Abstract
The stomatogastric nervous system (SNS) of Drosophila is a simply organized neural circuitry that innervates the anterior enteric system. Unlike the central and the peripheral nervous systems, the SNS derives from a compact epithelial anlage in which three invagination centers, each giving rise to an invagination fold headed by a tip cell, are generated. Tip cell selection involves lateral inhibition, a process in which Wingless (Wg) activity adjusts the range of Notch signaling. Here we show that RTK signaling mediated by the Drosophila homolog of the epidermal growth factor receptor, DER, plays a key role in two consecutive steps during early SNS development. Like Wg, DER signaling participates in adjusting the range of Notch-dependent lateral inhibition during tip cell selection. Subsequently, tip cells secrete the DER ligand Spitz and trigger local RTK signaling, which initiates morphogenetic movements resulting in the tip cell-directed invaginations within the SNS anlage.
INTRODUCTION
The stomatogastric nervous system (SNS) of Drosophila derives through the formation of a compact epithelial anlage at the roof of the stomodeum (González-Gaitán and Jäckle, 1995; Hartenstein, 1997) in response to maternal Torso activity in the terminal region of the embryo (González-Gaitán and Jäckle, 1995). Torso is a receptor tyrosine kinase (RTK) (Sprenger et al., 1989). Upon activation, it causes local Ras/Raf signaling that leads to the spatially restricted expression of Krüppel, wingless (wg) and proneural genes of the Achaete-Scute Complex (AS-C), including achaetae (ac), covering the SNS anlage (Hartenstein et al., 1994; González-Gaitán and Jäckle, 1995; Hartenstein, 1997). Once the SNS anlage is formed at the roof of the stomodeum, AS-C gene expression becomes restricted to three cells (González-Gaitán and Jäckle, 1995). These cells, termed tip cells, define invagination centers from which three invagination folds, each headed by a single ac-expressing tip cell, are generated (González-Gaitán and Jäckle, 1995). Subsequently, the invagination folds pinch off from the stomodeal epithelium and form separate vesicles that give rise to a stereotyped pattern of four SNS ganglia, termed frontal ganglion, esophagial ganglion 1 and 2 and proventricular ganglion, including their nerve tracts (Hartenstein et al., 1994; González-Gaitán and Jäckle, 1995; Hartenstein, 1997).
Singling out of the three tip cells involves Notch-dependent lateral inhibition in which wg activity adjusts the range of Notch signaling (González-Gaitán and Jäckle, 1995). In the absence of wg or components of the Wg pathway, such as dishevelled or armadillo, only one achaete-expressing tip cell is selected and it gives rise to only a single invagination fold in the center of the SNS anlage (González-Gaitán and Jäckle, 1995). These findings show that wg activity adjusts the range of Notch-dependent lateral inhibition required for the singling out of the three tip cells, whereas morphogenetic movements underlying the invagination process are independent of wg activity. Here we show that RTK signaling, which depends on the Drosophila homolog of the epidermal growth factor receptor (DER), plays a key role in two consecutive steps of early SNS development. The results demonstrate that RTK signaling functions in parallel or in concert with wg to adjust properly the range of Notch-dependent lateral inhibition within the SNS anlage and that DER signaling initiates morphogenetic movements. The DER ligand Spitz (Rutledge et al., 1992) emanates from the tip cells and triggers RTK signaling in the neighboring cells that participate in the tip cell-dependent invagination process.
RESULTS AND DISCUSSION
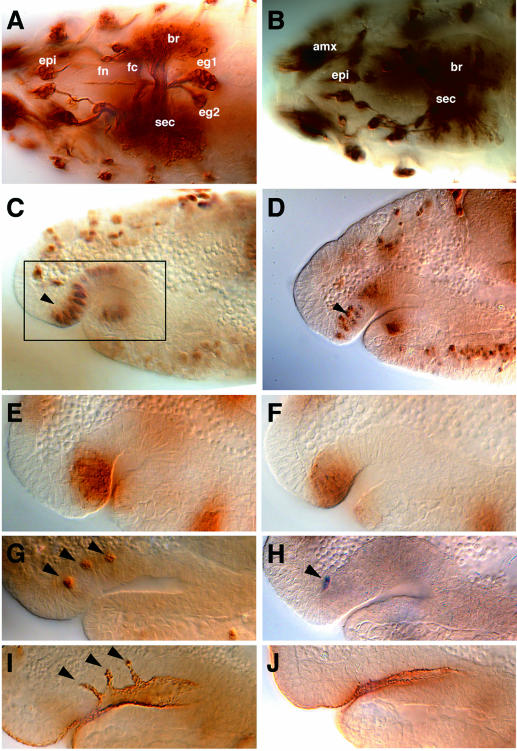
A recent study aimed at the identification of genes required for SNS development suggested that Ras/Raf signaling is not only required to establish the SNS anlage (González-Gaitán and Jäckle, 1995), but may also participate in later aspects of SNS formation (Forjanic et al., 1997). In order to investigate this role of RTK signaling, we examined SNS development in lack-of-function mutants of the DER ligand Spitz (Rutledge et al., 1992). In spitz mutants, the formation of the four SNS ganglia is strongly impaired (Figure 1A and B). The SNS anlage, however, forms normally (Figure 1C and D). In addition, the expression domain of wg (Figure 1E and F) and proneural AS-C genes (not shown) was indistinguishable from a wild-type SNS anlage. At the stage when the three ac-expressing cells were singled-out within the wild-type SNS anlage (Figure 1G), we found only one ac positive cell in spitz mutants (Figure 1H). The same phenotype had been observed in wg mutants or mutants lacking an integral component of the wg pathway (González-Gaitán and Jäckle, 1995). Since no altered wg pattern was found in the spitz mutant SNS anlage, Spitz-dependent RTK signaling may act in parallel or in combination with wg to adjust the proper range of Notch-dependent lateral inhibition. In contrast to wg mutants, however, no invagination fold is observed (compare Figure 1I and J). This observation indicates that the singled-out ac-expressing cell of spitz mutants (Figure 1H) has lost the ability to function as a tip cell and possibly fails to induce morphogenetic movements within the SNS anlage.
Fig. 1. spitzIIT mutant SNS phenotype. (A and B) Mab22c10 immunostaining showing the wild-type SNS (A) and the disrupted SNS of a spitzIIT mutant embryo (B) at stage 17 (dorsal view). Abbreviations: epi, epiphysis; amx, antennomaxillary complex; br, brain hemisphere; sec; supraesophageal commissure; fn, frontal nerve; fc, frontal commissure; eg1 and 2, esophageal ganglia 1 and 2. Note that PNS (epi, amx) and CNS (sec, br) structures are present in spitz mutants, whereas SNS ganglia and nerves are absent. (C and D) Krüppel immunostaining showing the SNS anlage (arrowheads) in wild-type (C) and spitz mutant (D) embryos at stage 10. The box in (C) corresponding to the stomodeal invagination within the cephalic region shows the enlarged area shown in (E–J). (E and F) Wingless immunostaining to show the SNS primordium of a wild-type (E) and a spitz mutant embryo (F) at stage 10. (G and H) Achaete immunostaining showing the three singled-out cells (arrowheads) in wild-type (G) and only one singled-out cell in spitz mutant embryos (H) at stage 10. (I and J) Crumbs immunostaining showing three invaginations (arrowheads) within the stomodeum of wild-type (I) and their absence in spitz mutant embryos (J) at stage 11 (lateral views). Orientation: anterior is left, dorsal is up. Staging of embryos was according to Campos-Ortega and Hartenstein (1997).
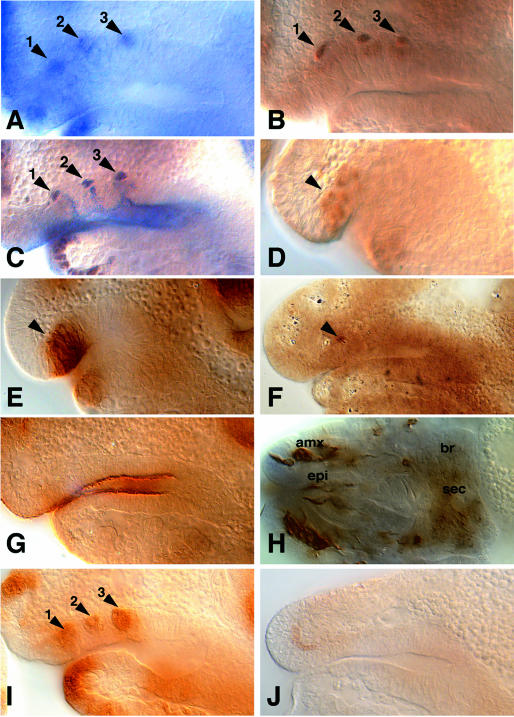
spitz, like other genes encoding components of the DER signaling pathway such as DER, Ras, Raf and the cascade of MAP kinases, is ubiquitously expressed (Rutledge et al., 1992). Local activation of DER signaling requires the transmembrane protein Star, which is necessary for the secretion of Spitz (Schweitzer et al., 1995; Golembo et al., 1996; Pickup and Banerjee, 1999; Bang and Kintner, 2000). Star is expressed in restricted patterns corresponding to the Spitz secreting cells (Kolodkin et al., 1994). In the SNS anlage, we noted that Star becomes restricted to the three tip cells and is maintained in these cells when invagination takes place (Figure 2A–C). As in spitz mutants, the Star mutant SNS anlage is established normally (Figure 2D–E), only one ac-expressing cell is selected (Figure 2F) and no invagination occurred (Figure 2G). Consistently, Star mutants fail to develop the proper set of SNS ganglia and the associated nerves (Figure 2H). These observations suggest that tip cells are a Star-dependent source of Spitz activity that triggers DER-dependent RTK signaling in the neighboring cells within the SNS anlage. This conclusion is supported by the finding that phosphorylated MAPK, a cellular marker for RTK signaling activity (Gabay et al., 1997), is indeed activated in cells of the invagination folds (Figure 2I), whereas phosphorylated MAPK did not appear in the Star mutant (Figure 2J) or in the spitz mutant SNS anlage (not shown).
Fig. 2. Star SNS expression and S54 mutant SNS primordium. (A) Star RNA in situ hybridization to show expression of Star in the three single cells of the SNS primordium (1,2,3). (B) β-galactosidase immunostaining to show reporter gene expression in an embryo heterozygous for a P-element [l(2)05671] insertion at the Star locus (1,2,3). (C) Double immunostaining showing Star-expressing tip cells labelled by β-galactosidase (brown) and the three SNS invaginations (blue) labelled by Crumbs in a l(2)05671/+ embryo. (D) Krüppel immunostaining showing the SNS primordium (arrowhead) in an S54 mutant embryo. (E–H) Immunostainings of S54 mutant embryos with Wingless (E), Achaete (F), Crumbs (G) and Mab22c10 antibodies (H). In Star mutant embryos the SNS anlage is established normally as monitored by early Wg expression (E; arrowhead). A single Achaete-expressing cell (F; arrowhead) is unable to drive SNS invagination within the stomodeum (G), leading to a lack of SNS ganglia and nerves (H). (I and J) Immunostaining showing phosphorylated MAPK (1,2,3) within the wild-type (I) and an S54 mutant (J) SNS anlage. (A–G, I and J) Lateral views (dorsal is up). (H) Dorsal view. (D and E) Stage 10; (A–C, F, G, I and J) stage 11, (H) stage 17. For staging, orientation and position of the enlarged area see legend of Figure 1.
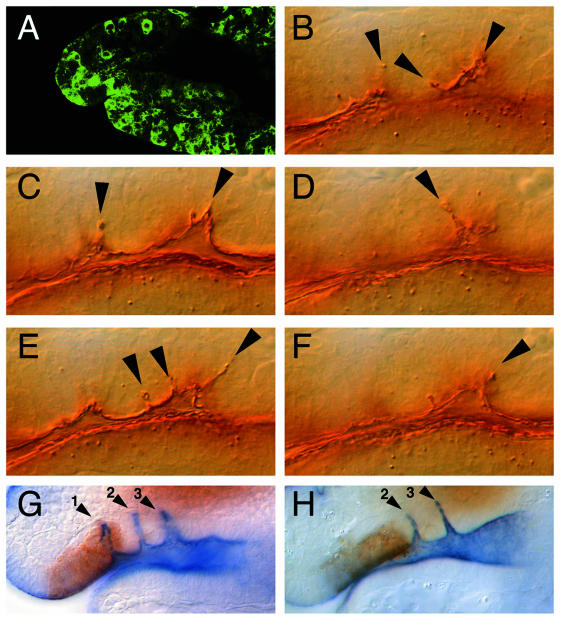
To examine whether activated Spitz is sufficient to induce cell movements within the SNS anlage, we made use of the GAL4/UAS system (Brand and Perrimon, 1993) to misexpress secreted Spitz in an ectopic pattern. This was achieved through the expression of activated Spitz from a UAS promotor driven transgene (Schweitzer et al., 1995) that was activated by Gal4 under the control of the actin promotor. Under the conditions applied (see Methods), we observed scattered UAS-dependent transgene expression throughout the early embryo, including the SNS anlage (Figure 3A). When activated Spitz was expressed in such a pattern, a variable number of supernumerary infoldings within the SNS anlagen were observed (Figure 3B–F), indicating that activated Spitz is sufficient to initiate cell movements. This result, in conjunction with the observation that the invaginated cells express phosphorylated MAPK (Figure 2I), provides evidence that tip cell-derived activated Spitz triggers RTK signaling to initiate the invagination process. We tested this proposal by blocking DER signaling in the anterior most region of the SNS anlage that gives rise to the first invagination fold. For this, we used a GAL4 driver (SNS1–Gal4; see Methods) that causes UAS-dependent gene expression in the corresponding region of the SNS anlage (Figure 3G). SNS1–Gal4-mediated expression of a dominant-negative DER mutant form (Buff et al., 1998) from a UAS-controlled transgene causes a specific suppression of the anterior most invagination fold without affecting the others (Figure 3H).
Fig. 3. Expression of secreted Spitz and DERDN at the invaginating SNS. (A) GFP immunolabeling to show embryonic expression of GFP under the control of an actin promotor during stage 11. Genotype Actin-Gal4/UAS-GFP. At this stage, the actin promotor in the Gal4 driver activates scattered embryonic expression of the reporter gene. (B–F) Crumbs immunolabeling of an embryo (genotype: actin-Gal4/UAS-sspi) in which secreted Spitz was expressed under the control of the actin promotor. The different focal planes (five consecutive Nomarski optical sections) through the SNS anlage show the supernumerary SNS invaginations (arrowheads). (G) Double immunolabeling showing GFP reporter gene expression (brown) covering the first of the three SNS invaginations (1,2,3) stained with anti-Crumbs antibodies (blue) in a transgene containing wild-type embryo (genotype: SNS1–Gal4 UAS-GFP). (H) GFP and Crumbs double immunolabeling in embryos expressing a dominant-negative version of DER in the area of the first invagination (genotype: SNS1–Gal4 UAS-GFP/UAS-DERDN). Note that the first invagination is absent, whereas the second and third (2,3) are formed normally. Lateral views of stage 11 embryos (dorsal is up). For staging, orientation and position of the enlarged area see legend of Figure 1.
The results demonstrate that RTK signaling participates in the selection of tip-cell-dependent invagination centers in the SNS anlage and is subsequently required to initiate morphogenetic movements resulting in invagination folds. We have not focussed on how RTK signaling ties into the wg-modulated Notch signaling process previously shown to be necessary for the selection of the three SNS invagination centers (González-Gaitán and Jäckle, 1995). Our data indicate, however, that RTK signaling acts either in parallel or in combination with wg signaling to adjust the proper range of Notch-dependent lateral inhibition. Although in both wg and DER signaling mutants, only one ac-expressing cell is singled-out, the selected cells differ with respect to whether they function as tip cells or not. In wg mutants, the single cell causes an invagination, whereas in DER signaling mutants, the selected cell fails to provide this feature of SNS invagination centers. The results, therefore, consistently argue that tip cell-derived Spitz triggers local RTK signaling and thereby initiates the formation of invagination folds each headed by the Spitz-secreting tip cell. Thus, DER-dependent RTK signaling in Drosophila does not only participate in cell fate decisions (Sprenger et al., 1989; Perrimon, 1993; Raz and Shilo, 1993; Schweitzer et al., 1995; Freeman, 1996; Golembo et al., 1996; Tio and Moses, 1997), cell migration (Klämbt et al., 1992; Beiman et al., 1996; Gisselbrecht et al., 1996) and cell proliferation (Díaz-Benjumea and García-Bellido, 1990; Simcox, 1997; Nagaraj et al., 1999), but also triggers morphogenetic movements within an epithelium, as has been recently demonstrated for fibroblast growth factor (FGF) signaling (Glazer and Shilo, 1991). It will be interesting to see whether the role of the EGF pathway in cell migration differs at the cellular level from cell migration events triggered by activated FGF receptors.
METHODS
Mutant strains, immunostainings and in situ hybridization. Mutant flies were maintained using CyO, hb-lacZ blue balancer as described (González-Gaitán and Jäckle, 1995). l(2)05671 is a P-element insertion in the Star gene (Freeman, 1994; Forjanic et al., 1997), and spiIIT and S54 are strong lack-of-function mutants (Lindsley and Zimm, 1992). SNS1–Gal4 is an enhancer-trap P-element insertion from G. Technau’s laboratory collection (Ito et al., 1995) (Mz798hII:gal4) carrying Gal4 as a reporter gene. Insertion is on the second chromosome and reproduces the embryonic pattern of expression of goosecoid, leaving open if the P-element is inserted in this gene. UAS–sspi (Schweitzer et al., 1995) and UAS–DERDN (Buff et al., 1998) are UAS constructs driving activated spitz and dominant-negative DER, respectively. For Gal4 driven expression, embryos developed at 25°C. Under these conditions actin–Gal4 drives embryonic expression in a scattered pattern (Figure 3A). Other mutants, Gal4 and UAS flies are described in Lindsley and Zimm (1992) and flybase.
Immunostainings and in situ hybridization were performed as previously described (González-Gaitán and Jäckle, 1995, 1996). Primary antibodies were used at the following dilutions: Mab22C10 (Fujita et al., 1982) (Hybridoma bank), 1:50; rabbit anti-Krüppel (Gaul and Jäckle, 1987), 1:500; mouse anti-Achaete (Skeath and Carroll, 1992), 1:3; mouse anti-Crumbs (Tepass and Knust, 1993), 1:20; mouse anti-Wingless (Brook and Cohen, 1996) (Hybridoma), 1:5; mouse anti-MAPK-P (Gabay et al., 1997) (Sigma), 1:40; rabbit anti-GFP (Brock et al., 1999), 1:3. Star in situ was performed using a full-length Star cDNA as an RNA probe.
Acknowledgments
ACKNOWLEDGEMENTS
We thank R. Fernández de la Fuente for excellent technical assistance; G. Vorbrüggen and S. Fellert for help with the phosphorylated MAPK staining and the Star in situ; G. Technau and J. Urban for addressing our attention to the SNS expression of one of their Gal4 lines and G. Vorbrüggen, T. Wucherpfennig, A. Schwabedissen, B. Linder and O. Piepenburg for fruitful discussions and comments on the manuscript. The work was supported by the Max-Planck Society.
REFERENCES
- Bang A.G. and Kintner, C. (2000) Rhomboid and Star facilitate presentation and processing of the Drosophila TGFα homolog Spitz. Genes Dev., 14, 177–186. [PMC free article] [PubMed] [Google Scholar]
- Beiman M., Shilo, B.Z. and Volk, T. (1996) Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev., 10, 2993–3002. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon, N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Brock R., Hamelers, I.H.L. and Jovin, T.M. (1999) Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry, 35, 353–362. [DOI] [PubMed] [Google Scholar]
- Brook W.J. and Cohen, S.M. (1996) Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science, 273, 1373–1377. [DOI] [PubMed] [Google Scholar]
- Buff E., Carmena, A., Gisselbrecht, S., Jimenez, F. and Michelson, A.M. (1998) Signalling by the Drosophila epidermal growth factor receptor is required for the specification and diversification of embryonic muscle progenitors. Development, 125, 2075–2086. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J.A. and Hartenstein, V. (1997) The Embryonic Development of Drosophila melanogaster. 2nd edn. Springer Verlag, Berlin, Germany.
- Díaz-Benjumea F.J. and García-Bellido, A. (1990) Behaviour of cells mutant for an EGF receptor homologue of Drosophila in genetic mosaics. Proc. R. Soc. Biol. Sci., 242, 36–44. [DOI] [PubMed] [Google Scholar]
- Forjanic J.P., Chen, C.K., Jäckle, H. and González Gaitán, M. (1997) Genetic analysis of stomatogastric nervous system development in Drosophila using enhancer trap lines. Dev. Biol., 186, 139–154. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1994) The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech. Dev., 48, 25–33. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell, 87, 651–660. [DOI] [PubMed] [Google Scholar]
- Fujita S.C., Zipursky, S.L., Benzer, S., Ferrús, A. and Shotwell, S.L. (1982) Monoclonal antibodies against the Drosophila nervous system. Proc. Natl Acad. Sci. USA, 79, 7929–7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay L., Seger, R. and Shilo, B.Z. (1997) MAP kinase in situ activation atlas during Drosophila embryogenesis. Development, 124, 3535–3541. [DOI] [PubMed] [Google Scholar]
- Gaul U. and Jäckle, H. (1987) Pole region-dependent repression of the Drosophila gap gene Krüppel by maternal gene products. Cell, 51, 549–555. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S., Skeath, J.B., Doe, C.Q. and Michelson, A.M. (1996) heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev., 10, 3003–3017. [DOI] [PubMed] [Google Scholar]
- Glazer L. and Shilo, B.Z. (1991) The Drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev., 5, 697–705. [DOI] [PubMed] [Google Scholar]
- Golembo M., Raz, E. and Shilo, B.Z. (1996) The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development, 122, 3363–3370. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M. and Jäckle, H. (1995) Invagination centers within the Drosophila stomatogastric nervous system anlage are positioned by Notch-mediated signaling which is spatially controlled through wingless. Development, 121, 2313–2325. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M.A. and Jäckle, H. (1996) In situ localization of proteins in whole mounted tissue. In Crampton, J.M., Beard, C.B. and Lewis, C. (eds), The Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapmann and Hall, London, UK, pp. 283–294.
- Hartenstein V. (1997) Development of the insect stomatogastric nervous system. Trends Neurosci., 20, 421–427. [DOI] [PubMed] [Google Scholar]
- Hartenstein V., Tepass, U. and Gruszynski-Defeo, E. (1994) Embryonic development of the stomatogastric nervous system in Drosophila. J. Comp. Neurol., 350, 367–381. [DOI] [PubMed] [Google Scholar]
- Ito K., Urban, J. and Technau, G.M. (1995) Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux Arch. Dev. Biol., 204, 284–307. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer, L. and Shilo, B.Z. (1992) breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev., 6, 1668–1678. [DOI] [PubMed] [Google Scholar]
- Kolodkin A.L., Pickup, A.T., Lin, D.M., Goodman, C.S. and Banerjee, U. (1994) Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development, 120, 1731–1745. [DOI] [PubMed] [Google Scholar]
- Lindsley D.L. and Zimm, G.G. (1992) The Genome of Drosophila melanogaster. Academic Press Inc., San Diego, CA.
- Nagaraj R., Pickup, A.T., Howes, R., Moses, K., Freeman, M. and Banerjee, U. (1999) Role of the EGF receptor pathway in growth and patterning of the Drosophila wing through the regulation of vestigial. Development, 126, 975–985. [DOI] [PubMed] [Google Scholar]
- Perrimon N. (1993) The torso receptor protein-tyrosine kinase signaling pathway: an endless story. Cell, 74, 219–222. [DOI] [PubMed] [Google Scholar]
- Pickup A.T. and Banerjee, U. (1999) The role of Star in the production of an activated ligand for the EGF receptor signaling pathway. Dev. Biol., 205, 254–259. [DOI] [PubMed] [Google Scholar]
- Raz E. and Shilo, B.Z. (1993) Establishment of ventral cell fates in the Drosophila embryonic ectoderm requires DER, the EGF receptor homolog. Genes Dev., 7, 1937–1948. [DOI] [PubMed] [Google Scholar]
- Rutledge B.J., Zhang, K., Bier, E., Jan, Y.N. and Perrimon, N. (1992) The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev., 6, 1503–1517. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Shaharabany, M., Seger, R. and Shilo, B.Z. (1995) Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev., 9, 1518–1529. [DOI] [PubMed] [Google Scholar]
- Simcox A. (1997) Differential requirement for EGF-like ligands in Drosophila wing development. Mech. Dev., 62, 41–50. [DOI] [PubMed] [Google Scholar]
- Skeath J.B. and Carroll, S.B. (1992) Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development, 114, 939–946. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens, L.M. and Nüsslein-Volhard, C. (1989) The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature, 338, 478–483. [DOI] [PubMed] [Google Scholar]
- Tepass U. and Knust, E. (1993) Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol., 159, 311–326. [DOI] [PubMed] [Google Scholar]
- Tio M. and Moses, K. (1997) The Drosophila TGFα homolog Spitz acts in photoreceptor recruitment in the developing retina. Development, 124, 343–351. [DOI] [PubMed] [Google Scholar]