Summary
Background
We aimed to evaluate the cost-effectiveness of an artificial intelligence-(AI) based diabetic retinopathy (DR) screening system in the primary care setting for both non-Indigenous and Indigenous people living with diabetes in Australia.
Methods
We performed a cost-effectiveness analysis between January 01, 2022 and August 01, 2023. A decision-analytic Markov model was constructed to simulate DR progression in a population of 1,197,818 non-Indigenous and 65,160 Indigenous Australians living with diabetes aged ≥20 years over 40 years. From a healthcare provider's perspective, we compared current practice to three primary care AI-based screening scenarios—(A) substitution of current manual grading, (B) scaling up to patient acceptance level, and (C) achieving universal screening. Study results were presented as incremental cost-effectiveness ratio (ICER), benefit-cost ratio (BCR), and net monetary benefits (NMB). A Willingness-to-pay (WTP) threshold of AU$50,000 per quality-adjusted life year (QALY) and a discount rate of 3.5% were adopted in this study.
Findings
With the status quo, the non-Indigenous diabetic population was projected to develop 96,269 blindness cases, resulting in AU$13,039.6 m spending on DR screening and treatment during 2020–2060. In comparison, all three intervention scenarios were effective and cost-saving. In particular, if a universal screening program was to be implemented (Scenario C), it would prevent 38,347 blindness cases, gain 172,090 QALYs and save AU$595.8 m, leading to a BCR of 3.96 and NMB of AU$9,200 m. Similar findings were also reported in the Indigenous population. With the status quo, 3,396 Indigenous individuals would develop blindness, which would cost the health system AU$796.0 m during 2020–2060. All three intervention scenarios were cost-saving for the Indigenous population. Notably, universal AI-based DR screening (Scenario C) would prevent 1,211 blindness cases and gain 9,800 QALYs in the Indigenous population, leading to a saving of AU$19.2 m with a BCR of 1.62 and NMB of AU$509 m.
Interpretation
Our findings suggest that implementing AI-based DR screening in primary care is highly effective and cost-saving in both Indigenous and non-Indigenous populations.
Funding
This project received grant funding from the Australian Government: the National Critical Research Infrastructure Initiative, Medical Research Future Fund (MRFAI00035) and the NHMRC Investigator Grant (APP1175405). The contents of the published material are solely the responsibility of the Administering Institution, a participating institution or individual authors and do not reflect the views of the NHMRC. This work was supported by the Global STEM Professorship Scheme (P0046113), the Fundamental Research Funds of the State Key Laboratory of Ophthalmology, Project of Investigation on Health Status of Employees in Financial Industry in Guangzhou, China (Z012014075). The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. W.H. is supported by the Melbourne Research Scholarship established by the University of Melbourne. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Keywords: Cost-effectiveness, Artificial intelligence, Diabetic retinopathy, Screening
Research in context.
Evidence before this study
We searched PubMed using the keywords “cost-effectiveness”, “cost-utility”, “health economics”, “artificial intelligence”, “diabetic retinopathy” and “Australia” until April 01, 2023. We found no publications pertaining to the cost-effectiveness analysis of applying AI for DR screening in Australia.
Added value of this study
We modelled different scenarios of AI-based DR screening in Australian primary care settings for Indigenous population and non-Indigenous population respectively, including (A) replacing clinicians with AI-based DR screening, (B) and scaling up the coverage to the level of patient acceptance and (C) ultimately universal screening. This study found that in Australian settings, implementing AI-based DR screening would be cost-saving compared to the current practice in all scenarios. For the Australian health care system, the screening program will generate total net savings consisting of screening, link-to-care, direct medical costs and blindness care costs, as well as health benefits of timely detection of additional cases, reduction in blindness cases and gain of quality-adjusted life years.
Implications of all the available evidence
Our cost-effectiveness study provides economic evidence for making an informed decision to implement AI-based DR screening. It showed to be highly cost-saving for Indigenous and non-Indigenous Australians. AI-based DR screening could provide a novel point-of-care approach in the primary care setting facilitating large-scale DR screening. This approach would bridge the skill gap of GPs in performing DR screening and potentiate the efficiency of the primary care system in Australia. Furthermore, in rural and remote areas where people are more vulnerable to DR, AI-based DR screening would improve equity in both access to care and health outcomes. Lastly, the evidence of the cost-effectiveness of AI-based DR screening may inform the potential value of implementing other AI-based diagnostic tools.
Introduction
Diabetic retinopathy (DR), a visually debilitating condition resulting from hyperglycaemia, continues to be a leading cause of low vision and blindness in the working-age population in Australia.1,2 This condition imposes a significant burden in terms of the affected individuals’ well-being, healthcare expenditure and social productivity.3 Over the past three decades, the prevalence of diabetes in Australia increased tremendously, accompanied by a two-fold increase in mortality rate.4 In Australia, more than one million people are living with diabetes and up to one-third of these patients are affected by DR.5,6 In 2015, the economic cost associated with diabetic macular edema alone was estimated to be AU$2.07 billion and disability-adjusted life years (DALYs) exceeded 7720 years.3 Urgent and proactive measures are needed to mitigate the detrimental impact of DR on the Australian population and healthcare system.
The World Health Organization (WHO) recommends regular screening for DR as an effective intervention to prevent vision impairment and blindness in people with diabetes.7 Regular screening enables early identification, prompt management and slowing down disease progression.8 The English National Programme for DR demonstrated the effectiveness of universal DR screening by removing DR as the leading cause of blindness in the UK.9 These findings highlight the importance of implementing similar screening programs to combat DR in other global regions.
Retinal photography has gained popularity as a DR screening technique and presents a valuable opportunity to enhance DR screening in Australia. Nevertheless, the availability of active screening programs remains limited in Australia, particularly in rural and remote areas where access to eye care services is constrained.10,11 Primary healthcare providers, such as general practitioners (GPs), are ideally positioned to bridge this gap, given that 85% of the Australian population visits a GP at least once a year.12 To support this approach, the Australian Government has introduced new Medicare Benefits Schedule (MBS) item numbers to promote the use of retinal cameras by GPs for DR screening.13 Despite the ready availability of retinal cameras, their utilisation remains suboptimal, evident from low rates of DR screening MBS item number claims, with only 1506 claims during November 2016–December 2017.14 Previous studies have identified several barriers, including a lack of awareness regarding the DR screening services, cost of retinal cameras, time constraints, and insufficient training in the interpretation of retinal images among GPs,15 hindering the success of the initiative. Therefore, it is essential to address these challenges to facilitate greater uptake of DR screening in Australia.
Recent years have witnessed significant advancements in AI-based screening for DR, which offer an efficient, cost-effective, and labour-saving approach to improving DR screening on a large scale. Multiple prospective studies conducted in real-world settings have demonstrated that AI-based tools achieve comparable performance to human experts,16, 17, 18 highlighting their potential for enhancing DR screening efforts. In Australia, pilot studies have validated the real-world accuracy, feasibility and patient acceptability of automated real-time AI-based DR screening systems.19,20 The large-scale implementation of this technology requires careful consideration of health economics. While previous research has investigated the cost-effectiveness of implementing AI-assisted DR screening in Brazil, China, Singapore, the United Kingdom and the United States,21, 22, 23, 24, 25, 26 the results remain inconsistent, partially due to variations in healthcare systems among these countries. Furthermore, in previous studies, AI was considered as an alternative strategy to be compared with the current practice on the same scale. The real-world and lifetime impact of implementing AI-based screening in increasing the accessibility of service to diabetic patients under different levels of care has not been investigated.
The unique population structure in Australia encompasses both Indigenous and non-Indigenous populations. The risk of developing diabetes in Indigenous people doubled compared to the non-Indigenous population as a result of health inequities such as less access to health resources, higher prevalences of health risk factors, and socioeconomic disadvantage.27 Therefore, Indigenous people have demonstrated a significantly higher disease burden of diabetes and DR.5 In addition, Australia has its universal public insurance scheme that provides access to free and quality care to everyone,28 which would be a crucial factor that drives the implementation and execution of a universal screening program for conditions such as DR. Therefore, our study aims to investigate the cost-effectiveness of implementing AI-based DR screening for Indigenous and non-Indigenous Australians in primary care.
Methods
Model construction
We performed a cost-effectiveness analysis between January 01, 2022 and August 01, 2023. We constructed a decision-analytical Markov model to investigate the cost-effectiveness of implementing AI-based DR screening in Australian primary care settings from the health provider’s perspective.29, 30, 31, 32, 33, 34 The model was constructed using TreeAge Pro 2022 (TreeAge Software, Williamstown, MA, USA). The findings were reported according to the Consolidated Health Economic Evaluation Reporting Standards statement.35
The workflow of DR screening in Australia is demonstrated in Fig. 1. In the status quo, diabetic individuals were screened for DR by optometrists, ophthalmologists or GPs.36 As per the National Health and Medical Research Council’s (NHMRC’s) Guidelines, non-Indigenous diabetic participants having no DR need to be screened biennially and Indigenous diabetic individuals should be screened annually.37 All participants graded as mild DR would be suggested to be screened annually and those with moderate DR and worse and/or macular edema, would require referral to an ophthalmologist for further treatment.37,38 In the intervention scenarios, participants will be screened by an automated AI-based DR screening system and provided with a real-time report with referral recommendations.19
Fig. 1.
Flowchart of diabetic retinopathy screening and follow-upmanagement.
The Markov model was constructed to simulate the progression of DR in 1,197,818 non-Indigenous and 65,160 Indigenous diabetic individuals in Australia aged 20 years and older in 2020.5,39 The cohort was followed up for 40 years because it can model the lifetime of this cohort with a baseline mean age of 65 years old. DR states were determined by the International Clinical Disease Severity Scale for DR40 and the clinically relevant classification for diabetic macular edema (DME),41 including mild non-proliferative diabetic retinopathy (NPDR), moderate NPDR, severe NPDR, proliferative diabetic retinopathy (PDR), non-central-involved diabetic macular edema (NCIDME), and central-involved diabetic macular edema (CIDME), along with blindness and death. Each DR state was divided into undetected and detected states (Supplementary Figure S1). The blindness state included unilateral and bilateral blindness. The proportions of unilateral and bilateral blindness in the Indigenous and non-Indigenous populations were derived from the Blue Mountains Eye Study and the National Eye Health Survey (Supplementary Figure S1).42,43 Blindness was defined as the best-corrected visual acuity of less than 3/60 in both eyes and/or a corresponding visual field of less than 10° or no light perception.44 As this is a static model, it did not include the newly onset diabetic individuals after 2020. Details of the management plan of each DR state were described in Supplementary Methods. The compliance rate to follow-up treatment after screening was estimated from the Australian Institute of Health and Welfare data45 and prevalence of DR,6,46 resulting in 20.9% and 67.1% for Indigenous and non-Indigenous people, respectively. Assumptions of no regression to previous disease states were applied.
Definition of intervention scenarios
We defined four scenarios including the status quo and three intervention scenarios (Fig. 2). For the status quo, 75% of the Australian population living with diabetes has been diagnosed.47 50% of the individuals with diagnosed diabetes (37.5% of the diabetic patients) undertake regular follow-up48 and of these, 42% of non-Indigenous people and 34% of Indigenous people undertake diabetic eye checks,36 resulting in an estimated 15.75% of the total non-Indigenous diabetic population and 12.75% of the Indigenous diabetic population undertaking DR screening. For individuals currently under DR screening, 89.77% were screened by optometrists, 7.05% by ophthalmologists and 3.17% by GPs.36
Fig. 2.
Scenarios of different diabetic retinopathy screening rates among the diabetic population. Figure captions: Status quo demonstrated the proportion of undiagnosed diabetic patients, diagnosed but not followed-up diabetic patients, regularly followed-up without eye screening patients, and the professions that conveyed DR screening in patients who are currently under DR screening. Scenario A modelled the replacement of manual screening of DR by the automated AI system. Scenario B modelled the replacement and the increased screening rate in the patients who are not undertaking DR screening. 62.0% of the patients who are regularly followed up for diabetes are assumed to undertake DR screening by the AI. 85% of the patients with undiagnosed or unfollowed-up diabetes will have access to healthcare and 62.0% of those will undertake DR screening using the AI system. In Scenario C, a universal screening scenario was modelled where 80% of the patients who are unscreened for DR currently will be screened by the AI.
As shown in Fig. 2, Scenario A modelled the replacement of clinician screening with AI in the sub-population that is currently under screening. As automated AI-based screening negates the need for healthcare professionals and when implemented in general practice clinics, the first key point that links all patients to the healthcare system, this approach holds the potential to increase the screening rate and formulate a screening program. Therefore, we modelled the increased screening rate of AI-based DR screening to the patient acceptance level in Scenario B and to universal screening in Scenario C. The increased screening rate was determined by the patient satisfaction rate of 62.0% from our pilot study (unpublished) and the fact that 85% of Australians visited their GPs at least once a year.49 We assumed in Scenario B that 62.0% of the diabetic patients with regular follow-up would be screened; 85% of the diagnosed diabetic patients without follow-up would visit a GP in one year for a general health issue (link to care) and 62.0% of them would be screened; 85% of the undiagnosed diabetic patients would visit GP and complete diagnostic tests for diabetes (link to care) and 62.0% of them would be screened. This resulted in a total screening rate of 62.17% and 61.03% in the non-Indigenous and Indigenous population, respectively. Scenario C modelled a universal AI-based DR screening scenario that would cover 100% of the patients who were under DR screening and 80% of the rest of the unscreened population,50 resulting in a screening rate of 83.15% in the non-Indigenous population and 82.55% in the Indigenous population.
Disease burden of diabetic retinopathy
The prevalence of any DR, PDR and DME in the Indigenous and non-Indigenous population was derived from a meta-analysis of the Australian population and further breakdown of the prevalence of mild NPDR, moderate NPDR, severe NPDR, NCIDME and CIDME was calculated based on the prevalence reported in the National Eye Health Survey (Supplementary Table S1). As DME can occur at any stage of DR, the initial probability of mild, moderate, and severe NPDR and PDR in the Markov model has excluded DME and any DR with DME was considered as a separate DME state in the Markov model (Supplementary Figure S1).
Screening sensitivity and specificity
The sensitivity and specificity of DR screening by the AI system, optometrists, ophthalmologists and GPs were shown in Table 1. Consistent with previous studies, referable DR includes moderate DR or worse and/or DME, and vision-threatening diabetic retinopathy (VTDR) includes severe DR and worse and/or DME.57,58
Table 1.
Variable ranges and distributions for the sensitivity and specificity of AI, general practitioners, optometrists and ophthalmologists.
| Base case value | References | Range | Distribution | |
|---|---|---|---|---|
| Sensitivity | ||||
| AI for any DR | 0.870 | 51 | ±25% (0.653–1) | Triangular |
| AI for VTDR | 0.970 | 52 | ±25% (0.728–1) | Triangular |
| AI for DME | 0.950 | 52 | ±25% (0.713–1) | Triangular |
| Optometrists for any DR | 0.750 | 53 | ±10% (0.675–0.825) | Triangular |
| Optometrists for referable DR | 0.730 | 53 | ±10% (0.657–0.803) | Triangular |
| GP for any DR | 0.433 | 54, 55 | ±10% (0.390–0.476) | Triangular |
| GP for referable DR | 0.560 | 56 | ±10% (0.504–0.616) | Triangular |
| Ophthalmologists for any DR | 0.848 | 16 | 95% CI (0.794–0.933) | Triangular |
| Ophthalmologists for VTDR | 0.848 | 16 | 95% CI (0.794–0.933) | Triangular |
| Specificity | ||||
| AI for any DR | 0.907 | 51 | ±25% (0.680–1) | Triangular |
| AI for VTDR | 0.914 | 52 | ±25% (0.686–1) | Triangular |
| AI for DME | 0.929 | 52 | ±25% (0.697–1) | Triangular |
| Optometrists for any DR | 0.940 | 56 | ±10% (0.846–1) | Triangular |
| Optometrists for referable DR | 0.930 | 56 | ±10% (0.837–1) | Triangular |
| GP for any DR | 0.940 | 56 | ±10% (0.846–1) | Triangular |
| GP for referable DR | 0.980 | 56 | ±10% (0.882–1) | Triangular |
| Ophthalmologists for any DR | 0.955 | 16 | 95% CI (0.941–0.967) | Triangular |
| Ophthalmologists for VTDR | 0.955 | 16 | 95% CI (0.941–0.967) | Triangular |
Transition probabilities
All transitions between different DR disease states were based on a 1-year cycle (Supplementary Table S2). If transition probability was not available, it was translated from the incident rate using the formula , where r represents the incident rate over a period time of t. Age-standardised mortality was calculated from the Australian Bureau of Statistics (ABS) in 2021.59 The age-specific increased risk of mortality in the diabetic population was derived from the study by Tancredi et al.60 The Indigenous people had a 1.8 increased risk of mortality compared to the non-Indigenous people.61 In addition, the risk of mortality in the blindness population was 1.08 times higher compared to non-blindness people.62
Utility values
Utility values were obtained from previous studies (Supplementary Table S3). Patients with no DR were assigned the utility value of diabetes. The annual discount rate of utility was 3.5% according to the recommendations from the National Institute for Health and Clinical Excellence (NICE).63
Screening, intervention and healthcare costs
The costs of screening, consultation and treatment were obtained from the MBS and Pharmaceutical Benefits Scheme (PBS) in July 2022.13,64 The costs for blindness care were derived from published studies.65 As data was limited for unilateral blindness, we adopted an assumption that the costs associated with rehabilitation, equipment, education, training, and daily life support for unilateral blindness was 30% of the corresponding costs for bilateral blindness.66 Supplementary Table S4 shows the detailed cost breakdown. The cost of DR screening using the AI system was derived from our empirical study (unpublished; Supplementary Table S5). In detail, the patent cost was suggested by Eyetelligence Pty Ltd., which charges AU$5 per patient, covering patent licensing, software development and maintenance, and organization overhead. The hardware cost and depreciation included the automated fundus cameras, personal computer and maintenance fee, resulting in a unit cost of AU$2.2 per person under the conservative assumption that 1000 patients per year would be screened by the system. The labour cost included the one-time training and wages for the operators’ assistance with image acquisition. This resulted in an estimated unit cost of AU$12.2 for the AI-based screening. All The costs were discounted at an annual rate of 3.5%.63
Cost-effectiveness analysis
The effectiveness measures included the incremental detected VTDR cases, decremental blindness cases (i.e., blindness cases prevented), and the incremental quality-adjusted life years (QALYs) in the intervention scenarios relative to the status quo. We estimated the cost-effectiveness of implementing AI-based DR screening compared to the status quo using several measurements. The incremental cost-effectiveness ratio (ICER) indicates the additional cost of a unit increase in the QALY. A Willingness-to-pay (WTP) threshold of AU$50,000 per QALY was adopted in this study.67 We also evaluated the cost-effectiveness by quantifying the cost per blindness case prevented, benefit-cost ratio (BCR) and net monetary benefit (NMB). In this study, BCR was calculated as the total cost savings divided by the total investment in screening costs and link-to-care costs related to launching the AI system for DR screening.
Sensitivity analysis
To assess the uncertainties of the main outcomes, we performed one-way sensitivity analysis, two-way sensitivity analysis (on sensitivity and specificity of the AI) and probabilistic sensitivity analysis (PSA). The ranges for the variables were shown in Table 1 and Supplementary Tables S1–S4. A range of ±25% was used for the sensitivity and specificity of the AI system68 and a range of ±50% was applied to the cost of AI, with the aim of providing a conservative estimation for the uncertainties associated with AI performance and cost in the real world. When available, the upper and lower limits of the 95% CI were used for the other variables. Lower and upper limits of ±10% from the base case values were used for the rest of the variables.26 Tornado diagrams were plotted to demonstrate the factors that exert the most significant influence on the ICER. In PSA, triangular distributions were assigned to all the variables. 10,000 Monte Carlo simulations were conducted where each random sampling of the input variables from the probability distribution generated an ICER.
Ethics
Ethics approval is waived for this cost-effectiveness analysis as it does not involve human subjects. The analysis solely relies on aggregated and unidentifiable data obtained from published literature or open-source data sources.
Role of the funding source
All authors confirmed that they had full access to all data involved in this study and accepted responsibility for the decision to submit for publication. The sponsor or funding organisation had no role in the design or conduct of this research.
Results
Population impact and DR-related costs with the status quo
The results of the cost-effectiveness analyses in non-Indigenous and Indigenous populations can be found in Table 2 and 3. With the status quo, the cohort of 1,197,818 non-Indigenous diabetic individuals projected a total of 15,102,882 quality-adjusted life years (QALYs) over a 40-year period. Within this timeframe, 304,865 cases of VTDR would be detected by screening and 96,269 cases of blindness would occur. The estimated cost for the non-Indigenous cohort over 40 years would be $13,039.6 m, including $139.8 m for screening costs, $2,875.0 m for direct medical costs, and $10,024.8 m for blindness care. In particular, the direct medical costs included $330.5 m consultation fees, $92.4 m angiogram fees, $81.0 m OCT fees, $639.0 m photocoagulation fees, $1,687.6 m anti-vascular endothelial growth factor (anti-VEGF) injection fees, and $44.7 m vitrectomy surgery fees.
Table 2.
Cost-effectiveness analysis of applying AI in DR screening in primary care settings in non-Indigenous diabetic patients over a time horizon of 40 years.
| Status quo | Scenario A | Scenario B | Scenario C | Incremental cost or effectiveness (Scenario A) | Incremental cost or effectiveness (Scenario B) | Incremental cost or effectiveness (Scenario C) | |
|---|---|---|---|---|---|---|---|
| QALY | 15,102,882 | 15,119,359 | 15,250,035 | 15,274,972 | 16,477 | 147,154 | 172,090 |
| Total cost (AU$, million) | 13,039.6 | 12,860.0 | 12,510.6 | 12,443.8 | −179.6 | −529.0 | −595.8 |
| Screening cost | 139.8 | 24.3 | 58.5 | 67.0 | −115.4 | −81.3 | −72.8 |
| Link to care cost | 0.0 | 0.0 | 62.9 | 83.5 | 0.0 | 62.9 | 83.5 |
| Direct medical cost | 2,875.0 | 3,344.8 | 5,861.0 | 6,220.1 | 469.8 | 2985.9 | 3345.0 |
| Consultation cost | 330.5 | 428.5 | 783.0 | 836.9 | 98.0 | 452.6 | 506.5 |
| Fluorescein angiography cost | 92.4 | 107.9 | 188.9 | 200.1 | 15.5 | 96.6 | 107.7 |
| OCT cost | 81.0 | 94.5 | 165.6 | 175.4 | 13.6 | 84.6 | 94.4 |
| Photocoagulation cost | 639.0 | 746.3 | 1,308.4 | 1,384.3 | 107.3 | 669.4 | 745.4 |
| Anti-VEGF injection cost | 1,687.6 | 1,967.7 | 3,415.0 | 3,623.3 | 280.1 | 1,727.5 | 1,935.7 |
| Vitrectomy cost | 44.7 | 52.1 | 90.4 | 95.9 | 7.4 | 45.7 | 51.2 |
| Cost for blindness care | 10,024.8 | 9,490.8 | 6,528.2 | 6,073.2 | −533.9 | −3,496.6 | −3,951.6 |
| VTDR detected (cases) | 304,865 | 348,186 | 509,209 | 521,692 | 43,321 | 204,344 | 216,827 |
| Blindness (cases) | 96,269 | 89,645 | 60,884 | 57,922 | −6,624 | −35,385 | −38,347 |
| ICER | – | – | – | – | −10,897 (Dominating) | −3,595 (Dominating) | −3,462 (Dominating) |
| Incremental cost/blindness averted (AU$) | – | – | – | – | −27,108 (Dominating) | −14,950 (Dominjating) | −15,537 (Dominating) |
| Benefit-cost ratioa | – | – | – | – | 7.37 | 4.36 | 3.96 |
| NMB (AU$, million)b | – | – | – | – | 1,003 | 7,887 | 9,200 |
Benefit-cost ratio is calculated as the total cost savings divided by the sum of screening costs and link-to-care costs.
NMB, net monetary benefit is calculated as the QALYs gained multiplied by willingness-to-pay minus total incremental costs.
Table 3.
Cost-effectiveness analysis of applying AI in DR screening in primary care settings in Indigenous diabetic patients over a time horizon of 40 years.
| Status quo | Scenario A | Scenario B | Scenario C | Incremental cost or effectiveness (Scenario A) | Incremental cost or effectiveness (Scenario B) | Incremental cost or effectiveness (Scenario C) | |
|---|---|---|---|---|---|---|---|
| QALY | 647,551 | 648,219 | 655,751 | 657,350 | 669 | 8,200 | 9,800 |
| Total cost (AU$, million) | 796.0 | 787.7 | 778.4 | 776.7 | −8.3 | −17.6 | −19.2 |
| Screening cost | 6.7 | 1.1 | 3.5 | 4.2 | −5.6 | −3.2 | −2.5 |
| Link to care cost | 0.0 | 0.0 | 5.7 | 7.7 | 0.0 | 5.7 | 7.7 |
| Direct medical cost | 101.7 | 116.8 | 251.2 | 276.1 | 15.1 | 149.5 | 174.4 |
| Consultation cost | 10.4 | 13.1 | 30.5 | 33.8 | 2.7 | 20.1 | 23.4 |
| Fluorescein angiography cost | 2.9 | 3.4 | 7.4 | 8.1 | 0.5 | 4.5 | 5.2 |
| OCT cost | 2.6 | 3.0 | 6.5 | 7.1 | 0.4 | 3.9 | 4.5 |
| Photocoagulation cost | 18.5 | 21.6 | 47.6 | 52.3 | 3.1 | 29.1 | 33.8 |
| Anti-VEGF injection cost | 65.6 | 75.7 | 159.2 | 174.8 | 10.1 | 93.6 | 109.2 |
| Vitrectomy cost | 1.7 | 2.0 | 4.2 | 4.6 | 0.3 | 2.5 | 2.9 |
| Cost for blindness care | 687.6 | 669.9 | 518.1 | 488.8 | −17.7 | −169.5 | −198.8 |
| VTDR detected (cases) | 5,010 | 5,932 | 11,846 | 12,522 | 922 | 6,836 | 7,512 |
| Blindness (cases) | 3,396 | 3,260 | 2,313 | 2,185 | −136 | −1,083 | −1,211 |
| ICER | – | – | – | – | −12,360 (Dominating) | −2,143 (Dominating) | −1,964 (Dominating) |
| Incremental cost/blindness averted (AU$) | – | – | – | – | −60,837 (Dominating) | −16,232 (Dominating) | −15,890 (Dominating) |
| Benefit-cost ratioa | – | – | – | – | 7.70 | 1.91 | 1.62 |
| NMB (AU$, million)b | – | – | – | – | 42 | 428 | 509 |
Benefit-cost ratio is calculated as the total cost savings divided by the sum of screening costs and link-to-care costs.
NMB, net monetary benefit is calculated as the QALYs gained multiplied by willingness-to-pay minus total incremental costs.
The cohort of 65,150 Indigenous diabetic individuals is projected to gain 647,551 QALYs. 5,010 VTDR cases would be detected and 3,396 cases of blindness would develop over 40 years. The total cost would be $796.0 m, including $6.7 m screening costs, $101.7 m direct medical costs, and $687.6 m for care for blindness. The details of the direct medical costs breakdown can be found in Table 3.
Population impact and cost-effectiveness of replacing manual DR screening with AI-based DR screening
Replacing manual DR screening with AI-based DR screening (Scenario A) in the non-Indigenous people was projected to increase the number of detected VTDR cases to 348,186, resulting in 43,321 more detected VTDR cases than the status quo. The number of blindness cases would reduce to 89,645, preventing 6,624 cases of blindness compared with the status quo, leading to an additional 16,477 QALYs gained by the cohort over 40 years. The projected cost for the non-indigenous people over 40 years was $12,860.0 m, including $24.3 m for screening, $3,344.8 m for direct medical costs, and $9,490.8 for blindness care. The direct medical costs included $428.5 m for consultation, $107.9 m for angiogram, $94.5 m for OCT, $746.3 m for photocoagulation, $1,967.7 m for anti-VEGF injection, and $52.1 m for vitrectomy surgeries. Although this scenario required an additional $469.8 m for direct medical care, it would save $115.4 m on screening and $533.9 m on blindness care, resulting in net savings of $179.6 m. The net savings and health benefits resulted in a BCR of 7.37 and NMB of $1,003 m.
For the Indigenous people, the total QALYs gained would increase by 669 compared to the status quo. An additional 922 VTDR cases would be detected and 136 people would be prevented from blindness. The projected total cost for Indigenous people would be $787.7 m, saving $8.3 m in total. It is projected to increase the direct medical cost by $15.1 m compared to status quo, but would save $5.6 m in screening and $17.7 m in care for blindness, resulting in a BCR of 7.70 and NMB of $42 m.
Population impact and cost-effectiveness of scaling-up AI-based DR screening to potential population acceptance level
Increasing AI-based DR screening coverage to the potential patient’s acceptance level (62.17% for non-Indigenous, Scenario B) was projected to detect a total of 509,209 VTDR cases over the 40 years in the non-Indigenous cohort, which was 204,344 more than the status quo. A total of 60,884 blindness cases would develop, preventing 35,385 blindness cases and increasing the total QALYs gained by 147,154. This scenario would have cost the healthcare system a total of $12,510.6 m for the non-Indigenous people, including $58.5 m in screening costs, $62.9 m that linked the unscreened population to primary healthcare, $5,861.0 m in direct medical costs, and $6,528.2 m blindness care cost. The direct medical costs would consist of a $783.0 m consultation fee, $188.9 m angiogram fee, $165.6 m OCT fee, $1,308.4 m photocoagulation fee, $3,415.0 m anti-VEGF injection fee, and $90.4 m vitrectomy surgery fee. While this scenario would lead to an increase of $2,985.9 m in direct medical care, it could save $81.3 m in DR screening and $3,496.6 m in blindness care, resulting in a net saving of $529.0 m compared to the status quo, resulting in a BCR of 4.36 and NMB of $7,887 m.
For the Indigenous cohort, when the screening coverage increased to 61.03% in Scenario B, it was projected to detect 11,846 VTDR cases, resulting in 6,836 more cases detected compared to the status quo. In addition, a total of 2,313 blindness cases would develop, which is 1,083 less than the status quo. This scenario is projected to cost the health system $778.4 m, including $3.5 m screening costs, $5.7 m link-to-care costs, $251.2 m direct medical costs and $518.1 m cost for blindness care. The net saving would be $17.6 m, resulting in a BCR of 1.91 and NMB of $428 m.
Population impact and cost-effectiveness of achieving universal AI-based DR screening
Scaling up AI-based DR screening to universal coverage (83.15%for non-Indigenous people, Scenario C), would identify 521,692 cases of VTDR over 40 years, 216,827 cases more compared to the status quo. A total of 57,922 blindness cases would develop and an additional 38,347 cases of blindness would be prevented compared with the status quo, leading to an additional gain of 172,090 QALYs. This universal screening scenario was estimated to cost the healthcare system $12,443.8 m for non-Indigenous people, including $67.0 m for screening, $83.5 m for link-to-care costs, $6,220.1 m for direct medical costs, and $6,073.2 m for blindness care costs. It was projected to save the healthcare system $595.8 m over 40 years. Despite an increase in direct medical costs by $3,345.0, this scenario would reduce screening costs by $72.8 m and blindness care costs by $3,951.6 m, resulting in a BCR of 3.96 and NMB of $9,200 m.
When the AI-based DR screening covers 82.55% of the Indigenous population, it is projected to detect 12,522 cases of VTDR over 40 years, which would be 7,512 more cases compared to the status quo. 2,185 cases of blindness would develop, which would be 1,211 less compared to the status quo. The universal screening program for the Indigenous people would cost $776.7 m, including $4.2 m screening costs, $7.7 m link-to-care costs, $276.1 m direct medical costs, and $488.8 m costs for blindness care. This scenario would save $19.2 m in total relative to the status quo, and would result in a BCR of 1.62 and NMB of $509 m.
Sensitivity analysis
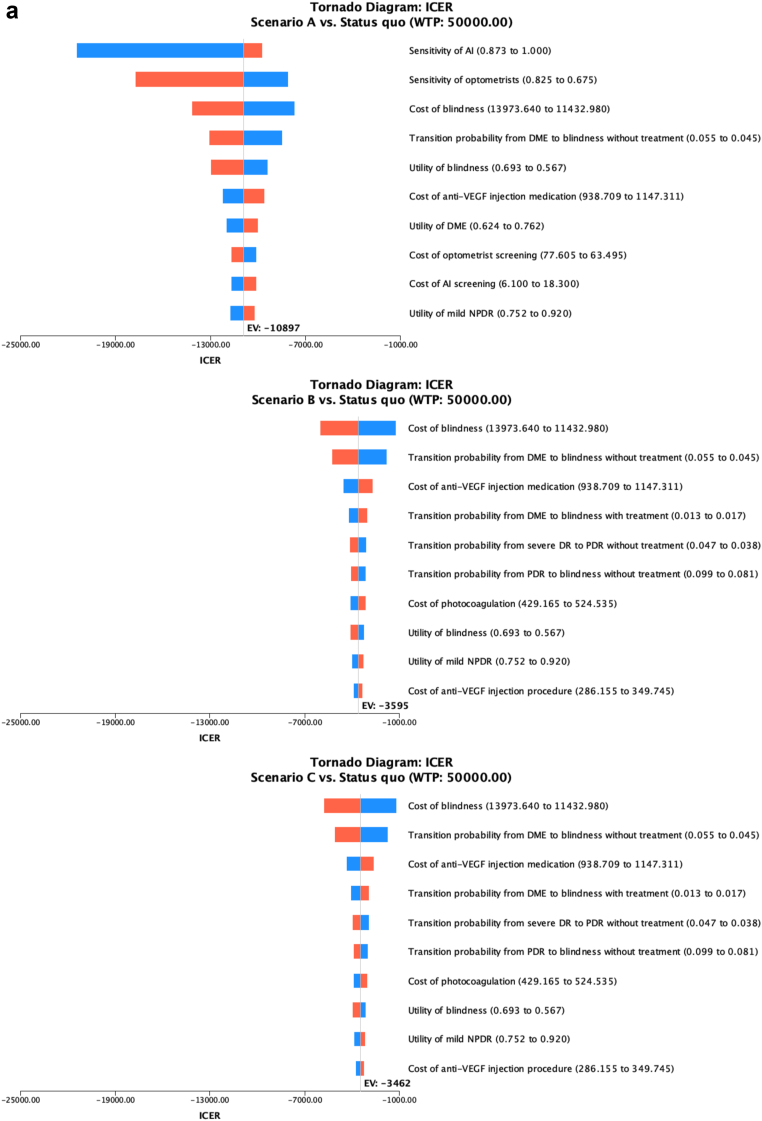
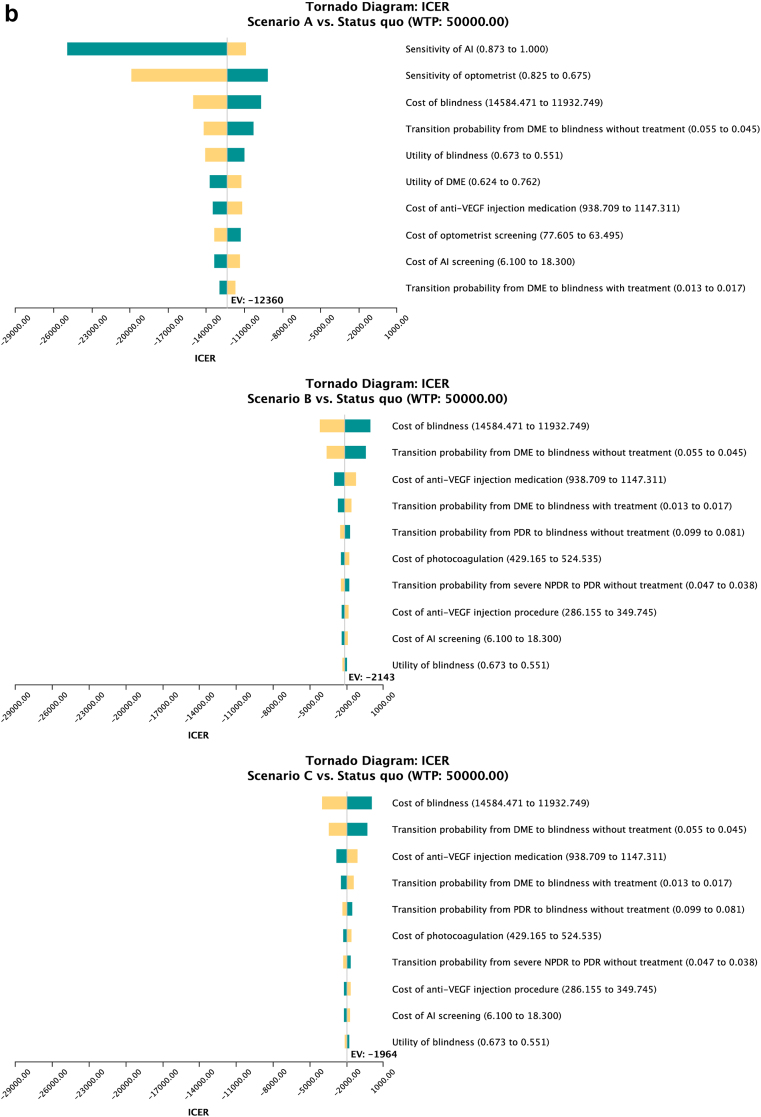
Fig. 3a and b shows the results from the one-way sensitivity analysis for non-Indigenous and Indigenous people respectively as Tornado diagrams. In Scenario A, the most important parameters influencing the ICER included the sensitivity of the AI, sensitivity of optometrists, cost of blindness, utility of blindness, and transition probability from DME to blindness without treatment in both Indigenous and non-Indigenous populations. In Scenario B and C, the cost of blindness, the transition probability from DME to blindness with treatment, and without treatment, the cost of anti-VEGF injection, and the transition probability from severe NPDR to PDR without treatment were the five most impactful parameters that affected the ICER in the non-Indigenous population. The Indigenous population shared four of those parameters, except for the transition probability from severe NPDR to PDR without treatment. Instead, the transition probability from PDR to blindness without treatment was among the most impactful parameters.
Fig. 3.
The tornado diagram from one-way sensitivity analysis in the non-Indigenous population (A) and the Indigenous population (B). Figure captions: The Tornado diagram demonstrated the ten parameters from one-way sensitivity analysis that have the most significant influence on ICER. The analysis compared scenarios A, B and C with different screening rates with status quo. The bars on the plot represent the potential effect of each parameter on the ICER, with the width of the bar indicating the range of the parameter. The red part of the bar represents high input values of the variables, while the blue part represents low values.
Results from two-way sensitivity analysis on the sensitivity and specificity of the AI system showed that if manual screening would be replaced by the AI system at the same screening rate, the AI system should have a sensitivity of at least 80.1% for the non-indigenous population, and 79.5% for the indigenous population to become a cost-effective measure compared to the status quo. The specificity of the AI did not show an apparent influence on cost-effectiveness.
The scatter plot depicted the PSA results (Fig. 4a and b). For Scenario A, all 10,000 simulations demonstrated cost-saving results in both non-Indigenous and Indigenous people. Among the non-Indigenous population, Scenario B exhibited cost-saving outcomes in 99.7% of all iterations, with the remaining 0.3% being cost-effective. Similarly, in Scenario C, 99.7% of all iterations resulted in cost-saving results, while 0.3% were categorised as cost-effective. For the Indigenous population, 94.7% of all iterations in Scenario B were identified as cost-saving, with 5.3% deemed cost-effective. In Scenario C, 93.3% were cost-saving iterations and the rest 6.7% were cost-effective.
Fig. 4.
Probabilistic sensitivity analysis in the non-Indigenous population (A) and Indigenous population (B). Scatter plot from the probabilistic sensitivity analysis represented the incremental cost and incremental effectiveness of Scenario A, B and C compared to the status quo under 10,000 simulations.
Discussion
Our study has demonstrated that implementing an AI-based DR screening system in primary care settings was a highly cost-saving strategy in Australia. This would be attributed to the lower DR screening cost and higher accessibility and sensitivity of the AI system allowing for increased early detection, prompt treatment and slowing down the disease progression. Despite the increase in direct medical costs, the overall health benefits and monetary savings for the health system are significant.
The use of AI-based DR screening is a new model of care that is gaining popularity. Investigating the health economics of this technology would facilitate its further clinical application. To the best of our knowledge, this is the first study to investigate the cost-effectiveness of implementing AI-based DR screening in Australia, where optometrists and GPs play key roles in providing primary eye care under the national publicly-funded health insurance scheme.
Several studies have previously assessed the health economics of using AI for DR screening in various settings.21, 22, 23, 24, 25, 26 For example, Xie et al. conducted a cost-minimisation analysis to demonstrate that the annual unit cost of AI-assisted DR screening in fully-automated or semi-automated approaches is less expensive than human graders in Singapore.21 In terms of short-term cost-effectiveness, the UK National Health Services Diabetic Eye Screening Program indicated that AI-assisted DR grading saves costs in terms of screening, however, the diagnostic accuracy of the AI systems will largely affect the effectiveness compared to human graders.25 A one-year cost-effectiveness analysis was conducted in pediatric diabetes population in the United States, and the findings suggested the cost-effectiveness of AI would be largely associated with adherence to DR screening.23 As DR is a chronic condition that requires lifelong management, long-term cost-effectiveness analysis found that AI-based DR screening would be cost-saving compared to clinician screening over the lifetime horizon in hypothetical cohorts in China and Singapore.22 Our study demonstrated similar cost-saving findings in both real-world non-Indigenous and Indigenous cohorts. Moreover, we measured the effectiveness not only in terms of QALYs but also VTDR detected and blindness prevented, which provided more clinical relevance. However, inconsistent findings were reported in Brazil, where the AI-based DR screening cost more than the standard care model, with QALYs remaining similar.24 The potential reason is that the costs of specialist consultations and DR management were inexpensive.
Our findings suggest that using AI-based DR screening in primary care could be an effective way to increase efficiency and reduce barriers to DR screening. According to the National Eye Health Survey, a significant proportion of Australians are not adhering to DR screening guidelines.69 A plethora of potential barriers to performing DR screening may explain the low adherence, such as lack of time in routine practice, lack of awareness, and inadequate training of primary care providers in DR screening.15 The assistance of AI could help to bridge the skill gaps of GPs, thus providing the point-of-care screening service to the large number of eligible patients visiting the GP clinics who were not previously screened. Furthermore, in rural and remote areas, there is a higher proportion of indigenous Australians who have poorer overall health and greater disease burdens. The high cost of retinal cameras and limited access to eye care may exacerbate the problem in these areas.15,69 Introducing AI-based automated screening facilities to rural and remote areas helps to increase the accessibility of healthcare to these residents who are more vulnerable but are underserved, therefore improving the health of this population. Although no AI-based DR screening program has been launched in Australia, the technology has been proven through pragmatic trials to be a feasible and accurate approach in Australian healthcare settings and participants demonstrated reasonably good satisfaction.19,20 These findings shed light on the potential of AI-based DR screening to be translated into a routine screening practice and integrated into the full-cycle management of DR.
Nonetheless, the real-world implementation and scaling up of AI-based screening is challenging. Firstly, it is crucial to ensure the technology is well-validated before its application, as the algorithms may perform differently in distinct populations and devices used in practice. Secondly, the acceptability of the technology by end-users should be considered. Measures such as training, workshops, and informative materials could help shift the mindset of clinicians, patients, and policymakers and familiarise them with the technology. Thirdly, further research on health policy is necessary to establish a model of care that integrates AI-based DR screening into existing practices while upholding ethical standards, affordability and quality of care. Lastly, infrastructure updates are essential to the implementation of large-scale screening, such as setting up screening camps or point-of-care screening sites in primary care clinics.
Several limitations of the study need to be acknowledged. Firstly, when limited data is available on some parameters, such transition probabilities between DR states and utility values for the Australian population, we used data from other populations but have prioritised data from countries with similar socioeconomic status and ethnicity background. Nonetheless, we performed sensitivity analyses to confirm the robustness of our results. Secondly, as data was limited for a finer breakdown of disease states (i.e., unilateral and bilateral blindness) and population demographics (i.e., remoteness index), it has prevented us from performing more detailed analyses for these characteristics. Thirdly, further validation of the AI parameters is needed, as the cost data were obtained from our primary data in the empirical study and the sensitivity/specificity was based on published data from experimental settings.52 The real-world performance of the AI system may be affected by various factors, necessitating additional validation in prospective studies. Fourthly, the workflow of DR screening in our model was constructed based on the best available guideline in Australia in 2008. Although we have incorporated the updated treatment strategies according to the literature and guidelines in other countries, we did not take into consideration the impact of policy updates on diagnosis and treatment in this study. Lastly, as a modelling study, we simulated the ideal scenarios of scaling up the AI-based DR screening using primary care attendance and patient satisfaction rate from our pilot study, but the real-world feasibility was not considered, which requires further research into the end-user acceptability, health policies and availability of infrastructure.
In conclusion, our study showed that introducing an AI-based DR screening system in Australian primary care settings would be both effective and cost-effective. This novel approach has the potential to overcome existing barriers to DR screening, such as high staffing costs, shortage of trained personnel, and difficulties accessing adequate eye care in remote regions. Further input and feedback from stakeholders and decision-makers are necessary to fully evaluate and implement this innovative screening method.
Contributors
LZ, MH, ZZ, WH, JS, and EW were responsible for the study concept and design. WH and RL were responsible for data acquisition, analysis, or interpretation. LZ, MH, and ZZ were responsible for verifying the underlying data. WH and SJ were responsible for drafting the manuscript. All authors were responsible for critical revision of the manuscript for important intellectual content. MH and ZZ were responsible for obtaining funding. LZ, MH, and ZZ were responsible for administrative, technical, or material support, and study supervision. All authors confirmed that they had full access to all data involved in this study and accepted responsibility for the decision to submit for publication.
Data sharing statement
All data is provided in the Supplementary Materials.
Declaration of interests
We declare no competing interests.
Acknowledgements
The present work was supported by Medical Research Future Fund (MRFF) (MRFAI000035), National Health and Medical Research Council Investigator Grants (APP1175405, 2010072), and Australian Government Research Training Program Scholarship. The sponsor or funding organisation had no role in the design or conduct of this research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102387.
Contributor Information
Zhuoting Zhu, Email: lisa.zhu@unimelb.edu.au.
Mingguang He, Email: mingguang.he@polyu.edu.hk.
Lei Zhang, Email: lei.zhang1@monash.edu.
Appendix A. Supplementary data
References
- 1.Taylor H.R., Keeffe J.E., Vu H.T., et al. Vision loss in Australia. Med J Aust. 2005;182(11):565–568. doi: 10.5694/j.1326-5377.2005.tb06815.x. [DOI] [PubMed] [Google Scholar]
- 2.Tapp R.J., Shaw J.E., Harper C.A., et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003;26(6):1731–1737. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 3.The economic impact of diabetic macular oedema in Australia. Deloitte Access Economics; 2015. [Google Scholar]
- 4.Islam S.M.S., Siopis G., Sood S., et al. The burden of type 2 diabetes in Australia during the period 1990-2019: findings from the global burden of disease study. Diabetes Res Clin Pract. 2023;199 doi: 10.1016/j.diabres.2023.110631. [DOI] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare . AIHW, Australian Government; 2022. Diabetes: Australian facts. [Google Scholar]
- 6.Keel S., Xie J., Foreman J., van Wijngaarden P., Taylor H.R., Dirani M. The prevalence of diabetic retinopathy in Australian adults with self-reported diabetes: the national eye health survey. Ophthalmology. 2017;124(7):977–984. doi: 10.1016/j.ophtha.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Regional Office for Europe . World Health Organization. Regional Office for Europe; 2020. Diabetic retinopathy screening: a short guide: increase effectiveness, maximize benefits and minimize harm.https://apps.who.int/iris/handle/10665/336660 Available at: [Google Scholar]
- 8.Vashist P., Singh S., Gupta N., Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: an overview. Indian J Community Med. 2011;36(4):247–252. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanlon P.H. The English national screening programme for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54(6):515–525. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelaher M., Ferdinand A., Taylor H. Access to eye health services among indigenous Australians: an area level analysis. BMC Ophthalmol. 2012;12:51. doi: 10.1186/1471-2415-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiely P.M., Chakman J. Optometric practice in Australian standard geographical classification--remoteness areas in Australia, 2010. Clin Exp Optom. 2011;94(5):468–477. doi: 10.1111/j.1444-0938.2011.00590.x. [DOI] [PubMed] [Google Scholar]
- 12.Australian Institute of Health and Welfare . AIHW, Australian Government; 2023. General practice, allied health and other primary care services. [Accessed 04 October 2023] [Google Scholar]
- 13.Department of Health, Australian Government MBS online: medicare benefits schedule [homepage on the internet] http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home Canberra, ACT: Commonwealth of Australia. Available from:
- 14.Optometry Australia Retinal camera use clarified. https://www.optometry.org.au/workplace/retinal-camera-use-clarified/ Available at:
- 15.Watson M.J.G., McCluskey P.J., Grigg J.R., Kanagasingam Y., Daire J., Estai M. Barriers and facilitators to diabetic retinopathy screening within Australian primary care. BMC Fam Pract. 2021;22(1):239. doi: 10.1186/s12875-021-01586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruamviboonsuk P., Tiwari R., Sayres R., et al. Real-time diabetic retinopathy screening by deep learning in a multisite national screening programme: a prospective interventional cohort study. Lancet Digit Health. 2022;4(4):e235–e244. doi: 10.1016/S2589-7500(22)00017-6. [DOI] [PubMed] [Google Scholar]
- 17.Ipp E., Liljenquist D., Bode B., et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Shi J., Peng Y., et al. Artificial intelligence-enabled screening for diabetic retinopathy: a real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keel S., Lee P.Y., Scheetz J., et al. Feasibility and patient acceptability of a novel artificial intelligence-based screening model for diabetic retinopathy at endocrinology outpatient services: a pilot study. Sci Rep. 2018;8(1):4330. doi: 10.1038/s41598-018-22612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheetz J., Koca D., McGuinness M., et al. Real-world artificial intelligence-based opportunistic screening for diabetic retinopathy in endocrinology and indigenous healthcare settings in Australia. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-94178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y., Nguyen Q.D., Hamzah H., et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet Digit Health. 2020;2(5):e240–e249. doi: 10.1016/S2589-7500(20)30060-1. [DOI] [PubMed] [Google Scholar]
- 22.Huang X.M., Yang B.F., Zheng W.L., et al. Cost-effectiveness of artificial intelligence screening for diabetic retinopathy in rural China. BMC Health Serv Res. 2022;22(1):260. doi: 10.1186/s12913-022-07655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf R.M., Channa R., Abramoff M.D., Lehmann H.P. Cost-effectiveness of autonomous point-of-care diabetic retinopathy screening for pediatric patients with diabetes. JAMA Ophthalmol. 2020;138(10):1063–1069. doi: 10.1001/jamaophthalmol.2020.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez Rossi J., Rojas-Perilla N., Krois J., Schwendicke F. Cost-effectiveness of artificial intelligence as a decision-support system applied to the detection and grading of melanoma, dental caries, and diabetic retinopathy. JAMA Netw Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tufail A., Rudisill C., Egan C., et al. Automated diabetic retinopathy image assessment software: diagnostic accuracy and cost-effectiveness compared with human graders. Ophthalmology. 2017;124(3):343–351. doi: 10.1016/j.ophtha.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Liu H., Li R., Zhang Y., et al. Economic evaluation of combined population-based screening for multiple blindness-causing eye diseases in China: a cost-effectiveness analysis. Lancet Glob Health. 2023;11(3):e456–e465. doi: 10.1016/S2214-109X(22)00554-X. [DOI] [PubMed] [Google Scholar]
- 27.Australian Institute of Health and Welfare . AIHW, Australian Government; 2022. Australia's health 2022: in brief. [Accessed 23 November 2023] [Google Scholar]
- 28.The Commonwealth Fund International profiles of health care systems. https://www.commonwealthfund.org/international-health-policy-center/system-profiles Available at:
- 29.Su S., Wong W.C., Zou Z., et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10(2):e278–e287. doi: 10.1016/S2214-109X(21)00517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Z., Fairley C.K., Ong J.J., et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020;8(10):e1335–e1344. doi: 10.1016/S2214-109X(20)30277-1. [DOI] [PubMed] [Google Scholar]
- 31.Shen M., Zou Z., Bao H., et al. Cost-effectiveness of artificial intelligence-assisted liquid-based cytology testing for cervical cancer screening in China. Lancet Reg Health West Pac. 2023;34 doi: 10.1016/j.lanwpc.2023.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Liu H., Zou Z., et al. Shared-care models are highly effective and cost-effective for managing chronic hepatitis B in China: reinterpreting the primary care and specialty divide. Lancet Reg Health West Pac. 2023;35 doi: 10.1016/j.lanwpc.2023.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Phanuphak N., Henderson K., et al. Scaling up of HIV treatment for men who have sex with men in Bangkok: a modelling and costing study. Lancet HIV. 2015;2(5):e200–e207. doi: 10.1016/S2352-3018(15)00020-X. [DOI] [PubMed] [Google Scholar]
- 34.Zhao R., Fairley C.K., Cook A.R., et al. Optimizing HIV pre-exposure prophylaxis and testing strategies in men who have sex with men in Australia, Thailand and China: a modelling study and cost-effectiveness analysis. Lancet Glob Health. 2023 doi: 10.1016/S2214-109X(23)00536-3. Accepted. [DOI] [PubMed] [Google Scholar]
- 35.Husereau D., Drummond M., Augustovski F., et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20(1):23. doi: 10.1186/s12916-021-02204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Australian Institute of Health and Welfare . AIHW; Canberra: 2021. Indigenous eye health measures 2021. IHW 261. [Google Scholar]
- 37.National Health and Medical Research Council . Australian Diabetes Society for the Department of Health and Ageing; Canberra: 2008. Guidelines for the management of diabetic retinopathy. [Google Scholar]
- 38.Flaxel C.J., Adelman R.A., Bailey S.T., et al. Diabetic retinopathy preferred practice pattern(R) Ophthalmology. 2020;127(1):P66–P145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Australian Institute of Health and Welfare . AIHW; Canberra: 2023. Aboriginal and Torres Strait Islander Health Performance framework: summary report July 2023. [Accessed 04 October 2023] [Google Scholar]
- 40.Wilkinson C.P., Ferris F.L., 3rd, Klein R.E., et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 41.Bakri S.J., Wolfe J.D., Regillo C.D., Flynn H.W., Wykoff C.C. Evidence-based guidelines for management of diabetic macular edema. J Vitreoretin Dis. 2019;3(3):145–152. [Google Scholar]
- 42.Wang J.J., Foran S., Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains eye study. Clin Exp Ophthalmol. 2000;28(4):268–273. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 43.Foreman J., Xie J., Keel S., et al. Prevalence and causes of unilateral vision impairment and unilateral blindness in Australia: the national eye health survey. JAMA Ophthalmol. 2018;136(3):240–248. doi: 10.1001/jamaophthalmol.2017.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Australian Institute of Health and Welfare . 2nd ed. AIHW, Australian Government; 2009. A guide to Australian eye health data. [Accessed 23 November 2023] [Google Scholar]
- 45.Australian Institute of Health and Welfare . AIHW, Australian Government; 2023. Eye health measures for Aboriginal and Torres Strait Islander people 2022: interactive data. [Accessed 04 October 2023] [Google Scholar]
- 46.Chia M.A., Taylor J.R., Stuart K.V., et al. Prevalence of diabetic retinopathy in Indigenous and non-Indigenous Australians: a systematic review and meta-analysis. Ophthalmology. 2022;130(1):56–67. doi: 10.1016/j.ophtha.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 47.IDF diabetes atlas. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021. [Google Scholar]
- 48.Imai C., Li L., Hardie R.A., Georgiou A. Adherence to guideline-recommended HbA1c testing frequency and better outcomes in patients with type 2 diabetes: a 5-year retrospective cohort study in Australian general practice. BMJ Qual Saf. 2021;30(9):706–714. doi: 10.1136/bmjqs-2020-012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Department of Health . DoH; Canberra: 2021. Annual Medicare statistics: financial year 1984–85 to 2020–21. [Google Scholar]
- 50.Nash D.B., Fabius R.J., Skoufalos A. Preventing colorectal cancer: pathway to achieving an 80% screening goal in the United States: overview and proceedings of a population health advisory board. Popul Health Manage. 2021;24(2):286–295. doi: 10.1089/pop.2020.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramoff M.D., Lavin P.T., Birch M., Shah N., Folk J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Keel S., Liu C., et al. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care. 2018;41(12):2509–2516. doi: 10.2337/dc18-0147. [DOI] [PubMed] [Google Scholar]
- 53.Olson J.A., Strachan F.M., Hipwell J.H., et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med. 2003;20(7):528–534. doi: 10.1046/j.1464-5491.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- 54.Askew D., Schluter P.J., Spurling G., et al. Diabetic retinopathy screening in general practice: a pilot study. Aust Fam Physician. 2009;38(8):650–656. [PubMed] [Google Scholar]
- 55.Jackson C.L., Hirst L., de Jong I.C., Smith N. Can Australian general practitioners effectively screen for diabetic retinopathy? A pilot study. BMC Fam Pract. 2002;3:4. doi: 10.1186/1471-2296-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Hare J.P., Hopper A., Madhaven C., et al. Adding retinal photography to screening for diabetic retinopathy: a prospective study in primary care. BMJ. 1996;312(7032):679–682. doi: 10.1136/bmj.312.7032.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang O.S., Tay W.T., Ong P.G., et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol. 2015;99(12):1614–1621. doi: 10.1136/bjophthalmol-2014-306492. [DOI] [PubMed] [Google Scholar]
- 58.Ting D.S.W., Cheung C.Y., Lim G., et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Australian Bureau of Statistics . ABS; 2021. Deaths, Australia.https://www.abs.gov.au/statistics/people/population/deaths-australia/latest-release [Google Scholar]
- 60.Tancredi M., Rosengren A., Svensson A.M., et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 61.Australian Institute of Health and Welfare . AIHW, Australian Government; 2023. Deaths in Australia. [Accessed 04 October 2023] [Google Scholar]
- 62.Crewe J.M., Spilsbury K., Morlet N., et al. Health service use and mortality of the elderly blind. Ophthalmology. 2015;122(11):2344–2350. doi: 10.1016/j.ophtha.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 63.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence (NICE); 2013. Guide to the methods of technology appraisal 2013. [PubMed] [Google Scholar]
- 64.Department of Health, Australian Government PBS online. http://www.pbs.gov.au/info/about-the-pbs#Managing_the_cost_of_the_scheme Available at:
- 65.Wright S.E., Keeffe J.E., Thies L.S. Direct costs of blindness in Australia. Clin Exp Ophthalmol. 2000;28(3):140–142. doi: 10.1046/j.1442-9071.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 66.Tang J.J., Liang Y.B., O'Neill C., Kee F., Jiang J., Congdon N. Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Lancet Glob Health. 2019;7(7):E968–E978. doi: 10.1016/S2214-109X(19)30201-3. [DOI] [PubMed] [Google Scholar]
- 67.George B., Harris A., Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991 to 1996) Pharmacoeconomics. 2001;19(11):1103–1109. doi: 10.2165/00019053-200119110-00004. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen H.V., Tan G.S., Tapp R.J., et al. Cost-effectiveness of a national telemedicine diabetic retinopathy screening program in Singapore. Ophthalmology. 2016;123(12):2571–2580. doi: 10.1016/j.ophtha.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Foreman J., Keel S., Xie J., Van Wijngaarden P., Taylor H.R., Dirani M. Adherence to diabetic eye examination guidelines in Australia: the national eye health survey. Med J Aust. 2017;206(9):402–406. doi: 10.5694/mja16.00989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.