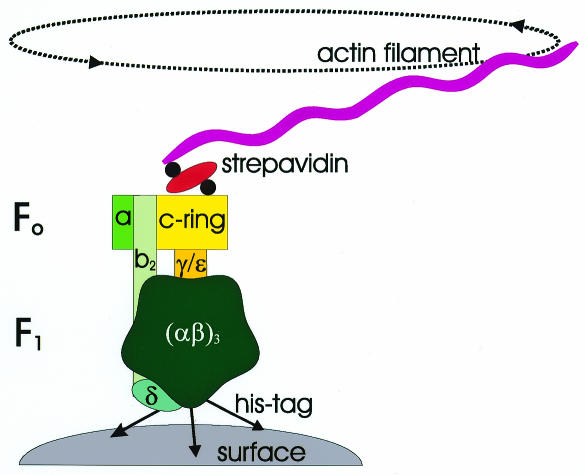
All living species use ATP as a general source of energy by hydrolysing it into ADP and phosphate. In most species the regeneration of this vital molecule is catalysed by the H+-transporting F1F0 ATP synthases, marvellous machines that couple the flow of protons down an electrochemical gradient to form ATP from ADP and phosphate. Under certain circumstances these enzymes can also work in the reverse direction, hydrolysing ATP and pumping protons. ATP synthases are composed of two parts, the membrane integrated F0 complex (in Escherichia coli: ab2c10–14?), and the cytoplasmic F1 complex (in E. coli (αβ)3γδɛ). In 1997, the existing data for the E. coli ATP synthase were combined into a ‘tentative structural model’ (Engelbrecht and Junge, 1997), in which the enzyme contains a rotary motor. According to this model, the rotor is formed by a ring of 12 copies of subunit c in the F0 complex, and the γ and ɛ subunits of the F1 complex (Figure 1). During proton translocation in ATP hydrolysis/synthesis, the rotor moves relative to the remaining ‘static’ subunits (the stator), causing conformational changes in the catalytical nucleotide binding sites in the subunits β. Although the rotation of subunit γ during ATP hydrolysis in F1 had already been shown directly (Noji et al., 1997), the rotation of the c-ring still needed to be verified.
Fig. 1. General experimental setup for the observation of F0-c-ring rotation. The F0-c-ring rotates together with subunits γ and ɛ in relation to subunits (αβ)3,δ, a and b2.
Sambongi et al. (1999) were the first to publish microvideograms of rotating filaments that were supposedly connected to the ring of subunits c. The group attached F1F0-ATPase to a surface via a histidine-tag at the α- or β-subunit, and fixed a fluorescent actin filament to the c-ring (Figure 1). Upon addition of ATP they found ∼0.4% of all filaments rotating. The fact that the percentage of rotating filaments was low, that rotation was only observed in the presence of the detergent Triton X-100, and that rotation could not be effectively inhibited by venturicidin (an inhibitor of F0), left open the question of whether the rotating filaments were actually attached to enzymes for which the coupling of proton transport and ATP hydrolysis remained intact.
Basically the same experiment was repeated by Tsunoda et al. (2000). Although this group also observed rotation of filaments, albeit by at an even lower percentage (0.12%) than in the study by Sambongi et al. (1999), it drew different conclusions. To Tsunoda’s group, it seemed possible that the observed rotation of the c-ring could be an artifact. Therefore, these investigators tested their system carefully for two possible limitations: (i) loss of coupling between ATP hydrolysis and proton transport; and (ii) labelling of the γ-subunit, instead of the c-ring, with fluorescent probe. Their demonstration that certain F0 inhibitors could not inhibit ATP-hydrolysis effectively suggested that the addition of detergents, which had been necessary to obtain filament rotation, leads to uncoupling of ATP hydrolysis and proton transport. To show that the few observed rotating filaments are not just accidentally labelled γ-subunits, they exchanged the F1 of the labelled enzyme with a ‘fresh’ F1 using a strip/reconstitution technique. If it were indeed the c-complex that was visualized in the original experiment, one would have expected to observe a similar number of rotating filaments. However, after reconstitution rotating filaments were no longer observed!
In another study, Pänke et al. (2000) optimized labelling conditions by engineering a streptavidin-tag into the C-terminal region of the c-subunit. This tag ensures the specific binding of the actin filaments to the c-ring, excluding non-specific labelling of other subunits. This technique resulted in 5% rotating filaments, a remarkably high yield. Although it was now certain that the fluorescent probe was exclusively fixed to the c-ring, it was not shown that the ATP synthase retained all of the subunits forming the stator, or that ATP hydrolysis remained coupled to proton transport.
Although these studies provide us with tantalizing pictures of the action of the motor of ATP synthases, whether or not the rotation of a small number of filaments seen in these experiments really relates to events involving c-ring rotation during proton-transport coupled ATP hydrolysis remains an open question. The fact that the γ- and ɛ-subunits and the c-complex form a tight complex that can co-rotate is insufficient to confirm the model (Engelbrecht and Junge, 1997). It remains possible that the co-rotation observed in these studies is caused solely by the loss or displacement of subunits forming the stator. Future experiments need to address this problem, as well as the question of whether or not the co-rotation of the c-ring with subunit γ involves a fully coupled enzyme.

References
- Engelbrecht S. and Junge, W. (1997) ATP synthase: a tentative structural model. FEBS Lett., 414, 485–491. [DOI] [PubMed] [Google Scholar]
- Noji H., Yasuda, R., Yoshida, M. and Kinosita, K., Jr (1997) Direct observation of the rotation of F1-ATPase. Nature, 386, 299–302. [DOI] [PubMed] [Google Scholar]
- Pänke O., Gumbiowski, K., Junge, W. and Engelbrecht, S. (2000) F-ATPase: specific observation of the rotating c subunit oligomer of EF0EF1. FEBS Lett., 472, 34–38. [DOI] [PubMed] [Google Scholar]
- Sambongi Y., Iko, Y., Tanabe, M., Omote, H., Iwamoto-Kihara, A., Ueda, I., Yanagida, T., Wada, Y. and Futai, M. (1999) Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science, 286, 1722–1724. [DOI] [PubMed] [Google Scholar]
- Tsunoda S.P., Aggeler, R., Noji, H., Kinosita, K., Jr, Yoshida, M. and Capaldi, R.A. (2000) Observations of rotation within the F0F1-ATP synthase: deciding between rotation of the F0c subunit ring and artifact. FEBS Lett., 470, 244–248. [DOI] [PubMed] [Google Scholar]