Abstract
Objective
Myofibrillar myopathies (MFM) are a group of sporadic and inherited progressive skeletal muscle disorders that can lead to physical disability and premature death. To date, pathogenic variants in different genes are associated with MFM. MFM induced by variants in the Desmin (DES) gene is the most common subtype of MFM.
Case presentation
A 15-year-old boy with MFM was described, whose symptoms first presented as cardiac symptoms. Enlarged right and left atria, thickened ventricular septal (IVS) and mild mitral (MR) and tricuspid regurgitation (TR) in the echocardiography were found. Atrial fibrillation, intermittent atrioventricular (AV) block, ST-T changes in the dynamic electrocardiogram (ECG) were shown. Mild myopathic changes in the electromyographic exam were detected. Ultrastructural analysis found slight Z-line changes and a few small myolysis lesions, but no abnormal inclusion bodies. Genetic testing detected a heterozygous missense variant (c.1216C > T) of DES, and 2 rare variants: TNNI3K (c.1102C > G) and PRDM16 (c.3074G > A). The patient's parents didn't show skeletal and cardiac muscle disorders. DNA sequencing analysis showed no variant of DES was carried by them. Thus, we detected a case of MFM caused by de novo DES variant c.1216C > T/p.Arg406Trp with predominantly myocardial alterations.
Keywords: Case report, Desmin, Hypertrophic cardiomyopathy, Myofibrillar myopathy, Variant
1. Introduction
Myofibrillar myopathy (MFM) (OMIM: #601419) is a clinically and genetically heterogeneous inherited muscle disease [1]. Despite its distinctive pathological features, the clinical manifestations of MFM are varied. This characteristic makes the clinical diagnosis of MFM difficult. MFM is transmitted in an autosomal dominant manner, but a few MFMs are also inherited through autosomal recessive inheritance [2]. Gene implicated in MFM includes DES [3], α-B-crystallin (CRYAB) [4], myotilin (MYOT) [5], Filamin C (FLNC) [6], Bcl-2-associated athanogene 3 [7], Four-and-a-half LIM protein-1 [8], Plectin [9], Titin [10] etc. Of these, MFM caused by abnormal accumulation of Desmin protein, known as desminopathy, is the most common form of MFM. DES is a cytoskeletal protein that is expressed abundantly in the heart or skeletal muscles of vertebrates [11]. This protein is involved in cellular integrity and mechanochemical signaling in muscle cells. Mutations in different functional domains of this gene can induce different clinical phenotypes. Here, we report a patient suffering from hypertrophic cardiomyopathy (HCM). Sanger sequencing identified a heterozygous variant c.1216C > T in the DES gene.
1.1. Patient information
A 15-year-old male boy was admitted to our hospital with mild post-activity intermittent palpitations. Two years ago, he would experience palpitations and discomfort after running about 100 m, and the symptoms would resolve after a few minutes of rest. The patient had fair mental and sleepiness, normal physical strength, and no significant abnormal changes in weight since the onset. Two weeks before this consultation, the patient's palpitations worsened. He carries no traditional cardiovascular risk factors. His parents had no history of heart disease or musculoskeletal problems.
His ECG exhibited ST-segment reduction in leads V 3–6, intermittent AV block, and episodic premature ventricular contractions (Supplemental Fig. 1). Echocardiography showed enlargement of both atria, asymmetric thickening of the IVS, slight aggravation of MR and TR, and a left ventricular ejection fraction (LVEF) of 70 % (Supplementary Fig. 2). The ratio of the thickness of the IVS to the left ventricular posterior wall was 1.4, and systolic anterior motion (SAM sign) of the anterior mitral was not observed. High-sensitivity cardiac troponin I (cTnI) was determined [cTnI: 0.097 ng/mL (Reference: <0.02 ng/mL)]. A diagnosis of HCM was made, followed by long-term medical advice of furosemide tablets (10 mg, BID), spironolactone tablets (20 mg, BID), and coenzyme Q10 tablets (10 mg, TID). The patient's parents also underwent a physical examination and echocardiographic evaluation, which revealed no signs associated with HCM.
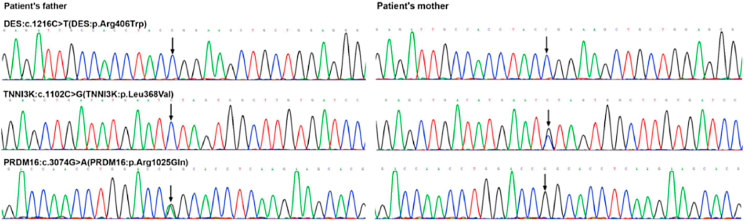
To further understand the pathogenic factors of the patient, the peripheral blood of the child and his parents was collected. Full-length cDNA was extracted and stored. Exome sequence enrichment of samples was performed by the Illumina Exome Enrichment protocol and captured libraries were sequenced using Illumina HiSeq 2000 Sequencers, which contains 130 genes known to be related to inherited cardiac diseases (see Supplementary Table 1). The pathogenic variants were confirmed by Sanger sequencing. Sanger sequencing identified a heterozygous missense variant (p. Arg406Trp) of the DES gene (Fig. 1), which is a common variant in MFM. This patient was also detected to carry two rare variants associated with hereditary cardiomyopathy (TNNI3K: c.1102C > G and PRDM16: c.3074G > A) (Table 1). Interestingly, his parents did not detect the DES gene variant and only carried TNNI3K and PRDM16 variants, respectively (Fig. 2).
Fig. 1.
DNA sequencing analysis showed a heterozygous missense variant in the exon 6 of the DES gene and two rare variants (black arrows).
Table 1.
Attributes of the variants identified in the study.
| Position (GRCh37) | Location | dbSNP rs# | CADD | ACMG | |
|---|---|---|---|---|---|
| DES(NM_001927.4):c.1216C > T/p.Arg406Trp | Chr2:220286254 | Exon 6 | rs121913003 | 27.1 | Pathogenic/Likely pathogenic (PP5, PP3, PM1, PM5, PS3, and PM2) |
| TNNI3K(NM_015978):c.1102C > G/p.Leu368Val | Chr1:74819738 | Exon 11 | – | 23.1 | VUS(PM2) |
| PRDM16(NM_022114):c.3074G > A/p.Arg4025Gln | Chr1:3342279 | Exon 13 | – | 25.7 | VUS(PM2) |
ACMG, American College of Medical Genetic Guidelines; VUS, uncertain significance; PM2/PM1/PM5, moderate pathogenicity evidence; PP3/PP5, supporting pathogenic evidence; PS3, strong pathogenicity evidence.
Fig. 2.
DNA sequencing chromatogram of the parents.
Ultrasound examination of the arteries and veins in the lower limbs did not reveal any abnormal blood flow signals. Electromyography showed no abnormalities in peripheral nerve movements of the limbs, but there was myotonic potential in the medial head of the gastrocnemius muscle. Skeletal muscle biopsy was performed on the right deltoid muscle (Supplemental Fig. 3C). Muscle biopsy did not reveal any abnormal material deposition in muscle tissue (Supplemental Fig. 3D), but small lipid droplets were observed in myocytes under electron microscopy (Supplemental Figs. 3A and 3B), and no abnormal inclusion bodies or other material deposition was found. The reporting of this study conformed to CARE guidelines [12].
2. Discussion
In the current case, a heterozygous missense variant of the DES gene led to HCM with arrhythmia, slight myogenic changes, and small lipid droplets in some muscle fibers. Interestingly, this patient was also detected to carry two rare variants associated with hereditary cardiomyopathy (TNNI3K and PRDM16). This case report can broaden the clinician's thinking to a greater extent to avoid misdiagnosis and underdiagnosis.
Desmin is an intermediate filament protein in which a highly conserved amino acid motif (YRKLLEGEE) at the Cterminal end of the 2 B helix is involved in filament assembly [13,14]. The Arg406Trp is located in this region, and interferes with Desmin function and affects the structural and functional integrity of intercalated discs, thereby affecting cardiac function [14]. Several reports have described the initial signs of heart involvement in young people caused by variants in the DES gene, and in particular, the variant that is the cause of this patient - c.1216C > T (p.R406W) - has been frequently reported to be deleterious (These patients are listed in Table 2) [14]. The variant has been also found in patients with other cardiomyopathies, such as dilated cardiomyopathy (DCM) [15,16]. One recent study conducted in Japan has reported the Arg406Trp variant in HCM patients with complete AV block and weakness in the soft palate and extremities [17]. In our case, the patient presented with HCM and intermittent AV block, without manifestations of skeletal muscle impairment. The Arg406Trp (c.1216C > T) variant may be the pathogenic variant for HCM and ST-T segment changes. Considering the phenotype of this case, we concluded that this case is a desminopathy case manifesting HCM caused by the Arg406Trp variant.
Table 2.
Previous literature on myofibrillar myopathy caused by pathogenic variants of R406W.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Current study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Male | Male | Female | Female | Female | Male | Female | Male | Male |
| Age of Onset | – | 24 | 27 | 15 | 24 | 15 | 23 | 18 | 15 | 9 | 13 |
| Cardiomyopathy | – | – | RCM | RCM | – | DCM | – | RCM | RCM | HCM | HCM |
| Paced rhythm | + | – | + | – | – | – | – | – | + | – | – |
| Atrial fibrillation | + | – | – | – | – | – | – | – | – | – | – |
| Pacemaker | + | – | + | – | + | + | + | + | – | – | – |
| RBBB | – | – | – | + | – | – | – | – | – | – | – |
| CAVB | – | + | + | – | + | + | + | + | + | + | + |
| Lower limb muscle weakness | – | – | – | – | + | + | + | + | + | – | – |
| Weakness in the soft palate | – | – | – | – | + | + | + | + | – | + | – |
| Dysphagia | – | – | – | – | – | + | + | – | – | + | – |
| Clinical classification | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic | pathogenic |
| Reference | Wahbi 2012 | Park 2000 | Arbustini 2006 | Arbustini 2006 | Dagvadorj 2004 | Dagvadorj 2004 | Dagvadorj 2004 | Dagvadorj 2004 | Olive 2004 | Takegami 2023 | – |
| Origin | Germline | Somatic | Germline | Germline | Germline | De novo | De novo | De novo | De novo | De novo | De novo |
| Frequency | – | – | – | – | – | – | – | – | – | – | – |
Abbreviations: CAVB: Complete a-v block; AF: Atrial fibrillation; RBBB: Right bundle branch block; RCM: restrictive cardiomyopathy; DCM: Dilated cardiomyopathy; HCM: Hypertrophic cardiomyopathy.
In addition, two rare variants (TNNI3K: c.1102C > G and PRDM16: c.3074G > A) were identified in this case. Genetic variants in TNNI3K have previously been associated with DCM and cardiac conduction disease [18]. Recent studies have revealed the role of PRDM16 in heart tissues. PRDM16 deficiency accelerated cardiac hypertrophy and fibrosis in aging mice [19]. A multi-institutional study showed that the most common cardiomyopathies in individuals with PRDM16 deleted were LVNC and DCM [20]. But two variants (TNNI3K: c.1102C > G and PRDM16: c.3074G > A) were not present in the Human Gene Mutation Database (HGMD). In addition, we analyzed the pathogenicity of the variants using ACMG evaluation criteria. Finally, both variants were identified as uncertain significance variants (PM2) (see Table 1). However, it is important to be alert to the possibility that multiple variants cause early onset of disease and severe clinical phenotype manifestations.
Previous studies have reported that patients carrying the Arg406Trp variant exhibit varying degrees of neuromuscular function or skin changes. Patients harboring the Arg406Trp variants carry varying degrees of neuromuscular and cutaneous disease [21]. However, no significant alterations in neuromuscular function were detected in the present case. This phenotype may have a confusing effect on clinical studies of the etiology of desminopathy patients. This case may suggest clinicians consider the possibility of demyelinating disease due to the Arg406Trp mutation in the presence of unexplained HCM combined with AV.
Ethics statement
Not applicable. Written informed consent was obtained.
Data availability statement
Data will be available on reasonable request.
Funding
None.
CRediT authorship contribution statement
Hongyan Xiao: Writing – original draft, Methodology, Data curation, Conceptualization. Laichun Song: Methodology, Conceptualization. Liang Tao: Writing – review & editing, Project administration, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank this patient.
Biographies
Hongyan Xiao: Division of cardiac surgery, Wuhan Asia Heart Hospital, Wuhan, 430022, China. E-mail address: xiaohy11@sina.com
Laichun Song: Division of cardiac surgery, Wuhan Asia Heart Hospital, Wuhan, 430022, China. E-mail address: songlaichun77@sina.cn
Liang Tao: Division of cardiac surgery, Wuhan Asia Heart Hospital, Wuhan, 430022, China. E-mail address: tliang72@sina.com
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e25009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Engel A.G. Myofibrillar myopathy. Ann. Neurol. 1999;46:681–683. doi: 10.1002/1531-8249(199911)46:5<681::aid-ana1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.van Spaendonck-Zwarts K.Y., van Hessem L., Jongbloed J.D., de Walle H.E., Capetanaki Y., van der Kooi A.J., et al. Desmin-related myopathy. Clin. Genet. 2011;80:354–366. doi: 10.1111/j.1399-0004.2010.01512.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Liu Y., Li B., Wang L., Zhang W. Case report: an unusual case of desmin myopathy associated with heart failure and arrhythmia. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.944459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodehl A., Gaertner-Rommel A., Klauke B., Grewe S.A., Schirmer I., Peterschroder A., et al. The novel alphaB-crystallin (CRYAB) mutation p.D109G causes restrictive cardiomyopathy. Hum. Mutat. 2017;38:947–952. doi: 10.1002/humu.23248. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi V., Pancheri E., Cannone E., Nigro V., Malatesta M., Vettori A., et al. A novel in-frame deletion in MYOT causes an early adult onset distal myopathy. Clin. Genet. 2023;104:705–710. doi: 10.1111/cge.14413. [DOI] [PubMed] [Google Scholar]
- 6.Verdonschot J.A.J., Vanhoutte E.K., Claes G.R.F., Helderman-van den Enden A., Hoeijmakers J.G.J., Hellebrekers D., et al. A mutation update for the FLNC gene in myopathies and cardiomyopathies. Hum. Mutat. 2020;41:1091–1111. doi: 10.1002/humu.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaba Y., Takeguchi R., Tanaka R., Makita Y., Kimura T., Yanagi K., et al. Wide Spectrum of cardiac phenotype in myofibrillar myopathy associated with a Bcl-2-associated athanogene 3 mutation: a case report and literature review. J. Clin. Neuromuscul. Dis. 2022;24:49–54. doi: 10.1097/CND.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 8.Poparic I., Schreibmayer W., Schoser B., Desoye G., Gorischek A., Miedl H., et al. Four and a half LIM protein 1C (FHL1C): a binding partner for voltage-gated potassium channel K(v1.5) PLoS One. 2011;6 doi: 10.1371/journal.pone.0026524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servidei S., Primiano G., Muto V., Cuccagna C., Bernardo D., Sauchelli D., et al. PLEC gene mutations cause familial disto-proximal myopathy and long QT syndrome mimicking mitochondrial disease. Neuromuscul. Disord. 2017;27:S150–S151. [Google Scholar]
- 10.Sano Y., Ota S., Oishi M., Honda M., Omoto M., Kawai M., et al. A Japanese patient with hereditary myopathy with early respiratory failure due to the p.P31732L mutation of Titin. Intern Med. 2022;61:1587–1592. doi: 10.2169/internalmedicine.7733-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulin D., Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D., et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob. Adv. Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagvadorj A., Olive M., Urtizberea J.A., Halle M., Shatunov A., Bonnemann C., et al. A series of West European patients with severe cardiac and skeletal myopathy associated with a de novo R406W mutation in desmin. J. Neurol. 2004;251:143–149. doi: 10.1007/s00415-004-0289-3. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann H., Cabet E., Chevalier N.R., Moosmann J., Schultheis D., Haas J., et al. Dual functional states of R406W-desmin assembly complexes cause cardiomyopathy with severe intercalated disc derangement in humans and in knock-in mice. Circulation. 2020;142:2155–2171. doi: 10.1161/CIRCULATIONAHA.120.050218. [DOI] [PubMed] [Google Scholar]
- 15.Kubanek M., Schimerova T., Piherova L., Brodehl A., Krebsova A., Ratnavadivel S., et al. Desminopathy: novel desmin variants, a new cardiac phenotype, and further evidence for secondary mitochondrial dysfunction. J. Clin. Med. 2020;9 doi: 10.3390/jcm9040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbustini E., Pasotti M., Pilotto A., Pellegrini C., Grasso M., Previtali S., et al. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur. J. Heart Fail. 2006;8:477–483. doi: 10.1016/j.ejheart.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Oka H., Nakau K., Imanishi R., Furukawa T., Tanabe Y., Hirono K., et al. A case report of a rare heterozygous variant in the desmin gene associated with hypertrophic cardiomyopathy and complete atrioventricular block. CJC Open. 2021;3:1195–1198. doi: 10.1016/j.cjco.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theis J.L., Zimmermann M.T., Larsen B.T., Rybakova I.N., Long P.A., Evans J.M., et al. TNNI3K mutation in familial syndrome of conduction system disease, atrial tachyarrhythmia and dilated cardiomyopathy. Hum. Mol. Genet. 2014;23:5793–5804. doi: 10.1093/hmg/ddu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cibi D.M., Bi-Lin K.W., Shekeran S.G., Sandireddy R., Tee N., Singh A., et al. Prdm16 deficiency leads to age-dependent cardiac hypertrophy, adverse remodeling, mitochondrial dysfunction, and heart failure. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108288. [DOI] [PubMed] [Google Scholar]
- 20.Kramer R.J., Fatahian A.N., Chan A., Mortenson J., Osher J., Sun B., et al. PRDM16 deletion is associated with sex-dependent cardiomyopathy and cardiac mortality: a translational, multi-institutional cohort study. Circ Genom Precis Med. 2023;16:390–400. doi: 10.1161/CIRCGEN.122.003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalakas M.C., Park K.Y., Semino-Mora C., Lee H.S., Sivakumar K., Goldfarb L.G. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N. Engl. J. Med. 2000;342:770–780. doi: 10.1056/NEJM200003163421104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available on reasonable request.