1. Introduction
The tegmental wall of the tympanic cavity is a thin plate of the temporal bone that separates the middle cranial fossa (MCF) from the ear. This anatomical region consists of two areas: an anterior one, comprised of the tegmen tympani (Tóth et al., 2007), and a posterior one, formed by the tegmen antri and the tegmen mastoideum (Makki et al., 2011). In some patients, the tegmental region of the temporal bone can be interrupted, causing a tegmen defect (TD). A TD is sometimes associated with a meningoencephalic herniation (MEH), in which brain tissue herniates through a TD. The etiology of TDs and MEH varies; most cases are due to inflammation linked to chronic pathological processes, such as the presence of cholesteatomatous matrix, whereas in other cases, the etiology may be congenital, traumatic, malignant, or iatrogenic (Marchioni et al., 2014). Because of the anatomical boundaries of this region, an interruption can lead to different clinical scenarios, ranging from a complete absence of symptoms (i.e., incidental findings during a computed tomography (CT) scan) to acute neurological disease, which can sometimes be associated with cerebrospinal fluid (CSF) leakage (Byrne et al., 2010; Golding-Wood DG et al., 1991; Grinblat et al., 2018; Nahas et al., 2008). Thus, TDs and/or MEH must be diagnosed promptly to ensure early intervention and avoid the risk of the pathological involvement of the central nervous system (CNS) (May et al., 1995).
Over the past several years, scientific research has extensively evaluated different surgical approaches to TD and/or MEH, offering numerous contributions and discussing both conservative and invasive approaches to treatment. According to these studies, the main conservative techniques are the middle cranial fossa (MCF) approach (Ahmed et al., 2017; Braca et al., 2013; Carlson et al., 2013; Hoang et al., 2018; Tolisano and Kutz, 2019), the transmastoid technique (Kim et al., 2014; Oliaei et al., 2012; Sergi et al., 2013), and the transmastoid technique with minicraniotomy (Adkins and Osguthorpe, 1983; Ahmed et al., 2017; Braca et al., 2013; Byrne et al., 2010; Carlson et al., 2013; Hoang et al., 2018; Kim et al., 2014; Kuhweide and Casselman, 1999; Marchioni et al., 2014; May et al., 1995; Oliaei et al., 2012; Ramalingam et al., 2008; Sergi et al., 2013; Tolisano and Kutz, 2019). A more invasive approach is subtotal petrosectomy (STP) (Sanna et al., 2008, 2009), which allows excellent control of the operating field (Grinblat et al., 2018; Magliulo et al., 2014; Prasad et al., 2017; Vincenti et al., 2014) in a safer procedure but leads to greater impairment in postoperative hearing ability. This paper presents our experience regarding surgical outcomes for the treatment of TD and/or MEH based on patient characteristics, such as age, preoperative hearing threshold, the presence of comorbidities, and history of recurrence despite previous surgery, considering TD and/or MEH characteristics in terms of size and location.
2. Material and methods
The study included 45 patients affected by TDs and/or MEH between January 2016 and January 2023. All patients received single-operator (GDD) procedures. Only patients over 18 years old were included. Patients who did not receive a preoperative and/or postoperative assessment were excluded from the study.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent prior to enrollment.
All patients underwent a complete audiological evaluation, which included anamnesis, otomicroscopy (followed by image collection using otoendoscopy), tonal audiometry, speech audiometry, and, when possible, an impedance test measurement. Moreover, a complete radiological evaluation was performed, including ear CT and brain magnetic resonance imaging (MRI) focused on the T1 and T2 sequences (Fig. 1, Fig. 2).
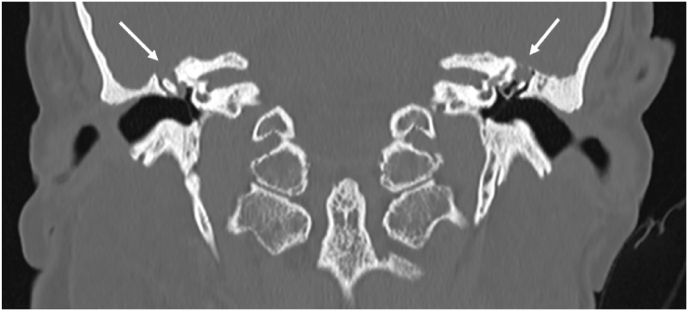
Fig. 1.
Preoperative CT evaluation showing large tegmen defect associated with meningoencephalic herniation at the level of both mastoids (indicated with white arrows).
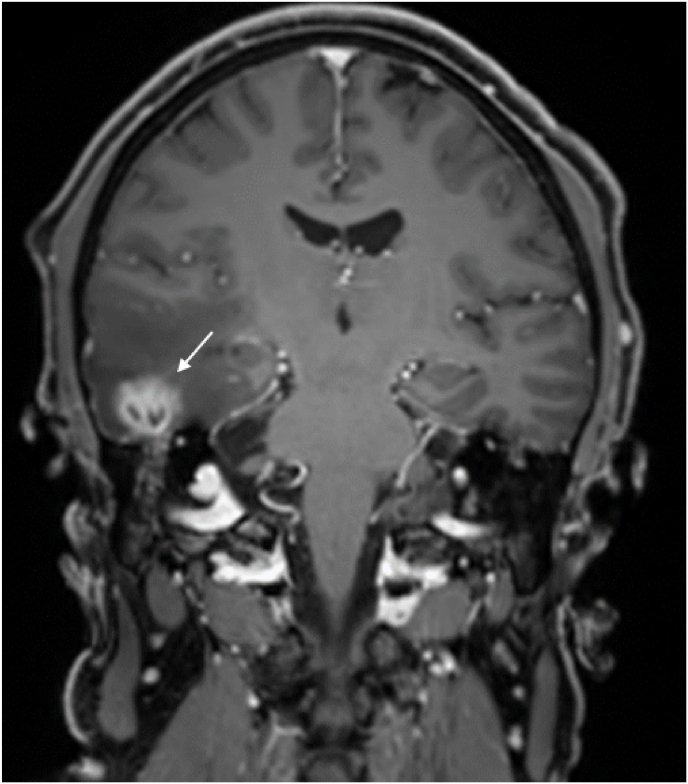
Fig. 2.
Preoperative MRI evaluation of the same patient of Fig. 1 which highlights the presence of a temporal abscess as a complication of the MEH on the right mastoid (indicated with white arrow).
The anamnestic evaluation focused on the presence of chronic disorders of the ear; the time of onset; clinical signs and referred symptoms, such as vertigo, ear pain, headache, and otorrhea; and familiarity with hearing pathologies. All previous audiological documentation was also collected when available.
In the audiometric evaluation, each ear and each pure-tone frequency stimulation (from 125 to 8000 Hz) was considered separately. An acoustic impedance test was performed to evaluate the intensity threshold of the acoustic reflex for each ear, using 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz stimulus tones. In patients with tympanic membrane perforation or acute disease (e.g., meningitis), an impedance test was not performed.
Population's data are summarized in Table 1 considering gender, age, ear side, etiology, preoperative hearing threshold, the size and location of the TD and/or MEH and the surgical technique.
Table 1.
Cohort of 45 patients, classified according to sex, side of the lesion, age at the time of surgery, diagnosis, location and size of the lesion.
| N. | Sex | Side | Age | Diagnosis | Etiology | Location | Size | Surgery | Preoperative PTA* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | left | 56 | TD | Iatrogenic (CSF leak in previous surgery for neurinoma) | AP | >10 mm | STP | 28 dB |
| 2 | F | left | 66 | MEH | Cholesteatoma | P | ≤10 mm | STP | 50 dB |
| 3 | M | right | 67 | TD | Cholesteatoma | P | >10 mm | STP | 55 dB |
| 4 | F | left | 81 | MEH | Cholesteatoma | P | >10 mm | STP | anacusis |
| 5 | M | left | 41 | TD | Cholesteatoma | AP | >10 mm | STP | 4 dB |
| 6 | F | left | 46 | MEH | Cholesteatoma | A | >10 mm | STP | anacusis |
| 7 | F | right | 52 | TD | Cholesteatoma | AP | >10 mm | STP | 82 dB |
| 8 | M | left | 44 | MEH | Cholesteatoma | A | ≤10 mm | MCF approach | 36 dB |
| 9 | F | right | 35 | MEH | Cholesteatoma | A | ≤10 mm | STP | 74 dB |
| 10 | F | left | 86 | MEH | Cholesteatoma | P | >10 mm | STP | 70 dB |
| 11 | F | left | 76 | TD | Cholesteatoma | AP | >10 mm | STP | 26 dB |
| 12 | M | right | 41 | TD | Cholesteatoma | AP | >10 mm | STP | 13 dB |
| 13 | M | left | 26 | MEH | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 26 dB |
| 14 | F | right | 65 | MEH | Cholesteatoma | P | >10 mm | STP | anacusis |
| 15 | M | left | 48 | MEH | Previous trauma + CSF leak | AP | >10 mm | STP | 24 dB |
| 16 | F | right | 48 | MEH | Cholesteatoma | AP | >10 mm | STP | 37 dB |
| 17 | F | right | 62 | MEH | Cholesteatoma | AP | >10 mm | STP | 18 dB |
| 18 | M | left | 33 | MEH | Cholesteatoma | P | >10 mm | STP | anacusis |
| 19 | M | right | 51 | MEH | Cholesteatoma | AP | >10 mm | STP | 15 dB |
| 20 | M | left | 65 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 32 dB |
| 21 | F | left | 67 | MEH | Cholesteatoma | AP | >10 mm | STP | 41 dB |
| 22 | F | right | 18 | MEH | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 10 dB |
| 23 | M | right | 33 | TD | Cholesteatoma | P | >10 mm | STP | anacusis |
| 24 | M | left | 75 | TD | Cholesteatoma | P | >10 mm | STP | 100 dB |
| 25 | F | right | 49 | MEH | Previous traumatic injury | AP | >10 mm | STP | 28 dB |
| 26 | F | left | 80 | MEH | Cholesteatoma | AP | >10 mm | STP | 41 dB |
| 27 | F | left | 31 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 11 dB |
| 28 | M | left | 79 | TD | Cholesteatoma | P | >10 mm | STP | 57 dB |
| 29 | F | right | 54 | MEH | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 20 dB |
| 30 | M | left | 59 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 9 dB |
| 31 | F | left | 44 | MEH | Acute meningitis + CSF leak | P | ≤10 mm | Transmastoid technique | 5 dB |
| 32 | M | left | 67 | MEH | Cholesteatoma | AP | >10 mm | STP | 69 dB |
| 33 | M | left | 59 | TD | Cholesteatoma | P | >10 mm | STP | anacusis |
| 34 | F | right | 60 | TD | Cholesteatoma | AP | >10 mm | STP | 29 dB |
| 35 | M | right | 18 | TD | Cholesteatoma | P | >10 mm | STP | anacusis |
| 36 | F | right | 49 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 10 dB |
| 37 | M | right | 42 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 24 dB |
| 38 | F | right | 70 | MEH | Cholesteatoma | P | >10 mm | STP | 58 dB |
| 39 | F | left | 76 | TD | Cholesteatoma | P | >10 mm | STP | 63 dB |
| 40 | M | right | 62 | TD | Cholesteatoma | P | ≤10 mm | Transmastoid technique | |
| 41 | M | left | 76 | MEH | Cholesteatoma | A | ≤10 mm | STP | 45 dB |
| 42 | F | right | 54 | MEH | Cholesteatoma | A | ≤10 mm | MCF approach | 27 dB |
| 43 | F | right | 50 | MEH | Cholesteatoma | AP | >10 mm | STP | 20 dB |
| 44 | M | right | 75 | MEH | Cholesteatoma | AP | >10 mm | STP | 60 dB |
| 45 | M | left | 29 | MEH | Cholesteatoma | P | ≤10 mm | Transmastoid technique | 21 dB |
Abbreviations: M, male; F, female; MEH, meningoencephalic herniation; TD, tegmen defect; CSF, Cerebrospinal Fluid; PTA, pure tone audiometry; STP, subtotal petrosectomy; MCF, middel cranial fossa; AP, anteroposterior; P, posterior; A, anterior. *(considering bone conduction and calculating the mean at 500, 1000, 2000 and 4000 Hz).
Regarding the other parameters, preoperative hearing ability (of the pathological side) was defined as the average threshold frequency via bone conduction (considering the mean at 500, 1000, 2000, and 4000 Hz).
Defects were defined as anterior (A) if they were located at the level of the tegmen tympani, posterior (P) if they were located at the level of the tegmen antri or tegmen mastoideum, and anterior/posterior (A/P) if they involved both locations.
Finally, a TD/MEH was defined as small if it was less than or equal to 10 mm and large if it was over 10 mm.
During the first postoperative weeks, patients underwent serial dressings of the surgical wound; after this period, each patient received a complete otological evaluation, which included an ear, nose, and throat (ENT) visit and otomicroscopy and audiological examinations at 1 month, 3 months, 6 months, and 12 months after surgery. Subsequently, each patient was followed up every 6 months for the first 2 years, including a radiological follow-up with magnetic resonance and ear CT, according to the individual case.
3. Results
The final population of 45 patients included 22 men (48.9%) and 23 women (51.1%) between 18 and 86 years old (mean age: 51.7 years) at the time of surgery; at the time of enrollment, 31 patients were 65 years old or younger (68.9%), and 14 patients were older than 65 years (31.1%).
A total of 19 patients were affected by TD (42.2%), and the remaining 26 patients had MEH (57.8%). The right ear was affected in 21 cases (46.7%), and the left ear was involved in the remaining 24 cases (53.3%). No patients in our cohort showed bilateral involvement. Furthermore, 19 patients (42.2%) had previously undergone ipsilateral ear surgery, whereas 26 patients (57.8%) had never received previous ear operations.
We identified posterior TD/MEH in 24 patients (53.3%) and anterior TD/MEH in 5 patients (11.1%). The remaining 16 patients (35.6%) presented with an anterior/posterior defect. More than half of the patients (n = 29, 64.4%) presented with a large TD/MEH, and 16 patients (35.6%) had a small TD/MEH. In our cohort, most patients received STP (n = 32, 71.1%), whereas the transmastoid technique was employed in 11 patients (24.5%), and the MCF approach was performed in only 2 patients (4.4%). No patients underwent minicraniotomy with the transmastoid technique. All data are summarized in Table 1.
The main etiology of DT/MEH was chronic otitis media (COM) ± cholesteatoma (including recurrent cholesteatoma), which was diagnosed in 41 patients (91.1%). Regarding other etiologies, 2 patients had a temporal bone defect due to trauma (4.4%), 1 patient was diagnosed with a CSF fistula and neuroma of the seventh cranial nerve (2.2%), and 1 patient had an iatrogenic bone defect (2.2%)
3.1. Audiological outcomes
The audiological outcomes are presented in Table 2.
Table 2.
Hearing assessment considering preoperative and postoperative hearing tresholds calculating the mean at 500, 1000, 2000 and 4000 Hz of bone conduction of the affected side. All patients who underwent a conservative surgical procedure were highlighted in order to separately determine the audiological outcomes, as summarized in Materials and Methods section.
| N. | Preoperative hearing threshold | Postoperative hearing threshold | ABG |
|---|---|---|---|
| 1 | 28 dB | anacusis | >25 dB |
| 2 | 50 dB | anacusis | >25 dB |
| 3 | 55 dB | 60 dB | +5 dB |
| 4 | anacusis | anacusis | – |
| 5 | 4 dB | 18 dB | +14 dB |
| 6 | anacusis | anacusis | – |
| 7 | 82 dB | 91 dB | +9 dB |
| 8 | 36 dB | 45 dB | +9 dB |
| 9 | 74 dB | Anacusis | >25 dB |
| 10 | 70 dB | 78 dB | +8 dB |
| 11 | 26 dB | 60 dB | >25 dB |
| 12 | 13 dB | 24 dB | +11 dB |
| 13 | 26 dB | 23 dB | −3 dB |
| 14 | anacusis | anacusis | – |
| 15 | 24 dB | 24 dB | – |
| 16 | 37 dB | 40 dB | +3 dB |
| 17 | 18 dB | 22 dB | +4 dB |
| 18 | anacusis | anacusis | – |
| 19 | 15 dB | 27 dB | +12 dB |
| 20 | 32 dB | 35 dB | +3 dB |
| 21 | 41 dB | 55 dB | +14 dB |
| 22 | 10 dB | 20 dB | +10 dB |
| 23 | anacusis | anacusis | – |
| 24 | 100 dB | 100 dB | – |
| 25 | 28 dB | 39 dB | +11 dB |
| 26 | 41 dB | 50 dB | +9 dB |
| 27 | 11 dB | 25 dB | +14 dB |
| 28 | 57 dB | 70 dB | +13 dB |
| 29 | 20 dB | 40 dB | +20 dB |
| 30 | 9 dB | 23 dB | +14 dB |
| 31 | 5 dB | 15 dB | +10 dB |
| 32 | 69 dB | 80 dB | +11 dB |
| 33 | anacusis | anacusis | – |
| 34 | 29 dB | 45 dB | +16 dB |
| 35 | anacusis | anacusis | – |
| 36 | 10 dB | 19 dB | +9 dB |
| 37 | 24 dB | 27 dB | +3 dB |
| 38 | 58 dB | 53 dB | −5 dB |
| 39 | 63 dB | 65 dB | +2 dB |
| 40 | 35 dB | 30 dB | −5 dB |
| 41 | 45 dB | 42 dB | −3 dB |
| 42 | 27 dB | 14 dB | −13 dB |
| 43 | 20 dB | 17 dB | +3 dB |
| 44 | 60 dB | 61 dB | +1 dB |
| 45 | 21 dB | 21 dB | – |
Abbreviations: ABG, air bone gap.
In the 13 patients (28.9%) who underwent the conservative surgical approach (the transmastoid technique versus the MCF approach or combined techniques), the mean preoperative hearing threshold was 20.5 dB, and the postoperative threshold was 25.9 dB, with a mean air–bone gap (ABG) of +5.4 dB. In this group, 1 patient (patient 45) maintained the same audiological threshold (21 dB) in the preoperative and postoperative evaluation. By contrast, the ABG worsened in 9 out of 12 patients (75%) who received a conservative surgery, and only 3 patients (25%) experienced an improvement in their audiological outcomes (patient 13 gained 3 dB, patient 40 gained 5 dB, and patient 42 gained 13 dB) (Table 2).
3.2. Surgical outcomes
The mean postoperative follow-up period at the time of our statistical analysis was 48 months (range: 12–84 months). All patients exhibited resolution of their CSF leakage, and no clinical recurrences were observed during the follow-up period. After the surgery, two patients (4.4%) developed paralysis of the facial nerve; however, neither case exceeded House–Brackmann grade 3, and in both cases, the clinical condition was temporary and probably related to transient thermal damage of the facial nerve. Finally, one patient developed a hematoma after abdominal fat excision, which was resolved with serial dressings during the first two postoperative weeks.
4. Discussion
The scientific literature extensively describes different surgical approaches to manage TD and MEH. The primary objective of this surgery is to ensure patient safety, especially considering the possibility of serious complications related to severe neurological diseases, which include CFS leakage and meningitis (Byrne et al., 2010; Golding-Wood et al., 1991; Grinblat et al., 2018; May et al., 1995; Nahas et al., 2008). As mentioned in the introduction, in addition to avoiding life-threatening conditions, another surgical goal is to preserve hearing ability whenever possible. The MCF approach allows the surgeon to manage anterior tegmen defects. This technique is usually performed with an inverted U- or V-shaped incision cranial to the ear and a craniotomy bone flap. This allows access to the temporal lobe, which is gently retracted and elevated in a posterior-to-anterior direction, thus enabling the surgeon to treat the TD, with a reduced risk of damaging important anatomical, such as the greater superficial petrosal nerve (GSPN), the area of the geniculate ganglion, and the ossicular chain (Ahmed et al., 2017; Braca et al., 2013; Carlson et al., 2013; Hoang et al., 2018; Tolisano and Kutz, 2019).
The transmastoid technique (Kim et al., 2014; Oliaei et al., 2012; Sergi et al., 2013) is a valid alternative for the management of posterior and TD and/or MEH and is commonly achieved by retroauricular access, which permits access to the mastoid region and the tympanic cavity. This technique cannot treat anterior TD if the ossicular chain is still in place.
Finally, in selected cases, the surgeon can combine these techniques, opting for the transmastoid technique with minicraniotomy (Adkins and Osguthorpe, 1983; Kuhweide and Casselman, 1999; Marchioni et al., 2014; Ramalingam et al., 2008). As highlighted by Marchioni et al. (2014), combining these techniques allows the surgeon to repair anterior bone defects without manipulating the temporal lobe ossicular chain.
Regardless of the type of technique adopted, many reconstruction strategies are possible with autologous tissues (e.g., cartilage, temporalis fascia, and bone tissue) and heterologous tissues (e.g., hydroxyapatite cement and collagenous membrane) (Kveton and Goravalingappa, 2000; Sergi et al., 2013).
Dealing with large TD and/or TD with anterior/posterior localization may require STP petrosectomy, a more invasive approach that involves the complete removal of all air cells of the temporal bone, including those of the middle ear and mastoid, the ossicular chain and the tympanic membrane. This technique also involves the obliteration of the middle ear followed by a blind sac closure of the external auditory canal (EAC), resulting in worsened hearing (Magliulo et al., 2014; Prasad et al., 2017; Sanna et al., 2008, 2009; Vincenti et al., 2014).
Because each of these strategies has different topics, all patients should undergo a preoperative assessment considering individual characteristics—especially age, preoperative hearing threshold, and the location and size of the TD and/or MEH—to select the most suitable surgical technique to approach TD and/or MEH. In addition to these characteristics, comorbidities (e.g., diabetes and previous meningitis) and the history of recurrence of the disease despite previous surgical intervention should be considered for each patient.
During the preoperative assessment, patients with a good preoperative hearing threshold were considered differently from those with impaired hearing ability and/or anacusis. In our opinion, the maintenance of good hearing should be prioritized in all cases in which a conservative intervention does not expose the subject to a greater number of postoperative complications, which can be life threatening.
Along with the patient characteristics, we also considered the size and location of the TD and/or MEH. We choose the MCF approach for younger patients with a good preoperative hearing threshold and those who have an MEH of spontaneous etiology (Kim et al., 2014). This proposal agrees with the work of Tolisano and Kutz (2019), which suggested the use of the MCF technique to reach anterior defects (tegmen tympani), without the need to manipulate the ossicular chain, for total control of the cranial fossa floor. In our experience, the MCF approach has not been the optimal technique to manage intradural repair linked to TDs because the brain matrix is difficult to handle surgically; it is preferable to approach it through extradural techniques. Sergi et al. (2013) proposed the transmastoid approach to repair TDs limited to the tegmen mastoideum and the tympani using collagenous membranes and bone substitutes. By contrast, we propose the transmastoid technique (Kim et al., 2014; Oliaei et al., 2012; Sergi et al., 2013) as a valid strategy for the management of small TDs located at the level of the tegmen mastoideum and the tegmen antri in patients with a good preoperative audiometric threshold. This approach protects the integrity of the ossicular chain and does not exclude the possibility of subsequent prosthetic reconstruction of the ear ossicular chain (when the pathology involves the ossicular chain) (Kim et al., 2014).
If the bone defect is posterior and exceeds 10 mm and the patient has a good preoperative audiometric threshold, the transmastoid technique alone is not sufficient to approach the pathology; instead, it should be combined with the minicraniotomy approach (Adkins and Osguthorpe, 1983; Kuhweide and Casselman, 1999; Marchioni et al., 2014; Ramalingam et al., 2008).
Conversely, STP (Magliulo et al., 2014; Prasad et al., 2017; Sanna et al., 2008, 2009) is the safest surgical technique to treat TDs and MEH, as it guarantees superior surgical control and reduces the risk of complications, ensuring a lower relapse rate (Sanna et al., 2009).
In our experience, STP should be the approach of choice in patients with a reduced hearing threshold, as the auditory conduction component is sacrificed during the procedure, and the surgical cavity is ultimately occluded with abdominal fat and a double-blind closure of the EAC (Sanna et al., 2009). Moreover, STP is typically reserved for when the TD or MEH is too large to be approached with a more conservative technique.
Finally, STP was also performed in most patients with anteriorly localized TD/MEH, despite their good hearing ability. This technique was chosen to guarantee a safer procedure and lower the risk of neurological complications and possible life-threatening complications, especially in patients with chronic comorbidities and a compromised state of health; for these reasons, STP was a common surgical technique.
Outside of these parameters, other factors should be considered.
First, we must underline the need for experienced surgeons who can shift from a conservative approach to STP in cases with intraoperative evidence of more extensive disease than was apparent in the preoperative evaluation.
Second, each patient should be considered individually, and the current problem and the patient's history should be evaluated (i.e., our preoperative assessment could not be applied in patients affected by TD and/or MEH relapse who underwent STP in the past). Moreover, new hearing technologies enable the use of prosthetic aids in all patients who retain good postoperative bone conduction, such as bone-anchored hearing aids (BAHAs); in addition, through an accurate preoperative evaluation, a cochlear implant (CI) can be proposed to all patients who undergo STP (Magliulo et al., 2014), guaranteeing the possibility of auditory rehabilitation even in patients who undergo STP.
This study provides a useful initial perspective for providing criteria for approaching TDs and/or MEHs of the temporal bone; however, it also has several limitations. The major limitations are the small study population and the restricted audiological data. Moreover, we did not discuss the audiological outcomes in patients who underwent STP and subsequently received a CI or a postoperative prosthetic intervention (e.g., BAHA).
Finally, our study does not consider other technologies, such as 3D-printed materials (Oliaei et al., 2012) and new materials (e.g., S53P4 bioactive glass), which could change the therapeutic approach in the future, providing perspectives for audiological improvement in patients with postoperative hearing impairment.
5. Conclusions
Our study presents our experience in the approach to TDs of the temporal bone and MEH, considering patient characteristics and the size and location of the TDs and/or MEHs, to guide the selection of the optimal surgical technique and ensure better audiological outcomes and less exposure to the risk of complications and/or disease recurrence. In the future, we hope to collect more postoperative data over a longer follow-up period, considering audiological outcomes in a more complete and in-depth manner, along with the subsequent positioning of a CI in patients who undergo STP as well as new technologies.
We hope that our data and experience serve as valuable tools to guide surgical strategies for less experienced surgeons, and we emphasize that every surgeon must be able to manage all of the main possible complications related to the surgical intervention and perform all these techniques.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Emanuela Fuccillo, Email: emanuela.fuccillo@asst-santipaolocarlo.it.
Alberto Maria Saibene, Email: alberto.saibene@asst-santipaolocarlo.it.
Elena Ferrari, Email: el.ferrari.ef@gmail.com.
Giorgia Carlotta Pipolo, Email: carlotta.pipolo@unimi.it.
Antonia Pisani, Email: antonia.pisani@asst-santipaolocarlo.it.
Liliana Colletti, Email: liliana.colletti@unimi.it.
Luigi De Donato, Email: luigi.dedonato@unimi.it.
Giovanni Felisati, Email: giovanni.felisati@unimi.it.
References
- Adkins W.Y., Osguthorpe J.D. Mini-craniotomy for management of CSF otorrhea from tegmen defects. Laryngoscope. 1983;93:1038–1040. doi: 10.1288/00005537-198308000-00012. [DOI] [PubMed] [Google Scholar]
- Ahmed S., VanKoevering K.K., Kline S., Green G.E., Arts H.A. Middle cranial fossa approach to repair tegmen defects assisted by three-dimensionally printed temporal bone models. Laryngoscope. 2017;127(10):2347–2351. doi: 10.1002/lary.26438. [DOI] [PubMed] [Google Scholar]
- Braca J., Marzo S., Prabhu V.C. Cerebrospinal fluid leakage from tegmen tympani defects repaired via the middle cranial fossa approach. J. Neurol. Surg. B Skull. Base. 2013;74:103–107. doi: 10.1055/s-0033-1333616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W., Smith A.P., Roh D., Kanner A. Occult middle fossa encephaloceles in patients with temporal lobe epilepsy. World Neurosurg. 2010;73(5):541–546. doi: 10.1016/j.wneu.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Carlson M.L., Copeland W.R., Driscoll C.L., Link M.J., Haynes D.S., Thompson R.C., Weaver K.D., Wanna G.B. Temporal bone encephalocele and cerebrospinal fluid fistula repair utilizing the middle cranial fossa or combined mastoid-middle cranial fossa approach. J. Neurosurg. 2013;119(5):1314–1322. doi: 10.3171/2013.6.JNS13322. [DOI] [PubMed] [Google Scholar]
- Golding-Wood D.G., Williams H.O.L., Brookes G.B. Tegmental dehiscence and brain herniation into the middle ear cleft. J. Laryngol. Otol. 1991;105:477–480. doi: 10.1017/s0022215100116354. [DOI] [PubMed] [Google Scholar]
- Grinblat G., Dandinarasaiah M., Prasad S.C., Piras G., Piccirillo E., Fulcheri A., Sanna M. Temporal bone meningo-encephalic-herniation: etiological categorization and surgical strategy. Otol. Neurotol. 2018;39(3):320–332. doi: 10.1097/MAO.0000000000001693. [DOI] [PubMed] [Google Scholar]
- Hoang S., Ortiz Torres M.J., Rivera A.L., Litofsky N.S. Middle cranial fossa approach to repair tegmen defects with autologous or alloplastic graft. World Neurosurg. 2018;118:e10–e17. doi: 10.1016/j.wneu.2018.05.196. [DOI] [PubMed] [Google Scholar]
- Kim L., Wisely C.E., Dodson E.E. Transmastoid approach to spontaneous temporal bone cerebrospinal fluid leaks: hearing improvement and success of repair. Head Neck Surg. 2014;150:472–478. doi: 10.1177/0194599813518173. [DOI] [PubMed] [Google Scholar]
- Kuhweide R., Casselman J.W. Spontaneous cerebrospinal fluid otorrhea from a tegmen defect: transmastoid repair with minicraniotomy. Ann. Otol. Rhinol. Laryngol. 1999;108:653–658. doi: 10.1177/000348949910800706. [DOI] [PubMed] [Google Scholar]
- Kveton J.F., Goravalingappa R. Elimination of temporal bone cerebrospinal fluid otorrhea using hydroxyapatite cement. Laryngoscope. 2000;110:1655–1659. doi: 10.1097/00005537-200010000-00016. 2000. [DOI] [PubMed] [Google Scholar]
- Magliulo G., Iannella G., Ciniglio Appiani M., Re M. Subtotal petrosectomy and cerebrospinal fluid leakage in unilateral anacusis. J. Neurol. Surg. B Skull. Base. 2014;75(6):391–396. doi: 10.1055/s-0034-1376196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki F.M., Amoodi H.A., van Wijhe R.G., Bance M. Anatomic analysis of the mastoid tegmen: slopes and tegmen shape variances. Otol. Neurotol. 2011;32(4):581–588. doi: 10.1097/MAO.0b013e31820e75f7. [DOI] [PubMed] [Google Scholar]
- Marchioni D., Bonali M., Alicandri-Ciufelli M., Rubini A., Pavesi G., Presutti L. Combined approach for tegmen defects repair in patients with cerebrospinal fluid otorrhea or herniations: our experience. J. Neurol. Surg. B Skull. Base. 2014;75(4):279–287. doi: 10.1055/s-0034-1371524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.S., Mikus J.L., Matthews B.L., Browne J.D. Spontaneous cerebrospinal fluid otorrhea from defects of the temporal bone: a rare entity? Am. J. Otol. 1995;16(6):765–771. [PubMed] [Google Scholar]
- Nahas Z., Tatlipinar A., Limb C.J., Francis H.W. Spontaneous meningoencephalocele of the temporal bone: clinical spectrum and presentation. Arch. Otolaryngol. Head Neck Surg. 2008;134(5):509–518. doi: 10.1001/archotol.134.5.509. [DOI] [PubMed] [Google Scholar]
- Oliaei S., Mahboubi H., Djalilian H.R. Transmastoid approach to temporal bone cerebrospinal fluid leaks. Am. J. Otolaryngol. 2012;33:556–561. doi: 10.1016/j.amjoto.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Prasad S.C., Roustan V., Piras G., Caruso A., Lauda L., Sanna M. Subtotal petrosectomy: surgical technique, indications, outcomes, and comprehensive review of literature. Laryngoscope. 2017;127(12):2833–2842. doi: 10.1002/lary.26533. [DOI] [PubMed] [Google Scholar]
- Ramalingam K.K., Ramalingam R., SreenivasaMurthy T.M., Chandrakala G.R. Management of temporal bone meningo-encephalocoele. J. Laryngol. Otol. 2008;122(11):1168–1174. doi: 10.1017/S0022215108001990. [DOI] [PubMed] [Google Scholar]
- Sanna M., Dispenza F., Flanagan S., De Stefano A., Falcioni M. Management of chronic otitis by middle ear obliteration with blind sac closure of the external auditory canal. Otol. Neurotol. 2008;29(1):19–22. doi: 10.1097/MAO.0b013e31815dbb40. [DOI] [PubMed] [Google Scholar]
- Sanna M., Fois P., Russo A., Falcioni M. Management of meningoencephalic herniation of the temporal bone: personal experience and literature review. Laryngoscope. 2009;119(8):1579–1585. doi: 10.1002/lary.20510. [DOI] [PubMed] [Google Scholar]
- Sergi B., Passali G.C., Picciotti P.M., De Corso E., Paludetti G. Transmastoid approach to repair meningoencephalic herniation in the middle ear. Acta Otorhinolaryngol. Ital. 2013;33(2):97–101. [PMC free article] [PubMed] [Google Scholar]
- Tolisano A.M., Kutz J.W. Middle fossa approach for spontaneous cerebrospinal fluid fistula and encephaloceles. Curr. Opin. Otolaryngol. Head Neck Surg. 2019;27:356–360. doi: 10.1097/moo.0000000000000560. [DOI] [PubMed] [Google Scholar]
- Tóth M., Helling K., Baksa G., Mann W. Localization of congenital tegmen tympani defects. Otol. Neurotol. 2007;28(8):1120–1123. doi: 10.1097/MAO.0b013e31815aee0c. [DOI] [PubMed] [Google Scholar]
- Vincenti V., Pasanisi E., Bacciu A., Bacciu S. Long-term results of external auditory canal closure and mastoid obliteration in cochlear implantation after radical mastoidectomy: a clinical and radiological study. Eur. Arch. Oto-Rhino-Laryngol. 2014;271(8):2127–2130. doi: 10.1007/s00405-013-2698-3. [DOI] [PubMed] [Google Scholar]