Abstract
Objective
Fungiform papillae contain taste buds and play a critical role in mastication and the gustatory system. In this study, we report a series of sequential observations of organogenesis of fungiform papillae in miniature pigs, as well as changes in the expression of BMP2, BMP4, Wnt5a, Sox2, and Notch1 signaling pathway components.
Design
In this study, we investigated the spatiotemporal expression patterns of BMP, Wnt, Sox2 and Notch in the fungiform papillae of miniature pigs at the bud stage (E40), cap stage (E50) and bell stage (E60). Pregnant miniature pigs were obtained, and the samples were processed for histological staining. Immunohistochemistry and real-time PCR were used to detect the mRNA and protein expression levels of BMP2, BMP4, Wnt5a, Sox2, and Notch1.
Results
At E40, fungiform papillae were present on the anterior two-thirds of the tongue in a specific array and pattern. The fungiform papillae were enlarged and basically developed at E50 and were largest at the earlier stage (E60). Most of the BMP2 was concentrated in the epithelial layer and the connective tissue core of the fungal papilloma and gradually accumulated from E40-E60. BMP-4 was weakly expressed in the fungiform papillae epithelia, but BMP-4-positive cells were also observed in the developing tongue muscle at E50 and E60. Wnt5a-positive cells were observed in the fungiform papillae epithelia and developing tongue muscle at all three time points. Sox2-positive cells were observed only in fungiform papillae epithelial cells, and Notch1-positive cells could not be detected.
Conclusions
This study provides primary data regarding the morphogenesis and expression of developmental signals in the fungiform papillae of miniature pigs, establishing a foundation for further research in both this model and humans.
Keywords: Fungiform papilla, Development, Miniature pig, Immunohistochemistry
Abbreviations
- BMP
bone morphogenetic protein
- Wnt
wingless/integrated
- Sox2
SRY-box transcription factor 2
- E40
embryonic day 40
- E50
embryonic day 50
- E60
embryonic day 60
- H&E
hematoxylin and eosin
- BSA
bovine serum albumin
- RT
room temperature
- BMP2
bone morphogenetic protein 2
- BMP4
bone morphogenetic protein 4
- Wnt5a
Wnt family member 5A
- Notch1
Notch receptor 1
- HRP
horseradish peroxidase
- DAB
diaminobenzidine
- SEM
scanning electron microscope
1. Introduction
The development and periodic repair of organs (hair, teeth, skin, tongue papillae, etc.) are crucial for the regeneration and maintenance of normal function after organ wear and tear [1,2]. The activation of initiation signals for organ development and repair is crucial for inducing regeneration of cells or tissues from the remaining stage to the initial stage [3]. The activation of organ development or periodic repair initiation signals is programmed in a process that is not the same as the mechanism of immune repair in the body after organ damage. However, the mechanism of organ renewal has yet to be determined [4].
Lingual papillae are epithelial appendages that develop like other tissues, including hair, kidneys, teeth, feathers, and mammary glands [5]. Tongue papillae can be divided into filamentous papillae, fungal papillae, leaf-shaped papillae, and contoured papillae according to their shape [6]. Among them, the number of filamentous papillae is the highest, and the volume is the smallest, but there are no taste buds. The shape and function of leaf papillae gradually become vestigial, and the number of contour papillae is the lowest [7]. The bacterial papillae are the tongue papillae with the largest distribution area (2/3 of the front of the tongue) and the largest number of papillae, and each fungal papilla contains one or more taste buds [8]. Therefore, fungal papillae are the most important tongue papillae for taste perception [9,10]. However, little is known about the periodic regeneration mechanism of fungiform papillae.
Lingual papilla development might involve similar processes to those of other epithelial appendages, including feathers, hair, and teeth, which all require specific and complex interactions between the epithelium and mesenchyme [[11], [12], [13]]. Numerous studies have recently reported several important mechanisms for lingual papillae development in rodents, such as bone morphogenetic protein (BMP) and Wnt signaling [14,15]. Since the morphological, anatomical, and physiological features of the rodent tongue greatly differ from those of the human tongue, a large animal model is needed for the study of lingual papilla development [15].
The miniature pig is a good model for orofacial research because the organs of miniature pigs and humans not only are similar in morphology but are also basically the same in terms of physiological function, especially in the oral and maxillofacial systems, skin, cardiovascular system, gastrointestinal tract and urinary system [[16], [17], [18]]. In addition, the development of the miniature pig is also very close to that of humans, the development degree of each organ at birth is similar to that of humans, and the growth and development after birth is also similar to that of humans [19,20]. Therefore, minipigs are particularly suitable animal models for organ development research, and studying the development of lingual papillae in minipigs is highly important for the study of taste function and regeneration in humans. The research group established a small pregnant pig model as a research model for tooth development in the early stage and used a large animal model to define the development pattern of bidentate dentition and establish a gene expression network to explore in depth the mechanism of tooth regeneration and development [21]. Among them, BMP2, BMP4, Wnt5a, Sox2, and Notch1 have different distribution patterns in the epithelium and stroma of third molar embryos of small pigs at different stages of development and may serve as starting signals for organ development or periodic repair, inducing programmed organ repair [[21], [22], [23], [24], [25]]. Here, we used the miniature pig as a model to investigate the morphogenesis and expression of critical developmental signals of fungiform papillae in a large animal.
2. Materials and methods
2.1. Animals
This study was performed in accordance with a protocol approved by the Animal Care and Use Committees of Capital Medical University. Cesarean section was performed successfully on three pregnant miniature pigs under general anesthesia. Pregnant miniature pigs were obtained from the Institute of Animal Science at the Chinese Agricultural University. The embryonic development of the miniature pig is long, and the development of the miniature pig first molar includes three typical periods designated the bud stage (E40), cap stage (E50), and earlier bell stage (E60). Therefore, embryonic days E40, E50, and E60 were chosen as the time points for determining whether fungiform papilla development also included typical periods. The animals were allowed access to food and water ad libitum under normal conditions and were humanely sacrificed as necessary to ameliorate suffering. Pregnant miniature pigs were anesthetized and sacrificed as previously described [26]. In brief, pregnant sows were anesthetized with a combination of 6 mg/kg ketamine chloride and 0.6 mg/kg xylazine, and pregnancy and fetal state were roughly determined via B-mode ultrasonography. After the fetuses were removed by cesarean section, the pregnant sows were sacrificed by overanesthetization. The study was carried out in compliance with the ARRIVE guidelines.
2.2. Histological staining
The tongues of E40, E50, and E60 embryos were fixed in 4 % paraformaldehyde in PBS at 4 °C overnight, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E).
2.3. Scanning electron microscopy analysis
The tongues of E40, E50, and E60 embryos were prefixed with 2.5 % glutaraldehyde and postfixed with 1 % osmium tetroxide. The samples were then dehydrated in a graded series of ethanol, followed by critical point drying. The samples were mounted on stubs, coated with platinum, and examined by scanning electron microscopy (SEM, S-4800; Hitachi, Tokyo, Japan).
2.4. Immunohistochemistry for determining the Location of developmental signals
After deparaffinization, the sections were blocked with 5 % BSA for 1 h at RT and incubated with primary antibodies against Bmp-2 (1:100; Abcam; #ab82511), Bmp-4 (1:200; Abcam; #ab39973), Wnt5a (1:150; Abcam; #ab72583), Sox2 (1:100; Abcam; #ab97959), and Notch1 (1:200; Abcam; #ab8925). Secondary antibodies (Abcam; #ab6721) were used at a dilution of 1:200. The sections were developed with an anti-rabbit HRP/DAB detection kit (Abcam; #ab64261). Negative controls were obtained by omitting the primary antibody, and images were acquired with the same exposure.
2.5. Real-time PCR (RT‒PCR)
cDNA was obtained from E40, E50, and E60 pig embryos as described above. The primers used for BMP2/4, Wnt5a, Sox2 and Notch1 were designed using Primer Premier 5.0; the primers used are listed in Table S1. The relative expression of each gene was determined using the 2ΔΔCT method. The reactions were performed as follows: 50 μL of SYBR Green PCR Master Mix (Promega) containing 4 mmol/L Mg2+, 1 μL of upstream and downstream primers, 0.3 μL of Taq DNA polymerase (Promega), 2 μL of the cDNA template, and 20.7 μL of ddH2O. The PCR conditions were as follows: 95 °C denaturation for.
3 min; 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s; and a 72 °C extension for 10 min.
2.6. Statistical analysis
All of the experiments and data acquisitions involved at least three independent measurements. One-way ANOVA and the Newman–Keuls multiple comparison test were used to calculate statistical significance. The level of significance was defined as *p < 0.05, **p < 0.01, or ***p < 0.001. The statistical analysis was performed using SPSS 16.0 software.
3. Results
3.1. Morphological and histological Characterization of fungiform papillae development in the miniature pig
The number and distribution pattern of fungiform papillae were observed via SEM (Fig. 1). At E40, fungiform papillae were present on the anterior two-thirds of the tongue in a specific array and pattern. The lesions were small in size and did not fully develop in some areas of the tongue (Fig. 1A, B, and C). The fungiform papillae were enlarged and basically developed at E50 (Fig. 1D, E and F) and were largest at E60 (Fig. 1G, H and I). From E40 to E60, the fungiform papilla gradually increased (Fig. 1J) and the number of fungiform papillae per unit area (0.3 × 0.3 mm) gradually decreased, and there was a significant difference at different time points ((Fig. 1K).
Fig. 1.
SEM image of fungiform papillae during morphogenesis. At E40, E50, and E60, fungiform papilla morphology (arrows) was observed on the surface of the tongue dorsum. At different time points, the fungiform papilla morphology was basically consistent, with granular protrusions on the surface. The papilla gradually decreased in number per unit area (A–I). From E40 to E60, the fungiform papilla gradually increased, the area of a single papilla at E60 significantly increased, and there were significant differences at different time points, with units of square millimeters (J). From E40 to E60, the number of fungiform papillae per unit area (0.3 × 0.3 mm) gradually decreased, and there was a significant difference at different time points (K). Scale bar (A, D and G) 300 μm; (B, E and H) 40 μm; (C, F and I) 20 μm.
Transverse sections of the E40, E50, and E60 embryo tongues were labeled with H&E. At E40, the epithelial layer became concave on the dorsal surface of the tongue, and the condensed mesenchymal cells migrated upward, indicating that the fungiform papillae had begun to develop (Fig. 2A–D, G). Arch-like structures of fungiform papillae appeared on the dorsal surface of the tongue due to the continued upward migration of mesenchymal cells (Fig. 2B–E, H). The epithelial cells at the papillary surface continued to differentiate and became squamous at E60 (Fig. 2C–F, I).
Fig. 2.
Histology of fungiform papillae during morphogenesis. (A, D, G) The epithelium became concave, and the mesenchymal cells were condensed at E40. (B, E, H) Archlike structures arose, and the underlying mesenchymal cells continued to migrate upward at E50. (C, F, I) The epithelium continued to thicken, and squamous epithelial cells were detected on the surface of fungiform papillae at E60. The epithelium (EP), mesenchyme (MZ), lamina propria mucosae (M), apical papilla epithelium (APE), light superficial cells of the epithelium (LSC), and connective tissue core of the primordium of the fungiform papilla (star) were used. Scale bars: 100 μm (A, B, C), 50 μm (D, E, F) and 20 μm (G, H, I).
3.2. Activities of BMP, Wnt, Sox2 and Notch signals in fungiform papillae development
We observed the expression of BMP-2, BMP-4, Wnt5a, Sox2, and Notch1 in developing fungiform papillae through immunohistochemistry. BMP-2 was expressed in fungiform papillary epithelial cells and developing tongue muscles. Most of the BMP2 was concentrated in the epithelial layer and the connective tissue core of the fungal papilloma and gradually increased from E40-E60, with less expression in the mesenchymal and muscular layers (Fig. 3A, B, C, and P). BMP-4 was weakly expressed in the upper cortex of the fungiform papilla tip during E40-E60 development and was slightly expressed in the E60 mesenchymal layer. However, BMP-4-positive cells were also observed in the tongue muscles during E50 and E60 development (Fig. 3D, E, F, and Q). At all three time points, Wnt5a-positive cells were observed in the fungal apical papilla epithelium, stroma, connective tissue core of the fungal papilloma, and developing tongue muscles (Fig. 3G, H, I, and R). Sox2-positive cells were weakly expressed in the entire tongue epithelium and fungiform papilla, with only a small number of positive cells observed in the superficial cell layer of the epithelium (Fig. 3J, K, L, and S). In addition, the expression level of Notch1 was similar to that of Sox2, and Sox2 was only weakly expressed in the upper cortex of the fungiform papilla; however, Notch1-positive cells could not be detected in the superficial cell layer, mesenchymal layer, or muscular layer of the epithelium (Fig. 3M, N, O, and T).
Fig. 3.
Location of BMP-2, BMP-4, Wnt5a, Sox2, and Notch1. (A-C, P) BMP-2 expression was observed in the epithelial cells of fungiform papillae and the developing tongue muscle. (D-F, Q) BMP-4 was weakly expressed in fungiform papillae epithelia, and BMP-4-positive cells were observed in the developing tongue muscle at E50 and E60. (G-I, R) Wnt5a-positive cells were observed in fungiform papillae epithelia and the developing tongue muscle at all three timepoints. (J-L, S) Sox2-positive cells were observed throughout the lingual epithelium and fungiform papillae. (M − O, T) Notch1-positive cells could not be detected. Fungiform papillae are marked with arrowheads. Mesenchyme (MZ), lamina propria mucosae (M), apical papilla epithelium (APE), light superficial cells of the epithelium (LSC), and connective tissue core of the primordium of the fungiform papilla (star). Scale bar, 30 μm.
3.3. RT–PCR analysis of BMP2/4, Wnt5a, Sox2 and Notch1 mRNA expression
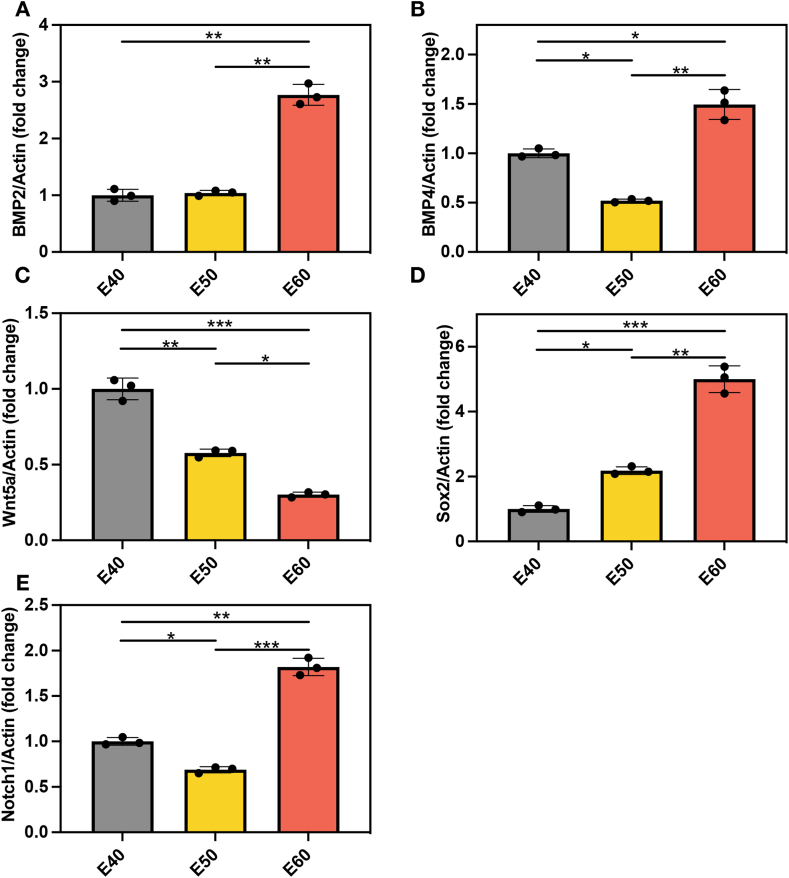
The RT‒PCR results were generally consistent with the immunohistochemistry results. BMP-2 and Sox2 expression increased with tongue development. BMP-2 and Sox2 expression was highest at E60 (Fig. 4a and d). BMP-4 and Notch1 expression was weaker at E50 than at E40, but BMP-4 and Notch1 expression increased again at E60 (Fig. 4b and e). Wnt5a expression decreased with tongue development (Fig. 4c).
Fig. 4.
Quantitative analysis of BMP-2, BMP-4, Wnt5a, Sox2, and Notch1 mRNA expression levels in the developmental stages of the fungiform papillae in miniature pigs. (A) Relative BMP2 mRNA expression in the fungiform papillae at E40-E60 determined by RT‒PCR. (B) Relative BMP4 mRNA expression in the fungiform papillae at E40-E60 determined by RT‒PCR. (C) Relative Wnt5a mRNA expression at E40-E60 determined by RT‒PCR in fungiform papillae. (D) Relative Sox2 mRNA expression in the fungiform papillae at E40-E60 determined by RT‒PCR. (E) Relative Notch1 mRNA expression in fungiform papillae at E40-E60 determined by RT‒PCR. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
The fungiform papilla of the tongue plays an important role in taste perception, but previous studies have mostly used mice as animal models [27]. In recent years, as the similarities between human and pig maxillofacial anatomy and oral diseases have become increasingly known, the application of small pigs in oral and neck research has become increasingly important. However, due to factors such as animal protection, the use of small pigs has significantly increased annually, and small pigs are expected to replace experimental monkeys and dogs as a new type of experimental animal widely used. Small pigs, as animal models, have broad application prospects and important positions in biomedical research [28]. The fungiform papillae emerge from the homogeneous epithelium of the early tongue by E13 in mice. Placodes are first identified morphologically at embryonic day 13 and occupy two bilateral rows adjacent to the midline. The epithelial thickening of the placode involves a series of invaginations and evaginations over a collection of mesenchymal cells to form a papilla with a distinctive epithelium over a core of connective tissue. A full complement of developing fungiform papillae is present on the tongue by embryonic day 14.5 [29]. In this study, the morphology and phase of the fungiform papillae of small pigs at different developmental stages were evaluated. We did not observe early development of the placodes in this study, but we detected the migration of condensed mesenchymal cells at E40. The epithelium and mesenchyme underwent subsequent morphogenesis at E50. Archlike structures were raised, and the papillae began to develop. At E60, the epithelial cells at the papillary surface became squamous, and the interaction between the epithelium and mesenchyme led to the formation of fungiform papillae with an epithelial covering a mesenchymal core.
Signaling factors involved in epithelial patterning in numerous tissues are also expressed on the tongue surface during development [30]. Bone morphogenetic proteins are multifunctional growth factors belonging to the transforming growth factor-beta superfamily [31]. The BMP signaling pathway is known to be important for regulating the shape of ectodermal organs, including teeth and tongue taste papillae [[32], [33], [34], [35], [36]]. Recent studies of developing mouse embryo tongues demonstrated that BMP-2 and BMP-4 are expressed within the epithelia of the tongue primordia, demonstrating the fundamental importance of these signaling factors in papillae morphogenesis [37]. In rats, BMP-2 and BMP-4 can inhibit the formation of fungiform papillae from placodes, and the BMP antagonist noggin was found to induce papilla formation and increase the number of fungiform papillae [36]. This finding suggested that the balance of BMPs and noggin is important for papilla integrity, number, and patterning in rats. In the present study, BMP2 was highly expressed at E60, but at the E60 stage, the number of papillae decreased, the area increased, and the development gradually matured. Therefore, it can be speculated that BMP2 may play a role in the development of papillae at E60. However, the expression of BMP4 increased from E40 to E60. The roles of BMP4 and BMP2 in the development of the pig lingual papilla may not be the same. BMP4 may play a regulatory role at E40 and E60. Wnts are a large ligand family that functions via multiple receptor-mediated pathways [38]. One of these factors involves β-catenin activation and results in transcriptional activation of the Lef1 and Tcf transcription factors [39]. Activating Wnt signaling in tongue cultures with LiCl addition increases papillae number, and disruption of β-catenin signaling blocks fungiform placode development [6,40]. Wnt5a is considered a representative ligand that activates the noncanonical Wnt signaling pathway because it does not signal by stabilizing β-catenin in many biological systems, and emerging evidence suggests that Wnt5a can inhibit Wnt/β-catenin signaling [[41], [42], [43]] and play a distinctive role in fungiform papilla patterning and development in mice [44]. In contrast to the temporal and spatial distribution of BMP2/4, Wnt5a was highly expressed at E60 during the development of lingual papillae in miniature pigs. At E40, there were more fungiform papillae in the unit area of the tongue, but no obvious papillary form had formed. Therefore, we speculate that Wnt5a may play an important role in regulating the number of fungiform papillae.
Sox2 is known to be a critical player in mammalian development and is needed for the development of sensory systems [45]. A recent study indicated that a reduction in Sox2 expression to 20 % of normal levels causes papilla placodes and early papillae to begin developing but fail to reach maturity, while filiform papillae develop normally [46]. In contrast, overexpression of Sox2 induces the development of atypical fungiform-like papillae, but filiform papillae do not develop [47]. This finding suggested that Sox2 regulates the differentiation of endodermal progenitor cells in the mouse tongue into taste bud cells rather than keratinocytes [46]. This finding is similar to the possible role of Sox2 in the development of fungiform papilla in miniature pigs because the expression of Sox2 gradually increases during the development of the fungiform papilla [48]. In the present study, SOX2 was expressed at relatively low levels from E40-E60, with only a small amount expressed in the superficial epithelium and some mesenchyme of the apical papilla. This finding is different from the expression of SOX2 in the fungiform papilla of mice. Moreover, Sox2 is expressed in the mesenchyme between the superior and inferior longitudinal and genioglossus muscles in the middle and posterior regions of the tongue as well as in the epithelium throughout the tongue [47]. Therefore, at which stage SOX2 plays a role in the development of tongue fungiform papillae in small pigs is unclear, and its developmental network still needs to be further constructed and explored.
The Notch pathway is involved in determining cell fate within the nervous system and in various sensory organs [[49], [50], [51]]. Previous studies demonstrated that Notch-associated genes were expressed both in the developing taste epithelia and in adult taste buds [52,53]. We detected a change in Notch1 gene expression, but we did not observe Notch1-positive cells in the tongue tissue of the miniature pigs. The possible reason is that the transcription of Notch1 mRNA is regulated by microRNAs, long noncoding RNAs, circular RNAs or other chemical modifications, which may inhibit the translation of mRNA into protein, resulting in the decreased expression of Notch1 [54]. In addition, the weak specificity of the Notch1 antibody may also be an important factor in the study of minipig development [55].
In this study, we identified BMP-2-positive cells, BMP-4-positive cells, Wnt5a-positive cells, and Sox2-positive cells in the fungiform papillae epithelium and investigated the changes in the expression of these genes. Our findings confirmed previous results on fungiform papillae development in mice [47,56,57]. These findings suggested that BMP signaling, Wnt signaling and Sox2 signaling are needed for fungiform papillae development in miniature pigs. Additionally, we found BMP-2, BMP-4, and Wnt5a expression in the developing tongue muscle of the miniature pig, suggesting that these proteins participate in the development of tongue muscle tissues [58].
5. Conclusions
This study bridges an important gap in understanding mammalian taste organ development and provides data describing the morphogenesis and expression of developmental signals in the fungiform papillae of the miniature pig. This could form the basis for further research on the functions and mechanisms of this model or human fungiform papillae.
Ethical approval
The animal experiments involved in the study were conducted with the permission of the Animal Care and Use Committee of Capital Medical University in accordance with relevant guidelines and regulations and with ARRIVE guidelines (Beijing, China) (permit number CMU-B2010010). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available for ethical reasons. Further inquiries can be directed to the corresponding author.
Funding
The design of the study; the collection, analysis and interpretation of the data; and the writing of the manuscript were supported by the Young Scientist Program of Beijing Stomatological Hospital, Capital Medical University, NO. YSP202208.
Data availability statement
The data of this study are available on reasonable request from the corresponding author.
CRediT authorship contribution statement
Lingxiao Wang: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jun Li: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Lingxiao Wang reports was provided by Beijing Stomatological Hospital, Capital Medical University. Lingxiao Wang reports a relationship with Beijing Stomatological Hospital, Capital Medical University that includes: funding grants. Lingxiao Wang has patent pending to Lingxiao Wang. No If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to our colleagues in the laboratory for providing assistance with sample collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24953.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Lu C.P., Polak L., Keyes B.E., Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354(6319) doi: 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X., Li Y., Wang F., Hu L., Li Y., Wang J., Zhang C., Wang S. Spatiotemporal expression of Wnt/beta-catenin signaling during morphogenesis and odontogenesis of deciduous molar in miniature pig. Int. J. Biol. Sci. 2017;13(8):1082–1091. doi: 10.7150/ijbs.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Feng J.Q. Signaling pathways critical for tooth Root formation. J. Dent. Res. 2017;96(11):1221–1228. doi: 10.1177/0022034517717478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sculean A., Gruber R., Bosshardt D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014;41(Suppl 15):S6–S22. doi: 10.1111/jcpe.12206. [DOI] [PubMed] [Google Scholar]
- 5.Chuong C.M., Chodankar R., Widelitz R.B., Jiang T.X. Evo-devo of feathers and scales: building complex epithelial appendages. Curr. Opin. Genet. Dev. 2000;10(4):449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F., Thirumangalathu S., Gallant N.M., Yang S.H., Stoick-Cooper C.L., Reddy S.T., Andl T., Taketo M.M., Dlugosz A.A., Moon R.T., Barlow L.A., Millar S.E. Wnt-beta-catenin signaling initiates taste papilla development. Nat. Genet. 2007;39(1):106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X., Liu Y., Zhao P., Dai Z., Yang X., Li Y., Qiu M., Zhang Z. Gpr177-mediated Wnt signaling is required for fungiform placode initiation. J. Dent. Res. 2014;93(6):582–588. doi: 10.1177/0022034514531985. [DOI] [PubMed] [Google Scholar]
- 8.Karikkineth A.C., Tang E.Y., Kuo P.L., Ferrucci L., Egan J.M., Chia C.W. Longitudinal trajectories and determinants of human fungiform papillae density. Aging (Albany NY) 2021;13(23):24989–25003. doi: 10.18632/aging.203741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Maksoud F.M., Inui-Yamamoto C., Kawano A., Honma S., Saeki N., Abe M., Kuraki M., Ohba S., Wakisaka S. Histological and immunohistochemical studies of the fungiform and the circumvallate papillae through the life stages from 6- to 72-week-old Sprague-Dawley male rats. Anat. Rec. 2023 doi: 10.1002/ar.25338. Hoboken. [DOI] [PubMed] [Google Scholar]
- 10.Hichami A., Saidi H., Khan A.S., Degbeni P., Khan N.A. In vitro functional Characterization of type-I taste bud cells as Monocytes/Macrophages-like which Secrete Proinflammatory Cytokines. Int. J. Mol. Sci. 2023;24(12) doi: 10.3390/ijms241210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuong C.M. The making of a feather: homeoproteins, retinoids and adhesion molecules. Bioessays. 1993;15(8):513–521. doi: 10.1002/bies.950150804. [DOI] [PubMed] [Google Scholar]
- 12.Chuong C.M., Jung H.S., Noden D., Widelitz R.B. Lineage and pluripotentiality of epithelial precursor cells in developing chicken skin. Biochem. Cell. Biol. 1998;76(6):1069–1077. doi: 10.1139/o99-015. [DOI] [PubMed] [Google Scholar]
- 13.Saunders J.W., Jr., Gasseling M.T., Gfeller M. Interactions of ectoderm and mesoderm in the origin of axial relationships in the wing of the fowl. J. Exp. Zool. 1958;137(1):39–74. doi: 10.1002/jez.1401370104. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki K., Porntaveetus T., Oommen S., Ghafoor S., Kawasaki M., Otsuka-Tanaka Y., Blackburn J., Kessler J.A., Sharpe P.T., Ohazama A. Bmp signalling in filiform tongue papillae development. Arch. Oral Biol. 2012;57(6):805–813. doi: 10.1016/j.archoralbio.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.Y., Mochizuki T., Akita K., Jung H.S. Morphological evidence of the importance of epithelial tissue during mouse tongue development. Exp. Cell Res. 2003;290(2):217–226. doi: 10.1016/s0014-4827(03)00319-7. [DOI] [PubMed] [Google Scholar]
- 16.Xiang L., Wang X., Li Y., Liu H.W., Zhang X., Mu X., Liu C., Hu M. Development of the temporomandibular joint in miniature pig embryos. J. Morphol. 2022;283(1):134–143. doi: 10.1002/jmor.21432. [DOI] [PubMed] [Google Scholar]
- 17.Sun L., Wang J., Liu H., Fan Z., Wang S., Du J. A Comprehensive study of palate development in miniature pig. Anat. Rec. 2017;300(8):1409–1419. doi: 10.1002/ar.23597. [DOI] [PubMed] [Google Scholar]
- 18.Schomberg D.T., Tellez A., Meudt J.J., Brady D.A., Dillon K.N., Arowolo F.K., Wicks J., Rousselle S.D., Shanmuganayagam D. Miniature Swine for Preclinical modeling of Complexities of human disease for translational Scientific Discovery and Accelerated development of Therapies and Medical Devices. Toxicol. Pathol. 2016;44(3):299–314. doi: 10.1177/0192623315618292. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman B.G., Zavaglia B., Beach M., Helgason C.D. Expression of Groucho/TLE proteins during pancreas development. BMC Dev. Biol. 2008;8:81. doi: 10.1186/1471-213X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A., Li Y., Song T., Wang F., Liu D., Fan Z., Cheng S., Zhang C., Wang J., He J., Wang S. Identification of differential microRNA expression during tooth morphogenesis in the heterodont dentition of miniature pigs, SusScrofa. BMC Dev. Biol. 2015;15:51. doi: 10.1186/s12861-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Z., Wang L., Wang F., Zhang C., Wang J., He J., Wang S. Expression of BMP2/4/7 during the odontogenesis of deciduous molars in miniature pig embryos. J. Mol. Histol. 2018;49(5):545–553. doi: 10.1007/s10735-018-9792-1. [DOI] [PubMed] [Google Scholar]
- 22.Gritli-Linde A., Bei M., Maas R., Zhang X.M., Linde A., McMahon A.P. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129(23):5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- 23.Liu B., Chen S., Cheng D., Jing W., Helms J.A. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J. Dent. Res. 2014;93(5):475–482. doi: 10.1177/0022034514528211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Feng J., Liu Y., Ho T.V., Grimes W., Ho H.A., Park S., Wang S., Chai Y. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell. 2015;33(2):125–135. doi: 10.1016/j.devcel.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X., Li Y., Alawi F., Bouchard J.R., Kulkarni A.B., Gibson C.W. An amelogenin mutation leads to disruption of the odontogenic apparatus and aberrant expression of Notch1. J. Oral Pathol. Med. 2011;40(3):235–242. doi: 10.1111/j.1600-0714.2010.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li A., Song T., Wang F., Liu D., Fan Z., Zhang C., He J., Wang S. MicroRNAome and expression profile of developing tooth germ in miniature pigs. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistretta C.M., Bradley R.M. The fungiform papilla is a complex, Multimodal, oral sensory organ. Curr Opin Physiol. 2021;20:165–173. doi: 10.1016/j.cophys.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F., Li G., Wu Z., Fan Z., Yang M., Wu T., Wang J., Zhang C., Wang S. Tracking diphyodont development in miniature pigs in vitro and in vivo. Biol Open. 2019;8(2) doi: 10.1242/bio.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez G.F., Krimm R.F. Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. J. Neurobiol. 2006;66(10):1033–1043. doi: 10.1002/neu.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumamoto H., Ohki K., Ooya K. Expression of Sonic hedgehog (SHH) signaling molecules in ameloblastomas. J. Oral Pathol. Med. 2004;33(3):185–190. doi: 10.1111/j.0904-2512.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 31.Halloran D., Durbano H.W., Nohe A. Bone morphogenetic protein-2 in development and bone Homeostasis. J. Dev. Biol. 2020;8(3) doi: 10.3390/jdb8030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beites C.L., Hollenbeck P.L., Kim J., Lovell-Badge R., Lander A.D., Calof A.L. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development. 2009;136(13):2187–2197. doi: 10.1242/dev.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirao T., Saga T., Kusukawa J., Yamaki K. Angiogenesis and developmental expression of vascular endothelial growth factor in rat lingual papillae. Kurume Med. J. 2007;54(1–2):9–24. doi: 10.2739/kurumemedj.54.9. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.Y., Cho S.W., Lee M.J., Hwang H.J., Lee J.M., Lee S.I., Muramatsu T., Shimono M., Jung H.S. Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res. 2005;320(3):409–415. doi: 10.1007/s00441-005-1091-y. [DOI] [PubMed] [Google Scholar]
- 35.Tucker A.S., Matthews K.L., Sharpe P.T. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282(5391):1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y., Liu H.X., Mistretta C.M. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev. Biol. 2006;297(1):198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Jung H.S., Oropeza V., Thesleff I. Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech. Dev. 1999;81(1–2):179–182. doi: 10.1016/s0925-4773(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 38.Hu K.J., Ianov L., Crossman D. Profiling and quantification of pluripotency reprogramming reveal that WNT pathways and cell morphology have to be reprogramed extensively. Heliyon. 2020;6(5) doi: 10.1016/j.heliyon.2020.e04035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Z.J., Liu M.X., Zhang Q., Tian Y.H., Wang L.Z., Yan X., Ren D.P., Yuan X. Wnt/?-catenin signaling pathway is activated in the progress of mandibular condylar cartilage degeneration and subchondral bone loss induced by overloaded functional orthopedic force (OFOF) Heliyon. 2022;8(10) doi: 10.1016/j.heliyon.2022.e10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwatsuki K., Liu H.X., Gronder A., Singer M.A., Lane T.F., Grosschedl R., Mistretta C.M., Margolskee R.F. Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci U S A. 2007;104(7):2253–2258. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikels A., Minami Y., Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 2009;284(44):30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P.J., Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y., Niu C.C., Deng G., Li Z.Q., Pan J., Zhao C., Yang Z.L., Si W.K. The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. Int. J. Mol. Med. 2011;27(1):63–69. doi: 10.3892/ijmm.2010.560. [DOI] [PubMed] [Google Scholar]
- 44.Liu H.X., Grosse A.S., Iwatsuki K., Mishina Y., Gumucio D.L., Mistretta C.M. Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development. Dev. Biol. 2012;361(1):39–56. doi: 10.1016/j.ydbio.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gou Y.Z., Vemaraju S., Sweet E.M., Kwon H.J., Riley B.B. Play unique roles in development of hair cells and neurons in the zebrafish inner ear. Dev. Biol. 2018;435(1):73–83. doi: 10.1016/j.ydbio.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okubo T., Pevny L.H., Hogan B.L. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20(19):2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa R., Kawasaki M., Kawasaki K., Yamada A., Trakanant S., Meguro F., Kitamura A., Kudo T., Maeda T., Ohazama A. Sox genes Show spatiotemporal expression during Murine tongue and Eyelid development. Int J Dent. 2018;2018 doi: 10.1155/2018/1601363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama A., Miura H., Ooki M., Harada S. During development intense Sox2 expression marks not only Prox1-expressing taste bud cell but also perigemmal cell lineages. Cell Tissue Res. 2015;359(3):743–753. doi: 10.1007/s00441-014-2076-5. [DOI] [PubMed] [Google Scholar]
- 49.Cau E., Gradwohl G., Casarosa S., Kageyama R., Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127(11):2323–2332. doi: 10.1242/dev.127.11.2323. https://www.ncbi.nlm.nih.gov/pubmed/10804175 [DOI] [PubMed] [Google Scholar]
- 50.Furukawa T., Mukherjee S., Bao Z.Z., Morrow E.M., Cepko C.L. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26(2):383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 51.Ito T., Udaka N., Yazawa T., Okudela K., Hayashi H., Sudo T., Guillemot F., Kageyama R., Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127(18):3913–3921. doi: 10.1242/dev.127.18.3913. https://www.ncbi.nlm.nih.gov/pubmed/10952889 [DOI] [PubMed] [Google Scholar]
- 52.Seta Y., Seta C., Barlow L.A. Notch-associated gene expression in embryonic and adult taste papillae and taste buds suggests a role in taste cell lineage decisions. J. Comp. Neurol. 2003;464(1):49–61. doi: 10.1002/cne.10787. [DOI] [PubMed] [Google Scholar]
- 53.Seta Y., Toyono T., Kataoka S., Toyoshima K. Regulation of taste bud cell differentiation by notch signaling pathway. Chem. Senses. 2005;30(Suppl 1):i48–i49. doi: 10.1093/chemse/bjh107. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich B., Haider S., Meinhardt G., Pollheimer J., Knofler M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell. Mol. Life Sci. 2022;79(6):292. doi: 10.1007/s00018-022-04285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casey L.M., Lan Y., Cho E.S., Maltby K.M., Gridley T., Jiang R. Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development. Dev Dyn. 2006;235(7):1830–1844. doi: 10.1002/dvdy.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawasaki K., Porntaveetus T., Oommen S., Ghafoor S., Kawasaki M., Otsuka-Tanaka Y., Blackburn J., Kessler J.A., Sharpe P.T., Ohazama A. Bmp signalling in filiform tongue papillae development. Arch. Oral Biol. 2012;57(6):805–813. doi: 10.1016/j.archoralbio.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H.X., Grosse A.M., Walton K.D., Saims D.A., Gumucio D.L., Mistretta C.M. WNT5a in tongue and fungiform Papilla development. Ann. N. Y. Acad. Sci. 2009;1170:11–17. doi: 10.1111/j.1749-6632.2009.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suga T., Fukui T., Shinohara A., Luan X., Diekwisch T.G., Morito M., Yamane A. BMP2, BMP4, and their receptors are expressed in the differentiating muscle tissues of mouse embryonic tongue. Cell Tissue Res. 2007;329(1):103–117. doi: 10.1007/s00441-007-0416-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The data are not publicly available for ethical reasons. Further inquiries can be directed to the corresponding author.
The data of this study are available on reasonable request from the corresponding author.