 1B–D).
Taken together with the data from Figure 1, this demonstrates that once caspase-3 activation is initiated this moves to completion within 5 min and, furthermore, that PARP cleavage must occur with a similarly rapid time course. Remarkably, this all appears to occur almost in parallel with the depolarization of the mitochondrial membrane potential (Figure 2A and B). Over four separate experiments, 76% of the cells exhibited ‘simultaneous’ caspase-3 activation and mitochondrial membrane depolarization, as exhibited by cell ‘b’ in Figure 2B (given the limitation of 2.5 min observation intervals). The majority of the remaining cells behaved like cell ‘c’ in which mitochondrial membrane depolarization preceded caspase-3 activation by ∼5 min and was slower to reach completion than caspase-3 activation.
Upon examination of the entire animated sequence of the experiment shown in Figure 2A, it is also clear that the activation of caspase-3 and mitochondrial membrane depolarization immediately precede a dramatic cell shrinkage, which is also typical of apoptotic cells (see supplementary data, available at EMBO Reports Online.
Mitochondrial membrane depolarization and caspase-3 activation have been reported to be causally related in a number of cell types (reviewed in Green and Reed, 1998; Kroemer and Reed, 2000). It should be noted that in our studies the broad-spectrum caspase-3 inhibitor, zVAD-fmk, inhibited staurosporine-induced CFP–DEVD–YFP cleavage but not mitochondrial membrane potential depolarization; furthermore, caspase-3 activation was not prevented by cyclosporin A, which has been reported to prevent the opening of the MPTP (data not shown). Thus, although caspase-3 activation and depolarization of the mitochondrial membrane potential may not be directly linked in staurosporine-induced apoptosis of COS-7 cells, they still appear to occur with very similar kinetics.
Once initiated, an almost complete release of cytochrome c from the mitochondrial inner membrane space was also recently reported to occur within 5 min (Goldstein et al., 2000). Taken together with our own work, these results demonstrate that many events crucial for the apoptotic response, and which take place in distinct subcellular compartments, occur within a very short time frame (<5 min) of induction of apoptosis in an individual cell; these events include caspase-3 activation in the cytoplasm, cytochrome c release from, and depolarization of, the inner mitochondrial membrane, and PARP cleavage in the nucleus (as inferred from the data in Figure 1). This is rapidly followed by changes in cell morphology and ultimate cell death. The reasons why a cell can apparently tolerate an apoptotic stimulus for several hours before this rapid chain of events occurs is not known and warrants further investigation; it may be related to differing degrees of drug resistance exhibited by individual cells but, equally, may depend on the stage in the cell cycle that the cell happens to be in. However, the sheer rapidity of the responses strongly suggests that at least some cell types may have mechanisms in place that are designed to prevent inadvertent reversion from the apoptotic pathway once it has been initiated.
METHODS
Construction of CFP–DEVD/G–YFP fusion protein expression vectors. ECFP (Clontech Laboratories Inc., Palo Alto, CA) was amplified using forward (5′-TCGACGGATCCGACGAGATGGAGCAGAAGCTGATCTCGGAGGAGGACCTGAAGGTGAGCAAGGGCGAGG-3′) and reverse (5′-TTTGGGGTACCATCGACCTCATCTCCGCTGAGCTCGGACGAGGACTTGTACAGCTCGTCCAT-3′) primers. EYFP (Clontech) was amplified using forward (5′-TTTGGGGTACCAGCGGAAGCGAATTCGTGAGCAAGGGCGAGGAG-3′) and reverse (5′-TTTCGCTCGAGAAGCTTGCGGCCGCTTACTTGTACAGCTCGTCCAT-3′) primers. The PCR products amplified from ECFP and EYFP were then digested with BamHI–KpnI and KpnI–HindIII, respectively, gel purified and three-way ligated into the BamHI–HindIII sites of the mammalian expression vector pcDNA3.1(–) (Invitrogen BV, Groningen, The Netherlands) to yield the pCFP–DEVD–YFP plasmid. This method of construction introduces an 18 amino acid linker peptide (SSSELSGDEVDGTSGSEF), which joins the C-terminus of ECFP to the N-terminus of EYFP, and also places a Myc-epitope tag for recognition by the 9E10 monoclonal antibody (Evan et al., 1985) at the N-terminus of the ECFP (Figure 1A). The critical aspartic acid residue in the caspase-3 cleavage site was substituted by a glycine residue (to yield the CFP–DEVG–YFP construct) by mutagenesis using a QuikChange‘ site-directed mutagenesis kit (Stratagene Cloning Systems, La Jolla, CA). The complete sequence integrity of all constructs was confirmed by DNA sequencing.
Transfection of COS-7 cells, lysis, western blotting and caspase-3 assays. COS-7 cells were maintained at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich Co. Ltd, Poole, UK) supplemented with 10% (v/v) fetal calf serum. Cells, in 35 mm dishes at 60–70% confluence, were transfected for 24 h at 37°C using 2 µg of plasmid DNA construct and 6 µl of FuGENE 6‘ (Roche Diagnostics Ltd., East Sussex, UK) according to the manufacturer’s instructions. The cells were then incubated in the presence of 1 µM staurosporine for the times indicated in the figure legends, washed in ice-cold phosphate-buffered saline (PBS) and lysed by mechanical scraping into 50 µl of lysis buffer [50 mM HEPES pH 7.4, 50 mM KCl, 2 mM MgCl2, 5 mM EDTA, 250 mM sucrose, 1% NP-40, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride, 0.5 µM DEVD.CHO caspase inhibitor (Biomol Research Lab Inc., Plymouth, PA), and 1 µg/ml each of pepstatin, antipain and leupeptin]. The supernatant, obtained by centrifugation at 14 000 g for 10 min at 4°C, was subjected to 10% SDS–PAGE and proteins were transferred onto Hybond C nitrocellulose (Amersham Pharmacia Biotech UK Ltd, Buckinghamshire, UK). Western blotting was performed using 0.04 µg/ml anti-GFP monoclonal antibody (Boehringer Mannheim, Germany), 0.7 µg/ml anti-Myc (9E10) monoclonal antibody (provided by Dr T. Harrison, University of Leicester) or 0.2 µg/ml anti-PARP polyclonal antibody (Santa Cruz Biotechnology Inc.) in PBS supplemented with 3% milk protein and 0.01% Tween-20, followed by ECL‘ detection (Amersham Pharmacia Biotech UK Ltd, Buckinghamshire, UK). Caspase-3 activity was also measured in the lysates (but prepared without DEVD.CHO in the lysis buffer) by determining the cleavage of the fluorogenic caspase-3 substrate Ac.DEVD–AMC (50 mM final concentration) in 50 mM HEPES pH 7.4, 1 mM EDTA, 10 mM DTT, 100 mM NaCl, 10% glycerol and 0.1% CHAPS. The extent of Ac.DEVD–AMC cleavage was measured as the increase in relative fluorescence units (RFU) resulting from the release of free fluorescent AMC, as previously described (Gurtu et al., 1997).
Live cell fluorescence confocal microscopy and image analysis. COS-7 cells expressing the CFP–DEVD–YFP fusion protein were grown to 60–70% confluence on collagen-coated 22 mm glass coverslips, and pre-incubated in 20 nM tetramethylrhodamine ethyl ester (TMREE) for 30 min at 37°C. The cells were then treated with 1 µM staurosporine for 90 min in a tissue culture incubator (37°C and 5% CO2) and then placed in a heated (37°C) chamber containing modified KRP buffer (136 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2, 5 mM Na3PO4, 2 mM NaHCO3, 25 mM HEPES pH 7.4). FRET was determined using a Leica Confocal Imaging Spectrophotometer by excitation using the 458 nm line supplied by an Ar-ion laser. Fluorescence emission was monitored at 1 frame per 2.5 min by collection across windows of 470–485 nm (CFP emission) and 520–540 nm (YFP emission). CFP/YFP ratios were calculated using the Leica TCS-NT4 physiology package and were manipulated for presentation using Metamorph (Universal Imaging Corp., West Chester, PA). Mitochondrial membrane potential was monitored simultaneously in the cells by excitation of TMREE with the 568 nm line of a Kr-ion laser, with emission being collected in a third channel across a 580–665 nm window. Imaging the cells with the confocal laser does not activate caspase-3 to any detectable extent (data not shown).
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
The authors are grateful to Keith Moore, Andrew Halestrap, Suchira Bose, Alison O’Toole and Ed Ainscow for discussions, and to Michelle Lazenby and Jason Stewart Clark for their excellent technical assistance. This work was supported in part by a grant from the Medical Research Council. J.M.T. is a Diabetes UK Senior Research Fellow. The confocal microscopy was performed using the MRC Cell Imaging Facility, which was funded by Infrastructure and JREI grants from the Medical Research Council.
REFERENCES
- Budihardjo I., Oliver, H., Lutter, M., Luo, X. and Wang, X. (1999) Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol., 15, 269–290. [DOI] [PubMed] [Google Scholar]
- Evan G.I., Lewis, G.K., Ramsay, G. and Bishop, J.M. (1985) Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol., 5, 3610–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.C., Waterhouse, N.J., Juin, P., Evan, G.I. and Green, D.R. (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol., 2, 156–162. [DOI] [PubMed] [Google Scholar]
- Green D.R. and Reed, J.C. (1998) Mitochondria and apoptosis. Science, 281, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Gurtu V., Kain, S.R. and Zhang, G. (1997) Fluorimetric and colorimetric detection of caspase activity associated with apoptosis. Anal. Biochem., 251, 98–102. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P., Doran, E., Gillespie, J.P. and O’Toole, A. (2000) Mitochondria and cell death. Biochem. Soc. Trans, 28, 170–177. [DOI] [PubMed] [Google Scholar]
- Heim R. and Tsien, R.Y. (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol., 6, 178–182. [DOI] [PubMed] [Google Scholar]
- Kroemer G. and Reed, J.C. (2000) Mitochondrial control of cell death. Nature Med., 6, 513–519. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Kaufmann, S.H., Desnoyers, S., Poirier, G.G. and Earnshaw, W.C. (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature, 371, 346–347. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan, D., Budihardjo, I., Srinivasula, S.M., Ahmad, M., Alnemri, E.S. and Wang, X. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Mahajan N.P., Harrison-Shostak, D.C., Michaux, J. and Herman, B. (1999) Novel mutant green fluorescent protein protease substrates reveal the activation of specific caspases during apoptosis. Chem. Biol., 6, 401–409. [DOI] [PubMed] [Google Scholar]
- Mitra R.D., Silva, C.M. and Youvan, D.C. (1996) Fluorescence resonance energy transfer between blue-emitting and red-shifted excitation derivatives of the green fluorescent protein. Gene, 173, 13–17. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Llopis, J., Heim, R., McCaffery, J.M., Adams, J.A., Ikura, M. and Tsien, R.Y. (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature, 388, 882–887. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. and Lazebnik, Y. (1998) Caspases: enemies within. Science, 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. et al. (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. J. Biol. Chem., 272, 17907–17911. [DOI] [PubMed] [Google Scholar]
- Tsien R.Y. (1998) The green fluorescent protein. Annu. Rev. Biochem., 67, 509–544. [DOI] [PubMed] [Google Scholar]
- Wolf B.B. and Green, D.R. (1999) Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem., 274, 20049–20052. [DOI] [PubMed] [Google Scholar]
- Xu X., Gerard, A.L., Huang, B.C., Anderson, D.C., Payan, D.G. and Luo, Y. (1998) Detection of programmed cell death using fluorescence energy transfer. Nucleic Acids Res., 26, 2034–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Li, Y., Liu, X. and Wang, X. (1999) An APAF-1·cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem., 274, 11549–11556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.