Abstract
Introduction
Children with chronic conditions have greater health care needs than the general paediatric population but may not receive care that centres their needs and preferences as identified by their families. Clinicians and researchers are interested in developing interventions to improve family‐centred care need information about the characteristics of existing interventions, their development and the domains of family‐centred care that they address. We conducted a scoping review that aimed to identify and characterize recent family‐centred interventions designed to improve experiences with care for children with chronic conditions.
Methods
We searched Medline, Embase, PsycInfo and Cochrane databases, and grey literature sources for relevant articles or documents published between 1 January 2019 and 11 August 2020 (databases) or 7–20 October 2020 (grey literature). Primary studies with ≥10 participants, clinical practice guidelines and theoretical articles describing family‐centred interventions that aimed to improve experiences with care for children with chronic conditions were eligible. Following citation and full‐text screening by two reviewers working independently, we charted data covering study characteristics and interventions from eligible reports and synthesized interventions by domains of family‐centred care.
Results
Our search identified 2882 citations, from which 63 articles describing 61 unique interventions met the eligibility criteria and were included in this review. The most common study designs were quasiexperimental studies (n = 18), randomized controlled trials (n = 11) and qualitative and mixed‐methods studies (n = 9 each). The most frequently addressed domains of family‐centred care were communication and information provision (n = 45), family involvement in care (n = 37) and access to care (n = 30).
Conclusion
This review, which identified 61 unique interventions aimed at improving family‐centred care for children with chronic conditions across a range of settings, is a concrete resource for researchers, health care providers and administrators interested in improving care for this high‐needs population.
Patient or Public Contribution
This study was co‐developed with three patient partner co‐investigators, all of whom are individuals with lived experiences of rare chronic diseases as parents and/or patients and have prior experience in patient engagement in research (I. J., N. P., M. S.). These patient partner co‐investigators contributed to this study at all stages, from conceptualization to dissemination.
Keywords: chronic conditions, family‐centred care, paediatrics, quality improvement, scoping review
1. INTRODUCTION
Children with chronic health conditions or disabilities have elevated health care needs, interact with health care systems more frequently or intensively over time and often have more unmet needs relative to the general paediatric population. 1 , 2 , 3 , 4 Health care systems are often ill‐equipped to respond effectively to the needs of this population, which may include interdisciplinary services, preventive care and timely interventions to support development. 5 , 6 It is now an accepted standard that health care be family‐centred, particularly for children with chronic conditions. 7 , 8 Family‐centred care refers to practices that engage patients and their families as integral, valued members involved in decision‐making and appraisal of their health care, and may contribute to higher levels of family satisfaction with care and lower levels of distress. 9 , 10 , 11 In paediatrics, family‐centred care recognizes that the family is the primary unit of support for a child's health care management and overall development. 12 Although there is no single definition of the term nor consensus on its processes or practices, the guiding principles of family‐centred care encompasses health care policies, strategies and interventions that treat patients and their families with dignity, promote collaboration with patients and families and focus on respectful interactions. 13 , 14 , 15 , 16 , 17 Meaningful engagement of patients and families in the co‐design or study of interventions that affect them is consistent with a family‐centred care approach and may improve intervention effectiveness and family satisfaction. 18 , 19
Confronted with a broadly defined topic, those interested in developing interventions to make care more family‐centred, such as policymakers, clinicians, and researchers and representatives of health care systems, may seek to learn more about, and build upon, existing interventions relevant to the health care setting of their interest. To adapt or build from such existing interventions, it would be important to understand how these similar interventions were developed, the key elements of the interventions and the aspects of family‐centred care that the interventions aim to improve. A scoping review by King and colleagues sought to identify ‘family‐oriented’ services that addressed the needs of parents of children with disabilities in paediatric rehabilitation in articles published between 2009 and 2014, leading to the development of a conceptual framework. 20 The authors described four main types of family‐oriented interventions for parents: education about disabilities and training to administer therapy; support groups; psychosocial interventions (e.g., counselling, coaching); and provision of information about disability and available resources. 20 Their proposed framework for family‐oriented services included six domains: information resources; education services; training/instruction services;, support groups; psychosocial services; and service coordination.
Building on King and colleagues' review, our focus for this scoping review was somewhat broader. Our overall aim was to identify and characterize recently published family‐centred health care interventions across a range of health care settings (including but not limited to rehabilitation settings) that sought to improve experiences with health care for children with chronic conditions and their families. More specifically, we sought to describe: (i) how the interventions were developed; (ii) which groups (children, caregivers, health care providers) were engaged in their development; (iii) the domains of family‐centred care that the interventions were designed to address; and (iv) the key components of the interventions that sought to address those domains.
With respect to domains of family‐centred care, we used an existing framework, the Picker principles, 21 , 22 as a guide. This set of principles was developed by the US Picker Institute to advocate for the needs and preferences of patients and families in health care interactions. 23 It has eight domains (access to care, coordination, communication and information provision, family involvement, respect for child and family, follow‐up and continuity of care, physical comfort and emotional support) and has been used or adapted in studies of family experiences with health care. 24 , 25 , 26
2. METHODS
Scoping reviews are ideal for characterizing a broad landscape of literature on a particular topic, aligned with our goal. 27 , 28 Our scoping review methods followed the Joanna Briggs Institute (JBI) Methodology for Scoping Reviews 29 and our published protocol (available at https://osf.io/cjyd9/?view_only=0077822cf9ef424290651ed7b5c80177). We report our review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) extension for scoping reviews (PRISMA‐ScR) (Appendix S1). 30
2.1. Patient and family engagement
Family caregivers of children with chronic conditions interact frequently with the health care system, becoming experts in their children's care needs. 31 , 32 Their partnership and engagement in research, especially about family‐centred care, can be expected to facilitate research questions that are more meaningful and relevant to children and their families and contribute to successful care improvement efforts. 33 , 34 This study was co‐developed with three patient partner co‐investigators, all of whom are individuals with lived experiences of rare chronic diseases as parents or patients and have prior experience in patient engagement in research (I. J., N. P., M. S.). These patient partner co‐investigators contributed to this study at all stages, from conceptualization to dissemination. Specifically, they participated in decision‐making about the concept of the review and its scope, contributed to the interpretation of the extracted data and critically reviewed the final manuscript.
2.2. Search strategy and information sources
An experienced medical information specialist (B. S.) developed and tested the search strategies through an iterative process in consultation with the research team, including review of a pre‐existing, extensive strategy covering a range of similar chronic conditions. 35 Another senior information specialist peer reviewed the strategies before execution using the PRESS Checklist. 36 Suggestions were reviewed and if applicable, incorporated into the final strategy. Our strategy was structured and designed to emphasize sensitivity over specificity (Appendix S2). Using the multifile option and deduplication tool on the OVID platform, we searched Ovid MEDLINE® ALL, including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Embase Classic+Embase, APA PsycInfo, and the following EBM Reviews Databases: Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Health Technology Assessment and the NHS Economic Evaluation Database (Appendix S2). We performed all searches on 11 August 2020. Results were downloaded and deduplicated using EndNote version X9.3.3 (Clarivate Analytics) and uploaded to Covidence for screening. The number of records generated by the database search (>15,000) exceeded the study team's resources to adequately screen those records. Before screening, we made a decision to restrict articles to those published 2019 or later rather than modifying the search by further restricting the population, concept or context criteria. This restriction returned a number of records that we deemed feasible to review.
Strategies used a combination of controlled vocabulary (e.g., ‘Disabled Children’, ‘Chronic Disease’, ‘Child Health Services’) and keywords (e.g., ‘complex need’, ‘partnership’, ‘paediatric program’). Vocabulary and syntax were adjusted across the databases. Where possible, animal‐only, opinion pieces and conference abstracts were excluded from the searches. To promote the retrieval of all relevant articles, we did not limit outcomes or study designs.
We also conducted a grey literature search. Two authors (B. K. P., A. J. C.) identified pertinent resources, including the websites ClinicalTrials.gov, the International Clinical Trials Registry Platform search portal, the Patient‐Centered Outcomes Research Institute and websites recommended by CADTH's Grey matters tool. 37 One reviewer (A. J. C.) conducted the grey literature search, performed 7–20 October 2020. A set of keywords and filters were adapted from the electronic database search strategy and tailored to the search capabilities and content of each grey literature source. Keywords included ‘child’, ‘family’, ‘caregiver’, ‘intervention’, ‘program’ and ‘health care’, among others. Search results were downloaded or copied to an Excel document; if that was not possible, the reviewer conducted stage 1 screening (see Section 2.4) by reviewing titles and document descriptions and downloaded or copied the records retained for stage 2 screening.
2.3. Eligibility criteria
To be eligible for inclusion, articles had to describe a family‐centred care intervention for children 12 years and younger with chronic conditions and/or their families (Table 1). Operationalization of these concepts is described below. These criteria were designed to balance a desire to include a wide range of recently published family‐centred care interventions.
Table 1.
Eligibility criteria.
| Inclusion | Exclusion | |
|---|---|---|
| Study design |
Primary studies of any design except those specifically excluded Clinical practice guidelines Theoretical articles (must describe family‐centred care model or framework AND indicate it was peer‐reviewed) |
Systematic reviews and other secondary review studies Case series Primary studies with <10 participants, including case studies |
| Population | Children (aged ≤12 years) with chronic conditions and/or their families (target of the intervention and study, or study alone) | Interventions or studies targeting solely adolescents (aged 13–18 years) |
| Articles where the age or the health care needs of the study population are not clear but possibly eligible will be included | Health care needs are related to short‐term disability or acute injury (reasonably expected to last <1 year); neonatal or infant conditions expected to last <1 year | |
| If <100% are aged ≤12 years, include if: | ||
| ||
| If <100% are children with ongoing, elevated health care needs, include if: | ||
| ||
| Concept | Family‐centred care interventions | Prevention or screening interventions for a condition if the population does not already have a condition requiring ongoing, elevated health care |
| The article, or a cited document, must clearly describe all of the following: | Clinical drug treatment regimens | |
|
Interventions targeting transition from paediatric to adult services | |
| Context | All settings, all health care professionals involved included | N/A |
| Outcomes | All outcomes | N/A |
| Publication date | Published in or after 2019 | N/A |
| Language | English | Anything other than English |
| Publication type | Published, full‐length articles or grey literature reports, policy documents |
Unpublished articles, books Protocols, conference abstracts, theses or dissertations, commentaries and letters |
Abbreviation: N/A, not available.
2.3.1. Study design types
Primary studies of any design were eligible except for case series and primary study designs with fewer than 10 participants, including case studies. Theoretical articles describing family‐centred care models or frameworks that have not yet been implemented were included if the article had been peer‐reviewed. Clinical practice guidelines were also included. Systematic and other secondary review studies were excluded as we anticipated that they would be repetitive.
2.3.2. Population
We included studies where the target of the intervention was children aged 12 years or younger with chronic conditions and/or their families. Interventions or studies that targeted solely adolescents (aged 13–18 years) were excluded as their health care needs are often distinct from those of younger children. ‘Chronic conditions’ were defined as requiring an elevated number or intensity of interactions with the health care system relative to the general paediatric population, expected to be required for 1 year or more.
2.3.3. Concept
We included articles that described family‐centred care interventions (activities, strategies or policies). 38 Family‐centred care was defined as an approach to the planning, delivery and evaluation of health care that was grounded in mutually beneficial partnerships among health care providers, patients and families. 13 We considered an intervention to be family‐centred if: the main objective of the intervention was to improve health care experiences (including timely access to care or treatment, coordination of care, communication with providers and emotional support) for the population; and both (i) health care providers and/or administrators and (ii) primary caregivers of children were actively engaged in the development or research of the intervention (e.g., in the study of implementation of the intervention). With respect to this latter criterion, we considered engagement of primary caregivers and providers/administrators to be critical given their roles as knowledge users positioned to benefit from or to implement respectively, interventions to improve family‐centred care. Included articles had to describe the intervention's activities or processes or cite other articles accessible to the authors that did. We excluded: screening or preventive interventions targeting children that did not have a pre‐existing, chronic condition; clinical drug treatment regimens, which are typically not generalizable and do not directly target improvement of health care experiences; interventions targeting transition from paediatric to adult health care services; institutional networks and partnerships; and existing services (longstanding, geographically widespread programmes that were not new interventions to improve care).
2.3.4. Context
We included interventions that addressed physical or mental health care for a child, were implemented in any setting (e.g., health care, school, home) for acute or nonacute care and involved any health care professional (e.g., physician, nurse, rehabilitation therapist).
2.3.5. Other
We excluded protocols, conference abstracts, theses and dissertations, commentaries and letters. We included articles irrespective of the outcomes studied. Only articles published in or after 2019 were included. As many grey literature websites and documents did not report a publication date, we chose to include all undated grey literature. Only articles published in English were included.
2.4. Study selection
In stage 1 of a two‐stage screening process, we used a liberal accelerated approach. 39 Two reviewers (among A. J. C., Z. A., R. I.) independently screened the titles and abstracts of identified records. A record was retained if at least one reviewer positively assessed that it met the eligibility criteria or was uncertain about its inclusion. For the grey literature, one author (A. J. C. or Ammar Saad) performed stage 1 screening.
In stage 2, the full‐text articles of all records retained in stage 1 were obtained. If an article could not be obtained electronically free of charge, we contacted the corresponding or lead study author by email up to two times and excluded the article if a copy could not be obtained. Two reviewers (among A. J. C., Z. A., R. I., Ammar Saad) independently screened all full‐text articles and grey literature in duplicate. We resolved disagreements by discussion and consensus; an arbitrator (B. K. P.) resolved disagreements that could not be resolved by the reviewers. Clinical experts (P. C., M. K.) provided input if the health care needs criterion could not be determined by the reviewers. If a full‐text article and its cited references did not report enough information to decide eligibility (e.g., regarding the population targeted by an intervention), we excluded the article. We did not contact study authors to request additional information.
2.5. Data charting
Key information from included articles was charted using verbatim information into a standardized, predefined, piloted Excel data charting form (Appendix S3) by one person (among Ammar Saad, Z. M., A. H., Z. A.), then verified by a second, different study team member (among A. J. C., Ammar Saad, R. I.). If multiple articles described the same intervention, we charted the information from each article separately and collated them during synthesis. If an article referenced a previous article published before 2019, we charted from the previous article only information related to the intervention.
We charted four main types of information: (1) the characteristics of each article (lead author, title, journal, year of publication); (2) study participant types for primary studies (e.g., children, family members, health care professionals), when applicable; (3) details about the development of each intervention (i.e., activities or processes used; engagement of groups such as patients, caregivers and health care providers) and characteristics of the children targeted by the intervention (i.e., age range, type of health care need); and (4) using an adapted version of the Template for Intervention Description and Replication (TIDieR) checklist. 40 We charted information about the reporting of each intervention including: the care delivery setting involved, the authors' stated objectives and rationale for, or theory grounding, each intervention, and the intervention's components (specific activities/processes). Our adapted checklist did not include TIDieR checklist items #11 and #12, about planned and actual fidelity to the intervention, as we did not aim to assess intervention effectiveness.
2.6. Synthesis
Using the data charted, we synthesized the development of the interventions by activity type (e.g., feedback seeking, pilot testing) and groups engaged in development (e.g., caregivers, children, health care providers). We also summarized the broad care delivery settings (i.e., inpatient, outpatient, community) and the chronic conditions of the target populations, grouping by pathophysiological manifestations and symptoms.
We summarized the activities/processes and objectives of each intervention and identified which among eight family‐centred care domains the intervention sought to address 21 , 22 (definitions, Appendix S4). For the latter characterization, we used an iterative coding approach, where one reviewer (Ammar Saad) coded and a second team member (A. J. C.) verified. We summarized the common types of intervention approaches within each family‐centred care domain.
3. RESULTS
3.1. Study selection
After deduplication and removal of records published before 2019, we screened 2882 records by their titles and abstracts; of those, 369 full‐text reports were assessed for eligibility (Figure 1). We further identified 127 records through grey literature searches, and after deduplication and removal of records published before 2019, we retrieved and assessed 59 full‐text reports for eligibility (Figure 1). A total of 63 reports were included in this review, representing 61 unique interventions. 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 Furthermore, we consulted additional publications, which were cited in the eligible reports, for additional details about 23 of the 61 included interventions during our data charting. 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132
Figure 1.
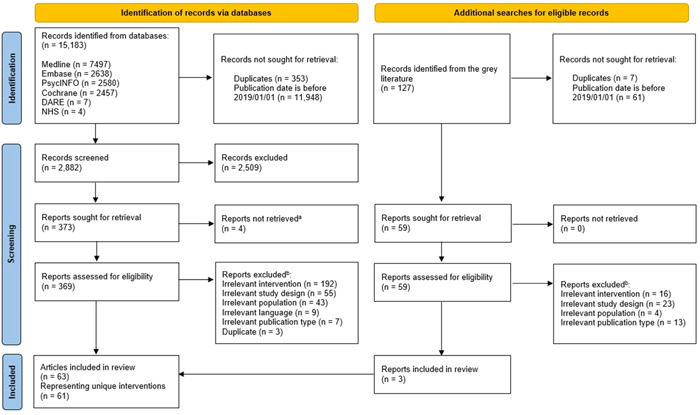
Preferred Reporting Items for Systematic reviews and Meta‐Analyses flow diagram of study screening and selection.
3.2. Study characteristics
Table 2 and Appendix S5 describe the 63 included articles, the majority of which reported on primary research studies (n = 57; 90.5%). The most common primary study design was quasiexperimental (n = 18; 28.6%), followed by randomized controlled trial (RCT) (n = 11; 17.5%). The sample size of the 57 included primary research studies ranged from 10 (per our inclusion criteria) to 6,259 participants, with almost a third reporting a sample size of 10–30 participants (n = 18/57; 31.6%). Seventy‐nine percent of articles described studies conducted in (or, if not stated, country of affiliation of lead author) North America (n = 40; 63.5%), as well as Europe (n = 6; 9.5%) and the United Kingdom (n = 4; 6.3%).
Table 2.
Characteristics of included articles.
| Study characteristics | N = 63, n (%) |
|---|---|
| Study design | |
| Primary studies | 57 (90.5) |
| Quasi‐experimental studies | 18 (28.6) |
| Randomized controlled trials | 13 (20.6) |
| Mixed methods studies | 10 (15.9) |
| Qualitative studies | 9 (14.3) |
| Cohort studies | 4 (6.3) |
| Cross‐sectional studies | 3 (4.8) |
| Clinical guidelines | 3 (4.8) |
| Theoretical articles | 3 (4.8) |
| Sample sizea | |
| 10–30 | 18 (28.6) |
| 31–90 | 17 (27.0) |
| 91–300 | 14 (22.2) |
| >300 | 7 (11.1) |
| Not reported | 1 (1.6) |
| Not applicable | 6 (9.5) |
| Geographic location | |
| North America | 40 (63.5) |
| South/East Asia | 7 (11.1) |
| Europe | 6 (9.5) |
| United Kingdom | 4 (6.3) |
| Australia | 1 (1.6) |
| South America | 1 (1.6) |
| Africa | 1 (1.6) |
| Unspecified (clinical guidelines) | 3 (4.8) |
| Health care conditions | |
| Multisystem, life‐limiting or complex conditions | 14 (22.2) |
| Developmental conditions and delays | 10 (15.9) |
| Pulmonary conditions | 9 (14.3) |
| Mental and behavioural conditions | 5 (7.9) |
| Oncological conditions | 4 (6.3) |
| Physical and motor disabilities | 4 (6.3) |
| Cardiological conditions | 3 (4.8) |
| Haematological conditions | 3 (4.8) |
| Neurological and cognitive conditions | 3 (4.8) |
| Autoimmune disorders | 2 (3.2) |
| Endocrinological conditions | 2 (3.2) |
| Renal conditions | 1 (1.6) |
| Uncategorized | 3 (4.8) |
Primary studies only.
3.3. Intervention development, including which groups were engaged in development
For the majority of the 61 interventions, authors provided or cited information about the activities or processes involved in intervention development (n = 51; 83.6%) and the groups engaged in their development (n = 43; 70.5%) (Figure 2A,B). Common activities included the elicitation of feedback from patients, caregivers or health care providers on intervention content or design (n = 30), the use of intervention theories or frameworks in conceptualization (n = 26) and piloting/refining interventions with end users (n = 24; Figure 2A and Appendix S5). Among interventions where authors described who was involved in development, caregivers (e.g., parents) were engaged in developing about half the interventions (n = 31; Figure 2B and Appendix S5). A similar proportion described involving health care providers (n = 28), whereas child patient involvement was described in the development of 15 interventions.
Figure 2.
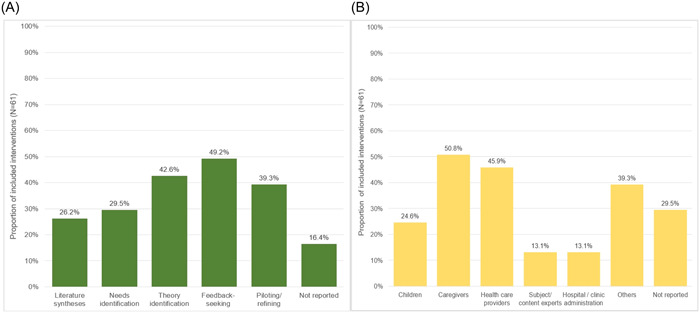
(A) Activities involved in intervention development. Feedback‐seeking activities: Quantitative and qualitative data collection of opinions and feedback from contributor groups on content or design. Piloting/refining: Feasibility testing and pilot implementation of interventions. (B) People and entities engaged in intervention development alongside researchers. Caregivers: Parents, caretakers and legal authority representatives. Subject/content: Clinical or intervention design expertise. Others: Allied health professionals, information technology specialists, insurers, teachers, nongovernmental organizations, community advocates and governmental health entities.
3.4. Intervention characteristics
Among the 63 articles, there were 61 unique interventions described (Table 3). Most interventions aimed to improve care experiences in one setting: outpatient (n = 22; 36.1%), inpatient (including emergency department) (n = 10; 16.4%) or community (n = 9; 14.8%) settings. A smaller number of interventions addressed shared or transitional care between outpatient and community (n = 11; 18.0%), inpatient and community (n = 7; 11.5%) or inpatient and outpatient settings (n = 2; 3.3%).
Table 3.
Characteristics of included interventions.
| References | Intervention description | Intended recipients | Timing (T) and duration (D) | Delivery mode (M) and format (F) |
|---|---|---|---|---|
| Interventions delivered in or from outpatient clinical settings (n = 22) | ||||
| [41] | Website to prepare parents to take part in decision‐making during their consultation with a rehabilitation physician; helps families identify their needs + access information | Caregivers |
T: As desired D: Not applicable |
M: Virtual F: Individual |
| [42, 43] | Collaborative meetings between families + therapists, at clinic or home (family preference), to codevelop + implement child development intervention, including family needs, activities + goals | Family, HCPs |
T: Six weekly sessions D: Approximately 40 min each |
M: IP F: Individual |
| [44] | Disease‐specific medical home care coordination programme that includes caregiver education (guidebook), team‐based care + clinical care plan | Children, caregivers |
T: U/S D: U/S |
M: U/S F: U/S |
| [46] | Online tool to engage caregivers in care planning. Caregivers identify + prioritize activities for participation and identify goals, attainment strategies, possible supports + barriers which are incorporated into a care plan report | Caregivers |
T: U/S D: U/S |
M: Virtual F: Individual |
| [49] | Online platform that: provides caregivers with information + resources on rehabilitation; facilitates communication (chat, video conference) with therapists + includes a forum for discussions among caregivers or with HCPs | Caregivers |
T: U/S D: U/S |
M: Virtual F: Individual |
| [56] | Educational video for caregivers about symptoms, treatment + side effects of leukaemia + testimonials, support from other families | Caregivers |
T: Watched when timely D: 23 min |
M: Virtual F: Individual |
| [58] | Tools + aids that HCPs use to facilitate communication with caregivers about care, family life + support needs; tool for caregivers to indicate care preferences | Children, caregivers, HCPs |
T: U/S D: U/S |
M: U/S F: U/S |
| [64] | Gamified, interactive digital communication tool for child use in waiting rooms to highlight their symptoms, worries + fears to the HCP | Children, HCPs |
T: U/S D: U/S |
M: IP; virtual F: U/S |
| [66] | Palliative care/end of life communication sessions incorporated into clinic visits to plan treatment, discuss prognosis, encourage caregiver articulation of goals + values + support caregivers emotionally | Caregivers |
T: Three sessions, 4–26 weeks after assessment D: Dependsa |
M: IP F: U/S |
| [69] | Collection of patient‐reported outcomes, collaboratively identified by families + HCPs, during routine care, followed by co‐development of personalized future care plans | Children, family, HCPs |
T: Access 1–2×/week D: 1 week to 8 months |
M: Virtual F: Individual |
| [71] | Paediatric rehabilitation coaching session in which child + family's views, values and needs are identified + used to individualize a care plan | Children, caregivers, family |
T: U/S D: U/S |
M: U/S F: U/S |
| [72] | Redesign of the clinic's booking system to give caregivers responsibility to book routine consultations; provides information to caregivers on ideal consultation frequency, advice on when + how to seek help as needed | Children, caregivers | Not applicable |
M: IP; virtual F: Individual |
| [77] | Tailored, structured decision coaching sessions to help families identify treatment preferences, with emphasis on child input before caregiver input | Children, caregivers |
T: U/S D: U/S |
M: IP F: Individual |
| [78] | A paper‐based tool to facilitate documentation + discussion of care plan specifics (i.e., actions, people responsible, timeline), completed by families with help from nurse practitioner | Children, family, HCPs |
T: 1 FU 2–3 weeks after first meeting D: First meeting 2 h, FU dependsa |
M: IP; virtual F: Individual |
| [82] | Care plan, developed by HCPs, is integrated into patient's electronic health record + made accessible via an online patient portal to facilitate communication between medical team + caregivers | Caregivers, HCPs |
T: U/S D: Not applicable |
M: Virtual F: Individual |
| [89] | Virtual platform to improve communication between families + HCPs: access to an individualized care plan, goal setting facilitation, medical history, educational resources. Families decide who may access sensitive information | Children, caregivers, HCPs | Not applicable |
M: Virtual F: Individual |
| [90] | Educational programme includes assessing information needs using a checklist before a tailored physician‐led education session (with booklet) on disease, treatment + complications | Children, caregivers |
T: 1 FU 4 weeks after first session D: First contact 20–30 min; FU U/S |
M: IP F: U/S |
| [92] | Online portal for families to log asthma symptoms, facilitating self‐monitoring + education and clinic awareness of changes, patient monitoring + timely follow‐up | Children, caregivers, HCPs |
T: Weekly D: 1 year |
M: Virtual F: Individual |
| [94] | Educational programme to improve HCP management of asthma, cross‐cultural communication + education practices via lectures, case studies, videos + interactive discussions | HCPs |
T: Two sessions over 2 weeks D: 2.5 h each |
M: IP F: Group |
| [97] | To encourage child involvement in clinic visits, waiting room educational videos delivered via iPad + question prompt list covering questions that the child/family may want to ask the HCP | Children, caregivers |
T: One video session; prompt list U/S D: Videos 11 min (1–2 min each); prompt list U/S |
M: IP F: Individual |
| [102] | Secure online system for communication among providers (so that caregivers do not have to act as messengers) + between providers + caregivers (communications shared with the child's care network) | Caregivers, HCPs |
T: Dependsa D: 6 months total |
M: Virtual F: Individual |
| [103] | A communication “passport” tool used by children + caregivers to track details of services received + outcomes; information is shared with other HCPs | Children, caregivers | Not applicable |
M: IP F: Individual |
| Interventions delivered in or from inpatient clinical settings and emergency departments (n = 10) | ||||
| [45] | Recommendations for hospital cost discussions with caregivers: where (at bedside), when (when child's medical status is stable) + how (should be transparent, optional + tailored to families' needs + preferences) | Caregivers |
T: U/S D: U/S |
M: IP F: U/S |
| [47] | Co‐development of early palliative care plan; support for caregivers to care for child's needs in the NICU; emotional + psychological support for caregivers + help to create visual memories | Children, siblings, caregivers |
T: Dependsa D: U/S |
M: U/S F: U/S |
| [53] | Perioperative programme responsive to the individual needs of children with ASD. All stages of surgery, from preparation to discharge, are designed to manage stress + provide comfort | Children, caregivers |
T: U/S D: Varied: presurgery to 1 day postsurgery |
M: IP; virtual F: U/S |
| [61] | NICU physical space redesign. Newborns are admitted to six‐bed pods + placed in single‐family rooms when they require stepped‐down care; family spaces accommodate family needs (e.g., showers + overnight stays) | Children, caregivers |
T: 24/7 access, caregiver meetings 1×/week D: Not applicable |
M: IP F: Individual |
| [75] | Cardiac surgery preparation to alleviate stress + anxiety in child patients through videos, games + toys + in caregivers through education + counselling | Children, caregivers |
T: Once before surgery D: U/S |
M: IP F: U/S |
| [76] | Mediation session with a transcultural team that facilitates: linguistic + cultural translation of medical information; families' articulation of their understanding + views of treatment options; collaboration between family + HCPs on a common story about the child's disease + life | Children, family, HCPs |
T: One session D: 2–4 h |
M: IP F: Individual |
| [79] | Enhancement of usual care with complementary therapies to alleviate pain + anxiety; therapies selected + tailored through collaboration between the child, family, nurse + integrative medicine specialist | Children, caregivers |
T: Dependsa D: Dependsa |
M: IP F: Individual |
| [81] | An animated robotic toy to provide medical play, offer distraction + facilitate emotional expression by children with cancer at the hospital or home | Children |
T: Dependsa D: 3 days |
M: IP F: Individual |
| [96] | Asthma care improvement initiative in the emergency department: processes to administer medication at admission + discharge; discharge instructions, care plan; team‐based communication; Spanish translation | Children, caregivers |
T: One session predischarge D: 8 min |
M: IP F: U/S |
| [99] | Two‐phase programme to support mothers by engaging them in safe infant care in the NICU + during transition to the general ward, providing information + negotiating mother participation preferences during NICU visiting hours | Caregivers |
T: 2×/day D: Phase 30 min each; total duration dependsa |
M: IP F: Individual |
| Interventions to improve experiences of care delivered in the community (e.g., home, school, community clinic) (n = 9) | ||||
| [48] | Primary care physicians can access rapid consultations with a child clinician/psychiatrist to answer questions+/or initiate referrals | HCPs |
T: U/S D: U/S |
M: IP; virtual F: U/S |
| [51] | HCPs + families co‐develop an action care plan; an online patient portal facilitates care follow‐up and family‐provider communication | Children, family |
T: U/S D: U/S |
M: IP; virtual F: U/S |
| [52] | School‐based psychiatric service to increase access: free assessment, treatment recommendations + follow‐up care support (e.g., wrap‐around needs, appropriate transition to community‐based mental health services) | Children, caregivers |
T: Depends.a 3–4 FU D: U/S |
M: IP F: U/S |
| [59] | Community‐based, ‘one‐stop‐shop’ allergy clinic; a general practitioner with allergy specialization, dietitian + nurse provide assessment, care coordination with primary care + care advice | Children |
T: Dependsa D: First assessment 30 min, FU dependsa |
M: IP F: U/S |
| [65] | Strategies to improve access to a school‐based asthma programme: (1) allow treatment to proceed with verbal caregiver consent; (2) give asthma care plan to caregivers; (3) support school staff to screen for potential cases | Children, caregivers | Not applicable |
M: IP F: U/S |
| [67] | Early childhood, home‐based intervention; involves family needs assessment, individualizing a care plan, five home visits to coach caregivers on behavioural management skills + support to implement learned skills | Children, caregivers, family |
T: Five home visits D: 1–1.5 h each over 3 months |
M: IP; virtual F: Individual |
| [83] | Team‐based, social worker‐led community care coordination, with regularly updated care coordination binder (care plan, medications, contact information) | Children |
T: ≥1×/3 months D: Initial 1 h, FU 40 min each |
M: IP; virtual F: Individual |
| [84] | Child‐centred care model that includes capacity‐building/training for health care workers, age‐appropriate education tools for children + child‐friendly spaces in clinics | Children, caregivers, HCPs | Not applicable |
M: IP F: U/S |
| [91] | Multidisciplinary team supports community care for children with neurodevelopmental disorders; educates + supports caregivers + preschool staff; delivers individualized developmental programmes for children | Children, caregivers |
T: Biweekly, number dependsa D: U/S |
M: IP F: U/S |
| Interventions shared or transitioned between outpatient clinical settings and the patient's community (n = 11) | ||||
| [54] | Training for caregivers on managing their child's conduct problems at home (educational videos followed by virtual sessions with a clinician) | Caregivers |
T: Videos before sessions; 6–10 weekly sessions D: Videos 7–19 min each, sessions 50–60 min each |
M: Virtual F: U/S |
| [55] | Telehealth consultation with a surgeon/specialist for remote families; includes education + coordination of lab testing | Children, family |
T: U/S D: 30–45 min each |
M: Virtual F: Individual |
| [62] | Epilepsy telemedicine programme connecting rural posts to a secondary care centre to facilitate tertiary referral, ensure follow‐up + improve consistent access to antileptics | Children, caregivers | Not applicable |
M: Virtual F: Individual |
| [68] | Telemedicine intervention connecting rural families from a remote clinic office to haematologist + other allied health professionals; on‐site physical exams | Children, caregivers | Not applicable |
M: IP; virtual F: Individual |
| [73, 74] | Collaborative management of health condition; coordinated by a care manager + primary care providers. After identification of family goals, coordination of on‐site services with specialists: education, training, support + coaching for caregivers on home management | Children, caregivers, HCPs |
T: Six to 12 sessions D: Up to 12 h over 6 months |
M: IP; virtual F: Individual; group |
| [85] | Group psychoeducation programme to improve children's knowledge of their medications + help caregivers support their children to take on a larger role in managing their medications | Children, caregivers |
T: Five sessions D: 90 min each |
M: IP F: Group |
| [86] | CPG recommends, inter alia: sharing information tailored to child capacity, family needs + preferences; development of care plan for caregivers' needs; access to trained advocate; HCP cultural competency training | Children, caregivers, HCPs |
T: U/S D: U/S |
M: U/S F: U/S |
| [87] | CPG recommends, inter alia: creating a shared treatment plan; tailoring information + support for children + families; identifying child concerns + preferences | Children, caregivers, HCPs |
T: U/S D: U/S |
M: U/S F: U/S |
| [95] | Care coordination by paediatric hospitals to enable children with medical complexity to remain with a local medical home + local services; includes home visit to assess needs + barriers to care, team review of the case, development of care plan + coordination strategy | Children, caregivers, HCPs |
T: Home visit, team review: 1 each, 2 FU 1 + 6 months after D: 45–120 min, FU U/S |
M: IP; virtual F: Individual |
| [98] | Telemedicine consultation connecting children + families at home to a renal dietitian to facilitate information sharing about diagnosis + treatment | Children, family |
T: U/S D: U/S |
M: Virtual F: Individual |
| [100] | Single point of access to mental health services. Children are assessed after referral + assigned to suitable providers. Care + recovery are tailored to child's needs. Programme includes crisis team + after‐hour services | Children, caregivers |
T: U/S D: U/S |
M: IP; virtual F: U/S |
| Interventions shared or transitioned between inpatient clinical settings and the patient's community (n = 7) | ||||
| [50] | Online care coordination to reduce system expenditures; includes: development of a care plan, advice on health systems navigation, access to a range of services, health education + consultations/coaching with community health workers | Children, caregivers |
T: First meeting + FUs every 2–12 weeks; Dependsa D: U/S |
M: IP; virtual F: U/S |
| [60] | Medical home programme following NICU discharge; predischarge needs assessment + home care education; post‐discharge primary care service, support to access care and services, emotional support + structured team care coordination | Children, caregivers |
T: Dependsa D: First meeting 30–60 min, FU U/S |
M: IP; virtual F: Individual |
| [63] | A nurse provides support for home care, including a 6‐month care plan, home management education, support after discharge + online platform for timely problem‐solving | Children, family |
T: Visits U/S, phone 1×/week D: 6 months |
M: IP; virtual F: Individual |
| [70] | Early neurorehabilitation programme in which a multidisciplinary specialist team conducts needs assessment, coordinates with community care, proactively plans discharge + supports transition to long‐term or local care | Children |
T: U/S D: Over course of 24 h |
M: IP F: U/S |
| [80] | Placement of a paediatric trauma nurse to coordinate care for high risk inpatients. Follows care from admission to postdischarge, coordinates follow‐up care services, liaises with child's care team, communicates with the family | Children, family, HCPs |
T: Dependsa D: Underreported (≥3 months) |
M: IP; virtual F: Individual |
| [88] | CPG recommends, inter alia: involving a multidisciplinary team in addressing care needs + developing family‐centred communication strategies | Children, caregivers, HCPs |
T: U/S D: U/S |
M: U/S F: U/S |
| [93] | Hospital‐to‐home transition programme ensures families receive medication before discharge, coordinates with primary care and schools, provides telephone access to a patient navigator postdischarge + makes a referral for a home evaluation of asthma triggers | Children, caregivers, HCPs |
T: Navigator contact 1–2/month; coordination, home evaluation U/S D: Over 6 months |
M: IP; virtual F: Individual |
| Interventions shared or transitioned between inpatient and outpatient clinical settings (n = 2) | ||||
| [57] | Care coordination by designated providers: Nurse is a member of the care team; provides continuity during hospitalization, accompanies families to outpatient visits, maintains care plan. Care coordination assistant provides logistical + emotional support to families | Children, caregivers | Not applicable |
M: IP; virtual F: U/S |
| [101] | Group programme to educate children with chronic pain and their caregivers about pain management strategies (relaxation, cognitive behavioural skills) through a video, workbook + goal‐setting exercises | Children, caregivers |
T: One session D: Session U/S, video 40 min |
M: IP F: Individual; group |
Abbreviations: CPG, clinical practice guideline; FU, follow‐up; HCP, health care provider; IP, in‐person; NICU, newborn intensive care unit; U/S, unspecified.
‘Depends’ defined as: Intervention timing and/or duration depends on the needs and preferences of the recipients and/or their families
We completed TIDieR checklists for each intervention (Appendix S6). This review's eligibility criteria for interventions were broader than what the TIDieR checklists were intended to address. Three included interventions were clinical practice guidelines 86 , 88 , 122 and one was a set of recommendations 45 ; we did not complete TIDieR checklists for these interventions. The quality of reporting for the remaining 57 included interventions varied: 10 interventions reported eight or nine TIDieR checklist items in sufficient detail, 26 interventions reported six or seven items, and 21 reported four or five items (Appendix S7). The checklist items least often reported were: whether the intervention was modified during the study (item #10, n = 2), whether the intervention was planned to be tailored (item #9, n = 22), the number of times the intervention was delivered and over what period (item #8, n = 44), and what materials were used in the intervention (item #3, n = 45) (Appendix S7).
Interventions targeted children (n = 48; 78.7%), their caregivers (n = 54; 88.6%), and health care providers (n = 20; 32.8%), with several interventions targeting multiple groups. Ten interventions (16.6%) specified ‘the family’ as a targeted recipient group without specifying further. One intervention (1.6%) targeted siblings. 47 Interventions were delivered in‐person (n = 23; 37.7%), virtually (n = 13; 21.3%) or using both modes (n = 18; 29.5%). Seven interventions (11.5%) did not specify a delivery mode.
3.5. Family‐centred care domains addressed by interventions and intervention components
Most interventions (n = 56; 91.8%) addressed more than one of the eight family‐centred care domains that we considered, with nearly half (n = 26, 42.6%) addressing at least four domains (Appendix S8). The most common domains addressed by interventions were communication and information provision (n = 45; 73.8%), family involvement (n = 37; 60.7%) and access to care (n = 30; 49.2%) (Figure 3). The least common domains addressed by interventions were physical comfort (n = 7; 11.5%) and emotional support (n = 18; 29.5%) (Figure 3).
Figure 3.
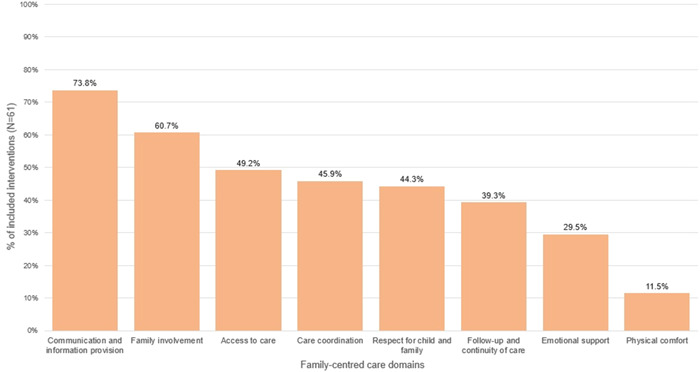
Proportion of interventions addressing selected family‐centred care domains.
Table 4 describes types of approaches taken to addressing each family‐centred care domain, with intervention examples. For example, care coordination was most commonly addressed by adopting a team‐based approach to care delivery, in which services are provided by a multidisciplinary team in the patient's medical home or via a single point‐of‐care (15/28 interventions). We identified more types of intervention approaches to the access to care domain (n = 6) than any other domain.
Table 4.
Approaches to addressing family‐centred care domains.
| Approaches | Number of interventions | Implementation examples |
|---|---|---|
| Communication and information provision | 45 41 , 42 , 43 , 44 , 45 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 58 , 60 , 61 , 63 , 64 , 66 , 67 , 73 , 74 , 75 , 76 , 77 , 78 , 80 , 82 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 | |
| In‐person interactions with providers | 27 42 , 43 , 44 , 45 , 51 , 52 , 53 , 57 , 60 , 61 , 63 , 66 , 67 , 73 , 74 , 75 , 76 , 77 , 80 , 85 , 86 , 87 , 88 , 90 , 91 , 94 , 96 , 99 , 101 |
Educational sessions to assess and address families' information needs Training sessions to coach children and their families on how to manage the disease |
| Tools and aids | 27 41 , 42 , 43 , 44 , 49 , 50 , 51 , 54 , 56 , 58 , 63 , 64 , 66 , 75 , 78 , 82 , 84 , 86 , 89 , 90 , 92 , 94 , 96 , 97 , 99 , 101 , 102 , 103 |
Educational aids (videos, printed materials) providing caregivers with information about the disease, symptoms and management Communication tools (online platforms, mobile apps, checklists) facilitating bidirectional information sharing with providers |
| Using technology to facilitate information sharing | 9 49 , 53 , 54 , 55 , 89 , 93 , 98 , 100 , 102 |
Telemedicine programmes to educate and train caregivers on how to manage symptoms |
| Family involvement | 37 41 , 42 , 43 , 45 , 46 , 47 , 50 , 51 , 53 , 54 , 57 , 58 , 60 , 61 , 66 , 67 , 69 , 71 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 82 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 95 , 97 , 99 , 100 , 101 | |
| Involving families in decision‐making about care or treatment | 22 41 , 42 , 43 , 45 , 46 , 51 , 57 , 58 , 66 , 67 , 69 , 71 , 73 , 74 , 76 , 77 , 78 , 79 , 82 , 88 , 89 , 90 , 95 , 100 |
Provide decision‐making tools (e.g., checklists, mobile applications) Adaptation of a decision‐making process (e.g., decision‐making discussions) Train families to be involved in decision‐making |
| Involving families as recipients of care and focusing on their well‐being | 13 47 , 50 , 53 , 54 , 57 , 58 , 60 , 66 , 73 , 74 , 75 , 86 , 87 , 88 |
Assess family members' wellbeing and include their needs in the care plan Train family members to self‐regulate their emotions Support family members emotionally and psychologically during and outside clinical visits |
| Supporting families to participate in the care of the child | 14 42 , 43 , 47 , 53 , 54 , 60 , 61 , 67 , 71 , 85 , 91 , 92 , 97 , 99 , 101 |
Train family members to provide care to the child (e.g., day‐to‐day support) Identify family members' roles and responsibilities within the care plan Ensure timely family presence during care (i.e., during hospitalization) |
| Access to care | 30 44 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 57 , 58 , 59 , 60 , 62 , 63 , 65 , 72 , 73 , 74 , 83 , 86 , 88 , 89 , 91 , 93 , 95 , 96 , 98 , 100 , 101 , 103 | |
| Using technology to connect families to providers | 12 49 , 53 , 54 , 55 , 57 , 72 , 73 , 74 , 83 , 88 , 89 , 93 , 98 |
Telemedicine‐based programme connecting remote families to providers Access to providers via telephone or email channels to receive support |
| Programme or care delivery in communities | 10 48 , 52 , 59 , 60 , 65 , 88 , 91 , 95 , 100 , 101 |
School‐based programme Medical home approach to care delivery in the community Home visits |
| Facilitating timely and affordable linkage to hospital or community services | 8 44 , 50 , 52 , 53 , 58 , 60 , 72 , 103 |
Checklists to assess service needs Booking system accessible to caregivers Designate community health workers to deliver care |
| Appointment scheduling and logistic support | 6 52 , 57 , 60 , 72 , 83 , 93 |
Transportation support Designate a team member to schedule appointments |
| Improving availability of and access to medications | 6 57 , 62 , 73 , 74 , 88 , 93 , 96 |
Medication supply to rural posts (connected via telemedicine) Dispense medications at discharge |
| Access to an online platform for treatment and support | 4 49 , 50 , 51 , 63 |
Websites or mobile applications to answer caregiver questions |
| Care coordination | 28 44 , 48 , 50 , 51 , 52 , 53 , 57 , 59 , 60 , 65 , 68 , 70 , 72 , 73 , 74 , 76 , 80 , 82 , 83 , 86 , 87 , 88 , 89 , 91 , 93 , 95 , 96 , 100 , 102 | |
| Adapting a team‐based approach to care delivery | 15 44 , 53 , 59 , 60 , 65 , 70 , 82 , 83 , 86 , 87 , 88 , 91 , 95 , 96 , 100 |
Medical home or ‘one‐stop‐shop’ clinic Coordination of care delivery responsibilities among a multidisciplinary team |
| Identification of one or more team members to coordinate care | 13 48 , 50 , 52 , 57 , 59 , 72 , 73 , 74 , 76 , 80 , 88 , 93 , 95 , 100 |
Identification of a team member to coordinate care professionals and/or liaise with the child and family Adapt a referral system to different providers |
| Documenting and sharing care plans and progress reports with other providers | 11 44 , 51 , 53 , 65 , 73 , 74 , 80 , 82 , 83 , 89 , 95 , 102 |
Accessible electronic health records where care plans can be shared Provide caregivers with progress reports to share with other providers |
| Using technology to enhance coordination | 8 50 , 51 , 53 , 60 , 68 , 82 , 89 , 102 |
Telemedicine programmes connecting families to multiple remote providers |
| Respect for child and family | 27 42 , 43 , 45 , 46 , 51 , 53 , 57 , 58 , 60 , 64 , 66 , 71 , 76 , 77 , 79 , 80 , 84 , 85 , 86 , 87 , 88 , 90 , 94 , 95 , 96 , 97 , 99 , 101 | |
| In‐person assessments of child and family values, preferences, and needs | 17 42 , 43 , 45 , 51 , 53 , 57 , 60 , 66 , 71 , 77 , 79 , 80 , 85 , 86 , 87 , 88 , 95 , 99 |
Home visit before intake to assess needs Discussion at initial contact about families' worldview and understanding of child's condition |
| Communication tools to promote families' expression of their values and needs | 6 46 , 58 , 64 , 66 , 90 , 97 |
Checklists Question prompt lists Mobile applications and gamified tools |
| Adjusting activities and communication to age, language, and culture | 5 76 , 84 , 94 , 96 , 101 |
Translators and interpreters Use child‐friendly language to explain the disease and its symptoms Adapt educational materials to the culture and language of the family |
| Follow‐up and continuity of care | 24 50 , 52 , 53 , 57 , 59 , 60 , 63 , 70 , 72 , 73 , 74 , 78 , 80 , 83 , 86 , 87 , 88 , 89 , 91 , 93 , 95 , 96 , 100 , 102 , 103 | |
| Ensuring access to follow‐up care | 22 50 , 52 , 53 , 57 , 60 , 63 , 70 , 72 , 73 , 74 , 78 , 80 , 83 , 86 , 87 , 88 , 89 , 93 , 95 , 96 , 100 , 102 , 103 |
Contact the family after discharge and schedule follow‐up appointments Provide discharge instructions or follow‐up care plans and review them periodically Assess and address child and family needs regularly after discharge (e.g., medical refills, financial needs) |
| Facilitating care transitions between different settings or levels of care | 8 52 , 60 , 70 , 86 , 88 , 91 , 93 , 100 |
From intensive or critical care to the regular ward From tertiary or secondary care to primary care From inpatient care in the hospital to outpatient care in the community |
| Designating a team member to support follow‐up and continuity of care | 8 52 , 57 , 59 , 63 , 73 , 74 , 80 , 83 , 95 |
Accompany families to outpatient clinic visits after discharge Monitor families' attendance of follow‐up appointments |
| Emotional support | 18 47 , 53 , 54 , 57 , 60 , 61 , 63 , 64 , 75 , 79 , 81 , 84 , 86 , 87 , 88 , 91 , 95 , 101 | |
| Designating an individual or group to provide emotional, psychological, or spiritual support | 11 47 , 53 , 54 , 57 , 60 , 63 , 75 , 86 , 87 , 88 , 91 |
Individual or group counselling by psychologists, social workers, health care providers or the clergy |
| Making changes to the visit process or adjusting the environment of the clinical setting | 6 53 , 61 , 75 , 81 , 84 , 95 |
Create child‐friendly spaces using toys and drawings Expedite the admission process to prevent busy and stressful waiting rooms |
| Physical comfort | 7 53 , 61 , 64 , 75 , 81 , 84 , 88 | |
| Making changes to the visit process or adjusting the environment of the clinical setting | 6 53 , 61 , 75 , 81 , 84 , 88 |
Give families light control or minimizing bright lights Schedule surgeries early in the morning to prevent long fasting times |
| Addressing or minimizing physical pain | 2 64 , 88 |
Tools that allow children to communicate their pain symptoms and location to providers Incorporate pain management strategies (e.g., medications, relaxation training) into the care plan |
4. DISCUSSION
4.1. Summary of findings
This scoping review aimed to identify and characterize recently described interventions designed to make care more family‐centred for children with chronic conditions and their families, so that practice and research can build on a synthesis and an understanding of how interventions have been developed to date, the domains of family‐centred care that these interventions have addressed and the key components of the interventions that have sought to address those domains. This was an important evidence gap, particularly since family‐centred care is widely agreed to be important but is broadly defined in the literature, rendering it challenging to understand which approaches have been developed to address specific domains, paediatric chronic disease patient populations and settings.
We identified 63 articles published over the course of fewer than 20 months that described family‐centred care interventions for children with chronic conditions. They encompassed a broad range of specific aims related to improving aspects of family‐centred care, activities and methods. Confirming family‐centred care as a multifaceted concept, nearly half of the included interventions addressed at least four family‐centred care domains while only a few addressed a single domain. Communication and information provision, access to care and family involvement were the most common domains addressed by included interventions' aims and activities, though all eight of the domains that we considered, based on the Picker principles of patient‐centred care, were addressed by multiple interventions.
Clear and comprehensive reporting of interventions is important 40 ; therefore, we assessed the reporting of included interventions using the TIDieR checklist (Appendices S6 and S7). In our assessment, reporting of many details recommended by the TIDieR checklist was strong, with >85% of studies adequately reporting an intervention name; a rationale, theory or goal of the intervention's elements; activities or procedures; facilitators or providers; and setting. We note that reporting of the intervention's goals and activities were among the inclusion criteria for our review, which likely led to a sample of interventions that were relatively better defined. We identified room for improvement in the reporting of intervention materials, mode of delivery, intervention timing and duration, planned tailoring and actual modifications.
Family and patient co‐design of interventions, particularly those aiming to be family‐centred, broadens the range of ideas and perspectives in design and may improve intervention effectiveness. 18 , 19 For close to 30% of included interventions, authors did not report or cite information on the groups engaged in the development of interventions. We anticipated a higher proportion as engagement of health care providers or administrators and of families in the development or research of an intervention was an inclusion criterion. Among the interventions that described such involvement in development, caregivers and child patients were reported to have been engaged in the development of 72.7% and 34.8% of the interventions, respectively. There is still room for improvement in engaging these groups in the development of family‐centred care interventions and the reporting of such engagement, particularly children, even with consideration of age and developmental capacity. There may also be a paucity of interventions addressing the needs of siblings of children with chronic conditions. Although 10 interventions described ‘families’ as a target of their activities, we identified only one intervention in which activities directly targeted siblings. 47 Two additional interventions included advising or educating caregivers to be aware of siblings' emotional needs after a diagnosis or medical event (data not shown). 56 , 88 Healthy siblings of children with chronic conditions may be more likely to experience adverse psychological effects, especially if the condition is more severe. 133 More interventions that directly address the needs of siblings are an important part of current efforts to make care more family‐centred.
4.2. Coordination of care among providers and with the family
Care coordination is a particularly important aspect of family‐centred care for children with chronic conditions, who often have ongoing relationships with care providers, regularly interact with multiple facets of the health care system beyond primary care, and need daily at‐home care management. 134 Health care systems often perform poorly in meeting the needs of this population, with fragmented or siloed institutions and providers and a lack of mechanisms for coordination of care and appointments and sharing of information across providers and systems. 6 , 134 , 135 Preferred approaches to health care coordination for children with chronic conditions that have been described include team‐based organization of care, designation of a care coordinator, digital means of information sharing among providers, care plans and patient registries to track and monitor patients. 136 , 137 Our review indicates that recent interventions adhere to those recommendations, as we identified four common approaches to address care coordination: medical home or team‐based models; designation of individuals or teams to coordinate aspects of care; processes for sharing information among providers; and using technology to enhance coordination. The most common of these was the medical home or team‐based model, which was examined or described by nearly a quarter of the interventions we reviewed. Such models aim to provide comprehensive, coordinated and accessible care, often through primary care, 138 though interventions we reviewed that addressed care coordination did so in both hospital and community settings. Several interventions identified one or more care team members to conduct care coordination activities or streamline a referral process. When these team members liaise directly with families, such points of contact also provide an opportunity for relational continuity, building familiarity and trust between families and health care teams. This continuity is consistently viewed as important to families of children with ongoing health care needs. 135 , 139
4.3. Technology for family‐centred care
The use of technology to improve family‐centred care was common among included interventions; for example, to facilitate access to care, communication or care coordination. However, the interventions included were likely developed and/or studied before the declaration of the COVID‐19 outbreak as a global pandemic on 11 March 2020: our searches were conducted shortly thereafter on 11 August 2020 (published literature) or 7–20 October 2020 (grey literature). Further, among articles that reported intervention implementation dates, all such dates occurred before 11 March 2020. Therefore, these interventions may need to be examined with consideration of needs and realities that have emerged as a result of the pandemic. Web‐based health care interactions, information sharing and education are becoming increasingly common; telehealth increased during the pandemic 140 , 141 , 142 and is likely to remain as a permanent facet of health care delivery in many parts of the world. Parent and family satisfaction with virtual health care or digital health interventions varies and may depend on the context of use.143, 144, 145, 146 Moreover, families with low digital literacy or without consistent access to high‐speed Internet may not have equal access to, or equally benefit from, technology‐based interventions, thereby increasing potential inequities in receiving family‐centred care. 147 , 148
4.4. Strengths and limitations
The high number of published articles returned in our search highlighted a need for a comprehensive, good‐quality review about family‐centred care interventions to provide policymakers, clinicians and researchers engaged in health care improvement for children with chronic conditions and their families with a useful summary of the literature. To create this resource of family‐centred care interventions, we aimed to identify and describe the scope of existing family‐centred care interventions, mapping their component activities and processes within established domains. We used rigorous methods, particularly in the search strategy and screening and extraction of articles, for example, including multiple reviewers and extractors to minimize bias; and we relied on widely accepted principles of family‐centred care to synthesize our findings.
We implemented two deviations from our published protocol: (i) we described the planned review as ‘rapid’ in our protocol but at this reporting stage we believe that the 2‐year timeframe needed to complete it renders that description inaccurate; and (ii) we changed the eligibility criteria regarding publication date for feasibility reasons. With respect to (ii) family‐centred care is a broad concept, necessitating a search strategy that was nonspecific and initially yielded a large number of citations (>15,000). Health care systems and paradigms of care change frequently, however, and our choice to narrow the eligibility to publications since 2019 enabled us to identify interventions that address the most current contexts and needs. The exclusion of articles published before 2019, as well as those published in languages other than English, may introduce selection bias into our review. 149 Interested readers may refer to Appendix S9 for a list of excluded non‐English articles. The English‐language eligibility criterion also likely affected the geographic distribution of articles included in this review: more than two‐thirds of articles described interventions implemented in North America or the United Kingdom. Finally, this review focused on a broad topic, interventions to improve family‐centred care. We strove to identify and include interventions of interest but some relevant interventions may have been missed or described in article types (e.g., commentaries) that were ineligible for our review.
5. CONCLUSION
As recognition of the unmet needs of children with chronic conditions grows, so does interest in improving family‐centred care. The identification of 61 family‐centred care interventions for children with chronic conditions, described in articles published 2019 to mid‐2020, demonstrates that this is an active area of research. The breadth of the concept of family‐centred care may present a challenge for individuals and groups interested in developing and evaluating interventions who want to build on previous work. This review is a concrete resource for health care providers, administrators and researchers, providing an inventory of interventions categorized by family‐centred care domain, setting and population, that describes the types of activities and processes that have been developed and/or implemented recently. It serves as a foundation for those engaged in practice and/or research to improve health care for children with high needs and their families by highlighting interventions that centre the needs of children and their caregivers, and to potentially advocate to governments and funding agencies for the resources to improve that care. The many interventions that have been the subject of RCTs and quasiexperimental designs underscores an opportunity for future systematic reviews to evaluate the effectiveness of interventions for a subset of domains, populations or settings, using our inventory as a starting point. Finally, we have highlighted two areas where future research on the development of future family‐centred care interventions may be improved: (1) involving patients and families in the development process and (2) improving the transparent reporting of intervention development and implementation, particularly with respect to clarifying aims and processes of engaging with patients, caregivers and providers.
AUTHOR CONTRIBUTIONS
Beth K. Potter, Andrea J. Chow, Zobaida Al‐Baldawi, Ryan Iverson, Isabel Jordan, Nicole Pallone, Maureen Smith, Becky Skidmore, Jamie Brehaut, Pranesh Chakraborty, Eyal Cohen, Sarah Dyack, Jane Gillis, Cheryl R. Greenberg, Robin Hayeems, Brian Hutton, Sara Khangura, Jennifer J. MacKenzie, John J. Mitchell, Stuart G. Nicholls, Amy Pender, Chitra Prasad, Andreas Schulze, Rebecca N. Sparkes, Kathy N. Speechley, Sylvia Stockler, Mari Teitlebaum, Yannis Trakadis, Clara Van Karnebeek, Jagdeep S. Walia and Kumanan Wilson contributed to the conception and design of the study. Becky Skidmore conducted the literature search. Andrea J. Chow, Zobaida Al‐Baldawi, Ryan Iverson, Ammar Saad, Beth K. Potter, and Pranesh Chakraborty contributed to the study selection, and Ammar Saad, Andrea J. Chow, Zeinab Moazin, Zobaida Al‐Baldawi and Ryan Iverson contributed to data extraction. Ammar Saad, Andrea J. Chow and Beth K. Potter analysed the data. Andrea J. Chow, Ammar Saad, Beth K. Potter, Zobaida Al‐Baldawi, Eyal Cohen, Sarah Dyack, Jane Gillis, Ryan Iverson, Isabel Jordan, Sharan Goobie, Nicole Pallone, Chitra Prasad, Maureen Smith and Monica Taljaard interpreted the data. Andrea J. Chow, Beth K. Potter and Ammar Saad drafted the manuscript. All authors critically revised the manuscript and approved the final version.
CONFLICTS OF INTEREST STATEMENT
John J. Mitchell has worked with pharmaceutical companies that market products for the treatment of inborn errors. This relationship did not have any impact on the design or review of this paper. Kumanan Wilson is a co‐founder and Chief Scientific Officer of CANImmunize Inc. He served on the Independent Data Monitoring Committee for Medicago and is a member of the Moderna Global Advisory Core Consultancy Group. The remaining authors declare no conflict of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGEMENTS
This work is supported by the Canadian Institutes of Health Research (Grant #PJT‐153230). The Canadian Institutes of Health Research did not have a role in the design of the study or the writing of this manuscript. The authors thank Alison Howie for data extraction assistance, Dr Maria Karaceper for expertise on health conditions to support screening, Ben Martinez for assistance with health condition taxonomies, Christine Neilson, MLIS (University of Manitoba) for peer review of the MEDLINE search strategy and Dr Brenda Wilson for expertise on study conceptualization.
Chow AJ, Saad A, Al‐Baldawi Z, et al. Family‐centred care interventions for children with chronic conditions: a scoping review. Health Expect. 2023;27(1);e13897. 10.1111/hex.13897
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hoefgen ER, Andrews AL, Richardson T, et al. Health care expenditures and utilization for children with noncomplex chronic disease. Pediatrics. 2017;140(3):e20170492. 10.1542/peds.2017-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuo DZ, Melguizo‐Castro M, Goudie A, Nick TG, Robbins JM, Casey PH. Variation in child health care utilization by medical complexity. Matern Child Health J. 2015;19(1):40‐48. 10.1007/s10995-014-1493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parasuraman SR, Anglin TM, McLellan SE, et al. Health care utilization and unmet need among youth with special health care needs. J Adolesc Health. 2018;63(4):435‐444. 10.1016/j.jadohealth.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 4. Silver EJ, Stein REK. Access to care, unmet health needs, and poverty status among children with and without chronic conditions. Ambul Pediatr. 2001;1(6):314‐320. [DOI] [PubMed] [Google Scholar]
- 5. Dewan T, Cohen E. Children with medical complexity in Canada. Paediatr Child Health. 2013;18(10):518‐522. 10.1093/pch/18.10.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toomey SL, Chien AT, Elliott MN, Ratner J, Schuster MA. Disparities in unmet need for care coordination: the national survey of children's health. Pediatrics. 2013;131(2):217‐224. 10.1542/peds.2012-1535 [DOI] [PubMed] [Google Scholar]
- 7. Johnson BH. Family‐centered care: four decades of progress. Fam Syst Health. 2000;18(2):137‐156. 10.1037/h0091843 [DOI] [Google Scholar]
- 8. Shelton TL, Jeppson ES, Johnson BH. Family‐Centered Care for Children with Special Health Care Needs. Association for the Care of Children's Health; 1987. [Google Scholar]
- 9. Epstein RM, Street RL. The values and value of patient‐centered care. Ann Fam Med. 2011;9(2):100‐103. 10.1370/afm.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenbaum P, King S, Law M, King G, Evans J. Family‐centred service. Phys Occup Ther Pediatr. 1998;18(1):1‐20. 10.1300/j006v18n01_01 [DOI] [Google Scholar]
- 11. Kuhlthau KA, Bloom S, Van Cleave J, et al. Evidence for family‐centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11:136‐143.e8. 10.1016/j.acap.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 12. Wertlieb D. Converging trends in family research and pediatrics: recent findings for the American Academy of Pediatrics Task Force on the Family. Pediatrics. 2003;111(suppl_2):1572‐1587. 10.1542/peds.111.S2.1572 [DOI] [PubMed] [Google Scholar]
- 13. Johnson BH, Abraham MR. Partnering with Patients, Residents, and Families: A Resource for Leaders of Hospitals, Ambulatory Care Settings, and Long‐Term Care Communities. Institute for Patient‐ and Family‐Centered Care; 2008. http://www.hqontario.ca/Portals/0/modals/qi/en/processmap_pdfs/articles/partnering [Google Scholar]
- 14. King S, Rosenbaum P, King G. The Measure of Processes of Care (MPOC): A Means to Assess Family‐centered Behaviours of Health Care Providers. McMaster University; 1995. [Google Scholar]
- 15. Kokorelias KM, Gignac MAM, Naglie G, Cameron JI. Towards a universal model of family centered care: a scoping review. BMC Health Serv Res. 2019;19(1):564. 10.1186/s12913-019-4394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Registered Nurses' Association of Ontario. Person‐ and Family‐Centred Care Clinical Best Practice Guidelines. Registered Nurses' Association of Ontario; 2022. https://rnao.ca/sites/rnao-ca/files/FINAL_Web_Version_0.pdf [Google Scholar]
- 17. Wells N, Bronheim S, Zyzanski S, Hoover C. Psychometric evaluation of a consumer‐developed family‐centered care assessment tool. Matern Child Health J. 2015;19(9):1899‐1909. 10.1007/s10995-015-1709-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brett J, Staniszewska S, Mockford C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient. 2014;7(4):387‐395. 10.1007/s40271-014-0065-0 [DOI] [PubMed] [Google Scholar]
- 19. Gustavsson SM, Andersson T. Patient involvement 2.0: experience‐based co‐design supported by. Action Res. 2019;17(4):469‐491. 10.1177/1476750317723965 [DOI] [Google Scholar]
- 20. King G, Williams L, Hahn Goldberg S. Family‐oriented services in pediatric rehabilitation: a scoping review and framework to promote parent and family wellness. Child Care Health Dev. 2017;43(3):334‐347. 10.1111/cch.12435 [DOI] [PubMed] [Google Scholar]
- 21. Picker Institute. Principles of Patient‐Centered Care. Picker Institute; 2018. http://pickerinstitute.org/about/picker-principles/ [Google Scholar]
- 22. Picker Institute Europe. Principles of Person Centred Care. Picker Institute Europe; 2018. https://picker.org/who-we-are/the-picker-principles-of-person-centred-care/ [Google Scholar]
- 23. Picker Institute Europe. Our History. Picker Institute Europe; 2023. https://picker.org/who-we-are/our-history/ [Google Scholar]
- 24. Arthur KC, Mangione‐Smith R, Meischke H, et al. Impact of English proficiency on care experiences in a pediatric emergency department. Acad Pediatr. 2015;15(2):218‐224. 10.1016/j.acap.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 25. Byczkowski TL, Gillespie GL, Kennebeck SS, Fitzgerald MR, Downing KA, Alessandrini EA. Family‐centered pediatric emergency care: a framework for measuring what parents want and value. Acad Pediatr. 2016;16(4):327‐335. 10.1016/j.acap.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 26. Klingshirn H, Gerken L, Hofmann K, et al. Comparing the quality of care for long‐term ventilated individuals at home versus in shared living communities: a convergent parallel mixed‐methods study. BMC Nurs. 2022;21(1):224. 10.1186/s12912-022-00986-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141‐146. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 29. Peters MDJ, Godfrey C, McInerney P, Soares CB, Khalil H, Parker D. Chapter 11: Scoping reviews (2020 version). In: Aromataris E, Munn Z, eds. JBI Reviewer's Manual. JBI; 2017. [Google Scholar]
- 30. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 31. Potter BK, Khangura SD, Tingley K, Chakraborty P, Little J. Translating rare‐disease therapies into improved care for patients and families: what are the right outcomes, designs, and engagement approaches in health‐systems research? Genet Med. 2016;18(2):117‐123. 10.1038/gim.2015.42 [DOI] [PubMed] [Google Scholar]
- 32. Nuutila L, Salanterä S. Children with a long‐term illness: parents' experiences of care. J Pediatr Nurs. 2006;21(2):153‐160. 10.1016/j.pedn.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 33. Bate P, Robert G. Experience‐based design: from redesigning the system around the patient to co‐designing services with the patient. Qual Saf Health Care. 2006;15(5):307‐310. 10.1136/qshc.2005.016527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batalden M, Batalden P, Margolis P, et al. Coproduction of healthcare service. BMJ Qual Saf. 2016;25(7):509‐517. 10.1136/bmjqs-2015-004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez B, Pechlivanoglou P, Meng D, et al. Clinical health outcomes of siblings of children with chronic conditions: a systematic review and meta‐analysis. J Pediatr. 2022;250:83‐92.e8. 10.1016/j.jpeds.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 36. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 37. CADTH. CADTH's Grey Matters: A Practical Tool for Searching Health‐Related Grey Literature. CADTH; 2020. https://www.cadth.ca/resources/finding-evidence/grey-matters [Google Scholar]
- 38. World Health Organization. International Classification of Health Interventions (ICHI). WHO; 2022. http://www.who.int/classifications/ichi/en/ [Google Scholar]
- 39. Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1(1):10. 10.1186/2046-4053-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 41. Alsem MW, Verhoef M, Braakman J, et al. Parental empowerment in paediatric rehabilitation: exploring the role of a digital tool to help parents prepare for consultation with a physician. Child Care Health Dev. 2019;45(5):623‐636. 10.1111/cch.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. An M, Palisano RJ, Yi CH, Chiarello LA, Dunst CJ, Gracely EJ. Effects of a collaborative intervention process on parent empowerment and child performance: a randomized controlled trial. Phys Occup Ther Pediatr. 2019;39(1):1‐15. 10.1080/01942638.2017.1365324 [DOI] [PubMed] [Google Scholar]
- 43. An M, Palisano RJ, Yi C, Chiarello LA, Dunst CJ, Gracely EJ. Effects of a collaborative intervention process on parent–therapist interaction: a randomized controlled trial. Phys Occup Ther Pediatr. 2019;39(3):259‐275. 10.1080/01942638.2018.1496965 [DOI] [PubMed] [Google Scholar]
- 44. Barriteau CM, Murdoch A, Gallagher SJ, Thompson AA. A patient‐centered medical home model for comprehensive sickle cell care in infants and young children. Pediatr Blood Cancer. 2020;67(6):e28275. 10.1002/pbc.28275 [DOI] [PubMed] [Google Scholar]
- 45. Beck J, Wignall J, Jacob‐Files E, et al. Parent attitudes and preferences for discussing health care costs in the inpatient setting. Pediatrics. 2019;144(2):e20184029. 10.1542/peds.2018-4029 [DOI] [PubMed] [Google Scholar]
- 46. Bosak DL, Jarvis JM, Khetani MA. Caregiver creation of participation‐focused care plans using participation and environment measure plus (PEM+), an electronic health tool for family‐centred care. Child Care Health Dev. 2019;45(6):791‐798. 10.1111/cch.12709 [DOI] [PubMed] [Google Scholar]
- 47. Callahan K, Steinwurtzel R, Brumarie L, Schechter S, Parravicini E. Early palliative care reduces stress in parents of neonates with congenital heart disease: validation of the “baby, attachment, comfort interventions. J Perinatol. 2019;39(12):1640‐1647. 10.1038/s41372-019-0490-y [DOI] [PubMed] [Google Scholar]
- 48. Cama S, Knee A, Sarvet B. Impact of child psychiatry access programs on mental health care in pediatric primary care: measuring the parent experience. Psychiatr Serv. 2020;71(1):43‐48. 10.1176/appi.ps.201800324 [DOI] [PubMed] [Google Scholar]
- 49. Camden C, Couture M, Pratte G, et al. Recruitment, use, and satisfaction with a web platform supporting families of children with suspected or diagnosed developmental coordination disorder: a randomized feasibility trial. Dev Neurorehabil. 2019;22(7):470‐478. 10.1080/17518423.2018.1523243 [DOI] [PubMed] [Google Scholar]
- 50. Caskey R, Moran K, Touchette D, et al. Effect of comprehensive care coordination on Medicaid expenditures compared with usual care among children and youth with chronic disease: a randomized clinical trial. JAMA Network Open. 2019;2(10):e1912604. 10.1001/jamanetworkopen.2019.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chakravorty S, Knapp CA. The impact of the patient‐centered medical home on asthma‐related visits to the emergency room: a fixed effects regression approach. Matern Child Health J. 2019;23:369‐376. 10.1007/s10995-018-2661-4 [DOI] [PubMed] [Google Scholar]
- 52. Cho E, Herman KC, Salau M, Young‐Walker L. Increasing access to psychiatric services in schools: the Bridge Program. J Psychiatr Pract. 2019;25(3):227‐236. 10.1097/PRA.0000000000000381 [DOI] [PubMed] [Google Scholar]
- 53. Clark LA, Whitt S, Lyons K. Improving communication between health care providers, families, and children with autism spectrum disorder: the linked program. J Perianesth Nurs. 2019;34(5):889‐899. 10.1016/j.jopan.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 54. Dadds MR, Thai C, Mendoza Diaz A, et al. Therapist‐assisted online treatment for child conduct problems in rural and urban families: two randomized controlled trials. J Consult Clin Psychol. 2019;87(8):706‐719. 10.1037/ccp0000419 [DOI] [PubMed] [Google Scholar]
- 55. Dean P, O'Donnell M, Zhou L, Skarsgard ED. Improving value and access to specialty medical care for families: a pediatric surgery telehealth program. Can J Surg. 2019;62(6):436‐441. 10.1503/cjs.005918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Di Giuseppe G, Pole JD, Abla O, Punnett A. Impact of videotaped information on the experience of parents of children with acute lymphoblastic leukemia. J Cancer Educ. 2020;35:479‐484. 10.1007/s13187-019-1485-2 [DOI] [PubMed] [Google Scholar]
- 57. Donnelly S, Shaw E, Timoney P, Foca M, Hametz P. Parents' assessment of an advanced‐practice nurse and care coordination assistant model medical care coordination program for children with medical complexity. J Pediatr Health Care. 2020;34(4):325‐332. 10.1016/j.pedhc.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 58. Eberhart A, Slogeris B, Sadreameli SC, Jassal MS. Using a human‐centered design approach for collaborative decision‐making in pediatric asthma care. Public Health. 2019;170:129‐132. 10.1016/j.puhe.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 59. El‐Shanawany IR, Wade C, Holloway JA. The impact of a general practitioner‐led community paediatric allergy clinic: a service evaluation. Clin Exp Allergy. 2019;49(5):690‐700. 10.1111/cea.13375 [DOI] [PubMed] [Google Scholar]
- 60. Feehan K, Kehinde F, Sachs K, et al. Development of a multidisciplinary medical home program for NICU graduates. Matern Child Health J. 2020;24(1):11‐21. 10.1007/s10995-019-02818-0 [DOI] [PubMed] [Google Scholar]
- 61. Feeley N, Robins S, Genest C, Stremler R, Zelkowitz P, Charbonneau L. A comparative study of mothers of infants hospitalized in an open ward neonatal intensive care unit and a combined pod and single‐family room design. BMC Pediatr. 2020;20(1):38. 10.1186/s12887-020-1929-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fortini S, Espeche A, Caraballo R. Telemedicine and epilepsy: a patient satisfaction survey of a pediatric remote care program. Epilepsy Res. 2020;165:106370. 10.1016/j.eplepsyres.2020.106370 [DOI] [PubMed] [Google Scholar]
- 63. Geng W, Tao N, Wang T, et al. Continuous nursing reduces postoperative complications and improves quality of life of patients after enterostomies. Int J Clin Exp Med. 2019;12(5):5895‐5901. [Google Scholar]
- 64. Gilljam BM, Nygren JM, Svedberg P, Arvidsson S. Impact of an electronic health service on child participation in pediatric oncology care: quasiexperimental study. J Med Internet Res. 2020;22(7):e17673. 10.2196/17673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Green LA, Ailey SH. Increasing childhood asthma care appointments on a mobile asthma van. J Sch Nurs. 2021;37(3):209‐219. 10.1177/1059840519857143 [DOI] [PubMed] [Google Scholar]
- 66. Hendricks‐Ferguson VL, Haase JE. Parent perspectives of receiving early information about palliative and end‐of‐life care options from their child's pediatric providers. Cancer Nurs. 2019;42(4):E22‐E30. 10.1097/NCC.0000000000000589 [DOI] [PubMed] [Google Scholar]
- 67. Hsieh YH, Liao HF, Jeng SF, et al. Collaborative home‐visit program for young children with motor delays in rural Taiwan: a pilot randomized controlled trial. Phys Ther. 2020;100(6):979‐994. 10.1093/ptj/pzaa033 [DOI] [PubMed] [Google Scholar]
- 68. Jacob SA, Carroll AE, Bennett Jr., WE . A feasibility study of telemedicine for paediatric sickle cell patients living in a rural medically underserved area. J Telemed Telecare. 2021;27(7):431‐435. 10.1177/1357633X19883558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jerome RN, Pulley JM, Edwards TL, et al. We're not all cut from the same cloth: TAILORing treatments for children with chronic conditions. J Patient‐Rep Outcomes. 2019;3(1):25. 10.1186/s41687-019-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Keetley R, Kelly L, Whitehouse WP, et al. Early discharge and rehabilitation in paediatric acquired brain and neurological injury: a transferable model. Arch Dis Child. 2020;105(1):41. 10.1136/archdischild-2018-315096 [DOI] [PubMed] [Google Scholar]
- 71. King G, Schwellnus H, Servais M, Baldwin P. Solution‐focused coaching in pediatric rehabilitation: investigating transformative experiences and outcomes for families. Phys Occup Ther Pediatr. 2019;39(1):16‐32. 10.1080/01942638.2017.1379457 [DOI] [PubMed] [Google Scholar]
- 72. Kofoed PE, Thomsen J. Leaving the responsibility of booking appointments to parents in a paediatric diabetes outpatient clinic resulted in a deterioration of metabolic control. Pediatr Diabetes. 2020;21(2):390‐394. 10.1111/pedi.12968 [DOI] [PubMed] [Google Scholar]
- 73. Kolko DJ, Hart JA, Campo J, et al. Effects of collaborative care for comorbid attention deficit hyperactivity disorder among children with behavior problems in pediatric primary care. Clin Pediatr. 2020;59(8):787‐800. 10.1177/0009922820920013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hsiung KS, Hart J, Kelleher KJ, Kolko DJ. Impact of stressful climates on provider perceptions of integrated behavioral health services in pediatric primary care: an exploratory study. J Dev Behav Pediatr. 2019;40(9):686‐695. 10.1097/DBP.0000000000000712 [DOI] [PubMed] [Google Scholar]
- 75. Kumar A, Das S, Chauhan S, Kiran U, Satapathy S. Perioperative anxiety and stress in children undergoing congenital cardiac surgery and their parents: effect of brief intervention—a randomized control trial. J Cardiothorac Vasc Anesth. 2019;33(5):1244‐1250. 10.1053/j.jvca.2018.08.187 [DOI] [PubMed] [Google Scholar]
- 76. Lachal J, Escaich M, Bouznah S, et al. Transcultural mediation programme in a paediatric hospital in France: qualitative and quantitative study of participants' experience and impact on hospital costs. BMJ Open. 2019;9(11):032498. 10.1136/bmjopen-2019-032498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lawson ML, Shephard AL, Feenstra B, Boland L, Sourial N, Stacey D. Decision coaching using a patient decision aid for youth and parents considering insulin delivery methods for type 1 diabetes: a pre/post study. BMC Pediatr. 2020;20(1):1‐8. 10.1186/s12887-019-1898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lindstrom K, Cady R, Bushaw A. Family‐centered care for children with medical complexity: a goal‐planning initiative. Nurse Pract. 2020;45(8):49‐55. 10.1097/01.NPR.0000681796.57869.a0 [DOI] [PubMed] [Google Scholar]
- 79. Mayan M, Alvadj T, Punja S, Jou H, Wildgen S, Vohra S. A caregiver, an expert, a patient: how complementary therapies support the roles of parents of children with life threatening conditions in hospital settings. Explore. 2021;17(4):297‐302. 10.1016/j.explore.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 80. McRoberts CM, Bohlen N, Wills HE. Bridging the gap: utilizing a pediatric trauma care coordinator to reduce disparities for pediatric trauma follow‐up care. J Trauma Nurs. 2019;26(4):193‐198. 10.1097/JTN.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 81. Miller TP, Klosky JL, Zamora F, Swift M, Mertens AC. Feasibility and acceptability of an animatronic duck intervention for promoting adaptation to the in‐patient setting among pediatric patients receiving treatment for cancer. Pediatr Blood Cancer. 2019;66(12):e27984. 10.1002/pbc.27984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ming DY, Jackson GL, Sperling J, et al. Mobile complex care plans to enhance parental engagement for children with medical complexity. Clin Pediatr. 2019;58(1):34‐41. 10.1177/0009922818805241 [DOI] [PubMed] [Google Scholar]
- 83. Moeenuddin Z, Kim‐Kupfer C, Owchar E, Baker J, Duffield A, Santoro T. The influence of care coordination on patients with special health care needs in a pediatric residency continuity clinic. Glob Pediatr Health. 2019;6:2333794. 10.1177/2333794x19848677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mutambo C, Shumba K, Hlongwana KW. User‐provider experiences of the implementation of KidzAlive‐driven child‐friendly spaces in KwaZulu‐Natal, South Africa. BMC Public Health. 2020;20(1):91. 10.1186/s12889-019-7712-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nagae M, Tokunaga A, Morifuji K, et al. Efficacy of a group psychoeducation program focusing on the attitudes towards medication of children and adolescents with ADHD and their parents: a pilot study. Acta Med Nagasaki. 2019;62(3):77‐86. 10.11343/amn.62.77 [DOI] [Google Scholar]
- 86. National Institute for Health and Care Excellence. Bipolar Disorder: Assessment and Management (NICE Guideline CG185). NICE; 2021. https://www.nice.org.uk/guidance/cg185 [PubMed] [Google Scholar]
- 87. National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management (NICE Guideline NG87). NICE; 2021. https://www.nice.org.uk/guidance/ng87 [PubMed] [Google Scholar]
- 88. National Institute for Health and Care Excellence. End of Life Care for Infants, Children and Young People with Life‐Limiting Conditions: Planning and Management (NICE Guideline NG61). NICE; 2021. https://www.nice.org.uk/guidance/NG61 [Google Scholar]
- 89. Niemann MH, Alvarado MC, Camayd‐Muñoz C, Jonas RR, Wenger JK, Douglass LM. A multimodal telehealth strategy to improve pediatric epilepsy care. Pediatr Clin North Am. 2020;67(4):629‐634. 10.1016/j.pcl.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 90. Niemitz M, Schrader M, Carlens J, et al. Patient education for children with interstitial lung diseases and their caregivers: a pilot study. Patient Educ Couns. 2019;102(6):1131‐1139. 10.1016/j.pec.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 91. Nilses Å, Jingrot M, Linnsand P, et al. Experiences of immigrant parents in Sweden participating in a community assessment and intervention program for preschool children with autism. Neuropsychiatr Dis Treat. 2019;15:3397‐3410. 10.2147/NDT.S221908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nkoy FL, Fassl BA, Wilkins VL, et al. Ambulatory management of childhood asthma using a novel self‐management application. Pediatrics. 2019;143(6):e20181711. 10.1542/peds.2018-1711 [DOI] [PubMed] [Google Scholar]
- 93. Parikh K, Richmond M, Lee M, et al. Outcomes from a pilot patient‐centered hospital‐to‐home transition program for children hospitalized with asthma. J Asthma. 2021;58(10):1384‐1394. 10.1080/02770903.2020.1795877 [DOI] [PubMed] [Google Scholar]
- 94. Patel MR, Song PXK, Bruzzese JM, et al. Does cross‐cultural communication training for physicians improve pediatric asthma outcomes? A randomized trial. J Asthma. 2019;56(3):273‐284. 10.1080/02770903.2018.1455856 [DOI] [PubMed] [Google Scholar]
- 95. Sadof M, Carlin S, Brandt S, Maypole J. A step‐by‐step guide to building a complex care coordination program in a small setting. Clin Pediatr. 2019;58(8):897‐902. 10.1177/0009922819849057 [DOI] [PubMed] [Google Scholar]
- 96. Santos Malavé C, Diggs D, Sampayo EM. Spanish‐speaking caregivers' experience with an emergency department pediatric asthma‐care bundle quality initiative. J Racial Ethn Health Disparities. 2019;6(4):660‐667. 10.1007/s40615-019-00564-1 [DOI] [PubMed] [Google Scholar]
- 97. Sleath B, Carpenter D, Sayner R, et al. Questions and reported medication problems from pediatric patients and caregivers after intervention. Am J Health Syst Pharm. 2019;76(6):366‐373. 10.1093/ajhp/zxy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Trace SL, Collinson A, Searle AJ, Lithander FE. Using videoconsultations to deliver dietary advice to children with chronic kidney disease: a qualitative study of parent and child perspectives. J Hum Nutr Diet. 2020;33(6):881‐889. 10.1111/jhn.12750 [DOI] [PubMed] [Google Scholar]
- 99. Uhm JY, Kim HS. Impact of the mother–nurse partnership programme on mother and infant outcomes in paediatric cardiac intensive care unit. Intens Crit Care Nurs. 2019;50:79‐87. 10.1016/j.iccn.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 100. Vusio F, Thompson A, Laughton L, Birchwood M. After the storm, solar comes out: a new service model for children and adolescent mental health. Early Interv Psychiatry. 2021;15(3):731‐738. 10.1111/eip.13009 [DOI] [PubMed] [Google Scholar]
- 101. Wihak T, Burns M, Miranda J, et al. Development and feasibility testing of the Comfort Ability Program for sickle cell pain: a patient‐informed, video‐based pain management intervention for adolescents with sickle cell disease. Clin Pract Pediatr Psychol. 2020;8(2):150‐163. 10.1037/cpp0000326 [DOI] [Google Scholar]
- 102. Yamada J, Ballantyne M, Kron AT, Sidani S. Parents' perceptions of the acceptability of evidence‐based interventions to support transition from neonatal to rehabilitation services. CJNR. 2021;53(3):292‐302. 10.1177/0844562120931661 [DOI] [PubMed] [Google Scholar]
- 103. Young E, Aiyadurai R, Jegathesan T, et al. Increasing access to developmental services for children with autism spectrum disorder: the pediatric developmental passport pilot randomized trial. J Autism Dev Disord. 2019;49(12):4867‐4876. 10.1007/s10803-019-04199-3 [DOI] [PubMed] [Google Scholar]
- 104. Alsem MW, Van Meeteren KM, Verhoef M, et al. Co‐creation of a digital tool for the empowerment of parents of children with physical disabilities. Res Involv Engagem. 2017;3(1):26. 10.1186/s40900-017-0079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. An M, Palisano RJ. Family–professional collaboration in pediatric rehabilitation: a practice model. Disabil Rehabil. 2014;36(5):434‐440. 10.3109/09638288.2013.797510 [DOI] [PubMed] [Google Scholar]
- 106. Arvidsson S, Gilljam BM, Nygren J, Ruland CM, Nordby‐Bøe T, Svedberg P. Redesign and validation of Sisom, an interactive assessment and communication tool for children with cancer. JMIR Mhealth Uhealth. 2016;4(2):e76. 10.2196/mhealth.5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baldwin P, King G, Evans J, McDougall S, Tucker MA, Servais M. Solution‐focused coaching in pediatric rehabilitation: an integrated model for practice. Phys Occup Ther Pediatr. 2013;33(4):467‐483. 10.3109/01942638.2013.784718 [DOI] [PubMed] [Google Scholar]
- 108. Bouznah S, Larchanché S. Transcultural mediation in the management of cancer patients in the tropical area. In: Droz JP, Carme B, Couppié P, eds. Tropical Hemato‐Oncology. 1st ed. Springer; 2015:55‐64. [Google Scholar]
- 109. Dvir Y, Wenz‐Gross M, Jeffers‐Terry M, Metz WP. An assessment of satisfaction with ambulatory child psychiatry consultation services to primary care providers by parents of children with emotional and behavioral needs: the Massachusetts Child Psychiatry Access Project University of Massachusetts parent satisfaction study. Front Psychiatry. 2012;3:7. 10.3389/fpsyt.2012.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Feenstra B, Lawson ML, Harrison D, Boland L, Stacey D. Decision coaching using the Ottawa family decision guide with parents and their children: a field testing study. BMC Med Inform Decis Mak. 2015;15(1):5. 10.1186/s12911-014-0126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gulmans J, Vollenbroek‐Hutten MM, Visser JJ, Nijeweme‐d'Hollosy WO, van Gemert‐Pijnen JL, van Harten WH. A web‐based communication system for integrated care in cerebral palsy: design features, technical feasibility and usability. J Telemed Telecare. 2010;16(7):389‐393. 10.1258/jtt.2010.091013 [DOI] [PubMed] [Google Scholar]
- 112. Hendricks‐Ferguson VL, Pradhan K, Shih CS, et al. Pilot evaluation of a palliative and end‐of‐life communication intervention for parents of children with a brain tumor. J Pediatr Oncol Nurs. 2017;34(3):203‐213. 10.1177/1043454216676836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hendricks‐Ferguson VL, Kane JR, Pradhan KR, et al. Evaluation of physician and nurse dyad training procedures to deliver a palliative and end‐of‐life communication intervention to parents of children with a brain tumor. J Pediatr Oncol Nurs. 2015;32(5):337‐347. 10.1177/1043454214563410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Herman KC, Cho E, Marriott BR, Walker LY. Bridging the gap in psychiatric care for children with a school‐based psychiatry program. Sch Ment Health. 2018;10:181‐189. 10.1007/s12310-017-9222-7 [DOI] [Google Scholar]
- 115. Jarvis JM, Gurga A, Greif A, et al. Usability of the Participation and Environment Measure Plus (PEM+) for client‐centered and participation‐focused care planning. Am J Occup Ther. 2019;73(4):7304205130p1‐7304205130p8. 10.5014/ajot.2019.032235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Khetani MA, Lim HK, Corden ME. Caregiver input to optimize the design of a pediatric care planning guide for rehabilitation: descriptive study. JMIR Rehabil Assist Technol. 2017;4(2):e10. 10.2196/rehab.7566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Khetani MA, Cliff AB, Schelly C, Daunhauer L, Anaby D. Decisional support algorithm for collaborative care planning using the participation and environment measure for children and youth (PEM‐CY): a mixed methods study. Phys Occup Ther Pediatr. 2015;35(3):231‐252. 10.3109/01942638.2014.899288 [DOI] [PubMed] [Google Scholar]
- 118. Knapp C, Madden V, Baron‐Lee J, et al. Florida Pediatric Medical Home Demonstration Project Evaluation 2011. Institute for Child Health Policy at the University of Florida; 2020. https://www.healthmanagement.com/wp-content/uploads/florida-pediatric-medical-home-demonstration-report-year-4.pdf [Google Scholar]
- 119. Kolko DJ, Campo J, Kilbourne AM, Hart J, Sakolsky D, Wisniewski S. Collaborative care outcomes for pediatric behavioral health problems: a cluster randomized trial. Pediatrics. 2014;133(4):e981‐e992. 10.1542/peds.2013-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kolko DJ. Doctor‐office collaborative care for pediatric behavioral problems: a preliminary clinical trial. Arch Pediatr Adolesc Med. 2012;166(3):224‐231. 10.1001/archpediatrics.2011.201 [DOI] [PubMed] [Google Scholar]
- 121. May J, Kazee N, Castillo S, et al. From silos to an innovative health care delivery and patient engagement model for children in Medicaid. Healthcare. 2018;6(1):67‐73. 10.1016/j.hjdsi.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 122. National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Children and Young People: Recognition and Management (NICE Guideline CG155). NICE; 2021. https://www.nice.org.uk/guidance/cg155 [PubMed] [Google Scholar]
- 123. National Institute for Health and Care Excellence. How we Develop NICE Guidelines. NICE; 2021. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/how-we-develop-nice-guidelines [Google Scholar]
- 124. Nkoy FL, Stone BL, Fassl BA, et al. Development of a novel tool for engaging children and parents in asthma self‐management. Annu Symp Proc. 2012;2012:663‐672. [PMC free article] [PubMed] [Google Scholar]
- 125. Nuss S, Camayd‐Muñoz C, Jonas R, et al. Defining the virtual patient‐centered medical home. Clin Pediatr. 2018;57(13):1592‐1596. 10.1177/0009922818793355 [DOI] [PubMed] [Google Scholar]
- 126. Parikh K, Hinds PS, Teach SJ. Using stakeholder engagement to develop a hospital‐initiated, patient‐centered intervention to improve hospital‐to‐home transitions for children with asthma. Hosp Pediatr. 2019;9(6):460‐463. 10.1542/hpeds.2018-0261 [DOI] [PubMed] [Google Scholar]
- 127. Patel MR, Thomas LJ, Hafeez K, Shankin M, Wilkin M, Brown RW. Study protocol for improving asthma outcomes through cross‐cultural communication training for physicians: a randomized trial of physician training. BMC Med Educ. 2014;14:118. 10.1186/1472-6920-14-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruland CM, Slaughter L, Starren J, Vatne TM, Moe EY. Children's contributions to designing a communication tool for children with cancer. Stud Health Technol Inform. 2007;129(2):977‐982. [PubMed] [Google Scholar]
- 129. Sleath B, Carpenter DM, Davis SA, et al. Acceptance of a pre‐visit intervention to engage teens in pediatric asthma visits. Patient Educ Couns. 2017;100(11):2005‐2011. 10.1016/j.pec.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sleath B, Carpenter DM, Lee C, et al. The development of an educational video to motivate teens with asthma to be more involved during medical visits and to improve medication adherence. J Asthma. 2016;53(7):714‐719. 10.3109/02770903.2015.1135945 [DOI] [PubMed] [Google Scholar]
- 131. Villegas V, Bosak D, Kaelin V, et al. Leveraging technology for family‐centered and participation‐focused care planning: a feasibility and pilot study of Participation and Environment Measure‐Plus (PEM+). Am J Occup Ther. 2020;74(S1):7411515374p1. 10.5014/ajot.2020.74S1-PO3119 [DOI] [Google Scholar]
- 132. Vohra S, Schlegelmilch M, Jou H, et al. Comparative effectiveness of pediatric integrative medicine as an adjunct to usual care for pediatric inpatients of a North American tertiary care centre: a study protocol for a pragmatic cluster controlled trial. Contemp Clin Trials Commun. 2017;5:12‐18. 10.1016/j.conctc.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dinleyici M, Sahin Dagli F. Evaluation of quality of life of healthy siblings of children with chronic disease. Türk Pediatri Arş. 2019;53(4):205‐213. 10.5152/TurkPediatriArs.2018.6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Committee on Quality of Health Care in America, Institute of Medicine (US). Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. [PubMed] [Google Scholar]
- 135. Miller R, Tumin D, Hayes D, Uffman JC, Raman VT, Tobias JD. Unmet need for care coordination among children with special health care needs. Popul Health Manag. 2019;22(3):255‐261. 10.1089/pop.2018.0094 [DOI] [PubMed] [Google Scholar]
- 136. Turchi RM, Antonelli RC, Norwood KW, et al. Patient‐ and family‐centered care coordination: a framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451‐e1460. 10.1542/peds.2014-0318 [DOI] [PubMed] [Google Scholar]
- 137. Walton H, Simpson A, Ramsay AIG, et al. Development of models of care coordination for rare conditions: a qualitative study. Orphanet J Rare Dis. 2022;17(1):49. 10.1186/s13023-022-02190-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Primary Care Collaborative. Defining the Medical Home: A Patient‐Centered Philosophy that Drives Primary Care Excellence. Primary Care Collaborative; 2022. https://www.pcpcc.org/about/medical-home [Google Scholar]
- 139. Zanello E, Calugi S, Rucci P, et al. Continuity of care in children with special healthcare needs: a qualitative study of family's perspectives. Ital J Pediatr. 2015;41(1):7. 10.1186/s13052-015-0114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Belcher RH, Phillips J, Virgin F, et al. Pediatric otolaryngology telehealth in response to covid‐19 pandemic: lessons learned and impact on the future management of pediatric patients. Ann Otol Rhinol Laryngol. 2021;130(7):788‐795. 10.1177/0003489420976163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Howie F, Kreofsky BL, Ravi A, Lokken T, Hoff MD, Fang JL. Rapid rise of pediatric telehealth during Covid‐19 in a large multispecialty health system. Telemed e‐Health. 2022;28(1):3‐10. 10.1089/tmj.2020.0562 [DOI] [PubMed] [Google Scholar]
- 142. Johnson C, Dupuis JB, Goguen P, Grenier G. Changes to telehealth practices in primary care in New Brunswick (Canada): a comparative study pre and during the COVID‐19 pandemic. PLoS One. 2021;16(11):e0258839. 10.1371/journal.pone.0258839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Davis J, NeSmith A, Perkins R, et al. Patient and family perceptions of telehealth as part of the cystic fibrosis care model during COVID‐19. J Cyst Fibros. 2021;20(3):e23‐e28. 10.1016/j.jcf.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mörelius E, Robinson S, Arabiat D, Whitehead L. Digital interventions to improve health literacy among parents of children aged 0 to 12 years with a health condition: systematic review. J Med Internet Res. 2021;23(12):e31665. 10.2196/31665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Murphy A, Pinkerton LM, Bruckner E, Risser HJ. The impact of the novel coronavirus disease 2019 on therapy service delivery for children with disabilities. J Pediatr. 2021;231:168‐177.e1. 10.1016/j.jpeds.2020.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Waqar‐Cowles LN, Chuo J, Weiss PF, Gmuca S, LaNoue M, Burnham JM. Evaluation of pediatric rheumatology telehealth satisfaction during the COVID‐19 pandemic. Pediatr Rheumatol. 2021;19(1):170. 10.1186/s12969-021-00649-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Romain CV, Trinidad S, Kotagal M. The effect of social determinants of health on telemedicine access during the COVID‐19 pandemic. Pediatr Ann. 2022;51(8):e311‐e315. 10.3928/19382359-20220606-04 [DOI] [PubMed] [Google Scholar]
- 148. Turnbull S, Cabral C, Hay A, Lucas PJ. Health equity in the effectiveness of web‐based health interventions for the self‐care of people with chronic health conditions: systematic review. J Med Internet Res. 2020;22(6):e17849. 10.2196/17849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Higgins JPT, Thomas J, Chandler J, et al. eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. 10.1002/9781119536604 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


