Abstract
We examined the influence of thrombin-induced platelet microbicidal protein 1 (tPMP-1) on the progression and hematogenous dissemination of experimental endocarditis caused by isogenic Staphylococcus aureus strains differing in tPMP susceptibility (tPMPs) or resistance (tPMPr) in vitro. Following simultaneous challenge of animals with both strains, significantly higher tPMPr bacterial densities were present in vegetations (P < 0.0001), kidneys (P < 0.0001), and spleens (P < 0.0001) compared with those for the tPMPs strain. These data indicate that tPMP-1 limits the intravegetation proliferation and hematogenous dissemination of a tPMPs strain in experimental endocarditis, while the tPMPr phenotype confers a selective advantage associated with the enhanced progression of this infection.
The role of platelets in infective endocarditis (IE) has traditionally been viewed as facilitating the induction and evolution of this infection (10–12, 14). However, it is now evident that the antimicrobial host defense role of platelets is a key factor in limiting IE (6, 7, 19, 23). This salutary effect of platelets is believed to be mediated, in part, by the secretion of a low-molecular-weight, cationic protein, termed thrombin-induced platelet microbicidal protein 1 (tPMP-1 [22]). This peptide exerts potent microbicidal activity and prolonged growth-inhibitory effects in vitro against common blood-borne pathogens (15), including Staphylococcus aureus, Staphylococcus epidermidis, viridans group streptococci, and Candida albicans (20–22, 24).
The potential role of tPMP-1 resistance as a factor in microbial virulence was suggested by our recent studies with a rabbit model of IE. Utilizing an isogenic S. aureus strain pair differing only in susceptibility or resistance to tPMP-1 in vitro, we observed that animals separately infected with a tPMPr S. aureus strain (ISP479R) achieved significantly higher vegetation bacterial densities than those observed with its isogenic tPMPs counterpart strain (ISP479C [7]). The current study was designed to examine the potential competitive advantage afforded by tPMP-1 resistance in terms of progression and hematogenous dissemination of these same S. aureus strains when the same experimental model of IE was cochallenged.
(Part of this study was presented at the 35th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., September 1997 [6a].)
Strain ISP479R, the isogenic, tPMPr variant of the tPMPs parental strain ISP479, was constructed by transposon mutagenesis with Tn551 as previously described (7) and contained an erythromycin resistance determinant. Strain ISP479C, used in this study, is the plasmid-cured, erythromycin-susceptible, tPMPs variant of ISP479. Detailed genotypic and phenotypic comparison of ISP479C and ISP479R strains revealed no detectable differences other than susceptibility to tPMP-1 in vitro (7).
The rabbit model of experimental IE was used in this study, as previously detailed (14). In brief, anesthetized rabbits underwent transcarotid-transaortic valvular catheterization with an indwelling, polyethylene catheter to induce sterile valvular vegetations. IE was produced by the intravenous (i.v.) injection of 3 × 106 CFU of the staphylococcal strain (ISP479C or ISP479R) at 24 h postcatheterization. In pilot studies in our laboratory, this inoculum was shown to cause experimental IE in 100% of catheterized rabbits challenged with either strain. A distinct group of animals with aortic catheters were coinoculated i.v. with 3 × 106 CFU of both the ISP479C and ISP479R strains, in separate ear veins (competition study). As controls in this latter investigation, parallel groups of animals were separately challenged with either the ISP479C or ISP479R strain as previously described (7).
To confirm that there were no substantial differences in bacteremia clearance or adherence to vegetations between the infecting strains, animals were cochallenged i.v. at 24 h postcatheterization with 3 × 107 CFU of both S. aureus ISP479C and ISP479R (as previously described for individual strains [4, 5, 7]). At 30 min postchallenge, animals were sacrificed, and all vegetations from individual animals were removed. Parallel plating of the tissue homogenates was then performed on antibiotic-free or erythromycin-containing (10 μg/ml) medium. The fact that ISP479C is susceptible to erythromycin (while ISP479R is resistant to this agent) was the basis for the differential quantification of each strain within the vegetations. Also, blood samples were obtained from catheterized rabbits at 1 and 30 min postchallenge for differential quantitative cultures as described above.
Animals infected with either strain ISP479C or ISP479R were sacrificed at 48 or 96 h postchallenge. Cardiac vegetations from individual animals were removed and quantitatively cultured as described above, with intravegetation staphylococcal densities expressed as CFU per milliliter (mean log10 ± standard deviation [SD]). In addition, kidneys and spleen were removed and quantitatively cultured. In animals cochallenged with strains ISP479C and ISP479R, parallel plating of tissue homogenates was performed with both antibiotic-free and erythromycin-containing media as described above. Since we have previously documented retention of both the tPMPs and tPMPr phenotypes in vivo over a 6-day postinfection period (7), such studies were not repeated.
To address the possibility that potential differences in bacterial proliferation observed in vivo were due to organism-mediated mechanisms (2, 3, 16), the growth kinetics of strains ISP479C and ISP479R, alone or in coculture, were compared in vitro. For these studies, organisms were inoculated (∼103 CFU/ml) into brain heart infusion (BHI) broth, nutrient broth, or Trypticase soy broth (all media were from Difco Laboratories, Detroit, Mich.) and monitored for CFU/ml at selected times ranging from 1 to 24 h, with constant rotary shaking at 37°C. This technique allowed maximal physical contact between the strains and ensured exposure of each strain to potential secretory factors affecting growth kinetics. Quantification of the proportion of viable ISP479C versus ISP479R organisms in coculture experiments was achieved by parallel plating onto BHI agar with or without erythromycin (10 μg/ml).
Differences in tissue or bacteremia densities or growth kinetics of the two strains were compared by the Kruskal-Wallis test. P values of ≤0.05 were considered to be statistically significant.
There were no significant differences between strains ISP479C or ISP479R in the magnitudes of either early bacteremia clearance or early bacterial adherence to sterile vegetations (Table 1). These findings are consistent with our prior observations made when these strains were used as individual challenge inocula in experimental IE (7).
TABLE 1.
Clearance of bacteremia and valvular adherence following simultaneous challenge with tPMPs and tPMPr S. aureus strainsa
| Strain | Bacteremia (mean log10 CFU/ml ± SD)
|
Adherence at 30 min (mean log10 CFU/vegetation ± SD) | |
|---|---|---|---|
| 1 min | 30 min | ||
| ISP479C | 4.95 ± 0.53 | 1.5 ± 0.71 | 1.65 ± 0.42 |
| ISP479R | 4.31 ± 0.67 | 1.73 ± 0.68 | 1.56 ± 0.53 |
Strains (3 × 107 CFU of each) were simultaneously injected via marginal ear veins into eight animals with indwelling transaortic catheters.
Animals challenged separately with the tPMPr strain exhibited significantly higher mean vegetation densities than those challenged with the tPMPs strain at both the 48 h (P = 0.008) and 96 h (P = 0.003) time points (Table 2). Similarly, in the cochallenge study, vegetation densities of tPMPr were significantly higher than those of the tPMPs strain at both 48 and 96 h after challenge (P = 0.0001) (Table 3).
TABLE 2.
Comparative S. aureus densitiesa
| Source of culture and time (h) cultured | Log10 CFU/g of tissue ± SD (n) for indicated strain
|
P valueb | |
|---|---|---|---|
| ISP479C | ISP479R | ||
| Vegetation | |||
| 48 | 6.5 ± 0.84 (11) | 7.8 ± 0.76 (8) | 0.008 |
| 96 | 6.47 ± 1.56 (7) | 8.71 ± 0.05 (11) | 0.003 |
| Kidney | |||
| 48 | 5.18 ± 3.4 (11) | 4.61 ± 0.9 (8) | NS |
| 96 | 3.94 ± 2.1 (7) | 5.4 ± 4.0 (11) | NS |
| Spleen | |||
| 48 | 4.34 ± 2.4 (11) | 4.6 ± 0.78 (8) | NS |
| 96 | 4.73 ± 2 (7) | 6.85 ± 0.85 (11) | 0.014 |
Animals were infected separately with a 95% infective dose of inoculum for either strain (∼3 × 106 CFU).
NS, nonsignificant.
TABLE 3.
Comparative S. aureus densitiesa
| Source of culture and time (h) cultured | Log10 CFU/g of tissue ± SD (n = 9) for indicated strain
|
|
|---|---|---|
| ISP479C | ISP479R | |
| Vegetation | ||
| 48 | 0.57 ± 0.50 | 8.12 ± 0.72 |
| 96 | 0.52 ± 0.11 | 7.70 ± 1.42 |
| Kidney | ||
| 48 | 0.41 ± 0.19 | 5.34 ± 0.54 |
| 96 | 0.91 ± 0.17 | 5.60 ± 0.84 |
| Spleen | ||
| 48 | 0.28 ± 0.04 | 5.33 ± 0.25 |
| 96 | 0.80 ± 0.60 | 4.60 ± 2.44 |
Animals were infected simultaneously with a 95% infective dose of inoculum for each strain (∼3 × 106 CFU). P was <0.0001 for all comparisons.
Among animals challenged individually with either the tPMPs or tPMPr strain, there were no statistically significant differences between the frequencies (data not shown) or magnitudes of renal or splenic dissemination for the two strains at 48 h postchallenge (Table 2). However, the tPMPr strain achieved significantly higher splenic densities than the tPMPs strain by 96 h postchallenge (P = 0.014). Following coinoculation, bacterial densities of the tPMPr strain were significantly higher than those of the tPMPs strain, both in the kidneys and the spleen, (P = 0.0001) at 48 and 96 h postchallenge.
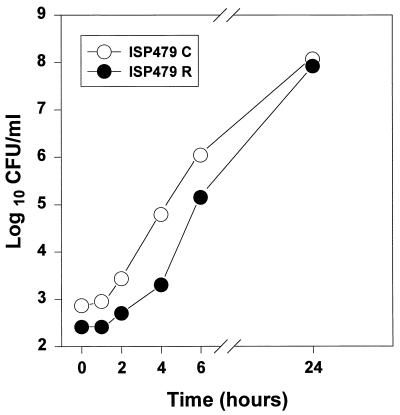
No differences in the growth kinetics of either strain ISP479C or strain ISP479R were observed during coculturing in vitro versus individual growth curves (Fig. 1).
FIG. 1.
Comparison of S. aureus ISP479C and ISP479R growth kinetics in vitro. Log-phase organisms were dually inoculated (103 CFU of each strain) into 10 ml of BHI broth, and the cultures were incubated on a rotary shaker (for aeration) at 37°C. At selected time points, aliquots were quantitatively cultured on BHI agar with or without erythromycin (10 μg/ml; as described in Materials and Methods). Data points represent the mean values from two independent experiments performed in duplicate. In no case was the deviation from the mean greater than 0.2 log CFU/ml; error bars were intentionally excluded to improve clarity. These results mirrored those of identical dual-culture experiments performed with nutrient or Trypticase soy broth (see Materials and Methods). These results were not significantly different from those for either organism cultured alone (data not shown).
The antimicrobial host defense function of platelets is believed to occur principally through the release of endogenous platelet microbicidal proteins (PMPs) (6, 17, 19, 22). This may be amplified at sites of endothelial cell colonization or damage by microbial pathogens, leading to thrombin generation and subsequent procoagulant activity (1, 8, 9). Thrombin is, in turn, a potent agonist for PMP release (tPMP-1) from platelets (22). Conceptually, it follows that pathogens intrinsically resistant to tPMP-1 may circumvent the mitigating effects of tPMP-1 in vivo and achieve relatively greater proliferation within cardiac vegetations. Several lines of experimental and clinical evidence support this hypothesis. The antimicrobial function of platelets in IE (presumably through tPMP-1 secretion) was suggested by the finding that thrombocytopenic animals challenged with a tPMPs viridans streptococcal strain had higher bacterial densities in endocardial vegetations than did animals with normal platelet counts (17). In addition, a tPMPs Candida strain caused significantly less-severe IE than its genetically related tPMPr counterpart (23). Furthermore, clinical bloodstream isolates, which are susceptible to tPMP-1 in vitro, are infrequently associated with IE (18).
We recently studied an isogenic S. aureus strain pair differing solely in their in vitro susceptibility or resistance to tPMP-1 to show a selective advantage for the proliferation of the tPMPr strain within endocardial vegetations (7). These prior studies, and those of Dankert et al. (6), strongly suggested that the fundamental influence of tPMP-1 resistance in IE involved one or more postvalvular adherence events. Our current study focused on the impact of the tPMP-1 resistance phenotype on intravegetation microbial proliferation as well as on hematogenous dissemination to target organs. A key strategy in the current study was to use the same isogenic strain pair and animal model described above (7) but to examine the outcome of cochallenge with both the tPMPs and tPMPr strains in this model. This approach facilitated a direct comparison of the relative competitive survival advantage in the setting of IE conferred to S. aureus by resistance to tPMP-1.
Results of the current investigations support the concept that the influence of tPMP-1 in mediating antimicrobial host defense may differ in distinct vascular compartments. In these studies, the tPMPr strain significantly outcompeted the tPMPs strain in all tissues examined, but to different extents. There are several potential explanations for these observations. Our studies confirmed that the differences between tPMPs and tPMPr strain proliferation in vegetation, kidney, or spleen were not due to disparities in initial valvular colonization or in hematogenous seeding to these organs. Furthermore, we found no in vitro evidence that the tPMPr strain directly inhibits the growth of the tPMPs strain. Therefore, the most likely explanation for the dramatic differences in the proliferation of the tPMPs and tPMPr strains within target tissues in this model is the difference in their abilities to survive in the presence of tPMP-1 when they are competing for the same anatomic niche. Thus, it is reasonable to speculate that even modest tPMP-1-mediated growth inhibition of the tPMPs strain would significantly magnify the capacity of the tPMPr strain to utilize available colonization sites and nutrients to achieve unimpeded proliferation.
The splenic environment represents a rich and diverse repertoire of host defense modalities (e.g., macrophages, antibody, complement). Our finding that the tPMP-1 resistance phenotype also provided the infecting strain a time-dependent survival advantage in the spleen underscores the likely importance of this phenotype in establishing infection in multiple and distinct vascular sites. The fact that equivalent densities of the tPMPs and tPMPr strains were found in the spleen in the individual challenge studies at 48 h argues against the possibility that this organ was differentially seeded with distinct tPMPr versus tPMPs populations.
Hypertonic conditions, such as those that exist in the kidney, are known to inhibit PMP antimicrobial activities in vitro (13). Thus, the microenvironment of the kidney may dampen the effects of PMPs on microbial survival and proliferation in this milieu. These concepts provide a possible explanation for the observations that, when present individually, the tPMPr strain achieved greater proliferation in cardiac vegetations and spleen, but not in the kidney, than the tPMPs strain.
Collectively, our current findings support the evolving concept that the tPMP-1 susceptibility phenotype plays an integral role in determining the overall virulence of an organism in distinct vascular environments.
Acknowledgments
We thank Nimee Bhat and Deborah Kahler for assistance in the studies. We thank Ambrose Cheung (Rockefeller University, New York, N.Y.) for assistance in the genotypic and phenotypic characterization of the strains used in this study.
This research was supported in part by the following grants from the National Institutes of Health: RCMI G12 RR03026-09 (to V.K.D.), RO1-AI39108 (to A.S.B.), and R29-AI39001 (to M.R.Y.). M.R.Y. was also supported by a grant-in-aid from the American Heart Association (National Center; 95-01-2620).
REFERENCES
- 1.Azizi N, Li C, Shen A J, Bayer A S, Yeaman M R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Staphylococcus aureus elicits release of platelet microbicidal proteins in vitro, abstr. G-54; p. 153. [Google Scholar]
- 2.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramley A J, Patel A H, O’Reilly O, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M C, Bayer A S. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung A L, Yeaman M R, Sullam P M, Witt M D, Bayer A S. Role of sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect Immun. 1994;62:1719–1725. doi: 10.1128/iai.62.5.1719-1725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dankert J, van der Werff J, Zaat S A J, Joldersma W, Klein D, Hess J. Involvement of bactericidal factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect Immun. 1995;63:663–671. doi: 10.1128/iai.63.2.663-671.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Dhawan V K, Bayer A S, Yeaman M R. Program and Abstracts of the 35th Annual Meeting of the Infectious Diseases Society of America. Calif: San Francisco; 1997. Influence of in vitro susceptibility to thrombin-induced platelet microbicidal protein (t-PMP-1) on the progression of experimental Staphylococcus aureus endocarditis, abstr. 266; p. 120. [Google Scholar]
- 7.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake T A, Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989;57:507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 10.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 11.Herzberg M, MacFarlane G D, Gong K E, Amstrong N N, Witt A R, Erickson P R, Meyer M W. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect Immun. 1992;60:4809–4818. doi: 10.1128/iai.60.11.4809-4818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzberg M C, Gong K, MacFarlane G D, Erickson P R, Soberay A H, Kresbach P H, Manjula G, Schilling K, Bowen W H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990;58:512–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo S-P, Yeaman M R, Bayer A S. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect Immun. 1996;64:3758–3764. doi: 10.1128/iai.64.9.3758-3764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlman B B, Freedman L S. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet D, Wenzel R P. Nosocomial blood stream infections—secular trends in rates, mortality and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 16.Sahl H-G, Brandis H. Production, purification, and chemical properties of an anti-staphylococcal agent produced by Staphylococcus epidermidis. J Gen Microbiol. 1991;127:377–384. doi: 10.1099/00221287-127-2-377. [DOI] [PubMed] [Google Scholar]
- 17.Sullam P M, Frank U, Tauber M G, Yeaman M R, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among staphylcoccal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 20.Yeaman M R, Ibrahim A, Filler S G, Bayer A S, Edwards J E, Jr, Ghannoum M A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effects in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial purification and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman M R, Soldan S S, Ghannoum M A, Edwards J E J, Filler S G, Bayer A S. Resistance to platelet microbicidal protein (tPMP) results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman M R, Sullam P M, Dazin P F, Bayer A S. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect Immun. 1994;62:3416–3423. doi: 10.1128/iai.62.8.3416-3423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]