Abstract
The demand for protein is increasing with an expanding world population and is influencing the rapid growth of fish and animal agriculture. These sectors are becoming a significant source of water pollution and need to develop environmentally sustainable techniques that are cost-effective, ideally with potential for downstream value-added production. This study investigated the potential of one of the fastest-growing cyanobacterial species, Synechococcus elongatus UTEX 2973, for bioremediation of mixed wastewater (combination of sturgeon and swine wastewater). Three different mixing ratios (25:75, 50:50, and 75:25 sturgeon:swine) were compared to find a suitable combination for the growth of S. elongatus as well as carbohydrate accumulation in biomass. The final biomass production was found to be 0.65 ± 0.03 g Dry cell Weight (DW)/L for 75%–25 %, 0.90 ± 0.004 g DW/L for 50%–50 %, and 0.71 ± 0.04 g DW/L for 25%–75 % sturgeon-swine wastewater combination. Cyanobacteria cultivated in 50%–50 % sturgeon-swine wastewater also accumulated 70 % total carbohydrate of DW, whereas 75%–25 % sturgeon-swine and 25%–75 % sturgeon-swine accumulated 53 % and 45 %, respectively. Subsequently, the S. elongatus cells were grown in a separate batch of 50%–50 % sturgeon-swine wastewater and compared with cells grown in BG11 synthetic growth media. Cultivation in BG11 resulted in higher biomass production but lower carbohydrate accumulation than 50%–50 % mixed wastewater. Final biomass production was 0.85 ± 0.08 g DW/L for BG11 and 0.65 ± 0.04 g DW/L for 50%–50 % sturgeon-swine wastewater. Total carbohydrate accumulated was 75 % and 64 % of DW for 50%–50 % sturgeon-swine mixed wastewater and BG11 growth media, respectively, where glycogen was the main carbohydrate component (90 %). The nutrient removal efficiencies of S. elongatus were 67.15 % for orthophosphate, 93.39 % for nitrate-nitrite, and 97.98 % for ammonia. This study suggested that S. elongatus is a promising candidate for enabling simultaneous bioremediation of mixed wastewater and the production of value-added biochemicals.
Keywords: Wastewater treatment, Nutrient removal, Cyanobacteria, Carbohydrate, Biomass growth
Graphical abstract
Highlights
-
•
S. elongatus has the potential to be used as a feedstock for sugar production.
-
•
Combinations of swine and aquaculture wastewater were used as the growth media.
-
•
50%–50 % mixed wastewater resulted in higher carbohydrate content in biomass.
-
•
NH4+, NO3−, and PO43−contents were reduced in bioremediation using S. elongatus.
1. Introduction
Producing enough food to feed the global population is one of humanity's most substantial challenges in the upcoming decades. Rapid growth has led experts to estimate that by 2050, global food production must be doubled to meet consumption needs [1]. Production from traditional agriculture, such as swine and other livestock, will need to increase due to changes in meat-rich diets. Pork comprised about 34.46 % of the total meat consumed worldwide in 2021 (122.5 million tonnes, mmt) [2]. According to United States Department of Agriculture (USDA), pig consumption is projected to increase 17.3 % between 2021 and 2030 [3]. The increase in production will increase wastewater generation, which has a negative effect on the environment as it contains fecal matter, pig urine, and floor-washing water [4]. Earth-walled lagoons are the most widely used treatment and storage of wastewater from swine farms. Bacterial consortia degrade organic wastes in the lagoons into fertilizer and biogas [5]. However, lagoon wastewater contains high concentrations of inorganic nutrients such as ammonia and phosphate, which can cause water contamination due to soil permeability or runoff during storms.
Aquaculture is also a growing agricultural field, which will play a vital role in meeting the increasing seafood demand worldwide. It is the fastest-growing agricultural sector and has great potential to produce protein-rich food on a large scale [6]. In particular, finfish such as sturgeon are widely cultivated around the world, and managing nutrients in this and similar effluents is critical to ensure sustainability. With the increase in production required to support this rapidly growing protein sector [6], aquaculture is inclining towards the intensive practice known as recirculating aquaculture systems (RAS). As this intensive practice is developed and implemented, an inevitable increase in wastewater also potentially harms the environment [7,8]. The wastewater contains inorganic nutrients, suspended solids, and dissolved organic matter that are predominantly sourced from fish excreta and uneaten fish feed [9,10]. RAS are used by farmers across Europe, the United States, and China [[11], [12], [13]]. These systems use biofiltration to remove ammonia from wastewater effectively [11,14]. Biofiltration uses nitrifying bacteria on plastic media for nitrogen removal in RAS to reduce the inorganic nitrogen to molecular nitrogen (N2) [13]. While this method can obtain a satisfactory reduction of nutrients, the filtration process still results in some wastewater discharge along with intensive electric costs, plastic waste, and leaching of microplastics into the environment [13,15].
Hence, one of the major concerns regarding wastewater generated from the above-mentioned agricultural sectors is the harmful inorganic nutrients such as ammonia, nitrate, and phosphate. The ammonia concentration in swine wastewater is generally higher than in aquaculture wastewater [16,17], whereas the nitrate concentration is higher in aquaculture wastewater [18]. Moreover, the concentrations of these different nutrients may vary with respect to time, animal numbers, feed quality, and waste treatment facility. The existing techniques for wastewater mitigation are effective, but as these sectors grow, environmental concerns continue to increase. The need for effective and sustainable wastewater treatments is of great importance for these systems to ensure the safe disposal of wastewater into the environment. Improper disposal of these wastewaters can cause groundwater contamination, odor problems, health hazards including the release of harmful pathogens and hormones, and eutrophication resulting in harmful algal blooms and ‘dead zones’ in aquatic ecosystems [5,[19], [20], [21], [22]]. Though this untreated wastewater is detrimental to the environment, it can be viewed as a resource if the nutrients are appropriately utilized [23]. Therefore, mitigating these wastes and producing value-added commodities in the same process is desirable. Hence, new technologies to treat these different wastewaters effectively and sustainably are being investigated [[24], [25], [26]].
Photosynthetic microorganisms such as algae and cyanobacteria (also known as blue-green algae) have been increasingly used in recent decades to treat different types of wastewaters such as aquaculture [[27], [28], [29], [30], [31]], swine [32], and sewage wastewater [33,34] to reduce inorganic nutrients, total dissolved solids, biological oxygen demand, and other harmful material. They can effectively uptake atmospheric carbon dioxide and nutrients from wastewater and convert them to cellular storage materials such as carbohydrates, lipids, and proteins in their biomass. The use of photosynthetic microorganisms in wastewater treatment has the combined advantage of bioremediation of wastewater and the production of valuable biomass enriched in carbohydrates and protein. As an added benefit, this biomass can provide economic benefits in downstream biotechnological applications, such as biofuel (biodiesel, bioethanol, bio-oil) and bioplastic production [31,35,36]. Cyanobacteria and microalgae also have unique secondary metabolisms that can be utilized in production of biological hydrogen, bioactive compounds, fertilizer, emulsifiers, and food [31,35,37,38]. Moreover, replacing the expensive growth media with wastewater can reduce biomass production costs for synthesizing these value-added compounds [39,40].
The focus of this study was to convert waste into a valuable resource. In this study, the fast-growing cyanobacterial species Synechococcus elongatus UTEX 2973 [41] was used for the bioremediation of wastewater. This species has previously been studied for enhanced sugar production [42] and salt tolerance [43]. The growth of this cyanobacterial species in wastewater sources has not yet been tested. Rueda et al. [44] studied cyanobacterial bioremediation of agricultural runoff and reported significant removal of nutrients along with carbohydrate accumulation. Agricultural runoff could vary widely depending on landscape, source, and region [45] whereas this study examined mixing very specific agricultural waste sources (sturgeon and swine wastewater). Sturgeon was selected as a representative and widely cultivated finfish and used as a proxy for aquaculture effluent more generally. The present study investigated three different sturgeon and swine wastewater mixtures as the growth media to find a balanced combination to support growth. Carbohydrate accumulation was also examined as a potential feedstock for valorization and compared with synthetic growth medium BG11. Subsequently, the nutrient removal efficiency of this species from mixed wastewater was also evaluated.
2. Methodology
2.1. Cyanobacterial cultivation
The cyanobacterial species S. elongatus UTEX 2973 was obtained from the Culture Collection of Algae at the University of Texas, Austin (UTEX) and was maintained in BG11 growth media at 30 °C (as the lowest feasible cultivation temperature for this strain) and irradiance of 65 μE. BG11 medium consists of the following components: NaNO3 (1.5 g), CaCl2.2H2O (0.036 g), MgSO4.7H2O (0.075 g), K2HPO4 (0.04 g), citric acid (0.006 g), ferric ammonium citrate (0.006 g), EDTA (0.001 g), Na2CO3 (0.02 g), and trace metal mix A5 (1 mL) per 1 L deionized water. Trace metal mix contains H3BO3 (2.86 g), MnCl2.4H2O (1.81 g), ZnSO4.7H2O (0.222 g), NaMoO4.2H2O (0.39 g), CuSO4.5H2O (0.079 g), and Co(NO3)2.6H2O (49.4 mg) per 1 L deionized water [46]. The inoculum used for experiments was prepared using BG11 growth media.
2.2. Wastewater collection and preparation
Aquaculture wastewater was collected from the North Carolina Marine Aquaculture Research Center (MARC) situated in Smyrna, North Carolina. This facility cultures red drum, white drum, hybrid striped bass, and sturgeon. Wastewater from this facility goes through a treatment process consisting of a mechanical filter followed by a biofilter. Wastewater was collected during the summer of 2021 through backwashing from the sturgeon tank.
Swine wastewater was collected from the primary lagoon of the swine education unit of the Lake Wheeler Road Field Laboratory at North Carolina State University. The wastewater from the barns flows directly into the primary lagoon.
Pretreatment of wastewater was carried out by sedimentation and centrifugation to separate large, non-soluble particulate solids. The supernatant from the centrifuge was then sterilely filtered using a two-step filtering process, first with 0.45 μm and then 0.2 μm, to remove microorganisms.
2.3. Experimental design
The study was comprised of two steps (Fig. 1), where the first step was to select a suitable ratio of a mixture of sturgeon wastewater and swine wastewater to support the growth and carbohydrate accumulation of S. elongatus. In the first set of experiments, S. elongatus UTEX 2973 was cultivated in three different ratios (25%–75 %, 50%–50 %, and 75%–25 %) of mixed wastewater containing sturgeon and swine wastewater. Preliminary experiments found that 100 % sturgeon wastewater was insufficient to support growth, necessitating supplementation with an alternative nutrient source. The different wastewater ratios were used as the growth medium for S. elongatus cultivation and were cultured for 13 days at a temperature of 30 °C and irradiance of 65 μE on a shaker at 150 rpm inside an AlgaeTron 230 photo incubator (Photon Systems Instruments) under atmospheric CO2. 100 mL of wastewater and 2 mL of seed culture were introduced to a 250 mL Erlenmeyer flask. The seed inoculum was prepared in BG11 media using the culture conditions described in section 2.1 and used on day 10 at an optical density of 1.18. A lower ratio of seed: wastewater was used to obtain a high-resolution, detailed growth curve over 13 days. All experiments were performed in triplicate using the same S. elongatus inoculum source.
Fig. 1.
Experimental design to examine biomass production and carbohydrate accumulation of S. elongatus firstly, comparing three different combinations of swine and aquaculture wastewater, and secondly, comparing the selected optimal wastewater combination from the first step with BG11.
Secondly, growth and carbohydrate accumulation in S. elongatus biomass in the selected optimal mixed wastewater growth media was compared with a synthetic growth media BG11. The swine wastewater used in this experiment was collected from different points of the same lagoon with approximately one month interval between sampling during summer 2021. The potential of S. elongatus for nutrient removal from wastewater was also studied in this step (Fig. 1). The biomass was grown for 14 days. The experiments were performed in quadruplicate with culture conditions such as temperature, irradiance, and shaking speed remaining the same as the first experiment.
2.4. Analytical methods
2.4.1. Biomass growth and harvest
Growth curves were obtained by measuring the optical density of biomass at 730 nm (Genesys 10S UV–Vis). A biomass dry weight and optical density correlation was constructed by preparing a series of dilutions of S. elongatus cells and then measuring the corresponding optical densities and dry cell weight [47]. The correlation curve was used to determine the dry weight of biomass for carbohydrate analysis (data available). The average growth rate was determined from the slope of the linear trendline of the semi-log growth curve, and the maximum growth rate was determined using the first three to four days of the early exponential phase showing the most rapid growth. The biomass was harvested using a centrifuge (Eppendorf 5920R outfitted with Rotor S-4x1000) at 4 °C at 4000 rpm for 30 min and dried in a drying oven at 80 °C for 24 h for further analysis.
2.4.2. Bioremediation
Analysis of nutrients in the wastewater was measured before and after a 14 day culture period. The filtered wastewater was analyzed colorimetrically for ammonia nitrogen (NH4+-N), orthophosphate (PO43--P), and nitrate-nitrite (NOx−-N) with an AQ 400 Discrete Analyzer (EPA-114-C Rev. 1A, EPA-129-C Rev. 1, and EPA-146-A Rev. 0).
An uninoculated wastewater control was used to investigate the abiotic removal or conversion of nutrients present in the wastewater in the same culture conditions alongside inoculated cultures. It has been reported in previous studies that at a culture condition of nearly neutral pH, the chemical processes of volatilization of ammonia and precipitation of phosphates are typically insignificant [[48], [49]]. However, the pH of the wastewater was 8.93 in the beginning and 9.4 after the 14 day culture period, which was high enough that ammonia volatilization and phosphate precipitation could contribute to the decrease in ammonia and phosphate concentrations. Thus, the nutrient removal by abiotic processes was determined using equation (1) below:
| (1) |
where initial indicates the concentration of ammonia, nitrate, or phosphate at day 0, and available represents the concentrations present in the control wastewater at day 14. This calculation accounts for the significant removal of nutrients that could take place due to abiotic processes. These available concentrations were used to determine the biological nutrient removal rates according to equation (2) below:
| (2) |
here the available nutrients are the nutrients that were present in the uninoculated control after day 14, and the remaining nutrients were the nutrients present in the culture solution after day 14.
2.4.3. Carbohydrate analysis
The carbohydrate content in S. elongatus was measured using the anthrone method [47,50]. Carbohydrates were dehydrated with concentrated H2SO4 to form furfural (a colorless organic compound) and condensed with anthrone reagent (an aromatic ketone) to form a green color complex that was measured colorimetrically at 620 nm. The concentration of carbohydrates was determined from a standard curve with known concentrations of glucose.
2.4.4. Statistical analysis
An ANOVA test was used to determine the differences in biomass generation and carbohydrate accumulation among the three different wastewater mixtures tested. Based on the results of the ANOVA test, TukeyHSD tests were used to identify significantly different treatments at 95 % confidence interval (alpha = 0.05). The second set of experiments consisted of one treatment group (selected mixed wastewater) and one control group (BG11). T-tests were used to compare differences in biomass generation and carbohydrate accumulation.
3. Results and discussion
3.1. Characterization of sturgeon and swine wastewaters
In a recirculating aquaculture system, the nutrient concentration depends on several factors such as the species type, the fish density, and the type of fish feed. The sturgeon wastewater used in this study was characterized for ammonia, nitrate, and phosphate, which are crucial for the propagation of S. elongatus 2973. The nitrate-N + nitrite-N, ammonia-N, and orthophosphate-P concentrations were observed to be 20.905 ± 1.34 mg N/L, 10.074 ± 0.66 mg N/L, and 4.465 ± 2.07 mg P/L, respectively. However, initial testing found that the sturgeon wastewater was not adequate for S. elongatus growth beyond four days, likely due to nitrogen depletion (data available). A previous study has supplemented aquaculture wastewater with additional nitrate to maintain the growth of the microalgal species Chlorella sorokiniana [18]. Rather than directly adding nitrate in the form of a chemical salt, swine wastewater was used in this study to provide a supplemental nitrogen source. Swine wastewater was also characterized in a similar manner (Table 1). The concentration of ammonia and orthophosphate was substantially higher in swine wastewater than in sturgeon wastewater while nitrate-nitrite concentration was lower. No considerable difference was found in pH.
Table 1.
Characterization of sturgeon wastewater and swine wastewater.
| Growth Media | Ammonia-N (NH4+-N) mg N/L | Nitrate-N + Nitrite-N (NO2− –N/NO3− –N) mg N/L | Orthophosphate-P (PO43--P) mg P/L | pH |
|---|---|---|---|---|
| Sturgeon wastewater (Mean ± SDa) | 10.07 ± 0.66 | 20.90 ± 1.34 | 4.46 ± 2.07 | 8.99 |
| Swine wastewater (Mean ± SDa) | 126.48 ± 2.66 | 0.49 ± 0.10 | 16.08 ± 0.82 | 8.77 |
SD = Standard deviation.
3.2. Comparison of different compositions of wastewater as growth media
3.2.1. Analysis of S. elongatus UTEX 2973 growth in different mixed wastewater concentrations
S. elongatus UTEX 2973 is one of the fastest growing unicellular cyanobacterial species that has been reported [41,51] and has green pigment, mostly chlorophyll a [52]. Absorbance at 730 nm was used as a proxy for cell growth (turbidity). As previously stated in experimental design, this species was grown in three different combinations of sturgeon and swine wastewater for 13 days. These combinations of wastewater were characterized for ammonia nitrogen and nitrate-nitrite (Table 2).
Table 2.
Concentrations of Ammonia nitrogen (NH4+-N) and Nitrate-nitrite (NO2− –N/NO3−-N) in different wastewater ratios (phosphate measurements were not obtained for these samples).
| Wastewater ratios as growth media | NH4+-N (mg N/L) | NO2− –N/NO3− –N (mg N/L) |
|---|---|---|
| 25%–75 % Sturgeon-swine wastewater | 95.38 | 5.25 |
| 50%–50 % Sturgeon-swine wastewater | 67.43 | 10.47 |
| 75%–25 % Sturgeon-swine wastewater | 39.29 | 15.51 |
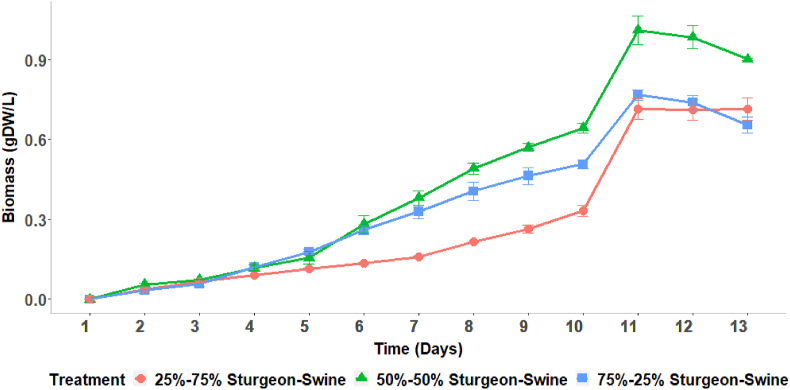
The 25%–75 % sturgeon-swine wastewater had high ammonia and low nitrate whereas 75%–25 % sturgeon-swine wastewater had low ammonia and high nitrate. As expected, 50%–50 % sturgeon-swine wastewater contained moderate concentrations of both ammonia and nitrate. Cell growth in these three wastewater mixes is shown in Fig. 2. In all three wastewater conditions, the lag phase was shorter than 24 h. Hence, the exponential growth phase started almost immediately and lasted three to four days. The growth rates were reported as mean ± standard deviation, and maximum growth rates were found to be 0.468 ± 0.018 day−1 for 75%–25 % sturgeon-swine during the first four days, 0.540 ± 0.013 day−1 for 50%–50 % sturgeon-swine during the first three days, and 0.418 ± 0.019 day−1 for 75%–25 % sturgeon-swine during the first four days. Growth continued more gradually until day 11 with a sudden spike in growth from day 10–11. Cells may have taken up some alternate nutrients in the wastewater after the decrease in ammonium, nitrate, and/or phosphate, or they may have been utilizing intracellular metabolites for growth [53]. Once these nutrients and/or metabolites were depleted, cell growth stabilized in stationary phase and began to decrease.
Fig. 2.
S. elongatus UTEX 2973 biomass accumulation on a dry weight basis over the growth period in three different mixed wastewater growth media in triplicates with standard deviation.
The average growth rate (from day 1–11) was observed to be 0.177 ± 0.003 day−1 when S. elongatus 2973 was grown in 75%–25 % sturgeon-swine wastewater. Relatively lower average growth rates were found for 50%–50 % sturgeon-swine and 25%–75 % sturgeon-swine (0.173 ± 0.002 day−1 and 0.154 ± 0.002 day−1, respectively). Biomass growth rates in 25%–75 % sturgeon-swine was significantly higher than the other two treatment processes (p < 0.05). However, the total biomass accumulation was higher for 50%–50 % sturgeon-swine wastewater than 75%–25 % sturgeon-swine wastewater at the end of the experiment. Some inconsistency in shaking, CO2 supply, and irradiance occurred during the first four days of this experiment due to power outages. These outages altogether were roughly 6 h during the 13-day experiment (∼2 % of the total experimental time period) and were not expected to substantially impact the results.
The concentration of NH4+-N in 75%–25 % sturgeon-swine wastewater was lower and nitrate-N was higher than in the other two treatment processes. Higher ammonia concentration appeared to negatively correlate with growth, as observed in the 25%–75 % sturgeon-swine wastewater. ANOVA analysis was carried out to compare the biomass accumulation among the treatment groups at the end of the experiment. It was found that different wastewater concentrations had a significant impact (p < 0.05) on biomass accumulation.
For further comparison, the TukeyHSD test was used, and it was observed that there was no significant difference in end of experiment biomass accumulation between 25% and 75 % sturgeon-swine and 75%–25 % sturgeon-swine wastewater (p > 0.05). However, 50%–50 % sturgeon-swine wastewater accumulated significantly higher biomass (p < 0.05). The concentrations of both nitrogen sources were adequate in 50%–50 % sturgeon-swine wastewater and offered the highest biomass accumulation among these three treatment groups. Thus, 50%–50 % sturgeon-swine was identified as the most suitable growth media for S. elongatus UTEX 2973 among the three mixed wastewater growth media.
3.2.2. Analysis of total carbohydrate storage in different mixed wastewater concentrations
Total carbohydrate content was measured from the harvested biomass to further investigate its potential for sugar production. Song et al. [42] and Lin et al. [54] studied S. elongatus UTEX 2973 as a potential for sugar feedstock production when grown in BG11 synthetic growth media. Synechococcus has been shown to accumulate carbohydrates as a response to stress such as nitrogen limiting conditions [55]. Therefore, it is essential to investigate the total carbohydrate accumulation in biomass when grown in wastewaters with different nitrogen sources and concentrations. The total carbohydrate content of S. elongatus UTEX 2973 varied significantly among the treatment groups (Fig. 3). Carbohydrate was significantly higher (approximately 70 % of the dry cell weight) when 50%–50 % sturgeon-swine wastewater was used as the growth media (p < 0.05) compared to the other two treatment groups, which were 45 % and 53 % (p > 0.05).
Fig. 3.
Comparison of percentage of total carbohydrate accumulation in S. elongatus UTEX 2973 biomass on a dry weight basis after 13 days of culturing in three different mixed wastewater treatment groups in triplicate (ns denotes p > 0.05, ***p < 0.001, and ****p < 0.0001).
Usually, there is a tradeoff between carbohydrate accumulation and biomass growth. Nitrogen stress can stimulate accumulation of more carbohydrates [42]. However, nitrogen is also a macro-nutrient to support biomass growth. As previously discussed, the concentration of both the nitrogen sources in the wastewater fell in the middle for 50%–50 % sturgeon-swine. This combination supported the growth of the species in the beginning, and after day 11 a decrease in cell growth was observed which indicated the cells were stressed and stored higher carbohydrates at the end (nutrient depletion has been shown to cause a similar tradeoff between growth rate and carbohydrate accumulation in the microalga Chlorella zofingiensis [56]). Therefore, after comparing biomass generation and total carbohydrate storage, 50%–50 % sturgeon-swine wastewater was selected as the optimal wastewater mixture and further compared with the synthetic growth medium BG11.
3.3. Comparison of optimal wastewater composition with synthetic growth medium BG11
3.3.1. Analysis of biomass growth
The nutrient concentrations and pH of 50%–50 % sturgeon-swine wastewater and BG11 are shown in Table 3. A key difference between these two growth media was the nitrogen sources. Nitrogen content was higher in BG11 with nitrate as the primary source, whereas 50%–50 % sturgeon-swine wastewater included both ammonia and nitrate. The orthophosphate concentration and pH were higher in wastewater.
Table 3.
Concentration of nutrients and pH in 50%–50 % sturgeon-swine wastewater and BG11.
| Growth Media | Ammonia-N (NH4+-N) mg N/L | Nitrate-N + Nitrite-N (NO2− –N/NO3− –N) mg N/L | Orthophosphate-P (PO43--P) mg P/L | pH |
|---|---|---|---|---|
| BG11 | 0.05 | 235.58 | 6.73 | 7.86 |
| 50%–50 % sturgeon-swine wastewater | 69.95 | 10.57 | 8.90 | 8.93 |
The performance of S. elongatus UTEX 2973 in 50%–50 % sturgeon-swine wastewater as a growth medium was compared with BG11, a synthetic growth medium commonly used to support the growth of cyanobacteria and microalgae (Fig. 4). The higher concentration of nitrate in BG11 compared to mixed wastewater could be one of the reasons for a higher growth rate of 0.162 ± 0.002 day−1 (p < 0.05). The growth rate was 0.146 ± 0.006 day−1 when 50%–50 % sturgeon-swine wastewater was used as the growth medium. This growth rate was statistically lower than the growth rate for the 50%–50 % mixture tested in the previous step (p < 0.05). As experimental parameters were kept the same across experiments, this difference is most likely due to differences among wastewater batches, which is an important observation for further applications to real-world scenarios where wastewater typically varies not only by source but also by season and batch. In this second batch, the concentration of ammonia was slightly higher in 50%–50 % sturgeon-swine wastewater than in the first batch collected (69.95 mg N/L in Table 3 compared with 67.43 mg N/L in Table 2). As previously observed, the higher ammonia concentration might negatively affect growth. The swine wastewater used to prepare the mixed wastewater for this experiment was collected from a different point of the lagoon at a different time; another possible reason could be that the wastewater used to culture S. elongatus contained another limiting substrate (e.g., differing concentrations of metals, ions, or other inhibitory compounds) that slowed down the growth process. The biomass produced at the end of the culturing period was 0.85 ± 0.08 g DW/L for BG11 and 0.65 ± 0.04 g DW/L for 50%–50 % sturgeon-swine wastewater. The biomass yield on N basis was 68.47 ± 32.36 g/g in BG11 and 44.86 ± 0.27 g/g in 50%–50 % sturgeon-swine wastewater. The biomass yield was higher in BG11 than 50%–50 % sturgeon-swine wastewater.
Fig. 4.
Comparison of S. elongatus UTEX 2973 biomass accumulation in 50%–50 % sturgeon-swine wastewater with control synthetic BG11 growth media: a) biomass accumulation on a dry weight basis over the growth period in quadruplicates with standard deviation and b) cell culture density visualization over the experimental time period.
The morphology of S. elongatus cells was investigated under a scanning electron microscope (SEM) (Fig. 5). The cells grown in BG11 (Fig. 5a) had a length of 2.0–2.4 μm and a width of 0.50–0.67 μm. Cells cultured in 50%–50 % sturgeon swine wastewater (Fig. 5b) were smaller (length of 1.3–1.5 μm and width of 0.4–0.5 μm). The presence of short pili enabled the bacteria to display an aggregation phenotype and colonize randomly [57] in wastewater while the cells from BG11 were unattached and floating freely, indicating that the cells in 50%–50 % sturgeon-swine wastewater were stressed.
Fig. 5.
Cell morphology of S. elongatus UTEX 2973: a) Scanning electron microscopy (SEM) image of cells grown in BG11 and b) SEM image of cells grown in 50%–50 % sturgeon-swine wastewater for 14 days.
3.3.2. Analysis of carbohydrate storage
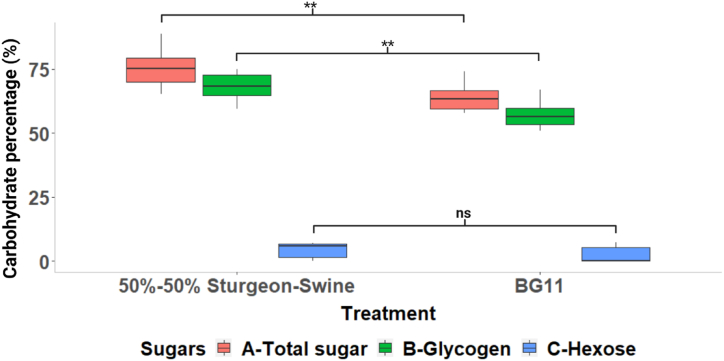
Harvested biomass from the two treatment groups was analyzed for total carbohydrate, glycogen, and non-glycogen hexoses. The 50%–50 % sturgeon-swine wastewater treatment group accumulated significantly higher total carbohydrate and glycogen (p < 0.05). However, no significant impact of treatment groups was found in hexose accumulation in biomass (p > 0.05). Total carbohydrate accumulated was 75 % and 64 % of dry biomass for 50%–50 % sturgeon-swine mixed wastewater and BG11 growth medium, respectively (Fig. 6). Glycogen was found to be the main carbohydrate present in biomass, almost 90 % of the total sugar. It was observed that glycogen content was 68 % and 57 % of dry cell weight for 50%–50 % sturgeon-swine mixed wastewater and BG11 growth medium, respectively.
Fig. 6.
Comparison of the percentage of carbohydrate accumulation (total carbohydrate, glycogen, and non-glycogen hexoses) on a dry weight basis in S. elongatus UTEX 2973 biomass grown in 50%–50 % sturgeon-swine wastewater and BG11 (ns denotes p > 0.05 and **p < 0.01).
To complement the experimental findings, scanning electron microscopy and energy dispersive X-ray spectroscopy (SEM-EDS) was performed to obtain an insight into the carbohydrate accumulation in terms of the percentage of carbon present in the cells (data available). EDS analysis can quantify all chemical elements in the cells except for hydrogen. Therefore, SEM-EDS does not provide absolute measurements but still provides useful relative comparisons across samples (Table 4). The difference between the percentages of carbon aligned with experimental findings; higher carbohydrates correlated with a higher carbon percentage. The percentage of nitrogen was 6.7 % for mixed wastewater biomass, whereas it was 5 % for BG11 biomass. Similarly, a higher percentage of phosphorus was also found in mixed wastewater biomass samples than BG11 biomass samples (1.3 % compared to 0.4 %). This indicated that S. elongatus had taken up a considerable amount of both nitrogen and phosphorus in the wastewater and stored in their biomass. Increased concentration of calcium (Ca), potassium (K), and magnesium (Mg) metal was observed in biomass grown in mixed wastewater.
Table 4.
SEM-EDS analysis of biomass in 50%–50 % sturgeon-swine wastewater and BG11.
| Elements | Biomass in 50%–50 % Sturgeon-swine wastewater (%) | Biomass in BG11 (%) |
|---|---|---|
| C | 67 | 60.3 |
| O | 23 | 32.7 |
| N | 6.7 | 5 |
| P | 1.3 | 0.4 |
| Al | 0.7 | 0.8 |
| Ca | 0.4 | 0.2 |
| Mg | 0.4 | 0.2 |
| S | 0.3 | 0.3 |
| K | 0.3 | 0 |
3.4. Nutrient removal from wastewater
S. elongatus UTEX 2973 utilized the nutrients present in the wastewater to support their growth during the 14 day culture period. The nutrient removal efficiency was also determined after harvesting the biomass. Before calculating biological removal potential, nutrient conversions due to abiotic processes were analyzed and factored into the calculation (see equation (1) in methods). Table 5 shows nutrient concentration initially on day 0, uninoculated wastewater control at day 14, and in wastewater after harvesting the biomass at day 14.
Table 5.
Concentration of nutrients in wastewater at the beginning (day 0), in uninoculated wastewater control at the end of the experiment (day 14), and in wastewater after harvesting the biomass (day 14).
| Nutrients | Ammonia-N (NH4+-N) mg N/L | Nitrate-N + Nitrite-N (NO2− –N/NO3− –N) mg N/L | Orthophosphate-P (PO43--P) mg P/L |
|---|---|---|---|
| Wastewater day 0 (Initial) | 69.95 | 10.57 | 8.90 |
|
Uninoculated wastewater control day 14 (Available) (Mean ± SDa) |
1.82 ± 0.032 | 13.65 ± 0.002 | 1.53 ± 0.005 |
|
Wastewater after harvesting biomass day 14 (Remaining) (Mean ± SDa) |
0.04 ± 0.002 | 0.90 ± 0.852 | 0.50 ± 0.172 |
SD = Standard deviation.
Ammonia is a volatile compound, and it was found that 97.4 % of ammonia was either evaporated or converted to nitrate, and only 2.6 % of the initial ammonia was present after 14 days in the uninoculated control sample (data available). It was also found that the concentration of nitrate-N in the uninoculated wastewater control increased from 10.57 mg N/mL to 13.64 mg N/mL after 14 days, indicating 4.4 % of ammonia was converted to nitrate [58]. It was also observed that the concentration of orthophosphate decreased over the culture period (from day 0 to day 11). The phosphorus in orthophosphate can react with other compounds present in the wastewater and precipitate [59]. In the uninoculated control, the 82.75 % decrease in concentration was attributed to abiotic removal (Fig. 7).
Fig. 7.
Percent removal of nutrients (ammonia nitrogen, nitrate-nitrite, and orthophosphate) from 50%-50 % sturgeon-swine wastewater by abiotic processes and S. elongatus UTEX 2973 assimilation.
The concentration of nutrients in the inoculated wastewater was measured after harvesting the biomass to quantify nutrients that were neither consumed by S. elongatus nor reacted abiotically by the end of the experiment (“Remaining” concentration in Table 5). Thus, the biological removal of nutrients was calculated as the difference between (1) the nutrients available after accounting for abiotic conversions and (2) the nutrients remaining in the wastewater at the end of the experiment. The biological removal efficiency was found to be 67.15 % for orthophosphate, 93.39 % for nitrate, and 97.98 % for ammonia (Fig. 8). These nutrients were utilized by S. elongatus to produce biomass and storage materials, mainly carbohydrates.
Fig. 8.
Percent removal of bioavailable nutrients (ammonia nitrogen, nitrate-nitrite, and orthophosphate) from 50%-50 % sturgeon-swine wastewater by S. elongatus UTEX 2973 assimilation.
4. Conclusion
This study highlighted the use of mixed wastewater (aquaculture and swine) as the growth medium to supply nutrients for S. elongatus UTEX 2973 growth and carbohydrate accumulation in biomass. It also demonstrated the nutrient removal potential of this species. Among the three different ratios of mixed wastewater tested, it was found that S. elongatus produced the maximum biomass and stored the highest amount of carbohydrate in 50%–50 % sturgeon-swine wastewater after 13 days of cultivation. Further comparison of a different batch of 50%–50 % sturgeon-swine wastewater with a synthetic growth medium, BG11, showed that a higher biomass growth was achieved in BG11 growth medium. However, the carbohydrate storage was significantly higher in the biomass grown in 50%–50 % sturgeon-swine wastewater. Moreover, considerable biological removal of ammonia nitrogen (≈98 %), nitrate-nitrite (≈93 %), and orthophosphate (≈67 %) from the mixed wastewater were achieved during this study. However, abiotic processes played major parts in the removal of ammonia and phosphate. In conclusion, this study demonstrated that S. elongatus UTEX 2973 could be used as a bioremediatory sustainable alternative in wastewater treatment, and the biomass generated during bioremediation could be used for sugar and other biochemical production downstream due to the high quantity of carbohydrate storage. Future work should explore additional optimization of the nutrient concentration and/or cultivation conditions (i.e., temperature, pH) of wastewater, as well as symbiotic interactions with native wastewater microbiota, to further improve growth and carbohydrate accumulation. The carbohydrate enriched S. elongatus biomass can be used in saccharification and/or fermentation to produce value-added bioproducts such as sugar, ethanol, and bioplastic.
Data availability statement
The data associated with this study has been deposited into Elsevier's Mendeley Data repository for public access: https://data.mendeley.com/datasets/wc4p9x5248.
CRediT authorship contribution statement
Rifat Hasan: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nitesh Kasera: Writing – review & editing, Investigation, Data curation. Ashley E. Beck: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization. Steven G. Hall: Writing – review & editing, Supervision, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for support from the Department of Biological and Agricultural Engineering at North Carolina State University, as well as funding support provided by the NCSU Provost's Doctoral Fellowship, USDA National Institute of Food and Agriculture, Hatch 1018813, and North Carolina Sea Grant, R/MG-2109. The authors would like to thank Dr. Aaron Bell, Dr. Jin Nakashima, and Dr. Charles Mooney for assistance with SEM imaging and EDS analysis. The authors are grateful to Connor Maycott for his assistance in the laboratory.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24646.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lahlou F.Z., Mackey H.R., Al-Ansari T. Wastewater reuse for livestock feed irrigation as a sustainable practice: a socio-environmental-economic review. J. Clean. Prod. 2021;294 doi: 10.1016/J.JCLEPRO.2021.126331. [DOI] [Google Scholar]
- 2.OECD-FAO . 2022. OECD-FAO Agricultural Outlook 2022-2031.https://www.fao.org/3/cb9427en/cb9427en.pdf Available online: [Google Scholar]
- 3.Dohlman E., Hansen J., Boussios D. United States Department of Agriculture; 2022. USDA Agricultural Projections to 2031 (No. 323859) [DOI] [Google Scholar]
- 4.Sakar S., Yetilmezsoy K., Kocak E. Anaerobic digestion technology in poultry and livestock waste treatment—a literature review. Waste Manag. Res. 2009;27(1):3–18. doi: 10.1177/0734242X07079060. [DOI] [PubMed] [Google Scholar]
- 5.Owusu-Twum M.Y., Sharara M.A. Sludge management in anaerobic swine lagoons: a review. J. Environ. Manag. 2020;271 doi: 10.1016/J.JENVMAN.2020.110949. [DOI] [PubMed] [Google Scholar]
- 6.FAO . Food and Agriculture Organization of the United Nations License: CC BY-NC-SA 3.0 IGO. 2022. The state of world fisheries and aquaculture. [Google Scholar]
- 7.Chen S., Yu J., Wang H., Yu H., Quan X. A pilot-scale coupling catalytic ozonation–membrane filtration system for recirculating aquaculture wastewater treatment. Desalination. 2015;363:37–43. doi: 10.1016/J.DESAL.2014.09.006. [DOI] [Google Scholar]
- 8.Mook W.T., Chakrabarti M.H., Aroua M.K., Khan G.M.A., Ali B.S., Islam M.S., Hassan M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination. 2012;285:1–13. doi: 10.1016/J.DESAL.2011.09.029. [DOI] [Google Scholar]
- 9.Crab R., Avnimelech Y., Defoirdt T., Bossier P., Verstraete W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture. 2007;270(1–4):1–14. doi: 10.1016/J.AQUACULTURE.2007.05.006. [DOI] [Google Scholar]
- 10.Godíneza J.R., Hernándeza R.I.B., Olivaresa C.C. 2013. Characterization of Aquaculture Effluents for in Situ Treatment and Reuse.https://www.researchgate.net/publication/236942991 [Google Scholar]
- 11.Bergman K., Henriksson P.J., Hornborg S., Troell M., Borthwick L., Jonell M.…Ziegler F. Recirculating aquaculture is possible without major energy tradeoff: life cycle assessment of warmwater fish farming in Sweden. Environ. Sci. Technol. 2020;54(24):16062–16070. doi: 10.1021/acs.est.0c01100. https://pubs.acs.org/doi/10.1021/acs.est.0c01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalsgaard J., Lund I., Thorarinsdottir R., Drengstig A., Arvonen K., Pedersen P.B. Farming different species in RAS in Nordic countries: current status and future perspectives. Aquacult. Eng. 2013;53:2–13. doi: 10.1016/j.aquaeng.2012.11.008. [DOI] [Google Scholar]
- 13.Martins C.I.M., Eding E.H., Verdegem M.C., Heinsbroek L.T., Schneider O., Blancheton J.P.…Verreth J.A.J. New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquacult. Eng. 2010;43(3):83–93. doi: 10.1111/raq.12392. [DOI] [Google Scholar]
- 14.Ruiz P., Vidal J.M., Sepúlveda D., Torres C., Villouta G., Carrasco C.…Urrutia H. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquacult. 2020;12(3):1478–1494. doi: 10.1111/raq.12392. [DOI] [Google Scholar]
- 15.Mnyoro M.S., Munubi R.N., Pedersen L.-F., Chenyambuga S.W. Evaluation of biofilter performance with alternative local biomedia in pilot scale recirculating aquaculture systems. J. Clean. Prod. 2022;366 doi: 10.1016/j.jclepro.2022.132929. [DOI] [Google Scholar]
- 16.Cheng D.L., Ngo H.H., Guo W.S., Liu Y.W., Zhou J.L., Chang S.W.…Zhang X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018;621:1664–1682. doi: 10.1016/j.scitotenv.2017.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Cripps S.J. Serial particle size fractionation and characterisation of an aquacultural effluent. Aquaculture. 1995;133(3–4):323–339. doi: 10.1016/0044-8486(95)00021-S. [DOI] [Google Scholar]
- 18.Guldhe A., Ansari F.A., Singh P., Bux F. Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017;99:47–53. doi: 10.1016/j.ecoleng.2016.11.013. [DOI] [Google Scholar]
- 19.Cheng D., Ngo H.H., Guo W., Chang S.W., Nguyen D.D., Liu Y.…Wei D. A critical review on antibiotics and hormones in swine wastewater: water pollution problems and control approaches. J. Hazard Mater. 2020;387 doi: 10.1016/j.jhazmat.2019.121682. [DOI] [PubMed] [Google Scholar]
- 20.Conley D.J., Paerl H.W., Howarth R.W., Boesch D.F., Seitzinger S.P., Havens K.E.…Likens G.E. Controlling eutrophication: nitrogen and phosphorus. Science. 2009;323(5917):1014–1015. doi: 10.1126/science.1167755. [DOI] [PubMed] [Google Scholar]
- 21.Thorne P.S. Environmental health impacts of concentrated animal feeding operations: anticipating hazards—searching for solutions. Environ. Health Perspect. 2007;115(2):296–297. doi: 10.1289/ehp.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bullers S. Environmental stressors, perceived control, and health: the case of residents near large-scale hog farms in eastern North Carolina. Hum. Ecol. 2005;33:1–16. doi: 10.1007/s10745-005-1653-3. [DOI] [Google Scholar]
- 23.Dan N.H., Rene E.R., Le Luu T. Removal of nutrients from anaerobically digested swine wastewater using an intermittent cycle extended aeration system. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.576438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasera N., Kolar P., Hall S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: a review. Biochar. 2022;4(1):17. doi: 10.1007/s42773-022-00145-2. [DOI] [Google Scholar]
- 25.Mohsenpour S.F., Hennige S., Willoughby N., Adeloye A., Gutierrez T. Integrating micro-algae into wastewater treatment: a review. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.142168. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S.N., Haider W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: a review. Nanotechnology. 2018;29(34) doi: 10.1088/1361-6528/aac6ea. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S., Khatoon H., Shariff M., Yusoff F. Immobilized periphytic cyanobacteria for removal of nitrogenous compounds and phosphorus from shrimp farm wastewater. Turkish Journal of Biology. 2015;39(3):388–395. doi: 10.3906/biy-1407-26. [DOI] [Google Scholar]
- 28.Kamilya D., Sarkar S., Maiti T.K., Bandyopadhyay S., Mal B.C. Growth and nutrient removal rates of Spirulina platensis and Nostoc muscorum in fish culture effluent: a laboratory‐scale study. Aquacult. Res. 2006;37(15):1594–1597. doi: 10.1111/j.1365-2109.2006.01588.x. [DOI] [Google Scholar]
- 29.Krasaesueb N., Incharoensakdi A., Khetkorn W. Utilization of shrimp wastewater for poly-β-hydroxybutyrate production by Synechocystis sp. PCC 6803 strain ΔSphU cultivated in photobioreactor. Biotechnology Reports. 2019;23 doi: 10.1016/J.BTRE.2019.E00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojsitthisak P., Burut‐Archanai S., Pothipongsa A., Powtongsook S. Repeated phosphate removal from recirculating aquaculture system using cyanobacterium remediation and chitosan flocculation. Water Environ. J. 2017;31(4):598–602. doi: 10.1111/WEJ.12288. [DOI] [Google Scholar]
- 31.Wuang S.C., Khin M.C., Chua P.Q.D., Luo Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016;15:59–64. doi: 10.1016/J.ALGAL.2016.02.009. [DOI] [Google Scholar]
- 32.Winayu B.N.R., Chuang H.P., Hsueh H.T., Chu H. Elimination of inorganic carbon and nitrogen resided in swine wastewater using Thermosynechococcus sp. CL-1 enriched culture. Bioresour. Technol. 2021;336 doi: 10.1016/j.biortech.2021.125325. [DOI] [PubMed] [Google Scholar]
- 33.Sharma G.K., Khan S.A., Shrivastava M., Bhattacharyya R., Sharma A., Gupta D.K.…Gupta N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J. Environ. Manag. 2021;287 doi: 10.1016/j.jenvman.2021.112295. [DOI] [PubMed] [Google Scholar]
- 34.Khan S.A., Sharma G.K., Malla F.A., Kumar A., Gupta N. Microalgae based biofertilizers: a biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019;211:1412–1419. doi: 10.1016/j.jclepro.2018.11.281. [DOI] [Google Scholar]
- 35.Abed R.M.M., Dobretsov S., Sudesh K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009;106(1):1–12. doi: 10.1111/j.1365-2672.2008.03918.x. [DOI] [PubMed] [Google Scholar]
- 36.Kandasamy S., Zhang B., He Z., Bhuvanendran N., EL-Seesy A.I., Wang Q.…Dar M.A. Microalgae as a multipotential role in commercial applications: current scenario and future perspectives. Fuel. 2022;308 doi: 10.1016/j.fuel.2021.122053. [DOI] [Google Scholar]
- 37.Abarzua S., Jakubowski S., Eckert S., Fuchs P. Biotechnological investigation for the prevention of marine biofouling II. Blue-green algae as potential producers of biogenic agents for the growth inhibition of microfouling organisms. Bot. Mar. 1999;42(5):459–465. doi: 10.1515/BOT.1999.053. [DOI] [Google Scholar]
- 38.Goswami R.K., Mehariya S., Obulisamy P.K., Verma P. Advanced microalgae-based renewable biohydrogen production systems: a review. Bioresour. Technol. 2021;320 doi: 10.1016/j.biortech.2020.124301. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros D.L., Sales E.A., Kiperstok A. Energy production from microalgae biomass: carbon footprint and energy balance. J. Clean. Prod. 2015;96:493–500. doi: 10.1016/J.JCLEPRO.2014.07.038. [DOI] [Google Scholar]
- 40.Rawat I., Kumar R.R., Mutanda T., Bux F. Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy. 2011;88(10):3411–3424. doi: 10.1016/J.APENERGY.2010.11.025. [DOI] [Google Scholar]
- 41.Ungerer J., Wendt K.E., Hendry J.I., Maranas C.D., Pakrasi H.B. Comparative genomics reveals the molecular determinants of rapid growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Proc. Natl. Acad. Sci. USA. 2018;115(50):E11761–E11770. doi: 10.1073/pnas.1814912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song K., Tan X., Liang Y., Lu X. The potential of Synechococcus elongatus UTEX 2973 for sugar feedstock production. Appl. Microbiol. Biotechnol. 2016;100(18):7865–7875. doi: 10.1007/s00253-016-7510-z. [DOI] [PubMed] [Google Scholar]
- 43.Cui J., Sun T., Li S., Xie Y., Song X., Wang F.…Zhang W. Improved salt tolerance and metabolomics analysis of Synechococcus elongatus UTEX 2973 by overexpressing Mrp antiporters. Front. Bioeng. Biotechnol. 2020;8:500. doi: 10.3389/fbioe.2020.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rueda E., García-Galán M.J., Ortiz A., Uggetti E., Carretero J., García J., Díez-Montero R. Bioremediation of agricultural runoff and biopolymers production from cyanobacteria cultured in demonstrative full-scale photobioreactors. Process Saf. Environ. Protect. 2020;139:241–250. doi: 10.1016/j.psep.2020.03.035. [DOI] [Google Scholar]
- 45.Xia Y., Zhang M., Tsang D.C., Geng N., Lu D., Zhu L.…Ok Y.S. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: current practices and future prospects. Applied Biological Chemistry. 2020;63(1):1–13. doi: 10.1186/s13765-020-0493-6. [DOI] [Google Scholar]
- 46.Hong J.W., Kim O.H., Jo S.W., Kim H., Jeong M.R., Park K.M.…Yoon H.S. Biochemical composition of a Korean domestic microalga Chlorella vulgaris KNUA027. Microbiology and Biotechnology Letters. 2016;44(3):400–407. doi: 10.4014/mbl.1512.12008. [DOI] [Google Scholar]
- 47.Beck A.E., Hunt K.A., Carlson R.P. Measuring cellular biomass composition for computational biology applications. Processes. 2018;6(5):38. doi: 10.3390/pr6050038. [DOI] [Google Scholar]
- 48.Gao F., Yang Z.H., Li C., Zeng G.M., Ma D.H., Zhou L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015;179:8–12. doi: 10.1016/J.BIORTECH.2014.11.108. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-Marin A., Mendoza-Espinosa L.G., Stephenson T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010;101(1):58–64. doi: 10.1016/J.BIORTECH.2009.02.076. [DOI] [PubMed] [Google Scholar]
- 50.Del Don C., Hanselmann K.W., Peduzzi R., Bachofen R. Biomass composition and methods for the determination of metabolic reserve polymers in phototrophic sulfur bacteria. Aquat. Sci. 1994;56:1–15. doi: 10.1007/BF00877431. [DOI] [Google Scholar]
- 51.Yu J., Liberton M., Cliften P.F., Head R.D., Jacobs J.M., Smith R.D., Pakrasi H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015;5(1):1–10. doi: 10.1038/srep08132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterbury J.B., Watson S.W., Guillard R.R., Brand L.E. Widespread occurrence of a unicellular, marine, planktonic, cyanobacterium. Nature. 1979;277(5694):293–294. doi: 10.1038/277293a0. [DOI] [Google Scholar]
- 53.Dechatiwongse P., Srisamai S., Maitland G., Hellgardt K. Effects of light and temperature on the photoautotrophic growth and photoinhibition of nitrogen-fixing cyanobacterium Cyanothece sp. ATCC 51142. Algal Res. 2014;5:103–111. doi: 10.1016/j.algal.2014.06.004. [DOI] [Google Scholar]
- 54.Lin P.C., Zhang F., Pakrasi H.B. Enhanced production of sucrose in the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Sci. Rep. 2020;10(1):390. doi: 10.1038/s41598-019-57319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aikawa S., Joseph A., Yamada R., Izumi Y., Yamagishi T., Matsuda F.…Kondo A. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ. Sci. 2013;6(6):1844–1849. doi: 10.1039/c3ee40305j. [DOI] [Google Scholar]
- 56.Zhu S., Wang Y., Huang W., Xu J., Wang Z., Xu J., Yuan Z. Enhanced accumulation of carbohydrate and starch in Chlorella zofingiensis induced by nitrogen starvation. Appl. Biochem. Biotechnol. 2014;174:2435–2445. doi: 10.1007/s12010-014-1183-9. [DOI] [PubMed] [Google Scholar]
- 57.Beaussart A., Baker A.E., Kuchma S.L., El-Kirat-Chatel S., O'Toole G.A., Dufrêne Y.F. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV pili. ACS Nano. 2014;8(10):10723–10733. doi: 10.1021/nn5044383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doane T.A. The abiotic nitrogen cycle. ACS Earth Space Chem. 2017;1(7):411–421. doi: 10.1021/acsearthspacechem.7b00059. [DOI] [Google Scholar]
- 59.Gao F., Li C., Yang Z.H., Zeng G.M., Feng L.J., Liu J.Z.…Cai H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016;92:55–61. doi: 10.1016/J.ECOLENG.2016.03.046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this study has been deposited into Elsevier's Mendeley Data repository for public access: https://data.mendeley.com/datasets/wc4p9x5248.