Graphical abstract
Keywords: Headspace–gas chromatography–ion mobility spectrometry, Flavor characteristics, Relative odor active values, Multivariate statistical analysis, Nutrient compositions, Byproduct utilization
Highlights
-
•
Volatiles and nutrition value of different chopped pepper seeds were firstly analyzed.
-
•
A total of 53 volatiles were identified by GC-IMS.
-
•
13 key volatiles were proposed to discriminate different chopped pepper seeds.
-
•
Chopped pepper seeds are rich in minerals, fatty acids and amino acids.
-
•
VOCs are closely correlated with amino acids and fatty acids by correlation analysis.
Abstract
Fermented-chopped pepper is a widely consumed condiment in China due to its attractive flavor. Chopped pepper seed (CPS) is the byproduct generated during the production of chopped pepper and is generally discarded as waste. In this study, the volatile organic compounds (VOCs) and nutritional value of three varieties of CPS were investigated. Results indicated that the nutritional compositions of the three CPS varieties exhibited significant differences. All CPS samples contained 17 amino acids and were rich in fatty acids, with unsaturated fatty acids being predominant and accounting for 79 % of the total fatty acids. A total of 53 VOCs were identified by gas chromatography–ion mobility spectrometry, which could be classified into 9 groups, with aldehydes, esters, and alcohols comprising the three largest groups. The three varieties of CPS had remarkably varied aromas whereas there are five key VOCs (i.e., 2-pentylfuran, methional, ethyl 3-methylbutanoate, dimethyl disulfide, and nonanal) in all CPS samples. Network correlation analysis revealed that VOCs are closely correlated with amino and fatty acids. Thus, this study provides a useful basis for understanding the nutritional values and flavor characteristics of different CPS varieties, which could be used as an ingredient and might have great potential in the food industry.
Introduction
Pepper (Capsicum annuum L.) belongs to the Solanaceae family. As a global vegetable, it ranks as the third-largest vegetable crop after legumes and tomato, and it is also an important condiment. China has an extensive pepper planting area, with reports indicating that the pepper planting area in the country reached 2 million hectares in 2019, which accounted for approximately 55 % of the world’s total pepper planting area. The total pepper output of China reached 40 million tons, which accounted for nearly 65 % of the world’s total output (Peng et al., 2019). Fermented-chopped pepper is one of the popular pepper processing products, which is widely consumed in China and received great interest worldwide due to its appealing aroma and nutritional value. To date, numerous reports on the aroma of pepper have been published (Lee et al., 2018, Li et al., 2023, Mi et al., 2022). The main types of volatile substances in chopped pepper include hydrocarbons, esters, alcohols, aldehydes, acids, ketones, and other compounds (Wang et al., 2019). The flavor components in chopped pepper are mainly flower and fruit aromas, as well as wood flavor. Unlike regular pepper, chopped pepper displays many distinct flavor substances due to microbial fermentation. Some flavors have been shown to be greatly connected with the core microbial structures in fermented samples (Xu et al., 2021).
The rapid development of the chopped pepper processing industry in recent years have facilitated the generation of a large amount of byproduct chopped pepper seeds (CPS) every year. The CPS are produced during the manufacturing of fermented-chopped pepper as follows: red pepper → cleaning and chopping → salting → fermentation → chopped pepper → production of a large amount of CPS (Liu et al., 2020). However, only a small part of CPS is used as animal feed (Cvetković et al., 2022); most of them are not effectively used and treated as solid waste, which results in environmental pollution and waste of resources. This situation simultaneously increases the load on the industry for waste treatment. Our previous work found that CPS are rich in phenolics, capsaicinoid compounds (e.g., capsaicin, dihydrocapsaicin, and nordihydrocapsaicin) and showed strong α-glucosidase inhibitory activity and antioxidant activity, which might have potential to be developed as a new nutraceutical ingredient (Liu et al., 2020). In addition, it is illustrated that the production of CPS involves fermentation, during which many microorganisms such as Pseudomonas, Rosenbergiella, Staphylococcus, Hyphopichia, and Kodamaea are involved in this process (Xu et al., 2021). Microorganisms can transform and break down macromolecules (e.g., protein, fat, and carbohydrates) (Lee et al., 2018). Thus, they might produce abundant volatile organic compounds (VOCs) and nutritional constituents in CPS. Given the urgent need for flavor compounds of natural origin to improve the flavor of food products, CPS can be used as a natural flavoring in the food industry to improve the quality of products. Thus, understanding what flavor components are contained in CPS is important, which is conducive to the high-value utilization of CPS. However, as far as we know, no studies are available on the flavor compounds and nutritional composition of CPS.
Headspace–gas chromatography–ion mobility spectrometry (HS–GC–IMS) is a powerful technique for the separation and sensitive detection of VOCs. It has a fast response, high sensitivity, easy operation, and low cost (Wang et al., 2020, Xiao et al., 2021). GC–IMS has been widely used in the past few years due to its outstanding ability in powerful separation and sensitive detection of VOCs, which provide easy work on food classification and adulteration, the evaluation of food freshness and spoilage, and monitoring the processing of food products, among other applications. For example, this technology has been widely used in chili sauces and other products to distinguish different varieties of samples (Chen et al., 2021). Furthermore, the result of Mi et al. (2022) indicated that GC–IMS can effectively reflect the flavor profile of chili peppers. Thus, this HS–GC–IMS technique could comprehensively analyze the flavor characteristics of CPS.
Thus, this study aimed to investigate the flavor characteristics and nutritional components (proximate composition, minerals, amino acids, and fatty acids) of three different types of CPS in various chopped pepper manufacturing factories. HS–GC–IMS, integrated with multivariate statistical analysis, was performed to systematically characterize and discriminate the VOCs of different CPS. The findings of this study are expected to present novel value for CPS, which makes them useful as ingredients in food products. Furthermore, these efforts may be advantageous for waste treatment and present a new possibility to assist sustainable development from an environmental and economic standpoint.
Materials and methods
Chemicals and materials
Three varieties of CPS, namely, bright-red pepper seed (BRPS), liner pepper seed (LPS), and wild pepper seed (WPS), were acquired from three different chopped pepper production companies as mentioned in our previous study (Liu et al., 2020). For further analysis, the collected CPS samples were first lyophilized and then crushed by an electric mill. Subsequently, a 60-mesh sieve was used to refine the resulting flour. Fatty acid and mineral analysis standards were obtained from Merck, Germany. Amino acid analysis standards were sourced from Sigma–Aldrich, USA. N-ketones C4–C9 were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd., China. All other chemicals and reagents were of analytical grade.
Proximate composition analysis
The proximate composition, including ash, crude fat, and crude protein, was determined based on AOAC methods (2005). The determination of crude protein was conducted using the Kjeldahl method. In this procedure, 0.2 g of pepper seed powder was placed into a digestion tube, to which 12 mL of 98 % concentrated sulfuric acid and a digestive stone were added. The sample was the digested at 420 °C for 1.5 h, cooled to room temperature, and subsequently evaluated with an automatic nitrogen analyzer. The content of crude fat was assessed using a Soxhlet system. The carbohydrate content in dry matter was calculated by subtracting the sum of crude fat, crude protein, and ash from 100. The results were reported as g/100 g dry matter.
Determination of amino acid composition
The amino acid composition assay was conducted using an automatic amino acid analyzer (HITACHI L-8900, Hitachi Ltd., Japan), following the method by Xiao et al. (2021). Briefly, CPS samples were accurately weighed (0.2 g) and added with 10 mL of hydrochloric acid solution (ultrapure water: analytical concentrated hydrochloric acid = 1:1 (v/v)), followed by incubation in a 110 °C blast drying oven for 22 h. After cooling to room temperature, hydrolysates were filtered into a 50 mL volumetric bottle, and the hydrolysis tube and filter paper were washed three times with ultrapure water. The washing solutions were collected into the volumetric flask, and the volume was adjusted to 50 mL. Subsequently, 0.2 mL of the sample was placed in a dish and dried in a 60 °C water bath, followed by dissolution with 2 mL of sample buffer. The mixture was then filtered with a 0.22 μm filter and analyzed in an automatic amino acid analyzer. The results were reported as mg/g dry matter.
Fatty acid composition assay
The fatty acid composition of CPS was evaluated following the method by Koncsek et al. (2018). Briefly, lipids were first extracted using a Soxhlet extractor and then transesterified into methyl esters. A GC-2010 Shimadzu GC (Tokyo, Japan) was used to determine the fatty acid composition. Fatty acids were qualitatively identified based on the retention time of an authentic standard mixture of methyl esters, and the relative amounts of each component were estimated from the peak area.
Determination of mineral composition
Mineral preparation was based on a previously reported method (Xiao et al., 2021). Briefly, 0.50 g of CPS was accurately weighed in the polytetrafluoroethylene digestion tank, and 2 mL of 65 % concentrated HNO3 was added. The mixture stood for 15 min in the fume hood and then sealed after the reaction was completed. The samples were digested in a microwave digestion instrument, transferred to a volumetric flask (25 mL), and made up to volume (50 mL) with distilled water. The major elements (P, K, Ca, Na, and Mg) and trace elements (Se, Mn, Zn, Fe, and Cu) were evaluated by atomic absorption spectrometry (AA 800, Perkin-Elmer Germany). Standard solution curves for different concentrations of each element were also determined using the same method.
Analysis of VOCs using HS–GC–IMS
The VOCs of CPS were evaluated according to the method by Mi et al. (2022) and performed on a GC–IMS instrument (Flavourspec ®, G.A.S, Dortmund, Germany) equipped with an MXT-5 capillary column (15 m × 0.53 mm × 1 μm). Briefly, 2.0 g of CPS was directly transferred into a 20 mL headspace vial, which was subsequently incubated at 60 °C for 15 min at an agitation speed of 500 rpm. After incubation, 300 μL of headspace was automatically injected into the injector by means of a heated syringe at 65 °C. High-purity nitrogen (purity ≥99.99 %) was employed as the carrier gas using the following programmed flow: starting at 2 mL/min for 2 min, and raised to 100 mL/min within 18 min. The drift tube was 9.8 cm, with a drift gas flow (nitrogen, purity ≥99.99 %) rate of 150 mL/min. The temperatures of the column and IMS were kept at 60 and 45 °C, respectively. Each sample was determined thrice in parallel. N-ketones C4–C9 were used as external references to calculate the retention index (RI) of VOCs. VOCs were tentatively identified by comparing RI and the drift time of standards in the GC–IMS library database obtained from G.A.S (Dortmund, Germany).
Relative odor activity value analysis
The relative odor activity value (ROAV), which measures the contributions of certain flavor components, are frequently utilized. A high ROAV suggests that a component made a significant impact to the sample’s overall flavor (Huang et al., 2023). Components with 0.1 ≤ ROAV < 1 have a significant modifying influence on the sample’s overall odor, while components with ROAV ≥ 1 are often regarded as essential aroma compounds for the samples. The formula used to calculate the ROAV for a VOC (i) is ROAVi = (OAVi/OAVmax) × 100. OAVi = Ci/OTi, and thus, ROAVi can be calculated as (Ci/OTi) × (OTmax/Cmax) × 100, where “max” is the VOC with the greatest value determined by dividing the relative content compound by the odor threshold concentration among the identified volatile compounds. OAVmax is the compound’s (“max”) odor activity value, which is equal to 100. Cmax is the compound’s (“max”) relative content, and OTmax is the compound’s (“max”) odor threshold. OAVi stands for a compound’s odor activity value, Ci indicates its relative concentration, and OTi indicates its odor threshold (Xiao et al., 2022).
Statistical analysis
All experiments were conducted three times, and data were reported as mean ± standard deviation. Statistical differences (p < 0.05) between the means were determined using SPSS 20.0 software with Duncan’s multiple range test. Multivariate statistical analyses, including principal component analysis (PCA), hierarchical cluster analysis (HCA), and variable importance factor (VIP), were performed by SIMCA-P + 12.0 software (Umea, Sweden). TBtools and Cytoscape software (version 3.5.1) were used to generate heatmaps and network plots. Reporter plug-in and Laboratory Analytical Viewer software (Dortmund, Germany) were employed to plot the GC–IMS data.
Results and discussion
Proximate composition
The proximate composition of three varieties of CPS is shown in Table 1a. The carbohydrate content in CPS ranged from 55.42 % to 67.92 %, which constituted more than 50 % of the dry matter. Carbohydrates are the main nutrient in CPS, and their level significantly exceeds those found in the eight kinds of CPS studied by Ma et al. (2017). This difference may be closely related to the type and growth environment of pepper. A significantly different content of carbohydrates was observed in the three kinds of CPS, with the lowest in LPS, followed by BRPS, and the highest in WPS. The carbohydrates in pepper seeds are mainly insoluble dietary fiber (Cvetković et al., 2022), with Capsicum annum seeds showing a high level of dietary fiber (60.96 %). Insoluble dietary fiber has the function of promoting gastrointestinal peristalsis and preventing constipation, which make it valuable for development and utilization as health food. The content of crude protein in pepper seeds ranged from 12.96 % to 16.09 %. BRPS had a significantly lower crude protein content than WPS and LPS. The crude fat content of CPS was in the range of 13.07 %–17.29 %. WPS had the lowest and BRPS had the highest crude fat content. The ash content in CPS ranged from 3.12 % to 12.54 %, and WPS had a significantly lower ash content than LPS and BRPS.
Table 1a.
Proximate composition (g/100 g) and mineral elements content (mg/Kg) of three varieties of chopped pepper seeds.
| WPS | LPS | BRPS | |
|---|---|---|---|
| Proximate composition (g/100 g) | |||
| Crude protein | 15.88 ± 0.44a | 16.09 ± 0.59a | 12.96 ± 0.20b |
| Crude fat | 13.07 ± 0.42c | 15.95 ± 0.41b | 17.29 ± 0.07a |
| Ash | 3.12 ± 0.19c | 12.54 ± 0.20a | 11.93 ± 0.35b |
| Carbohydrate | 67.92 ± 0.85a | 55.42 ± 0.84c | 57.83 ± 0.41b |
| Mineral elements content (mg/Kg) | |||
| Na | 1178.67 ± 149.74c | 22063.47 ± 1145.64b | 25462.83 ± 1270.28a |
| Ca | 460.00 ± 24.98a | 388.00 ± 25.53b | 389.00 ± 13.45b |
| K | 9213.33 ± 442.87a | 4526.67 ± 234.38b | 3186.67 ± 197.57c |
| P | 3410.00 ± 155.24a | 1220.00 ± 72.11c | 2786.67 ± 190.35b |
| Mg | 1780.00 ± 90.00b | 2012.33 ± 87.16a | 1418.00 ± 91.02c |
| Zn | 14.33 ± 0.40a | 13.23 ± 0.35b | 11.80 ± 0.56c |
| Cu | 7.17 ± 0.15b | 7.42 ± 0.11b | 9.09 ± 0.56a |
| Fe | 68.77 ± 6.37a | 44.53 ± 4.41b | 34.20 ± 1.85c |
| Mn | 40.47 ± 1.78a | 11.97 ± 0.65b | 10.83 ± 0.31b |
| Se | 0.046 ± 0.004a | 0.032 ± 0.004b | 0.021 ± 0.002c |
Data marked with different letters in a row show significant differences at p < 0.05.
Mineral composition
The mineral composition in CPS is also shown in Table 1a. A total of 10 essential mineral elements were measured in this study, which serve as cofactors of many metabolisms in the human body. These elements are important components of blood and bone in maintaining osmotic pressure and acid-base balance. The results show that CPS contained macronutrients, such as potassium, magnesium, sodium, phosphorus, and calcium. WPS showed a “high potassium and low sodium” profile, however, LPS and BRPS displayed a “high sodium and low potassium” attribute. Potassium and sodium play an important role in maintaining cellular osmotic pressure homeostasis in the human body, and significant differences in sodium were observed among the three types of CPS. During the processing of chopped pepper, high-salt pickling is necessary. The sodium chloride content in high-salt pepper produced by enterprises can vary significantly (Peng et al., 2023). Therefore, the “high potassium and low sodium” profile of WPS is presumably due to the lower salt content added during the processing of chopped pepper than that of the two other varieties. All three types of CPS contained trace elements such as iron, copper, zinc, manganese, and selenium, among which iron was the main element (34.20–68.77 mg/Kg). Iron promotes hemoglobin synthesis and plays an important role in oxygen transport in the human body. Table 1a reveals a drastic difference in the mineral composition of the three CPS, which may be related to the different varieties, growth environment, and the production and processing conditions.
Amino acid composition
A total of 17 amino acids had been identified in CPS samples (Table 1b and Fig. S1), including 10 essential amino acids: phenylalanine, isoleucine, tryptophan, leucine, lysine, methionine, threonine, valine, tyrosine, and cysteine. These CPS have a special cysteine compared with the amino acids in the eight varieties of pepper seeds previously reported (Ma et al., 2017). The protein in CPS is gradually degraded into amino acids by microorganisms, which results in a raise in the types of amino acids in CPS (Xiao et al., 2023). Leucine had the highest content among the essential amino acids, which ranged from 8.30 mg/g to 10.31 mg/g, as shown in Table 1b. Seven kinds of non-essential amino acids were found, namely, glutamic acid, arginine, serine, alanine, aspartic acid, glycine, and proline. Glutamic acid had the highest content among the amino acids in the three CPS, and it ranged from 24.38 mg/g to 36.68 mg/g. Glutamic acid, aspartic acid, glycine, alanine, and serine have a great impact on the taste of chopped pepper. The sodium salt formed by the interaction of glutamic acid and aspartic acid with salt could enhance the umami of fermented pepper (Li et al., 2023). In food nutrition, essential amino acids have always been of interest. According to the data analysis, the ratios of essential amino acids to total amino acids in CPS were 40.00 %, 38.41 %, and 39.77 %. WPS had the highest content of essential amino acids. The ratio of essential amino acids to total amino acids in red pepper has been shown to be 28.88 % in a previous study (Fei et al., 2022). Essential amino acids are crucial for humans, as the body cannot synthesize them. The findings demonstrate that CPS are rich in amino acids, particularly the eight essential amino acids necessary for the human body.
Table 1b.
Amino acid compositions of three varieties of chopped pepper seeds.
| WPS |
LPS |
BRPS |
||||
|---|---|---|---|---|---|---|
| content (mg/g) | Relative content | content (mg/g) | Relative content | content (mg/g) | Relative content | |
| Non-essential amino acids | ||||||
| Aspartic (Asp) | 15.58 ± 0.82a | 11.07 % | 14.22 ± 0.48b | 9.36 % | 12.24 ± 0.52c | 10.56 % |
| Serine (Ser) | 6.81 ± 0.30a | 4.84 % | 6.79 ± 0.20a | 4.47 % | 5.58 ± 0.45b | 4.81 % |
| Glutamic (Glu) | 28.50 ± 0.53b | 20.24 % | 36.68 ± 1.23a | 24.16 % | 24.38 ± 1.30c | 21.05 % |
| Glycine (Gly) | 7.41 ± 0.43a | 5.26 % | 7.71 ± 0.34a | 5.08 % | 6.17 ± 0.43b | 5.33 % |
| Alanine (Ala) | 6.80 ± 0.20a | 4.83 % | 6.85 ± 0.22a | 4.51 % | 5.78 ± 0.41b | 4.99 % |
| Arginine (Arg) | 13.30 ± 0.54b | 9.45 % | 15.17 ± 1.01a | 9.99 % | 10.57 ± 0.50c | 9.28 % |
| Proline (Pro) | 6.08 ± 0.38a | 4.32 % | 6.11 ± 0.70a | 4.03 % | 4.89 ± 0.53b | 4.22 % |
| Essential amino acids | ||||||
| Threonine (Thr) | 6.03 ± 0.23a | 4.28 % | 6.01 ± 0.28a | 3.96 % | 4.78 ± 0.33b | 4.13 % |
| Cysteine (Cys) | 4.59 ± 0.05b | 3.26 % | 5.26 ± 0.38a | 3.46 % | 4.13 ± 0.11c | 3.57 % |
| Valine (Val) | 7.56 ± 0.55a | 5.37 % | 7.60 ± 0.63a | 5.01 % | 6.01 ± 0.27b | 5.19 % |
| Methionine (Met) | 1.00 ± 0.05b | 0.71 % | 1.22 ± 0.06a | 0.80 % | 0.67 ± 0.04c | 0.58 % |
| Isoleucine (Ile) | 5.68 ± 0.41a | 4.03 % | 5.47 ± 0.20a | 3.61 % | 4.26 ± 0.46b | 3.68 % |
| Leucine (Leu) | 10.26 ± 0.45a | 7.29 % | 10.31 ± 0.35a | 6.79 % | 8.30 ± 0.35b | 7.17 % |
| Tyrosine (Tyr) | 4.12 ± 0.06b | 2.92 % | 4.43 ± 0.06a | 2.91 % | 4.08 ± 0.07b | 3.52 % |
| Phenylalanine (Phe) | 7.10 ± 0.45ab | 5.05 % | 7.32 ± 0.40a | 4.82 % | 6.55 ± 0.20b | 5.65 % |
| Lysine (Lys) | 7.52 ± 0.43a | 5.34 % | 8.01 ± 0.51a | 5.27 % | 5.37 ± 0.53b | 4.63 % |
| Histidine (His) | 2.46 ± 0.04b | 1.74 % | 2.69 ± 0.09a | 1.77 % | 1.92 ± 0.16c | 1.66 % |
| Total essential amino acids | 56.32 ± 2.08a | 40.00 % | 58.31 ± 2.38a | 38.41 % | 46.08 ± 1.71b | 39.77 % |
| Total amino acid | 140.79 ± 1.38b | 151.82 ± 2.08a | 115.86 ± 2.19c | |||
The values followed by different letters in a line are significantly different at p < 0.05.
Fatty acid composition
Table 1c shows the fatty acid composition of the three CPS, and the GC is shown in Fig. S2. CPS had 18 fatty acids, including 10 unsaturated fatty acids and 8 saturated fatty acids. The fatty acid compositions of the three types of CPS were similar. Koncsek et al. (2018) demonstrated that the fatty acid composition of pepper seed oils remained consistent across different varieties and growing seasons. However, we found that WPS contained pentadecenoic acid and lacked arachidonic acid. On the contrary, LPS and BRPS contained arachidonic acid but lacked pentadecenoic acid. The main saturated fatty acids in the three CPS were stearic acid (1.763 %–3.156 %) and palmitic acid (12.036 %–14.703 %). The unsaturated fatty acid content was high, with all samples exceeding 79 % of the total fatty acids. The main monounsaturated fatty acids were oleic acid (7.143 %–9.339 %) and palmitoleic acid (0.295 %–0.371 %). The polyunsaturated fatty acids were primarily linoleic acid (69.138 %–75.220 %) and linolenic acid (0.299 %–0.554 %). Linoleic acid, which is a critical fatty acid for the human body, accounted for the highest proportion of total fatty acids in CPS at more than 69 % (Table 1c). Recent investigations have indicated that pepper seeds have the potential to be a valuable source of edible oil, fiber-rich flour, and protein after processing (Cvetković et al., 2022). Linoleic acid was reported that displayed favorable nutritional implications and beneficial physiological effects, including anticancer, lipid-lowering, anti-diabetic, and anti-inflammatory activities (Fontes et al., 2017), with a high-value development potential as a health food. Thus, CPS has a great potential of high-value utilization due to their high content of linoleic acid.
Table 1c.
Relative content of fatty acids in different varieties of chopped pepper seeds.
| Fatty acid (%) | WPS | LPS | BRPS | |
|---|---|---|---|---|
| c14:0 | Myristic acid | 0.197 ± 0.012a | 0.135 ± 0.008b | 0.134 ± 0.001b |
| c15:0 | Pentadecanoic acid | 0.041 ± 0.004a | 0.013 ± 0.012a | 0.040 ± 0.034a |
| c15:1 | Pentadecenoic acid | 0.032 ± 0.007a | – | – |
| c16:0 | Palmitic acid | 14.408 ± 0.151b | 12.036 ± 0.111c | 14.703 ± 0.054a |
| c16:1 | Palmitoleic acid | 0.371 ± 0.028a | 0.304 ± 0.013a | 0.295 ± 0.063a |
| c17:0 | Heptadecanoic acid | 0.118 ± 0.003a | 0.093 ± 0.002c | 0.106 ± 0.004b |
| c17:1 | Heptadecenoic acid | 0.040 ± 0.002a | 0.046 ± 0.009a | 0.076 ± 0.036a |
| c18:0 | Stearic acid | 3.156 ± 0.065a | 2.007 ± 0.074b | 1.763 ± 0.004c |
| c18:1n9 | Oleic acid | 9.339 ± 0.232a | 7.265 ± 0.203b | 7.143 ± 0.077b |
| c18:2n6 | Linoleic acid | 69.138 ± 0.478c | 75.220 ± 0.690a | 72.633 ± 0.131b |
| c20:0 | Eicosanoic acid | 0.510 ± 0.015a | 0.285 ± 0.007b | 0.278 ± 0.004b |
| c20:1 | Eicosenoic acid | 0.103 ± 0.005a | 0.108 ± 0.005a | 0.113 ± 0.011a |
| c18:3n3 | Linolenic acid | 0.372 ± 0.009b | 0.299 ± 0.008c | 0.554 ± 0.010a |
| c20:2 | Eicosadienoic acid | 0.056 ± 0.001a | 0.064 ± 0.008a | 0.056 ± 0.003a |
| c22:0 | Behenic acid | 0.419 ± 0.004a | 0.294 ± 0.002c | 0.299 ± 0.002b |
| c20:3n3 | Arachidonic acid | – | 0.017 ± 0.015b | 0.046 ± 0.002a |
| c20:4n6 | Arachidonic acid | 0.053 ± 0.004a | 0.039 ± 0.004b | 0.049 ± 0.005a |
| c24:0 | Lignoceric acid | 0.491 ± 0.006a | 0.259 ± 0.007c | 0.284 ± 0.009b |
| Total saturated fatty acids | 19.340 ± 0.221a | 15.123 ± 0.163c | 17.607 ± 0.091b | |
| Total monounsaturated fatty acid | 9.884 ± 0.258a | 7.722 ± 0.228b | 7.627 ± 0.033b | |
| Total polyunsaturated fatty acid | 69.619 ± 0.483c | 75.639 ± 0.686a | 73.338 ± 0.135b | |
| Total unsaturated fatty acids | 79.503 ± 0.226c | 83.361 ± 0.458a | 80.965 ± 0.168b | |
Data marked with different letters show significant differences at p < 0.05.
–, not detected.
Analysis of VOCs in three varieties of CPS
Topographic plots of HS–GC–IMS results
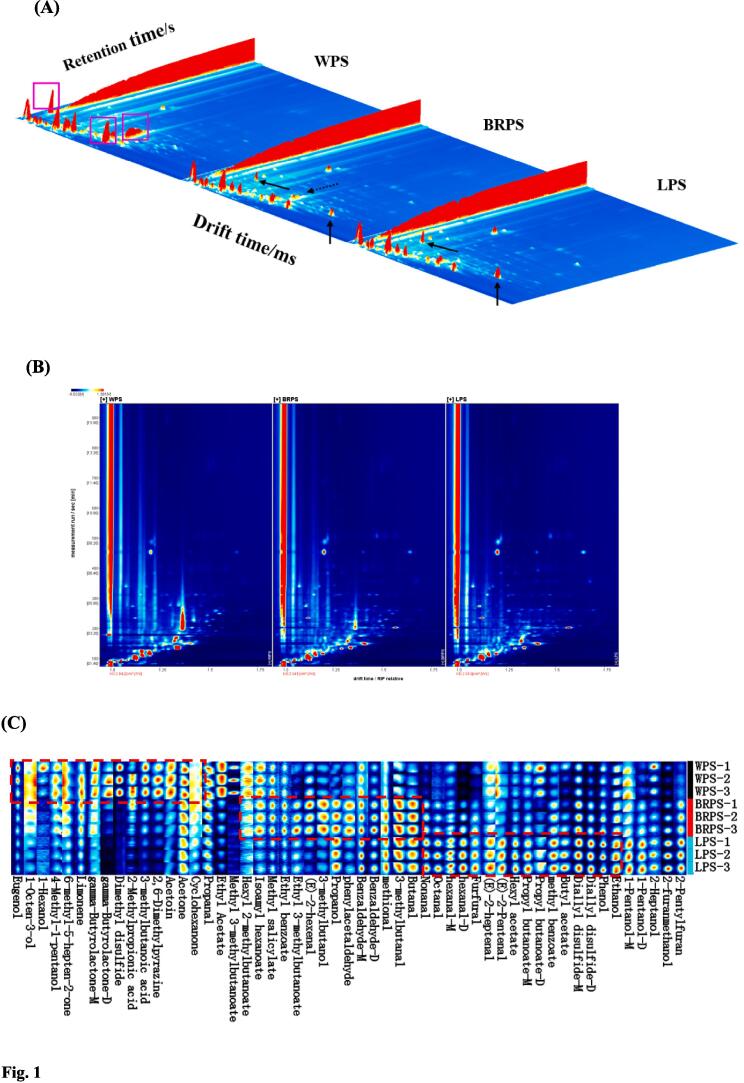
HS–GC–IMS is a powerful technique for the separation and sensitive detection of VOCs. It has a fast response, high sensitivity, easy operation, and low cost (Wang et al., 2020). Accordingly, HS–GC–IMS was conducted to clarify the variations in the VOCs of various CPS. A three-dimensional topographic picture of the VOCs in three CPS samples is shown in Fig. 1A. Some peaks in different CPS were clearly different, even though their VOCs profile were similar. This finding implies that the VOCs of the different varieties of CPS samples were substantially different. The VOCs specific to WPS, indicated by the red zone, were nearly nonexistent in the two other seeds. The substance displayed at the solid arrow in the diagram is specific to LPS and BRPS (Fig. 1A). BRPS had a higher concentration of these compounds than LPS. Fig. 1B shows the outcome of an overhead perspective used to more clearly observe the fluctuations in VOCs. Low and high amounts of VOCs are indicated in the sample by the colors blue and red, respectively. The majority of the signals had relative retention times (relative RIP) of 100–900 s and drift times of 1–1.75 s.
Fig. 1.
Three dimensional (A) and 2D top view (B) GC-IMS map of volatile components of chopped pepper seeds. (C) VOCs fingerprint comparisons of three varieties of chopped pepper seeds.
Variations in VOCs among CPS samples
The qualitative analysis of the VOCs, presented in topographic plots, is displayed in Supplementary Fig. S3. A total of 64 signal peaks were detected, and 53 typical VOCs were well characterized, including 14 esters, 15 aldehydes, 10 alcohols, 4 ketones, 3 thioethers, 2 acids, 2 hydrocarbons, 2 phenols, and 1 terpene in CPS. Owing to the limited information in the library databases, the remaining 11 peaks could not be identified (classified as the others). The identified 53 VOCs were evaluated in the form of a fingerprint plot (Fig. 1C), which could provide a summary difference among these CPS samples. The findings of the fingerprint analysis revealed significant variations in the VOCs of WPS, BRPS, and LPS. Table 2 lists detailed information on the 53 VOCs detected in the three CPS samples. These detected VOCs all had carbon chains between C2–C11, and some of them have been shown to produce several signals or spots (monomer or dimer) due to their various concentrations and properties (Xiao et al., 2022). These compounds include benzaldehyde, hexanal, 1-pentanol, gamma-butyrolactone, propyl-butanoate, and diallyl-disulfide. A heatmap is a way to reflect data information with color changes, which can visually represent the magnitude of data values by color shades. In the heat-map of GC–IMS data for three distinct types of CPS samples (Fig. 2C), each column represents a compound, each row represents a volatile component, and each cell represents a specific volatile component in a sample. The red color deepens with higher content of the relevant component in the corresponding sample, while the green hue darkens with lower amounts. The fingerprint and heatmap clearly show the drastic difference in the flavor profiles of the three varieties of CPS samples, with each sample exhibiting its own unique flavor composition. The contents of eugenol, 1-octen-3-ol, 6-methyl-5-hepten-2-one, limonene, γ-butyrolactone, dimethyl disulfide-M, 2-methylpropionic acid, 3-methylbutanoic acid, 2,6-dimethylpyrazine, acetoin, ethyl acetate, methyl 3-methylbutyrate, propanal, acetone, 4-methyl-1-pentanol, cyclohexanone, and hexyl 2-methylbutanoate in the WPS sample were higher than in the other samples. The contents of methyl salicylate, ethyl benzoate, ethyl 3-methylbutanoate, (E)-2-hexenal, 3-methylbutanol, phenylacetaldehyde, propanol, methional, 3-methylbutanal, butanal, and benzaldehyde were higher in BRPS than in WPS and LPS. The contents of nonanal, octanal, hexanal, furfural, (E)-2-heptenal, (E)-2-pentenal, hexyl acetate, propyl butanoate, methyl benzoate, butyl acetate, diallyl disulfide, phenol, 1-pentanol, 2-furanmethanol, 2-pentylfuran, and ethanol in the LPS sample were higher than in WPS and BRPS. These significant differences in VOCs among the three varieties of CPS may be due to factors such as the origin and variety of raw peppers and different production seasons of CPS. Niu et al. (2022) found through descriptive sensory analysis that the aroma intensities of pepper oil vary depending on the production area. Xiao et al. (2022) indicated that the aromatic components and volatile compounds of farmhouse chopped chili peppers differ significantly based on their production region in Hunan. The flavor and aroma intensity of the product are greatly affected by raw materials from different seasons and harvests, as suggested by Eggink et al. (2012). In addition, studies have reported that enhanced fermentation of pepper products using Kluyveromyces lactis and Lactobacillus plantarum resulted in significant differences in VOCs compared with naturally fermented pepper products (Tang et al., 2023). Notable variations in VOCs in chopped pepper were observed between products with sufficient fermentation (aw > 0.75) and those with slight fermentation (aw ≤ 0.75) (Peng et al., 2023). Therefore, the substantial differences in the VOCs of the three CPS samples could be attributed to factors such as pepper variety, different geographical production, and the fermentation method during the manufacturing of different chopped peppers.
Table 2.
GC–IMS integration parameters and peak intensity for the analysis of volatile components in three varieties of chopped pepper seeds by HS–GC–IMS.
| Compounds | Odor description | CAS | Formula | MW | RI | Rt [sec] | Dt [RIPrel] | peak intensity |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WPS | LPS | BRPS | |||||||||
| Aldehydes (15) | |||||||||||
| v1 | Nonanal | waxy aldehydic rose fresh orris orange peel fatty peely | 124-19-6 | C9H18O | 142.2 | 1111.3 | 505.72 | 1.47514 | 242.44 ± 8.43c | 751.71 ± 19.45a | 614.35 ± 24.70b |
| v2 | Phenylacetaldehyde | green sweet floral hyacinth clover honey cocoa | 122-78-1 | C8H8O | 120.2 | 1043.1 | 407.72 | 1.25135 | 55.89 ± 2.21c | 69.52 ± 1.99b | 111.74 ± 5.09a |
| v3 | Octanal | aldehydic waxy citrus orange peel green herbal fresh fatty | 124-13-0 | C8H16O | 128.2 | 1007.3 | 356.13 | 1.40466 | 83.98 ± 5.77c | 175.52 ± 2.16a | 135.01 ± 5.49b |
| v4 | Benzaldehyde-M | strong sharp sweet bitter almond cherry | 100-52-7 | C7H6O | 106.1 | 963.1 | 312.96 | 1.15351 | 595.93 ± 21.26a | 519.19 ± 34.07b | 569.93 ± 42.09ab |
| v5 | Benzaldehyde-D | strong sharp sweet bitter almond cherry | 100-52-7 | C7H6O | 106.1 | 962.0 | 312.08 | 1.47622 | 122.05 ± 11.31b | 164.75 ± 17.10a | 177.69 ± 22.02a |
| v6 | (E)-2-heptenal | pungent green vegetable fresh fatty | 18829-55-5 | C7H12O | 112.2 | 957.3 | 308.16 | 1.25734 | 56.25 ± 6.43b | 100.78 ± 8.86a | 64.53 ± 6.56b |
| v7 | (E)-2-hexenal | green banana aldehydic fatty cheesy | 6728-26-3 | C6H10O | 98.1 | 846.2 | 231.10 | 1.52364 | 223.80 ± 12.66b | 150.58 ± 27.17c | 294.98 ± 33.95a |
| v8 | Furfural | sweet woody almond fragrant baked bread | 98-01-1 | C5H4O2 | 96.1 | 826.2 | 221.09 | 1.08825 | 94.88 ± 26.04b | 258.63 ± 20.24a | 207.10 ± 47.90a |
| v9 | Hexanal-M | fresh green fatty aldehydic grass leafy fruity sweaty | 66-25-1 | C6H12O | 100.2 | 788.1 | 201.95 | 1.2548 | 146.97 ± 18.71c | 334.78 ± 20.45a | 221.30 ± 24.28b |
| v10 | Hexanal-D | fresh green fatty aldehydic grass leafy fruity sweaty | 66-25-1 | C6H12O | 100.2 | 789.8 | 202.84 | 1.57085 | 163.37 ± 53.99c | 1771.28 ± 204.17a | 1029.04 ± 223.80b |
| v11 | 3-Methylbutanal | ethereal aldehydic chocolate peach fatty | 590-86-3 | C5H10O | 86.1 | 622.5 | 148.36 | 1.41348 | 1497.18 ± 4.42c | 2203.28 ± 46.06b | 2365.84 ± 30.94a |
| v12 | Butanal | pungent cocoa musty green malty bready | 123-72-8 | C4H8O | 72.1 | 553.4 | 132.76 | 1.29414 | 425.82 ± 8.12c | 729.14 ± 6.69b | 782.69 ± 21.20a |
| v13 | Propanal | earthy alcohol wine whiskey cocoa nutty | 123-38-6 | C3H6O | 58.1 | 467.4 | 113.33 | 1.16169 | 3194.37 ± 44.55a | 2495.98 ± 3.03c | 2626.67 ± 27.36b |
| v14 | Methional | musty potato tomato earthy vegetable creamy | 3268-49-3 | C4H8OS | 104.2 | 908.8 | 267.92 | 1.09338 | 115.57 ± 7.95b | 164.53 ± 11.04a | 157.67 ± 7.11a |
| v15 | (E)-2-Pentenal | pungent green fruity apple orange tomato | 1576-87-0 | C5H8O | 84.1 | 741.6 | 183.64 | 1.36809 | 267.27 ± 45.02b | 375.07 ± 8.96a | 227.99 ± 20.54b |
| Alcohols (10) | |||||||||||
| v16 | 1-Octen-3-ol | mushroom earthy green oily fungal raw chicken | 3391-86-4 | C8H16O | 128.2 | 987.3 | 333.02 | 1.16333 | 149.21 ± 16.89b | 205.73 ± 19.98a | 175.25 ± 3.97ab |
| v17 | 2-Heptanol | fresh lemon grass herbal sweet floral fruity green | 543-49-7 | C7H16O | 116.2 | 879.7 | 247.88 | 1.71118 | 72.70 ± 8.40a | 63.24 ± 1.75ab | 51.10 ± 6.84b |
| v18 | 2-Furanmethanol | alcoholic chemical musty sweet caramel bread coffee | 98-00-0 | C5H6O2 | 98.1 | 853.8 | 234.93 | 1.12497 | 347.48 ± 83.86c | 1338.91 ± 84.47a | 864.77 ± 144.62b |
| v19 | 1-Hexanol | ethereal fusel oil fruity alcoholic sweet green | 111-27-3 | C6H14O | 102.2 | 870.3 | 243.17 | 1.63774 | 49.20 ± 10.33a | 25.68 ± 6.33b | 22.80 ± 4.56b |
| v20 | 4-Methyl-1-pentanol | nutty | 626-89-1 | C6H14O | 102.2 | 830.9 | 223.45 | 1.31644 | 351.23 ± 10.08a | 265.00 ± 20.50b | 345.86 ± 33.60a |
| v21 | 1-Pentanol-M | fusel oil sweet balsam | 71-41-0 | C5H12O | 88.1 | 760.1 | 190.76 | 1.2548 | 150.07 ± 18.35b | 177.01 ± 3.77a | 152.29 ± 4.27b |
| v22 | 1-Pentanol-D | fusel oil sweet balsam | 71-41-0 | C5H12O | 88.1 | 760.9 | 191.06 | 1.50922 | 69.27 ± 0.93c | 145.11 ± 1.25a | 115.12 ± 7.18b |
| v23 | 3-Methylbutanol | fusel oil alcoholic whiskey fruity banana | 123-51-3 | C5H12O | 88.1 | 721 | 175.75 | 1.49217 | 125.48 ± 5.36b | 132.74 ± 3.92b | 278.08 ± 15.13a |
| v24 | Propanol | alcoholic fermented fusel musty | 71-23-8 | C3H8O | 60.1 | 532.5 | 128.05 | 1.25087 | 235.44 ± 35.64b | 422.74 ± 14.11a | 376.86 ± 18.27a |
| v25 | Ethanol | strong alcoholic ethereal medical | 64-17-5 | C2H6O | 46.1 | 389.1 | 95.66 | 1.05284 | 1536.37 ± 5.60c | 2279.30 ± 70.49a | 1880.67 ± 54.05b |
| Esters (14) | |||||||||||
| v26 | Hexyl 2-Methylbutanoate | green waxy fruity apple spicy tropical | 10032-15-2 | C11H22O2 | 186.3 | 1290.3 | 763.24 | 1.52364 | 117.27 ± 6.46a | 104.16 ± 8.26b | 100.73 ± 4.25b |
| v27 | Isoamyl hexanoate | fruity banana apple pineapple green | 2198-61-0 | C11H22O2 | 186.3 | 1272.4 | 737.40 | 1.50262 | 240.18 ± 15.66a | 186.62 ± 10.73b | 235.31 ± 1.06a |
| v28 | Methyl salicylate | wintergreen mint | 119-36-8 | C8H8O3 | 152.1 | 1234.6 | 683.05 | 1.20188 | 203.41 ± 19.94ab | 181.09 ± 6.36b | 225.42 ± 5.77a |
| v29 | Ethyl benzoate | fruity dry musty sweet wintergreen | 93-89-0 | C9H10O2 | 150.2 | 1200.5 | 634.04 | 1.26009 | 252.95 ± 24.13b | 238.80 ± 12.40b | 293.07 ± 0.30a |
| v30 | Methyl benzoate | phenolic wintergreen almond floral cananga | 93-58-3 | C8H8O2 | 136.1 | 1098.1 | 486.78 | 1.21035 | 708.56 ± 11.43c | 1250.38 ± 10.94a | 1014.96 ± 36.18b |
| v31 | Hexyl acetate | fruity green apple banana sweet | 142-92-7 | C8H16O2 | 144.2 | 1033.8 | 394.26 | 1.41083 | 272.54 ± 14.11b | 425.91 ± 47.50a | 290.20 ± 20.30b |
| v32 | gamma-Butyrolactone-M | creamy oily fatty carame | 96-48-0 | C4H6O2 | 86.1 | 920.8 | 277.92 | 1.08432 | 423.88 ± 23.02a | 301.21 ± 25.24b | 324.50 ± 24.55b |
| v33 | gamma-Butyrolactone-D | creamy oily fatty carame | 96-48-0 | C4H6O2 | 86.1 | 920.5 | 277.62 | 1.3466 | 676.32 ± 215.79a | 68.99 ± 16.15b | 255.77 ± 77.78b |
| v34 | Propyl butanoate-M | fruity sweet apricot pineapple rancid sweaty | 105-66-8 | C7H14O2 | 130.2 | 898.8 | 259.66 | 1.26398 | 154.33 ± 36.49b | 229.40 ± 21.37a | 138.58 ± 25.52b |
| v35 | Propyl butanoate-D | fruity sweet apricot pineapple rancid sweaty | 105-66-8 | C7H14O2 | 130.2 | 898.8 | 259.66 | 1.69151 | 116.66 ± 12.31a | 116.79 ± 21.88a | 67.83 ± 15.84b |
| v36 | Ethyl 3-Methylbutanoate | fruity sweet apple pineapple tutti frutti | 108-64-5 | C7H14O2 | 130.2 | 844.5 | 230.22 | 1.65872 | 30.42 ± 3.87a | 47.96 ± 1.10b | 73.24 ± 7.79a |
| v37 | Butyl acetate | ethereal solvent fruity banana | 123-86-4 | C6H12O2 | 116.2 | 803.3 | 209.61 | 1.62725 | 56.55 ± 23.81b | 111.17 ± 17.49a | 63.81 ± 19.09b |
| v38 | Ethyl Acetate | ethereal fruity sweet weedy green | 141-78-6 | C4H8O2 | 88.1 | 570.4 | 136.59 | 1.34267 | 7888.77 ± 43.02a | 1898.37 ± 75.70c | 2667.27 ± 22.99b |
| v39 | Methyl 3-methylbutanoate | strong apple fruity pineapple | 556-24-1 | C6H12O2 | 116.2 | 761.6 | 191.33 | 1.53613 | 152.88 ± 13.04a | 62.30 ± 2.83b | 74.54 ± 4.17b |
| Phenols (2) | |||||||||||
| v40 | Eugenol | sweet spicy clove woody | 97-53-0 | C10H12O2 | 164.2 | 1381.1 | 893.80 | 1.29042 | 817.67 ± 63.37a | 676.37 ± 40.89b | 758.27 ± 43.79ab |
| v41 | Phenol | phenolic plastic rubber | 108-95-2 | C6H6O | 94.1 | 995.7 | 339.99 | 1.07915 | 677.68 ± 16.43b | 859.00 ± 26.30a | 727.51 ± 64.02b |
| Thioethers (3) | |||||||||||
| v42 | Diallyl disulfide-M | alliaceous onion garlic metallic | 2179-57-9 | C6H10S2 | 146.3 | 1074.7 | 453.14 | 1.20427 | 1630.49 ± 27.71c | 2782.77 ± 36.18a | 2285.77 ± 58.00b |
| v43 | Diallyl disulfide-D | alliaceous onion garlic metallic | 2179-57-9 | C6H10S2 | 146.3 | 1074.7 | 453.14 | 1.63865 | 304.88 ± 3.13c | 890.32 ± 17.37a | 603.11 ± 19.11b |
| v44 | Dimethyl disulfide | sulfurous vegetable cabbage onion | 624-92-0 | C2H6S2 | 94.2 | 726.4 | 177.81 | 0.9912 | 3902.85 ± 23.85a | 1645.49 ± 33.69b | 1248.37 ± 50.33c |
| Terpenes (1) | |||||||||||
| v45 | Limonene | citrus herbal terpene camphor | 138-86-3 | C10H16 | 136.2 | 1027 | 384.47 | 1.22366 | 524.04 ± 92.06a | 361.08 ± 20.24b | 484.56 ± 49.76a |
| Heterocyclics (2) | |||||||||||
| v46 | 2-Pentylfuran | fruity green earthy beany vegetable metallic | 3777-69-3 | C9H14O | 138.2 | 998.5 | 343.48 | 1.25734 | 59.36 ± 4.79c | 151.57 ± 2.99a | 128.97 ± 3.01b |
| v47 | 2,6-Dimethylpyrazine | ethereal cocoa nutty roasted roasted meaty beefy brown coffee buttermilk | 108-50-9 | C6H8N2 | 108.1 | 898.5 | 259.38 | 1.53905 | 360.32 ± 51.52a | 108.62 ± 18.56c | 179.41 ± 10.57b |
| Ketones (4) | |||||||||||
| v48 | 6-Methyl-5-hepten-2-one | citrus green musty lemongrass apple | 110-93-0 | C8H14O | 126.2 | 995.2 | 339.56 | 1.18157 | 362.76 ± 10.92a | 258.05 ± 15.58b | 352.62 ± 23.13a |
| v49 | Acetoin | sweet buttery creamy dairy milky fatty | 513-86-0 | C4H8O2 | 88.1 | 702.6 | 168.68 | 1.3348 | 1570.05 ± 46.02a | 773.33 ± 88.49b | 771.72 ± 88.76b |
| v50 | Acetone | solvent ethereal apple pear | 67-64-1 | C3H6O | 58.1 | 451.7 | 109.79 | 1.12104 | 7863.57 ± 70.34b | 7641.55 ± 77.76c | 8209.29 ± 134.49a |
| v51 | Cyclohexanone | minty acetone | 108-94-1 | C6H10O | 98.1 | 897.8 | 258.81 | 1.15621 | 58.47 ± 4.71b | 99.25 ± 9.19a | 58.60 ± 4.49b |
| Acids (2) | |||||||||||
| v52 | 2-Methylpropionic acid | acidic sour cheese dairy buttery rancid | 79-31-2 | C4H8O2 | 88.1 | 791 | 203.42 | 1.36365 | 4816.43 ± 443.70a | 1865.31 ± 481.87b | 3993.47 ± 425.70ab |
| v53 | 3-Methylbutanoic acid | sour stinky feet sweaty cheese tropical | 503-74-2 | C5H10O2 | 102.1 | 869.1 | 242.58 | 1.49348 | 351.62 ± 13.42a | 106.53 ± 13.27c | 191.19 ± 3.42b |
The values followed by different letters in a line are significantly different. Data marked with different letters show significant differences at p ≤ 0.05 using SPSS 20.0 software.
* Odor descriptions were from FEMA database.
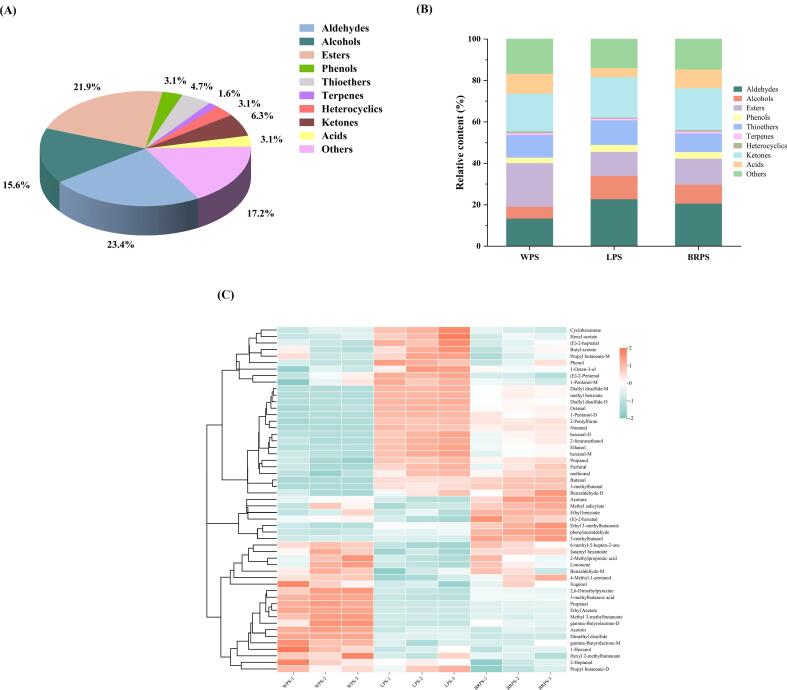
Fig. 2.
(A) Classification of all VOCs and (B) their relative content of different chopped pepper seeds. (C) Heat-map analysis of volatile components of different chopped pepper seeds.
In addition, aldehydes, esters, and ketones were identified as the most dominant groups in all CPS samples (Fig. 2A). They differ from the main volatile substances in pepper seed essential oil extracted by Ma et al. (2023) using the supercritical CO2 extraction process, where olefins (53.04 %), alcohols (29.83 %), and esters (9.95 %) were predominant. Table 2 shows that, among the 53 qualitatively detected VOCs, no difference was observed in the categories of VOCs discovered in the CPS of the three varieties, but a markedly variation was found in their contents (p < 0.05). The highest percentage of esters (20.95 %) was found in the VOCs of the WPS sample, followed by aldehydes (13.49 %). Among them, aldehydes (22.81 %) accounted for the highest proportion of VOCs in the LPS sample, followed by ketones (19.49 %). The BRPS sample exhibited the highest percentage of aldehydes (20.66 %), followed by ketones (20.23 %). Pepper samples contain an abundance of alcohols, aldehydes, ketones, and esters compounds, most of which are derived from the esterification and catabolism of fatty acids (Li et al., 2023). Terpenoids and aldehydes, which are mainly derived from the mevalonate metabolic pathway in plants (Muchlinski et al., 2020), and the decomposition of carotenoids (Lee et al., 2018), generally impart obvious floral and fruity aromas.
Aldehydes, with relatively low threshold values, can directly affect the flavor of chopped peppers or act as precursors for the synthesis of other aromatic compounds. Aldehydes constituted the largest number of detected compounds (15 compounds) in CPS samples (Fig. 2A), which varied in levels among the three CPS (13.49 %–22.81 %). They mainly included nonanal, benzaldehyde, hexanal, 3-methylbutanal, butanal, and propanal. Nonanal is identified as one of the dominant odor contributors to fermented chili pepper (Xiao et al., 2023). Specifically, in the BRPS sample, the content of 3-methylbutanal content was the highest at 5.09 %. In the LPS sample, hexanal had the highest content at 3.94 %. However, among the three varieties of CPS, the WPS sample had the lowest contents of 3-methylbutanal and hexanal at 2.77 % and 0.57 %, respectively, while having the highest propanal content (5.92 %). Therefore, hexanal, 3-methylbutanal and propanal might contribute to distinguishing the different varieties of CPS. A previous study has shown that lactic acid bacteria can biosynthesize the flavor compound 3-methylbutanal from leucine catabolism (Afzal et al., 2017). 3-Methylbutanal is a key volatile compound that imparts a nutty flavor (Chen et al., 2022). The presence and impact of this flavor compound in bread, meat, cheese, and certain beverages has been recently documented (Afzal et al., 2017).
The second-largest number of detected compounds (14 compounds) in CPS samples were esters (Fig. 2A), which impart sweet and fruity aroma attributes and enhance the flavor of fermented foods by reducing the intensity of unpleasant odors (Wang et al., 2019). Esters, with sweet or fruity flavors, were present at higher concentrations in WPS (20.95 %) than in LPS (11.61 %) and BRPS (12.56 %). Esters play a crucial role as fragrance components in the fermentation process of chopped pepper. During the fermentation of red peppers with Lactobacillus parabuchneri, the levels of most esters tended to increase (Lee et al., 2018). Esters are the most abundant volatile substances in chopped peppers (Xiao et al., 2020). The high levels of esters may result from the catalysis of acids and alcohols by esterases secreted by microbes during fermentation (Ardö, 2006).
For the three CPS samples, 10 alcohol compounds were identified and quantified. The alcohol content in WPS (5.72 %) was lower than that in LPS (11.22 %) and BRPS (9.18 %) (Fig. 2B). A previous study showed that the proportion of alcohols in chopped peppers ranged from 27.05 % to 62.70 % (Jiang et al., 2023). The alcohol content in CPS is lower than in chopped peppers. Microbial metabolism of sugars, amino acids, and other substances is the main source alcohol production. The detection of alcohols is mainly caused by the addition of liquor before fermentation in the manufacturing of chopped pepper (He et al., 2023). In addition, during the fermentation process, microorganisms such as yeasts utilize and transform carbohydrates to produce various alcohols through glycolysis (Lu et al., 2019), and most of these alcohols have pleasant aromas (Jiang et al., 2023). They serve as important precursors to the formation of esters (Xu et al., 2021).
The above obtained results show that CPS has a flavor characteristic similar to pepper or chili sauce products. For instance, CPS contains abundant 1-octen-3-ol (0.28 %–0.46 %), methyl salicylate (0.38 %–0.48 %), dimethyl disulfide (2.69 %–7.23 %), 6-methyl-5-hepten-2-one (0.57 %–0.76 %), acetone (14.57 %–17.68 %), and ethyl acetate (5.74 %–14.62 %). Notably, 1-octen-3-ol was also previously detected in horse bean-chili-paste (Lu et al., 2019). In particular, methyl salicylate, found in significant quantities in Capsicum pubescens and Capsicum chinense samples, is a phenol derivative that imparts peppermint-like aromas and is recognized as a key aroma component in chili sauce (Xu et al., 2021, Yan et al., 2023). Rotsatchakul et al. (2008) reported that dimethyl sulfide was among the most potent headspace odorants detected by dynamic headspace dilution analysis in three forms of Thai fried chili pastes. Moreover, 6-methyl-5-hepten-2-one and acetone were detected in Spice paprika (Cremer and Eichner, 2000). Ethyl acetate was detected in four different chili sauces, which provided a fruity aroma to the sauces (Chen et al., 2021). Pepper or chili sauce is widely used in the food industry as a natural favor to improve food products. This spice is capable of modifying the flavor of food to which it is added, which is due to its characteristic aroma and pungency. Thus, it is widely used in soups, sausages, cheeses, sauces, and snacks (Afzal et al., 2017). Accordingly, CPS shows a similar flavor characteristic to pepper or chili sauce and can be used as a natural spice/additive to replace some chili sauce in the food industry for quality improvement of food products.
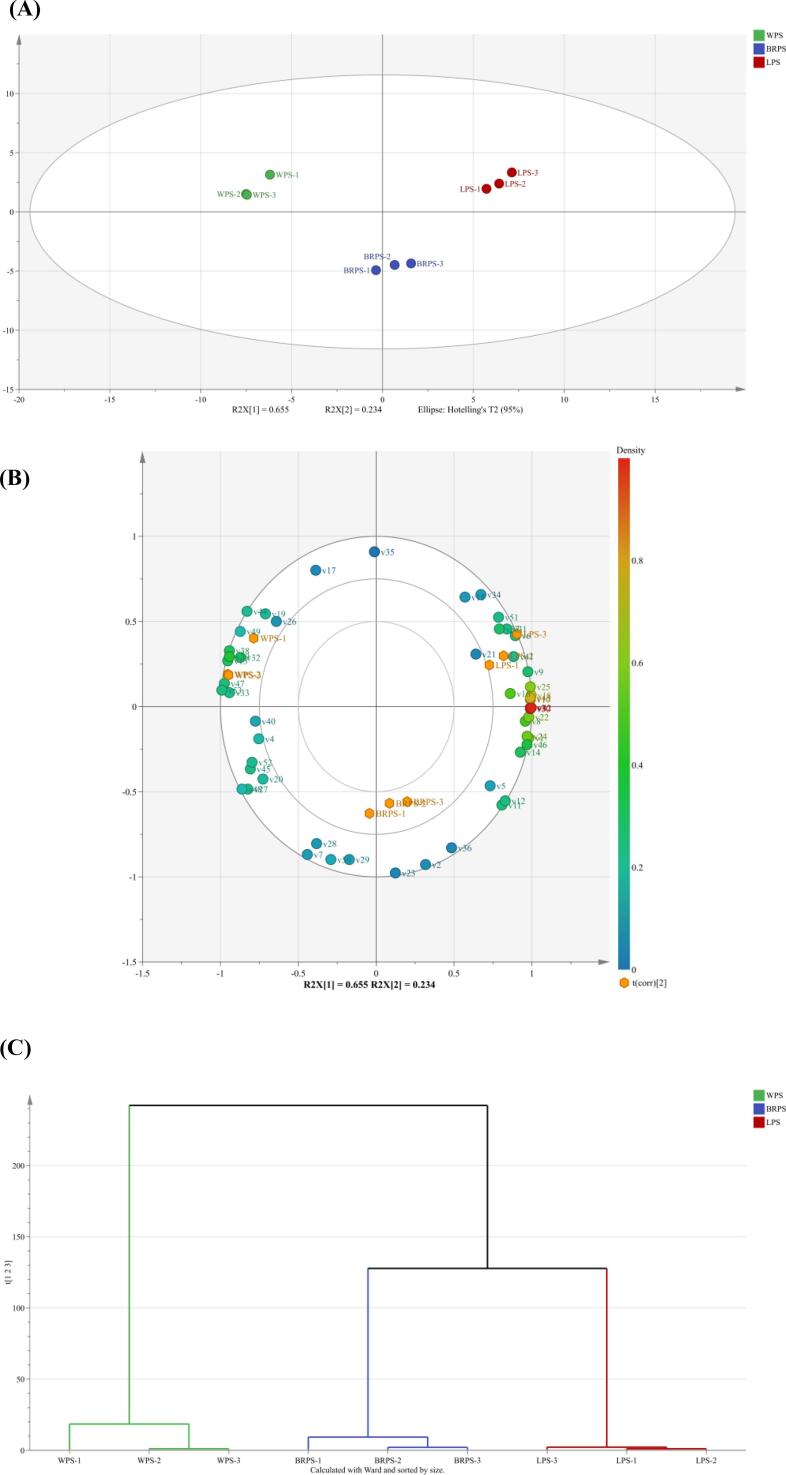
Multivariate statistical analysis of VOCs in CPS samples
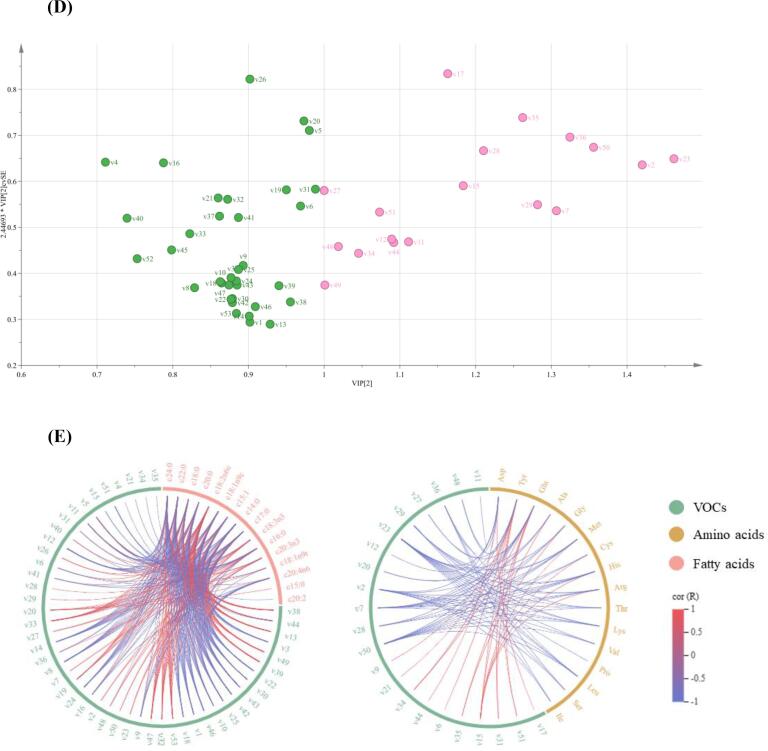
Multivariate statistical analysis was conducted on the VOCs in Fig. 3 to further support the differences among the CPS samples. The three different types of CPS were discovered to be easily distinguishable. The PCA plot clearly separated the three different types of CPS, which indicates that the VOCs in the CPS samples varied obviously. Two verified principal components (PCs), which together account for 88.9 % of the total variability information, could be used to represent the original variables’ information. Apparently, along with the vector of PC1 from left to right, all the CPS samples could be completely separated into three sections (LPS, BRPS, and WPS) (Fig. 3A). These findings demonstrate that the flavor characteristics of the CPS samples were significantly influenced by CPS variety. A partial least square model was used to explore the relationships between VOCs and CPS samples. Two latent variables were included in the PLS model, which accounted for 65.50 % of the variance in the X matrix and explained 23.30 % of the variance in the Y matrix. The loading plot (Fig. 3B) was drawn to observe the contribution of various VOCs to the flavor of different CPS. WPS samples were located at the negative values of PC1 and positive value of PC2, LPS samples were distributed at the positive values of PC2 and PC1, and BRPS samples were spread throughout the positive and negative axes of PC1 and were found in the negative values of PC2. The WPS was strongly associated with 2,6-dimethylpyrazine, propanal, gamma-butyrolactone-D, and 3-methylbutanoat. The BRPS was associated with 3-methylbutanol, ethyl benzoate, and phenylacetaldehyde. The LPS was associated with (E)-2-heptenal, phenol, hexyl acetate, and butyl acetate. The HCA result showed that the VOCs in the LPS and the BRPS were partially similar (Fig. 3C), which is different from the WPS sample. Therefore, the flavors of WPS were unique compared with those of the two other samples. The contribution of each variable to the classification can be quantified by the VIP. The variable can be defined as the key variable of the discriminant model if the VIP value is greater than 1. A total of 18 volatile compounds (VIP > 1) were screened in the partial least-square discrimination analysis model for the three analyzed CPS samples (Fig. 3D). These compounds were phenylacetaldehyde, (E)-2-hexenal, 3-methylbutanal, butanal, (E)-2-pentenal, 2-heptanol, 3-methylbutanol, isoamyl hexanoate, methyl salicylate, ethyl benzoate, propyl butanoate-M, propyl butanoate-D, ethyl 3-methylbutanoate, dimethyl disulfide, 6-methyl-5-hepten-2-one, acetoin, acetone, and cyclohexanone.
Fig. 3.
Principal component analysis score plot (A) and loading plot (B) and hierarchical cluster analysis (C), and variable importance for the projection (D) of three varieties of chopped pepper seeds. (E) Correlation analysis between volatile flavor compounds and fatty acids, amino acids (|r| > 0.8, p < 0.05).
ROAV analysis of CPS samples
The ROAV was applied to quantify the contribution of the volatile compounds in CPS to the overall fragrance. The contribution to the overall flavor of the sample was greater for the component with a higher ROAV. The component with ROAV ≥ 1 was classified as the key VOCs of the samples. The component with 0.1 ≤ ROAV < 1 had a crucial modifying influence on the overall aroma of the sample (Xiao et al., 2022). The contribution to the aroma characteristics in CPS is positively correlated with the ROAV. Table 3 shows that nonanal, methional, ethyl 3-methylbutanoate, dimethyl disulfide, and 2-pentylfuran had ROAV ≥ 1 in WPS, which were the characteristic VOCs of the sample. The VOCs in the range of 0.1 ≤ ROAV < 1 were octanal, hexanal-M, diallyl disulfide-M, 3-methylbutanal, butanal, 1-octen-3-ol, 3-methylbutanol, hexyl 2-methylbutanoate, eugenol, propyl butanoate-D, and diallyl disulfide-D. Thus, the 11 VOCs have an essential modifying impact on the whole aroma of the sample. Nonanal, octanal, hexanal-M, 3-methylbutanal, butanal, methional, 1-octen-3-ol, hexyl-2-methylbutanoate, ethyl-3-methylbutanoate, eugenol, dimethyl disulfide, and 2-pentylfuran were the 12 VOCs characterizing the flavor compounds of the sample in LPS with ROAV ≥ 1. The VOCs in the interval 0.1 ≤ ROAV < 1 were phenylacetaldehyde, (E)-2-heptenal, (E)-2-hexenal, hexanal-D, propanal, 1-hexanol, 3-methylbutanol, hexyl acetate, propyl butanoate-D, diallyl disulfide-M, diallyl disulfide-D, limonene, 2-pentylfuran, and 6-methyl-5-hepten-2-one. Therefore, the 14 VOCs consequently played a crucial role in the overall flavor of the samples. The ROAV ≥ 1 in BRPS were nonanal, octanal, 3-methylbutanal, butanal, methional, 1-octen-3-ol, hexyl 2-methylbutanoate, propyl butanoate-D, ethyl 3-methylbutanoate, eugenol, dimethyl disulfide, and 2-pentylfuran, which were the 12 VOCs characterizing the flavor compounds of this sample. The components in the interval of 0.1 ≤ ROAV < 1 were diallyl disulfide-D, hexanal-D, 1-hexanol, 3-methylbutanol, (E)-2-heptenal, hexyl acetate, ethyl acetate, diallyl disulfide-M, hexanal-M, and 6-methyl-5-hepten-2-one. Therefore, the 10 VOCs contributed to the whole sample flavor.
Table 3.
The ROAV values of main volatile components in three varieties of chopped pepper seeds.
| Compounds | Threshold value(mg/kg) | ROAV values |
|||
|---|---|---|---|---|---|
| WPS | LPS | BRPS | |||
| v1 | Nonanal | 0.0011 | 2.12 ± 0.25 | 5.81 ± 0.60 | 3.61 ± 0.24 |
| v2 | Phenylacetaldehyde | 0.0063 | 0.05 ± 0.00 | 0.16 ± 0.03 | 0.08 ± 0.00 |
| v3 | Octanal | 0.0008 | 0.85 ± 0.03 | 2.22 ± 0.33 | 1.54 ± 0.03 |
| v4 | Benzaldehyde-M | 0.75089 | <0.01 | <0.01 | <0.01 |
| v5 | Benzaldehyde-D | 0.75089 | <0.01 | <0.01 | <0.01 |
| v6 | (E)-2-heptenal | 0.013 | 0.05 ± 0.00 | 0.55 ± 0.05 | 0.25 ± 0.01 |
| v7 | (E)-2-hexenal | 0.0887 | 0.02 ± 0.00 | 0.13 ± 0.02 | 0.06 ± 0.00 |
| v8 | Furfural | 9.562 | <0.01 | <0.01 | <0.01 |
| v9 | Hexanal-M | 0.005 | 0.17 ± 0.01 | 1.69 ± 0.23 | 0.63 ± 0.02 |
| v10 | Hexanal-D | 0.005 | 0.03 ± 0.00 | 0.13 ± 0.02 | 0.12 ± 0.01 |
| v11 | 3-Methylbutanal | 0.0011 | 0.70 ± 0.03 | 3.70 ± 0.80 | 1.38 ± 0.11 |
| v12 | Butanal | 0.002–0.0022 | 0.68–0.74 | 1.55–1.70 | 1.15–1.27 |
| v13 | Propanal | 0.0151 | 0.02 ± 0.00 | 0.11 ± 0.01 | 0.05 ± 0.00 |
| v14 | Methional | 0.00045 | 4.29 ± 0.27 | 18.06 ± 1.64 | 8.47 ± 0.90 |
| v15 | (E)-2-Pentenal | 0.98 | <0.01 | <0.01 | <0.01 |
| v16 | 1-Octen-3-ol | 0.0015 | 0.69 ± 0.01 | 1.62 ± 0.10 | 1.23 ± 0.06 |
| v17 | 2-Heptanol | 0.065235 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.01 ± 0.00 |
| v18 | 2-Furanmethanol | 4.5005 | <0.01 | <0.01 | <0.01 |
| v19 | 1-Hexanol | 0.0056 | 0.06 ± 0.01 | 0.28 ± 0.06 | 0.17 ± 0.02 |
| v20 | 4-Methyl-1-pentanol | 0.82–4.1 | <0.01 | <0.01 | <0.01 |
| v21 | 1-Pentanol-M | 0.1502 | <0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| v22 | 1-Pentanol-D | 0.1502 | 0.01 ± 0.00 | <0.01 | <0.01 |
| v23 | 3-methylbutanol | 0.004 | 0.11 ± 0.03 | 0.55 ± 0.11 | 0.18 ± 0.04 |
| v24 | Propanol | 8.5056 | <0.01 | <0.01 | <0.01 |
| v25 | Ethanol | 950 | <0.01 | <0.01 | <0.01 |
| v26 | Hexyl 2-methylbutanoate | 0.0007 | 0.91 ± 0.02 | 2.01 ± 0.11 | 2.20 ± 0.21 |
| v27 | Isoamyl hexanoate | 0.32 | <0.01 | 0.04 ± 0.01 | 0.01 ± 0 |
| v28 | Methyl salicylate | 0.04 | <0.01 | 0.01 ± 0.00 | <0.01 |
| v29 | Ethyl benzoate | 0.05556 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.00 |
| v30 | Methyl benzoate | 0.073 | <0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| v31 | Hexyl acetate | 0.005 | 0.05 ± 0.02 | 0.49 ± 0.09 | 0.22 ± 0.05 |
| v32 | gamma-Butyrolactone-M | >1 | <0.01 | <0.01 | <0.01 |
| v33 | gamma-Butyrolactone-D | >1 | <0.01 | <0.02 | <0.01 |
| v34 | Propyl butanoate-M | 0.018 | <0.01 | 0.06 ± 0.02 | 0.02 ± 0.01 |
| v35 | Propyl butanoate-D | 0.018 | 0.76 ± 0.24 | 0.96 ± 0.13 | 1.16 ± 0.33 |
| v36 | Ethyl 3-methylbutanoate | 0.00001 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| v37 | Butyl acetate | 0.058 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| v38 | Ethyl Acetate | 0.005 | 0.04 ± 0.00 | 0.28 ± 0.03 | 0.12 ± 0.01 |
| v39 | Methyl 3-methylbutanoate | 0.0044–0.044 | 0.25–2.52 | 0.36–3.55 | 0.15–1.48 |
| v40 | Eugenol | 0.00071 | 0.50 ± 0.02 | 1.77 ± 0.17 | 2.05 ± 0.13 |
| v41 | Phenol | 58.58525 | <0.01 | <0.01 | <0.01 |
| v42 | Diallyl disulfide-M | 0.03 | 0.14 ± 0.01 | 0.70 ± 0.08 | 0.41 ± 0.01 |
| v43 | Diallyl disulfide-D | 0.03 | 0.75 ± 0.03 | 0.60 ± 0.05 | 0.47 ± 0.01 |
| v44 | Dimethyl disulfide | 0.0011 | 1.10 ± 0.03 | 6.28 ± 0.70 | 3.72 ± 0.16 |
| v45 | Limonene | 0.02 | 0.03 ± 0.01 | 0.20 ± 0.03 | 0.10 ± 0.01 |
| v46 | 2-Pentylfuran | 0.0058 | 1.57 ± 0.05 | 4.08 ± 0.48 | 2.37 ± 0.07 |
| v47 | 2,6-Dimethylpyrazine | 0.718 | 0.03 ± 0.00 | 0.10 ± 0.01 | 0.06 ± 0.00 |
| v48 | 6-methyl-5-hepten-2-one | 0.068 | 0.06 ± 0.00 | 0.32 ± 0.05 | 0.14 ± 0.01 |
| v49 | Acetoin | 0.014 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.00 |
| v50 | Acetone | 0.832 | <0.01 | <0.01 | <0.01 |
| v51 | Cyclohexanone | 0.28–0.67 | <0.01 | <0.01 | <0.01 |
| v52 | 2-Methylpropionic acid | 6.5505 | <0.01 | <0.01 | <0.01 |
| v53 | 3-Methylbutanoic acid | 0.49 | <0.01 | <0.01 | <0.01 |
As shown in Table 3, it was found that 13 VOCs with ROAV ≥ 1 in CPS. Among them, five VOCs of ROAV ≥ 1 were consistently present in all three different CPS samples, namely, nonanal, methional, ethyl 3-methylbutanoate, dimethyl disulfide, and 2-pentylfuran. These compounds are relatively abundant in each sample, which greatly contribute to the aroma of CPS due to their low flavor threshold. They also represent the main aroma components of WPS, LPS, and BRPS. Previous studies have reported that the five volatiles contribute to the flavor characteristics of many food products, which highlights their great potential in the food industry. For instance, nonanal, an aldehyde, imparting pepper seed rose, orange, and other aromas, is a key volatile flavor compound (ROAV ≥ 1) in Shanghai smoked fish, with a relative content of 4.84 % (Feng et al., 2021). Methional, which contributes to the aroma of potatoes, tomatoes, and vegetables, is one of the strongest odors in black olive extracts (Collin et al., 2008). Ethyl 3-methylbutanoate, which is a major contributor to the flavor characteristics of CPS, had the highest ROAV (ROAV = 100). It can influence the aroma of pepper seeds with its apple, pineapple, and tutti-frutti flavors and an extremely low odor threshold (0.00001 mg/Kg). Ethyl 3-methylbutanoate is a characteristic volatile flavor (ROAV > 1) of Mouding sufu, which provides the product with a fruity flavor (Chen et al., 2023). The odor of dimethyl disulfide is often described as a garlic-like aroma, which mainly originates from garlic volatile oils (Wang et al., 2019). Dimethyl disulfide can impart a vegetable flavor similar to onion and cabbage to pepper seeds. In addition, dimethyl disulfide and 2-methylbutanal, which are two of the 17 key odorants, were found to be positively correlated with the intensity of burnt and fermented soybean paste-like notes in heat-processed beef during storage (Zhang et al., 2022). Thus, dimethyl disulfide could improve the flavor of beef products. 2-Pentylfuran of CPS imparts fruity, green, earthy, beany, vegetable, and metallic odors, which could be generated from the Maillard reaction or linoleic acid oxidation reaction (Shahidi & Oh, 2020). Aroma recombination and omission experiments confirmed that 2-pentylfuran was a key odorant in prerigor and postrigor roasted mutton, yeast-fermented Chinese steamed bread (Mantou), and sourdough-fermented Chinese steamed bread (Xi et al., 2020, Liu et al., 2021). It contributes a wheat-like aroma to Chinese steamed bread (Xi et al., 2020). Therefore, 2-pentylfuran in CPS might serve as a valuable flavor enhancer in Chinese steamed bread products. In summary, CPS are abundant in 2-pentylfuran, methional, ethyl 3-methylbutanoate, dimethyl disulfide, and nonanal flavor substances, which make them valuable condiments in the processing of various food products to enhance these key flavors and provide foods with a richer aroma. Thus, the five volatiles hold great potential for the food industry.
Correlation analysis among fatty acids, amino acids, and volatile compounds in CPS
Fatty and amino acids are precursor components metabolized to form volatiles with low threshold values, and they contribute considerably to the overall flavor of food. It was reported that through dehydrogenation, decarboxylation and ammonia conversion processes, amino acids are transferred into certain aromatic compounds, such as alcohols, ketones, acids, aldehydes, phenols, etc (Zhang et al., 2023). The metabolism of fatty acids also has a crucial role on the formation of flavors. Short-chain fatty acids generate cheesy flavors while long-chain fatty acids serve as the precursors for flavors that can be broken down into aldehydes, alcohols, ketones, hydrocarbons, and aromatic compounds (Dinh et al., 2021). As the precursors of many volatiles, the oxidation and breakdown of fatty acids are crucial in the formation of aroma in pepper products (Xiao et al., 2023). CPS serve as a rich source of amino and fatty acids (Table 1a, Table 1b, Table 1c). Thus, the relationship among all identified VOCs, fatty acids, and amino acids was determined (Fig. 3E) to investigate the influence of fatty and amino acids on the formation of VOCs. A total of 370 strong correlation combinations, with Pearson correlation coefficients greater than 0.8, were identified. A total of 16 amino acids and 16 fatty acids were likely involved in the formation of 50 VOCs. Specifically, it was found that linoleic acid was strongly correlated with 30 VOCs, including nonanal (r = 0.968), (E)-2-heptenal (r = 0.840), 1-octen-3-ol (r = 0.928), 2-pentylfuran (r = 0.969), 1-pentanol (r = 0.985), and hexanal-M (r = 0.947) and hexanal-D (r = 0.973). Linolenic acid was strongly correlated with 8 VOCs, including 3-methylbutanol (r = 0.944) and (E)-2-hexenal (r = 0.922). Earlier studies have verified that unsaturated fatty acids such as linoleic acid and linolenic acid are the precursors of many volatiles including nonanal, (E)-2-octenal, hexenal, 1-octen-3-ol, 2-pentylfuran, butanol, (E)-2-pentenal, heptanal, pentanol, (E)-2-heptenal, hexanal, (E, E)-2,4-decadienal, (E)-2-hexenal, and (E, Z)-2,4-heptadienal (Nielsen et al., 2004). For instance, 1-octen-3-ol emits a mushroom-like odor, was regarded as an oxidation product of unsaturated fatty acids (Dinh et al., 2021). The oxidation of linoleic acid results in the production of aldehydes like heptanal and nonanal (imparting a fatty scent) and hexanal (giving a fresh grass odor) (Shahidi and Oh, 2020, Xu et al., 2018). 2-Pentylfuran has a relatively low perception threshold and contributes greatly to the overall aroma profile of all CPS (ROAV > 1), is also an oxidation product of linoleic acid (Shahidi & Oh, 2020). (E)-2-Hexenal gives a green and cheesy flavor, (E)-2-hexanal imparts green fruity flavor, which are produced by the oxidation of linolenic acid (Shahidi & Hossain, 2022). The oxidation of saturated fatty acids (SFA) results in the production of long-chain alkanals, alkanes, and ketones in a more intricate manner, when oxygen attacks the saturated carbon chain at the fourth or fifth carbon (C4 or C5), the formation of γ- or δ-lactones takes place (Dinh et al., 2021). Specifically, it was found in this study that gamma-butyrolactone-M and gamma-butyrolactone-D were strongly correlated with 7 and 5 saturated fatty acids, respectively. Thus, the oxidation of fatty acids is critical in the formation of volatiles. Additionally, it was well illustrated that unsaturated fatty acids are more prone to oxidation compared to saturated fatty acids, resulting in a greater influence on the production of lipid volatiles (Dinh et al., 2021). The catabolism of amino acids also plays crucial role on the formation of various volatiles, such as phenylacetaldehyde, 2-methylpropanol, 3-methylbutanol, 2-methylpropanal, 3-methylbutanal, phenylethanol (Ardö, 2006, Yang et al., 2021, Zhang et al., 2023). In this study, we have found that 3-methylbutanol, phenylacetaldehyde, 3-methylbutanal was greatly correlated with the amino acids (Fig. 3E). These three volatiles were also previously reported that produced by the breakdown of amino acids, namely leucine and phenylalanine (Yang et al., 2021). Besides, the metabolism of amino acids such as leucine, glutamic acid, glycine, alanine, and others can result in the production of 2-butanone, benzaldehyde, hexanal, and acetone (Zhang et al., 2023). In addition, it was found that dimethyl disulfide (ROAV > 1) was significantly correlated with aspartic acid. However, Liu et al. (2008) demonstrated that the primary generation of sulfur-containing compounds results from the removal of methionine and cysteine. The fermentation process, which creates an acidic environment, hydrolyzes proteins to produce various flavor amino acids and amino acid nitrogen. This process greatly enhances the umami of fermented CPS (Li et al., 2023). Peptides and free amino acids result from protein hydrolysis by Pichia spp. through amino acid catabolic pathways (Xiao et al., 2023). In addition, microbial transaminases can transfer amino acids into α-keto acids, which are further decomposed into ketone and aldehyde flavors by decarboxylases (Peng et al., 2023). Alcohols can be converted into esters by alcohol acetyltransferase, and acids can be transformed into esters by carboxymethyltransferase (Ardö, 2006). Thus, the metabolism of fatty and amino acids in fresh chili pepper plays a critical role in the flavor development of fermented chili pepper products.
Conclusion
The findings of this study indicate that CPS are rich in nutritional constituents, and the proximate composition, minerals, fatty acids, and amino acids of WPS, LPS, and BRPS exhibit remarkable differences. CPS is rich in trace elements necessary for the human body, such as iron, copper, zinc, manganese, and selenium trace, with iron being the predominant element (34.20–68.77 mg/Kg). A total of 17 amino acids were identified in CPS, with essential amino acids accounting for 38.41 %–40.00 % of the total amino acids, and leucine being the highest among essential amino acids. The fatty acids in CPS are mainly composed of unsaturated fatty acids, which account for 79.50 %–83.36 % of the total fatty acids. Linoleic acid, which is a crucial fatty acid for human health, constitutes the highest percentage of total fatty acids in CPS. HS–GC–IMS identified 53 VOCs in all CPS samples, which could be classified into 9 major groups. According to the ROAV analysis, the key VOCs of CPS are 2-pentylfuran, methional, ethyl 3-methylbutanoate, dimethyl disulfide, and nonanal. These VOCs are valuable in the food industry and can be added to many traditional Chinese fermented foods as condiments to enhance key flavors and provide a richer aroma. Furthermore, CPS has a flavor characteristic similar to chili sauce, which makes them suitable as a natural spice to replace some chili sauce in the food industry. The PCA and HCA results show that the three CPS varieties can be clearly distinguished, which suggests a wide range of aroma characteristics. In addition, the correlation analysis demonstrates that amino and fatty acids play a critical role in producing the distinctive flavor of CPS samples. This study is the first to explore the overall flavor of CPS. Based on the current research results, CPS are abundant in VOCs and nutritional components, which makes them a natural food spice. Given the growing demand in the food industry for natural flavors, this work provides a simple and effective approach to improve the application of CPS. This contribution supports the utilization of industrial byproducts, which leads to increased economic value and product utilization.
CRediT authorship contribution statement
Yulian Chen: Writing – original draft, Validation, Methodology, Investigation, Data curation. Xilu Zhang: Writing – original draft, Investigation. Xin Liu: Data curation. Yida Liu: Investigation. Aixiang Hou: Resources, Investigation. Yuanliang Wang: Resources. Luoming Li: Resources. Xiaozhen Peng: Writing – review & editing, Resources, Funding acquisition. Yu Xiao: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Key Research and Development Program of Hunan Province (No. 2023NK2025), National Natural Science Foundation of China (No. 32302611), the Science Research Project of Education Department of Hunan Province (No. 22C0108), and the Central Government Guides Local Science and Technology Development Fund Project (2023ZYQ138).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101150.
Contributor Information
Yulian Chen, Email: chenylhn@163.com.
Xiaozhen Peng, Email: peng112112@163.com.
Yu Xiao, Email: xiaoyu@hunau.edu.cn, yuxiao_89@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Afzal M.I., Ariceaga C.C.G., Boulahya K.A., Jacquot M., Delaunay S., Cailliez-Grimal C. Biosynthesis and role of 3-methylbutanal in cheese by lactic acid bacteria: Major metabolic pathways, enzymes involved, and strategies for control. Critical Reviews in Food Science and Nutrition. 2017;57:399–406. doi: 10.1080/10408398.2014.893502. [DOI] [PubMed] [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists; Washington, DC: 2005. Official Method of Analysis. [Google Scholar]
- Ardö Y. Flavour formation by amino acid catabolism. Biotechnology Advances. 2006;24(2):238–242. doi: 10.1016/j.biotechadv.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Chen C., Yuan J., Yu H., Wang B., Huang J., Yuan H., Tian H. Characterization of metabolic pathways for biosynthesis of the flavor compound 3-methylbutanal by Lactococcus lactis. Journal of Dairy Science. 2022;105(1):97–108. doi: 10.3168/jds.2021-20779. [DOI] [PubMed] [Google Scholar]
- Chen J., Huang X., Qian M., Zhao W., Li X., Bai W. Analysis of volatile flavor compounds in four chili sauces by GC-IMS. China Food Additives. 2021;32:173–182. doi: 10.19804/j.issn1006-2513.2021.11.024. [DOI] [Google Scholar]
- Chen Z., Liu L., Du H., Lu K., Chen C., Xue Q., Hu Y. Microbial community succession and their relationship with the flavor formation during the natural fermentation of Mouding sufu. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin S., Nizet S., Muls S., Iraqi R., Bouseta A. Characterization of odor-active compounds in extracts obtained by simultaneous extraction/distillation from Moroccan black olives. Journal of Agricultural and Food Chemistry. 2008;56(9):3273–3278. doi: 10.1021/jf073488x. [DOI] [PubMed] [Google Scholar]
- Cremer D.R., Eichner K. Formation of volatile compounds during heating of spice paprika (Capsicum annuum) powder. Journal of Agricultural and Food Chemistry. 2000;48(6):2454–2460. doi: 10.1021/jf991375a. [DOI] [PubMed] [Google Scholar]
- Cvetković T., Ranilović J., Jokić S. Quality of pepper seed by-products: A review. Foods. 2022;11(5):748. doi: 10.3390/foods11050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh T.T., To K.V., Schilling M.W. Fatty acid composition of meat animals as flavor precursors. Meat and Muscle Biology. 2021;5:1–16. doi: 10.22175/mmb.12251. [DOI] [Google Scholar]
- Eggink P.M., Maliepaard C., Tikunov Y., Haanstra J.P.W., Pohu-Flament L.M.M., de Wit-Maljaars S.C., Visser R.G.F. Prediction of sweet pepper (Capsicum annuum) flavor over different harvests. Euphytica. 2012;187:117–131. doi: 10.1007/s10681-012-0761-6. [DOI] [Google Scholar]
- Fei X., Hu H., Luo Y., Shi Q., Wei A. Widely targeted metabolomic profiling combined with transcriptome analysis provides new insights into amino acid biosynthesis in green and red pepper fruits. Food Research International. 2022;160 doi: 10.1016/j.foodres.2022.111718. [DOI] [PubMed] [Google Scholar]
- Feng M., Dai Z., Yin Z., Wang X., Chen S., Zhang H. The volatile flavor compounds of Shanghai smoked fish as a special delicacy. Journal of Food Biochemistry. 2021;45(1):e13553. doi: 10.1111/jfbc.13553. [DOI] [PubMed] [Google Scholar]
- Fontes A.L., Pimentel L.L., Simões C.D., Gomes A.M., Rodríguez-Alcalá L.M. Evidences and perspectives in the utilization of CLNA isomers as bioactive compounds in foods. Critical Reviews in Food Science and Nutrition. 2017;57(12):2611–2622. doi: 10.1080/10408398.2015.1063478. [DOI] [PubMed] [Google Scholar]
- He Z., He X., Hu N., Yi Y., Xia B., Zhu S., Zhu N. Research progress on the microorganisms and flavor substances in the fermented pepper. Modern Food Science and Technology. 2023;39(08):334–342. doi: 10.13982/j.mfst.1673-9078.2023.8.0984. [DOI] [Google Scholar]
- Huang Y., Chen R., Chen Y., Ho C.T., Hou A., Zhang X., Xiao Y. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Bioscience. 2023;55 [Google Scholar]
- Jiang X., Wang F., Zhou S., Su X., Li Q., Jiang L., Qin D. Effect of different chili varieties on the quality and flavor of fermented minced chili. China Condiment. 2023;48(04) doi: 10.3969/j.issn.1000-9973.2023.04.001. 1–6+19. [DOI] [Google Scholar]
- Koncsek A., Helyes L., Daood H.G. Bioactive compounds of cold pressed spice paprika seeds oils. Journal of Food Processing and Preservation. 2018;42(1) doi: 10.1111/jfpp.13403. [DOI] [Google Scholar]
- Lee S.M., Lee J.Y., Cho Y.J., Kim M.S., Kim Y.S. Determination of volatiles and carotenoid degradation compounds in red pepper fermented by Lactobacillus parabuchneri. Journal of Food Science. 2018;83(8):2083–2091. doi: 10.1111/1750-3841.14221. [DOI] [PubMed] [Google Scholar]
- Li Y., Luo X., Guo H., Bai J., Xiao Y., Fu Y., Gao H. Metabolomics and metatranscriptomics reveal the influence mechanism of endogenous microbe (Staphylococcus succinus) inoculation on the flavor of fermented chili pepper. International Journal of Food Microbiology. 2023;406 doi: 10.1016/j.ijfoodmicro.2023.110371. [DOI] [PubMed] [Google Scholar]
- Liu H., Hui T., Fang F., Ma Q., Li S., Zhang D., Wang Z. Characterization and discrimination of key aroma compounds in pre-and postrigor roasted mutton by GC-O-MS, GC E-Nose and aroma recombination experiments. Foods. 2021;10(10):2387. doi: 10.3390/foods10102387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Nauta A., Francke C., Siezen R.J. Comparative genomics of enzymes in flavor-forming pathways from amino acids in lactic acid bacteria. Applied and Environmental Microbiology. 2008;74(15):4590–4600. doi: 10.1128/AEM.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen Y., Wang Y., Chen J., Huang Y., Yan Y., Xiao Y. Total phenolics, capsaicinoids, antioxidant activity, and α-glucosidase inhibitory activity of three varieties of pepper seeds. International Journal of Food Properties. 2020;23(1):1016–1035. doi: 10.1080/10942912.2020.1775646. [DOI] [Google Scholar]
- Lu Y., Chi Y., Lv Y., Yang G., He Q. Evolution of the volatile flavor compounds of Chinese horse bean-chili-paste. LWT. 2019;102:131–135. doi: 10.1016/j.lwt.2018.12.035. [DOI] [Google Scholar]
- Ma L., Guo C., Hu T. Optimization ofsupercritical CO2 extraction process and volatile aroma components analysis of pepper seed essential oil. China Brewing. 2023;42(2):163–168. doi: 10.11882/j.issn.0254-5071.2023.02.027. [DOI] [Google Scholar]
- Ma Y., Xu Z., Zou H., Gao G., Wang Y., Cui J., Liao X. Analysis and comparison of constituents in hot pepper seeds of eight verities. Food Science. 2017;38(22):178–183. doi: 10.7506/spkx1002-6630-201722027. [DOI] [Google Scholar]
- Mi S., Zhang X., Wang Y., Zheng M., Zhao J., Gong H., Wang X. Effect of different genotypes on the fruit volatile profiles, flavonoid composition and antioxidant activities of chili peppers. Food Chemistry. 2022;374 doi: 10.1016/j.foodchem.2021.131751. [DOI] [PubMed] [Google Scholar]
- Muchlinski A., Ibdah M., Ellison S., Yahyaa M., Nawade B., Laliberte S., Tholl D. Diversity and function of terpene synthases in the production of carrot aroma and flavor compounds. Scientific Reports. 2020;10(1):9989. doi: 10.1038/s41598-020-66866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen G.S., Larsen L.M., Poll L. Formation of volatile compounds in model experiments with crude leek (Allium ampeloprasum Var. Lancelot) enzyme extract and linoleic acid or linolenic acid. Journal of Agricultural and Food Chemistry. 2004;52(8):2315–2321. doi: 10.1021/jf030600s. [DOI] [PubMed] [Google Scholar]
- Niu W., Tian H., Zhan P. The effects of Pepper (Zanthoxylum bungeanum) from different production areas on the volatile flavor compounds of fried pepper oils based on HS-SPME–GC–MS and multivariate statistical method. Molecules. 2022;27(22):7760. doi: 10.3390/molecules27227760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Luo Y., Xie T., Yi L., Mao D., Hu J., Yu C. Current situation, problems and countermeasures of the fusion of hot pepper industry and big data in China. Journal of China Capsicum. 2019;17(03):35–39. doi: 10.16847/j.cnki.issn.1672-4542.2019.03.009. [DOI] [Google Scholar]
- Peng S., Xu J., Xu J., Wang J., Zhang Y., Liao X., Zhao L. Microbial community and volatile metabolites related to the fermentation degree of salted fermented chili peppers. LWT. 2023;181 doi: 10.1016/j.lwt.2023.114752. [DOI] [Google Scholar]
- Rotsatchakul P., Chaiseri S., Cadwallader K.R. Identification of characteristic aroma components of Thai fried chili paste. Journal of Agricultural and Food Chemistry. 2008;56(2):528–536. doi: 10.1021/jf072499n. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Oh W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: An overview. Journal of Food Bioactives. 2020;10:20–31. doi: 10.31665/JFB.2020.10224. [DOI] [Google Scholar]
- Shahidi F., Hossain A. Role of lipids in food flavor generation. Molecules. 2022;27(15):5014. doi: 10.3390/molecules27155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Zhang Y., Liu W., Shi Q., Liu B., Li H., Luo Y. Effects of exogenous inoculums on microbial community and volatile flavor compounds of fermented hot pepper. Food Science. 2023;44(10):132–141. doi: 10.7506/spkx1002-6630-20220601-005. [DOI] [Google Scholar]
- Wang J., Wang R., Xiao Q., Liu C., Deng F., Zhou H. SPME/GC-MS characterization of volatile compounds of Chinese traditional-chopped pepper during fermentation. International Journal of Food Properties. 2019;22(1):1863–1872. doi: 10.1080/10942912.2019.1684320. [DOI] [Google Scholar]
- Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS) Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Xi J., Xu D., Wu F., Jin Z., Yin Y., Xu X. The aroma compounds of Chinese steamed bread fermented with sourdough and instant dry yeast. Food Bioscience. 2020;38 doi: 10.1016/j.fbio.2020.100775. [DOI] [Google Scholar]
- Xiao H., Wang R., Chen M., Wang X., Yin H., Liu Y., Jiang L. Comparison and analysis of the flavor components of chopped pepper from farmhouses in different region of Hunan. Science and Technology of Food Industry. 2020;43(22):310–318. doi: 10.13386/j.issn1002-0306.2022010125. [DOI] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Fan Z., Chen R., He C., Wang Y. Effects of solid-state fermentation with Eurotium cristatum YL-1 on the nutritional value, total phenolics, isoflavones, antioxidant activity, and volatile organic compounds of black soybeans. Agronomy. 2021;11(6):1029. doi: 10.3390/agronomy11061029. [DOI] [Google Scholar]
- Xiao Y., Huang Y., Chen Y., Zhu M., He C., Li Z., Liu Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT. 2022;169 doi: 10.1016/j.lwt.2022.113925. [DOI] [Google Scholar]
- Xiao Y., Zhang S., Liu Z., Wang T., Cai S., Chu C., Yi J. Effect of inoculating Pichia spp. starters on flavor formation of fermented chili pepper: Metabolomics and genomics approaches. Food Research International. 2023;173 doi: 10.1016/j.foodres.2023.113397. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li L., Mac Regenstein J., Gao P., Zang J., Xia W., Jiang Q. The contribution of autochthonous microflora on free fatty acids release and flavor development in low-salt fermented fish. Food Chemistry. 2018;256:259–267. doi: 10.1016/j.foodchem.2018.02.142. [DOI] [PubMed] [Google Scholar]
- Xu X., Wu B., Zhao W., Lao F., Chen F., Liao X., Wu J. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.) Food Chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127512. [DOI] [PubMed] [Google Scholar]
- Yan Y., Duan G., Tang X., Yao H., Du M. Comparative analysis of the quality of chili sauce made from different varieties of pepper. Food and Fermentation Industries. 2023;49:225–232. doi: 10.13995/j.cnki.11-1802/ts.033344. [DOI] [Google Scholar]
- Yang J., Wu S., Mai R., Lin L., Zhao W., Bai W. Formation of amino acid-derived volatile compounds in dry-cured mackerel (Scomberomorus niphonius): Metabolic pathways involving microorganisms, precursors, and intermediates. Food Chemistry. 2021;364 doi: 10.1016/j.foodchem.2021.130163. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang T.T., Guo R.R., Ye Q., Zhao H.L., Huang X.H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chemistry: X. 2023;19 doi: 10.1016/j.fochx.2023.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Blank I., Wang B., Cao Y. Changes in odorants and flavor profile of heat-processed beef flavor during storage. Journal of Food Science. 2022;87(12):5208–5224. doi: 10.1111/1750-3841.16363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.