Abstract
The vestibular system connects the inner ear to the midbrain and subcortical structures and can affect cognition. Patients with vertigo often experience cognitive symptoms such as attention deficits, memory problems, and spatial perception difficulties. This study aimed to explore the cognitive impairments associated with Benign paroxysmal positional vertigo (BPPV) and Meniere's Disease (MD). A non-experimental group comparison design was used with 107 participants divided into three groups: Group I (clinically normal), Group II (BPPV), and Group III (MD). Participants completed a questionnaire with 10 cognition-related questions, and their responses were scored. The data were found to be non-normally distributed. The analysis revealed a significant difference in scores between Group I and both Group II and Group III. Chi-square tests showed that the responses to cognition-related questions varied among the groups, with Group II exhibiting more cognitive problems. Associated conditions like hypertension, diabetes, and hearing loss did not significantly influence the responses within each group. This study suggests a significant relationship between cognitive problems and patients with BPPV and MD. However, there was no association found between the cognitive problems experienced in BPPV and MD patients. These findings align with previous research indicating that vestibular disorders can lead to deficits in spatial memory, attention, and other cognitive functions. By understanding the link between cognition and vestibular disorders, we can improve diagnosis and rehabilitation services to enhance the quality of life for these patients.
Keywords: BPPV, Meniere's disease, Cognition, Questionnaire
1. Introduction
Vestibular networks within subcortical structures travel through the midbrain and subsequently into the inner ear. Due to these diffused connections, different points along the routes are expected to affect vestibular function. Furthermore, it is made up of white matter and nerves, especially the vestibulocochlear nerve, a composite sensory nerve that makes it susceptible to various injuries and weakens cell signaling Thus, damage to the vestibular system can result in functional impairments and lead to symptoms like vertigo and dizziness. Recent clinical findings indicate that individuals experiencing vertigo often report associated cognitive issues. These problems include difficulties with attention, memory, spatial perception, navigation, mental rotation, and mental representation of three-dimensional space. Interestingly, these cognitive symptoms may not necessarily be linked to a specific vertigo episode (Gurvich et al., 2013a). Numerous animal studies conducted over the past few decades consistently demonstrate that animals with vestibular injuries exhibit spatial cognition deficits (Wallace et al., 2002; Zheng et al., 2012). Cognitive functions like spatial memory, navigation etc., are common impairments seen in the elderly population, especially with dementia. As with ageing, vestibular cells or neurons could be lost, leading to vestibular pathologies. Thus vestibular dysfunction may be one of the risk factors that cause such cognitive disorders, especially in the elderly (Jun et al., 2020).
It has been well established that long-standing, untreated hearing loss causes cognitive impairment; however, the relation between vestibular problems and cognition is less explored. Studies have shown that minimal hearing loss is linked to worse results on memory and executive function tests (Lin, 2011). Early treatment of age-related HL, such as hearing aids, may lessen the adverse functional effects on grey matter atrophy, leading to cognitive issues (Slade et al., 2022). Thus, a question arises if the long-standing hearing loss would cause sensory deprivation to the brain over time, leading to cognitive problems; similarly, could untreated and long-standing vestibular symptoms show the same?
Benign paroxysmal positional vertigo (BPPV) and Meniere's disease (MD) are the most common causes of vestibular vertigo, accounting for a maximum number of cases in specialized dizziness clinics (Neuhauser, 2016). Although the relation between BPPV and cognition has been less explored, there is some evidence of vestibular and cognitive symptoms in the case of Meniere's disease. Meniere's disease (MD) frequently reports abnormal emotional processing and vertigo symptoms. In Meniere's disease, uncertainty intolerance is most likely linked to anxiety and other emotional distress (Kirby and Yardley, 2009). Various studies have been carried out to evaluate the quality of life in patients dealing with vertigo. Several questionnaires, such as the Dizziness Handicap Inventory, Dizzy Factor Inventory, Vertigo Symptom Scale, and VertigoDizziness-Imbalance Questionnaire, have been developed to assess the physical limitations and emotional consequences (such as anxiety and depression) on the overall quality of life. However, these questionnaires tend to have limited focus on addressing the cognitive dysfunction associated with dizziness. Only a few questions in these assessments specifically target cognitive impairments resulting from vertigo (Lacroix et al., 2016; Liu et al., 2019).
Studying the association between vestibular problems and cognition could also help better rehabilitate and manage the particular disorder causing the vestibular issues if a correlation is found. Cognitive-behavioral therapy (CBT) is a form of psychotherapeutic treatment designed to assist individuals in recognizing and modifying harmful or disruptive thought patterns that have a negative impact on their behavior and emotions. Studies revealed that CBT and Vestibular rehabilitation therapy (VRT) could be coupled in treating dizziness-specific handicaps and discomfort, and CBT practitioners may be able to broaden their practice (Wei et al., 2018a). While the findings are preliminary, it can be recommended to the audiologist to consider CBT as a rehabilitation option and a supplement in treating dizzy patients along with VRT.
2. Method
A non-experimental standard group comparison research design was employed for the current study.
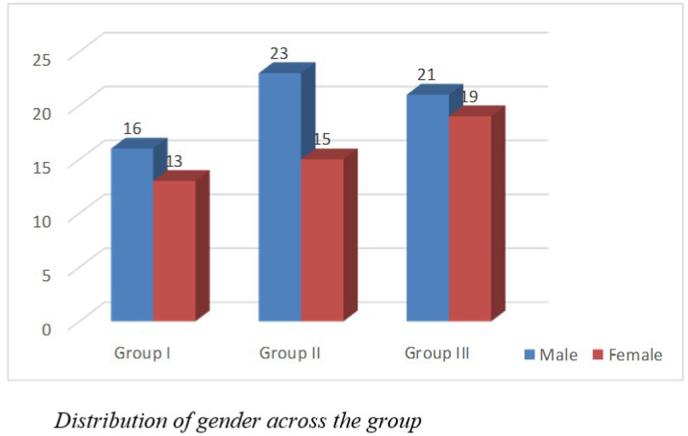
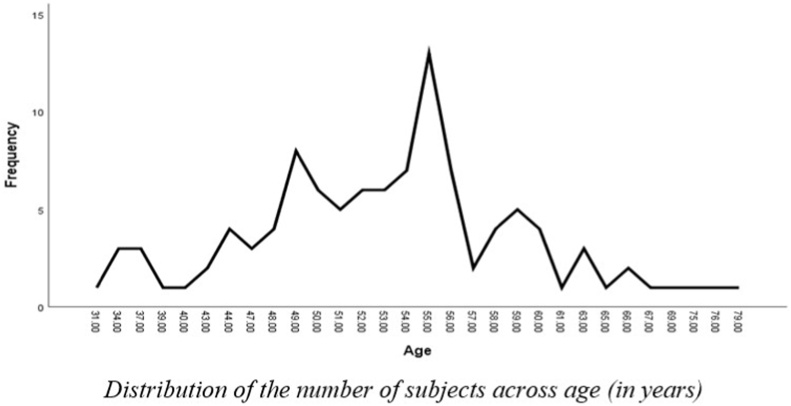
A total of 107 subjects participated in the study. Group-I had 29 subjects clinically normal, Group II had 38 subjects diagnosed with BPPV and Group III had 40 subjects diagnosed with Meniere's disease. Of the 107 subjects, 60 were male, and 47 were female as shown in Fig. 1. The age ranged from 49 to 60 years for Group I, from 31 to 66 years for Group II and 34–79 years for Group III. The mean age was 53.96 (SD = 3.39), 50.71(SD = 8.77) and 54.55 (SD = 9.82) years for the three groups, respectively as shown in Fig. 2. The clinical groups were diagnosed by ENT specialists and RCI-certified audiologists. Group I had clinically normal subjects with no history of vertigo or associated problems that could impact balance and equilibrium. Group II had patients diagnosed with BPPV (based on AAO-HNS Diagnostic criteria) with no other otological problem or associated problems like ear discharge, ear pain, history of surgery and had unilateral BPPV. Group III had patients diagnosed with Meniere's disease (based on AAO-HNS Diagnostic criteria). Unilateral MD cases were taken for the study. Patients with no other otological or associated problems like ear discharge, ear pain, or history of surgery were taken for the study. Patients with abnormal radiological findings or any history of CP angle tumor, microvascular disorders, vascular encephalopathy etc were excluded from the study.
Fig. 1.
Distribution of Gender across groups.
Fig. 2.
Distribution of the number of subjects across age (in years).
Subjects' consent and willingness to participate in the study were considered. The consent form had information regarding the study's title, a brief description of the research topic and the approximate amount of time it'll take to complete the questionnaire. The details were also explained verbally to the subjects. The Google form's initial section was the consent form, which included the alternatives “Yes” and “No” for subjects to select from. If the subjects voluntarily decided to participate in the study, they had to choose 'Yes', and further questions were followed. Other questions did not follow if the subjects responded with “No."
2.1. Sample selection
A total of 131 case files were screened from the institute's database from January 2017 to May 2022, out of which 10 subjects were rejected as they didn't fall into the inclusion criteria, which included bilateral BPPV cases, recurrent BPPV cases, bilateral MD cases, and 12 subjects did not respond to the phone call/email. Thus, the study was conducted on a total of 78 clinical subjects. The 29 non-clinical subjects were randomly selected. However, they underwent a quick GHQ-5 questionnaire (Shamasunder et al., 1986) for general health screening.
2.2. Procedure
The study was conducted in an online/tele-mode. After detailed instructions were provided to the participants, the questionnaire was sent via mail/WhatsApp. The instructions were in English in the consent form and the questionnaire; however, the details were also instructed in Hindi and Kannada as and when required. The first part of the questionnaire consisted the demographic details about the participants' names, ages, gender, and diagnosis. Questions related to the history of BP, diabetes, tinnitus, and if they were under any medication were asked. The next part had 10 cognitive related questions. These 10 questions were primarily obtained from the Neurobehavioral Cognitive Status Examination (NCSE) and were selected after consulting 3 experienced RCI certified audiologists (Annexure 1). For each response to the questions, a score of 0 for “Yes,” 1 for “Sometimes,” and 2 for “No” were assigned for the analysis.
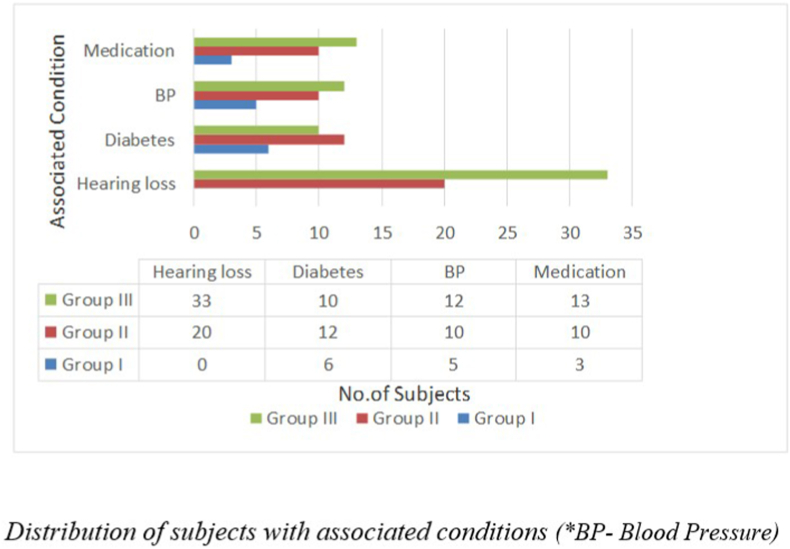
None of the subjects in the control group, i.e., Group I, had a history of hearing loss. 15 out of 38 individuals in Group II and 33 out of 40 individuals in Group III had been diagnosed with hearing loss, as shown in Fig. 3. Other than subjects with reduced hearing sensitivity, subjects with other otological complaints like otalgia, otorrhea, itching sensation or blockage were excluded from the study.
Fig. 3.
Distribution of subjects with associated conditions (*BP- Blood pressure).
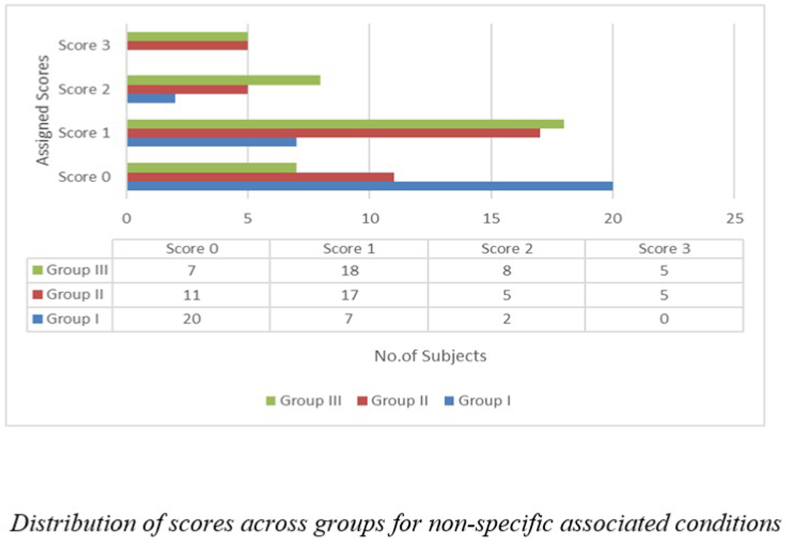
Fig. 4 depicts the groups with associated factors (i.e., hearing loss, diabetes, or BP). A score of ‘0′ was assigned for the subjects who reported none of these associated conditions. A score of ‘1′ was given if the subjects had any of the associated conditions, ‘2′ if they reported any two associated conditions or ‘3′ if the subjects reported all the three associated conditions. Thus an ‘Overall associated score’ was later used to compare cognitive abilities and non-specific associated conditions.
Fig. 4.
Distribution of scorers across groups for non-specific associated conditions.
3. Results
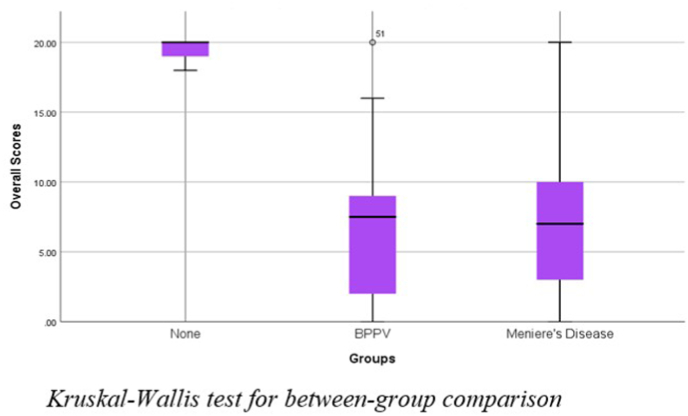
The data collected were organized into tables and subjected to statistical analysis using SPSS version 25.0 (Frey, 2017). It was observed that subjects diagnosed with BPPV, and Meniere's disease had mean overall scores of 6.76 and 7.67, respectively, significantly less than the control population, which had a mean score of 19.58. It has been tabulated in Table 1. Kruskal-Wallis H test was carried out to check if there was a significant difference between the groups. Fig. 5 shows a significant difference between the groups (p < 0.01). Thus, the Dunn-Bonferroni posthoc test was done for pairwise comparison between the three groups. The test revealed a significant difference between Group I, Group II, and Group I and Group III, but no significant difference between Group II and Group III, as shown in Table 2. The effect size was calculated between the groups using Cohen's d equation (Lakens, 2013), and it was found that there was no effect between Groups II and III. A medium effect between Groups I and II and Groups I and III was seen as the values were close to 0.5, as shown in Table 2.
Table 1.
Mean, Standard deviation (SD), median and Interquartile deviation (IQR) of cognitive scores across the groups.
| Mean | SD | Median | IQR | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Group I | 19.58 | 0.62 | 20.00 | 1.00 | 18 | 20 |
| Group II | 6.76 | 5.13 | 7.50 | 7.25 | 0 | 20 |
| Group III | 7.67 | 5.87 | 7.00 | 7.00 | 0 | 20 |
Fig. 5.
Kruskal-Wallis test for between group comparision.
Table 2.
Scores of the Dunn-Bonferroni posthoc test for pairwise comparison between the groups.
| H- value | p-value | Effect Size(ηH2) | |
|---|---|---|---|
| Group II-III | 3.69 | 0.59 | −0.01 |
| Group II-I | 51.70 | 0.01 | 0.45 |
| Group III-I | 48.01 | 0.01 | 0.42 |
Chi-square tests were done for the descriptive analysis of all the 10 questions, as depicted in Table 3. The Chi-square test revealed a significant association (p < 0.05) between the cognitive abilities and the groups, i.e., the responses to the cognitive-based questions changed according to the group. For Group I, the number of people with cognitive problems was less; for Group II and III, the number of people with cognitive problems was more, as seen in Table 3.
Table 3.
Chi-square test scores with respect to the cognitive-based questions across groups.
| Questions | Group I | Group II | Group III | Chi-square (significance) |
|---|---|---|---|---|
| 1. Does it seem that you can't think as quickly as before (i.e. before the diagnosis)? | Yes = 0 (0%) | Yes =23 (60.5%) | Yes = 23 (57.5%) | p < 0.01 |
| Sometimes = 5 (17.2%) | Sometimes = 10 (26.3%) | Sometimes = 8 (20.0%) | ||
| No = 24 (82.8%) | No = 5 (14.3%) | No = 9 (22.5%) | ||
| 2. Does it seem that you find it hard to think clearly? | Yes = 0 (0%) | Yes = 22 (57.9%) | Yes =21 (52.5%) | p < 0.01 |
| Sometimes = 0 (0%) | Sometimes = 12 (31.6%) | Sometimes = 12 (30.0%) | ||
| No = 29 (100%) | No = 4 (10.5%) | No = 7 (17.4%) | ||
| 3. Does it seem that you are easily more distracted? | Yes = 0 (0%) | Yes =24 (63.2%) | Yes =21 (52.5%) | p < 0.01 |
| Sometimes = 0 (0%) | Sometimes = 8 (21.1%) | Sometimes = 12 (30.0%) | ||
| No = 29 (100%) | No = 6 (15.8%) | No = 7 (17.5%) | ||
| 4. Does it seem that you can't concentrate? | Yes = 0 (0 %) | Yes =21 (55.3%) | Yes = 21 (52.5%) | p < 0.01 |
| Sometimes = 0 (33.3%) | Sometimes = 10 (26.3%) | Sometimes = 12 (30.0%) | ||
| No =29 (14.8%) | No = 7 (18.4%) | No = 7 (17.5%) | ||
| 5. Do you have trouble remembering the right words when talking? | Yes = 0 (0%) | Yes =20 (52.6%) | Yes =18 (45.0%) | p < 0.01 |
| Sometimes = 2 (6.9%) | Sometimes = 11 (28.9%) | Sometimes = 15 (37.5%) | ||
| No = 27 (93.1%) | No = 7 (18.4%) | No = 7 (17.5%) | ||
| 6. Do you have trouble understanding others? | Yes = 0 (0%) | Yes =20 (52.6%) | Yes =13 (45.0%) | p < 0.01 |
| Sometimes = 1 (3.4%) | Sometimes = 11 (28.9%) | Sometimes = 11 (37.5%) | ||
| No = 28 (96.6%) | No = 7(18.4%) | No = 3 (17.5%) | ||
| 7. Do you have trouble following conversations? | Yes = 0 (0%) | Yes =19 (50.0%) | Yes =21 (52.5%) | p < 0.01 |
| Sometimes = 2 (6.9%) | Sometimes = 16 (42.1%) | Sometimes = 11 (27.5%) | ||
| No = 27 (93.1%) | No = 3 (7.9%) | No = 8 (20%) | ||
| 8. Do you have trouble with your speech? | Yes = 0 (0%) | Yes = 13 (34.2%) | Yes=14 (35.0%) | p < 0.01 |
| Sometimes = 0 (0%) | Sometimes= 20 (52.6%) | Sometimes=14 (35.0%) | ||
| No= 29 (100%) | No = 5 (13.2%) | No = 12 (30.0%) | ||
| 9. Do you have trouble with reading? | Yes = 0 (0%) | Yes = 13 (34.2%) | Yes = 13 (32.5%) | p < 0.01 |
| Sometimes = 0 (0%) | Sometimes =18 (47.4%) | Sometimes =14 (35.0%) | ||
| No = 29 (100%) | No = 7 (18.4%) | No = 13 (32.5%) | ||
| 10. Do you have trouble with writing? | Yes = 0 (0%) | Yes = 11 (28.9%) | Yes = 13 (32.5%) | p < 0.01 |
| Sometimes = 2 (6.9%) | Sometimes =15 (39.5%) | Sometimes =14 (35.0%) | ||
| No = 27 (93.1%) | No = 12 (31.6%) | No = 13 (32.5%) |
Table 3 shows that patients with BPPV and MD significantly declined their cognitive abilities in the questions regarding quick thinking and decision-making (i.e., questions 1 and 2). Over 55% of patients with BPPV and over 50% of patients with MD reported having a problem, and roughly 20%–30% of these patients responded to having the problem “sometimes”. Similarly, over 55% of patients with BPPV and over 50% of patients with MD were often easily distracted and had trouble concentrating due to their problems (questions 3 and 4). In comparison, about 20–30% of the patients only occasionally experienced difficulties. However, the normal group displayed no issues in these cognitive domains. Nearly 45–50 % of patients with BPPV and MD also struggled with memory. They had trouble keeping up with conversations, remembering the appropriate words to use during a conversation, and understanding others (questions 5, 6 and 7). More than 90% of the patients in the normal group said they had no problems in this domain. Over 50% of patients with BPPV had occasionally encountered speech-related issues, and about 34% of patients had speech-related difficulties frequently. MD patients—approximately 35 percent—suffered from speech-related problems either frequently or occasionally. In contrast to BPPV and MD patients, none of the subjects in the normal group displayed any speech-related issues (question 8).
In contrast, only 13 percent of BPPV patients and 30 percent of MD patients had no speech-related difficulties. Patients with BPPV and MD occasionally reported reading and writing problems, with rates of 47 percent and 35 percent for reading and 39 percent and 35 percent for writing, respectively (question 9 and 10). However, fewer cognitive issues were overall seen in the reading and writing domain, and more participants chose to answer “No” to the questions than did the other subjects. Thus, the results show that cognitively relevant tasks like memory and concentration were more adversely impacted than other domains in Group II and Group III.
Item analysis was conducted to evaluate the effectiveness of the test's individual items (questions) and the test. All 10 questions had a good internal consistency and high item correlation as the Cronbach's alpha value (α) was around 0.7 to 0.8 (Ursachi et al., 2015) as shown in Table 4.
Table 4.
Item analysis of the cognitive-based questions.
| Questions | Cronbach's alpha value (α) |
|---|---|
| 1. Does it seem that you can't think as quickly as before (i.e. before the diagnosis)? | 0.74 |
| 2. Does it seem that you find it hard to think clearly? | 0.81 |
| 3. Does it seem that you are easily more distracted? | 0.82 |
| 4. Does it seem that you can't concentrate? | 0.71 |
| 5. Do you have trouble remembering the right words when talking? | 0.84 |
| 6. Do you have trouble understanding others? | 0.85 |
| 7. Do you have trouble following conversations? | 0.83 |
| 8. Do you have trouble with your speech? | 0.85 |
| 9. Do you have trouble with reading? | 0.82 |
| 10. Do you have trouble with writing? | 0.73 |
From Table 5, it can be inferred that positive history of diabetes had no significant effect (p > 0.05) on the cognitive scores for all three groups. Similarly, there was no significant effect (p > 0.05) on cognitive scores for subjects with a history of hearing loss for Group II and Group III. Group I had no subjects with a history of hearing loss. For subjects with a history of hypertension (BP) in Group II, a significant effect was observed (p = 0.02), i.e. subjects with a positive history of BP had significantly poorer cognitive abilities. However, no significant effect of any associated conditions was observed in the subjects in Groups I and III.
Table 5.
Mann-Whitney U test for subjects with Diabetes, BP, and Hearing loss within groups.
| Diabetes |
BP |
Hearing Loss |
||||
|---|---|---|---|---|---|---|
| |Z| | p value | |Z| | p value | |Z| | p value | |
| Group I | 0.99 | 0.41 | 1.07 | 0.38 | – | – |
| Group II | 1.26 | 0.20 | 2.25 | 0.02 | 0.38 | 0.71 |
| Group III | 1.07 | 0.56 | 1.14 | 0.26 | 0.76 | 0.46 |
3.1. Comparison of cognitive abilities (overall score) vs non-specific associated condition (overall associated scores)
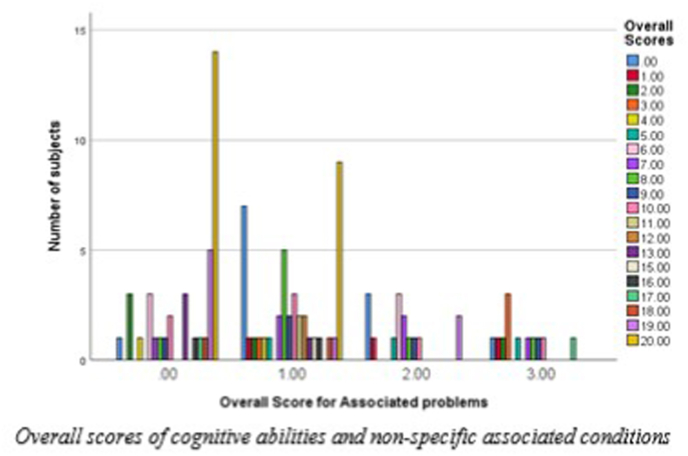
To analyze whether the presence or absence of any of the associated conditions (hearing loss, diabetes, hypertension) influenced the results, a score of ‘0′, ‘1′, ‘2′or ‘3′ was assigned when a subject had no history of any of the associated condition, had any one of the associated condition, had any two of the associated condition or had all of the three associated condition, respectively and ‘overall associated score’ was obtained for each subject. The ‘overall associated score’ was compared to the cognitive scores of the subjects, as depicted in Fig. 6. From Fig. 6, it can be inferred that although cognitive problems were widely spread across the subjects in the 3 groups, the problem was more in subjects having more associated conditions. Chi-square test results showed a significant association (p = 0.04) between the cognitive problems and associated conditions, i.e. subjects with all three associated conditions (diabetes, high blood pressure, and hearing loss) coupled with a diagnosis of BPPV or MD exhibited more cognitive problems than subjects in the same group who only had one or two associated conditions. Similarly, subjects with 2 associated conditions exhibited more cognitive problems than subjects with 1 or fewer associated conditions. The last group of subjects exhibited more cognitive problems than those with no associated conditions, even if only one condition existed. Therefore, the presence of a cognitive issue increases with comorbidity.
Fig. 6.
Overall scores of cognitive abilities and non-specific associated conditions.
4. Discussion
In recent years, substantial evidence has suggested a link between loss of vestibular function and cognitive issues. The current study reveals interesting finding with respect to vestibular problems and cognitive symptoms. The results showed significant differences in cognitive abilities between subjects with vertigo and subjects without vertigo. These results could hold immense clinical importance in better assessment and management.
4.1. BPPV and Cognition
The study's first objective was to determine if there is any cognitive-related problem in patients with BPPV. The BPPV group was compared to the normal group after administering the questionnaire. The results revealed a significant difference in cognitive scores between the BPPV and the normal groups. The BPPV group gave more “Yes” responses to the items pertaining to cognition, indicating that their disorder was causing them cognitive difficulties as compared to normal.
The results of the present study corroborate those of several studies on BPPV and cognitive issues. Kim et al. (2021) used a population cohort to evaluate the risk of dementia in patients with BPPV. The BPPV group had a greater risk of dementia than the matching control group. Male sex, high income, living in a rural area, having a history of hypertension, diabetes, or dyslipidaemia, as well as not having a history of ischemic heart disease, stroke, Meniere's disease, or head trauma, were all linked to an increased risk of dementia among BPPV patients. However, most of those factors were not considered in the current study. BPPV could be the first sign of degenerative alterations to the neurological system, and the possible explanation could be the otoconial separation resulting from macular and vestibular degeneration. Investigations have shown that BPPV patients have saccular macula neuronal degeneration (Yang et al., 2008). Patients with BPPV have been observed to have saccular ganglion cell loss and loss of ganglion cells in the superior or inferior vestibular nerve, affecting roughly 50% and 30% of the temporal bone, respectively (Gacek, 2003). In dementia patients, neuronal degeneration is known to impact the phylogenetically older neurons (Nandi and Luxon, 2009). Because the vestibular system is the phylogenetically first sensory system (Lyness et al., 2003), its degradation might be a prodromal or early stage of dementia. BPPV tends to be identified earlier than full-blown dementia because the symptoms of spinning-type vertigo are more noticeable than those linked to cognitive impairment. Thus, the current study also showed that BPPV was associated with an increased risk of cognitive issues and could be due to similar physiological reasons. The fear and anxiety that is associated with massive attacks of vertigo could also be a cause for distractibility and lack of concentration, however further evidences are required to arrive at a conclusion.
4.2. Meniere's disease and Cognition
A similar trend was observed for the study's second objective, where patients diagnosed with MD were compared with the normal group. A significant difference was observed between them, i.e., compared to the normal group, the MD group responded to questions about cognition with more “Yes” responses, indicating that their disorder was causing them to have problems with their cognitive abilities.
Chari et al. (2021) examined Dizziness Handicap Inventory (DHI) Performance and Subjective Cognitive Symptoms in individuals with Meniere's Disease and vestibular migraine. They concluded that patients with vestibular disorders like MD had a significant prevalence of cognitive impairment. Using a statewide cohort sample of data from the South Korean National Health Insurance Service, another study looked into the relationship between MD and the risk of dementia (Lee et al., 2022). The findings showed that people with Meniere's disease experienced higher rates of vascular dementia, Alzheimer's disease, and all-cause dementia than those in the comparison group. The hippocampus is well known for being involved in memory, learning, and emotion. As a result, the hippocampus has been the subject of investigations on neurodegenerative illnesses. According to a study by Brandt et al. (2005b), patients with bilateral vestibular loss had a considerably atrophying hippocampus compared to controls (16 percent decreased relative to controls). According to other research, the hippocampus volume of patients with Meniere's disease was considerably lower than that of controls (Van Cruijsen et al., 2009; Seo et al., 2016). The hippocampus is an area in the brain responsible for learning and memory; any structural or functional damage to it could be a reason for cognitive deficits in MD.
Additionally, Meniere's disease causes mental stress due to limits in everyday activities brought on by chronic dizziness and associated ear problems. Atrophic alterations in the hippocampus could come from the stress's activation of the hypothalamus-pituitary-adrenal axis, which increases cortisol output (Lupien et al., 1998; Dickerson et al., 2004). In patients with psychotic illnesses, increased emotional stress is also linked to a lower hippocampus volume (Collip et al., 2013). It suggests that the later onset of Meniere's disease may be connected to the emergence of dementia-related symptoms.
Patients from Meniere's disease also suffer from a condition called brain fog, a form of fluctuating cognitive impairment that many people with vestibular problems experience. The brain needs to work significantly more challenging to maintain equilibrium when the vestibular system is damaged, and this continuous effort has an adverse effect on cognitive performance. As a result, there could be a clouding of consciousness and a loss of mental energy, which can impede executive function, memory, recall, decision-making, and recall of words. Many patients struggle to remember people's names and the precise things they need to perform. As stated by Glenn Schweitzer (2020) in a case study, he reported difficulty in speech as words tend to slip off the tongue, and he lost track of why he entered rooms when Meniere's disease symptoms worsened, and brain fog made an appearance. Brain fog can severely interfere with the capacity to be productive in daily life, even if individuals aren't actively experiencing balance issues like vertigo or dizziness.
4.3. Comparison between BPPV and Meniere's disease and Cognitive abilities
The third objective of the study was to compare the cognitive abilities between BPPV and MD. The findings from the study revealed that the two clinical groups had a problem with cognitive abilities compared to the normal group. However, there was no significant difference between these two clinical groups regarding cognitive abilities. Using the Neuropsychological Vertigo Inventory (NVI), Liu et al. (2019) researched the quantification of cognitive dysfunction in patients with BPPV, MD and vestibular migraine (VM). Despite being substantially younger than BPPV patients, the study discovered that VM patients had significantly more cognitive issues.
Contrary to the current study's findings, MD patients also had higher cognitive impairment than BPPV patients. VM and MD did not significantly differ from one another, demonstrating that even peripheral vestibular disorders like MD can result in cognitive dysfunction comparable to central vestibular disorders like VM, which could be due to MD causing a reduction in hippocampus volume (Van Cruijsen et al., 2009; Seo et al., 2016). The results may have been influenced by the fact that MD and VM had lower mean ages than BPPV. Because younger patients could have greater demands in everyday life, such as those related to childcare and work performance, they may be more affected by cognitive impairment than older patients. Given that age is an essential factor in determining cognitive function (Harada et al., 2013) thus, in the current study, the mean ages of the two groups were almost similar and older compared to the study by Liu et al. (2019). Hence, similar cognitive scores were obtained for the two groups.
4.4. Cognitive problems in patients with BPPV and MD based on the questionnaire
Table 4 shows that patients with BPPV and MD significantly declined their cognitive abilities. Questions related to thinking skills, memory and distractibility were maximally affected. In BPPV, it could be due to the neural degeneration in the ganglion cells due to otoconia loss, as stated by Yang et al. (2008), which may affect the input travelling to the cerebral cortex for higher cognitive tasks like thinking, reasoning and memory. Research shows that otolithic functioning and cognition could have a link. Few studies have discovered a connection between saccular function and cognition (Bigelow and Agrawal, 2015; Xie et al., 2017; Wei et al., 2018).
Contrary to Bigelow and colleagues' research, Dobbels et al. (2019) study did not find a statistically significant link between saccular function and cognition. The variations in the study methodologies for the two studies could mitigate this. A dearth of literature discusses the saccule's role in cognitive function (Dobbels et al., 2019). In MD, cognitive functioning may be affected because of Brain fog. Due to this condition, executive function, memory, recall, decision-making, and verbal recall can all be affected by a possible clouding of consciousness and a lack of mental energy, which is also seen in the current study.
Studies have also shown that MD leads to hippocampal loss. The hippocampus is a crucial component of the limbic system of the brain. Learning, empathetic reactions, memory development, and storage are all significantly influenced by hippocampal function. Trouble in following conversation and speech were seen as less affected, and reading and writing problems were least affected in patients with BPPV and MD. The part responsible for speech in the brain is the frontal lobe (Broca's area). There is a lack of evidence that vestibular problem damages this part of the brain. It is also less likely to be affected as no significant vestibular network is innervating this brain area. The occasional trouble in speech and conversation could be due to the condition of Brain fog which leads to difficulty in word recall and cause words to get stuck in the mouth, as Glenn Schweitzer (2020), in MD patients. For BPPV patients, problems in speech and conversation could be due to neural degeneration due to otoconial loss, which may affect the firing rate of the nerve. The patients could miss out on input messages from other speakers. However, further investigation is required to come to a precise conclusion.
There are reports that vestibular problems could lead to dyscalculia. In 1990, Risey and Briner identified a connection between central vertigo and dyscalculia. Dyscalculia has been linked to thalamic lesions (Basso1 et al., 1987). Furthermore, it has been well-established that there exist numerous connections between the thalamus and the vestibular, auditory, and association cortices. (Streitfeld, 1980; Luethke et al., 1988). It is conceivable that an anatomical or physiological abnormality at the level of the thalamus or, more likely, the temporoparietal cortex is the cause of dyscalculia observed in individuals with central vertigo.
Furthermore, vestibular system disorders have been linked to learning disabilities in children (Bundy et al., 1987). The current study suggests that similar factors might contribute to reading and writing difficulties observed in the patients. However, it's essential to note that this study focused on an adult population, and further research is needed to draw definitive conclusions. To gain more insights, future investigations should explore the cognitive domains in the questionnaire, specifically related to memory, thinking, concentration, speech, and reading and writing abilities. This examination will shed light on whether individuals with BPPV or MD also experience cognitive challenges in these areas.
4.5. Influence of associated conditions on cognition
In the current study, several subjects in the 3 groups of clinically normal, BPPV and MD, with associated conditions of diabetes, hypertension (BP) or hearing loss, as depicted in Fig. 3, Fig. 4. The present research attempted to check whether any of these conditions affected cognitive abilities as there was evidence that diabetes, BP or hearing loss significantly affected cognitive performance.
It is clear from the current study that having a history of diabetes did not affect the cognitive scores for any of the three groups. There was no discernible impact on cognitive performance for patients with BPPV and MD with a history of hearing loss. A substantial effect was seen in BPPV patients with a history of hypertension (BP), i.e., subjects with a positive history of BP had a significantly worse cognitive performance. However, none of the accompanying conditions had a noticeable impact on the patients with MD.
Studies reported that individuals with diabetes might also suffer a cognitive decline over time (Gispen and Biessels, 2000; Biessels et al., 2008; Kumar et al., 2009; Ruis et al., 2009; Creavin et al., 2012). However, in the current study, the influence of diabetes on cognitive abilities was found to be insignificant in the groups. Studies also show that persons with hypertension may eventually decline their cognitive function (Skoog, 2003; Birns and Kalra, 2008; Knecht et al., 2009; Hajjar et al., 2011; Power et al., 2013). Similar findings have been evident in the current study, where subjects diagnosed with BPPV and associated conditions of BP reported significantly more cognitive problems than those without BP. However, a significant difference was not observed for the group with MD. This could be due to the difference in the severity and duration of the associated condition of BP, which was not accounted for in this study. Further analysis could be carried out to check for the association of BP in BPPV and MD with respect to the duration and severity of the problem.
A dearth of evidence explains how ailments like diabetes and high blood pressure are linked to cognitive issues in vestibular disorders.
There is ample evidence that hearing loss leads to dementia and other cognitive problems (Granick et al., 1976; Lin, 2011; Peracino, 2014; Dawes et al., 2015; Lawrence et al., 2018). Thus, in the current study, a within-group comparison was conducted to check whether hearing loss affected the cognitive problem. The results revealed no significant difference, i.e. hearing abilities had no influential role in patients who had BPPV and MD and were suffering from cognitive-related issues. Hence the cognitive problem in the subjects with BPPV and MD could be due to the vestibular problem or a combination of both. This result supports various studies where similar findings were reported. In an animal study conducted by Smith and Zheng (2013), the middle ear structures (tympanic membrane and the ossicles) were removed to partially achieve auditory control (partial sound transmission to the cochlea). They found that animals without vestibular lesions but with the tympanic membrane removed outperform animals with vestibular abnormalities in cognitive activities. Another supporting study claims that spatial memory issues in animals with BVD are not primarily caused by hearing loss and could be due to vestibular deficits (Brandt et al., 2005a).
Additionally, animal research has demonstrated that lesions to the auditory and vestibular systems have distinct effects on learning and memory (Schaeppi et al., 1991). Thus, from the current study, it can be concluded that hearing loss may not be influential in developing cognitive problems in patients with vestibular deficits. However, to arrive at a precise conclusion, further analysis needs to be conducted considering the type, degree, duration and mode of rehabilitation (HA, CI), etc. And compare the vestibular and hearing problem.
An attempt was also made to check whether more comorbid conditions influenced cognitive difficulties in the current study. Fig. 5 demonstrated a significant relationship between cognitive problems and associated conditions. The subjects with all three associated conditions (diabetes, high blood pressure, and hearing loss) and a diagnosis of BPPV or MD exhibited more cognitive problems than subjects in the same group who only had one or two associated conditions.
Studies regarding diabetes, hypertension and hearing loss is linked to cognitive abilities independently have been well established (Biessels et al., 2008; Kumar et al., 2009; Ruis et al., 2009; Birns and Kalra, 2008; Knecht et al., 2009; Hajjar et al., 2011; Power et al., 2013; Lin, 2011; Peracino, 2014; Dawes et al., 2015; Lawrence et al., 2018). Studies regarding cognitive impairment and comorbidities have been conducted. These studies reveal that people with cognitive impairment have more serious medical comorbidities than those without cognitive impairment (Doraiswamy et al., 2002; Lyketsos et al., 2005; Rise et al., 2016). Thus the progression of the cognitive problems may be impacted by the comorbidity in the current study. It may be possible to enhance cognition with the optimal management of medical comorbidities. The role of medical comorbidity in vestibular disorders and the development or evolution of the cognitive disorder, as well as the mechanisms behind its neuropathologic consequences, could be the subject of future research.
4.6. Clinical implications
The current study's findings suggest that patients with BPPV and MD are likely to have cognitive issues as both the groups' cognitive abilities are significantly lower than the normal groups.
The results also suggest that every patient with BPPV or MD should at least be screened for their cognitive abilities, if a complete diagnostic test battery is not possible.
The outcome of the current study highlights that patient with BPPV and MD might require training to improve their cognitive abilities and counselling to deal with their problems effectively. Cognitive behavioral therapy (CBT) along with Vestibular Rehabilitation Therapy (VRT) could be provided for holistic management and better quality of life for these patients. Thus, the study also emphasizes the importance of a team approach in managing vestibular disorders.
The study's results also add information to the existing literature regarding the association of cognitive problems in patients with vestibular disorders.
4.7. Limitations of the study
Purposive, non-random sampling was conducted in the study, subjected to researcher and sampling biases. Amidst the pandemic, performance-based cognitive tests couldn't be done. The data collection was done online, which might have affected the responses. The influence of tinnitus on Meniere's disease was not studied in the current research. The vestibular disorder's severity and duration and it's effect on cognitive abilities were not considered. Duration and severity of the comorbid factors were not considered. The influence of management of HL (untreated or treated) was not considered in the study. The study did not consider the impact of factors like age, gender, socio-economic status, or education level. Due to these limitations, further research is still necessary to validate and corroborate the conclusion of the current study.
4.8. Future research
The study can be done on a larger population. Research on different vestibular disorders and how they relate to cognitive issues could be explored. Along with questionnaires, performance-based tests related to cognition could be done along with the questionnaire. The vestibular disorder's severity and effect on cognitive abilities can be studied. Duration and severity of the comorbid factors and their association with cognitive problems could be studied. Factors like age, gender, socio-economic status and education level and their influence on the cognitive abilities of patients with vestibular disorders can be studied.
Ethics approval and consent to participate
Informed consent was obtained from all participants involved in the study. Participants were provided with detailed information about the study's purpose, procedures, and potential risks, and they voluntarily agreed to participate by signing a consent form. Confidentiality and anonymity measures were implemented to protect the privacy of the participants, ensuring that their identities are not disclosed.
Availability of data and material
The datasets and materials used in this study are available upon request.
Authors' contributions
All the authors have significant contribution and participation in this research.
Funding
There was no source of any external funding for the research.
Declaration of competing interest
The authors declare no competing interests that could potentially bias the research or create conflicts of interest.
Acknowledgements
We would like to express our gratitude to the HoD Audiology for their valuable contributions and support throughout this research. We also acknowledge Director of All India Institute of Speech & Hearing for providing access to necessary resources that facilitated this study.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joto.2023.11.001.
List of abbreviations
- BPPV
Benign Paroxysmal Positional Vertigo
- MD
Meniere's disease
- SD
Standard deviation
- CBT
Cognitive behavioral therapy
- VRT
Vestibular rehabilitation therapy
- NCSE
Neurobehavioral Cognitive Status Examinination
- BP
Blood Pressure
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- Basso1, A., Sala, S. Della, & Farabola, M. (n.d.). APHASIA ARISING FROM PURELY DEEP LESIONS. [DOI] [PubMed]
- Biessels G.J., Deary I.J., Ryan C.M. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- Bigelow R.T., Agrawal Y. Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J. Vestib. Res. : Equilibrium & Orientation. 2015;25(2):73–89. doi: 10.3233/VES-150544. [DOI] [PubMed] [Google Scholar]
- Birns J., Kalra L. Cognitive function and hypertension. J. Hum. Hypertens. 2008;23(2):86–96. doi: 10.1038/jhh.2008.80. 2009 23:2. [DOI] [PubMed] [Google Scholar]
- Brandt T., Schautzer F., Hamilton D.A., Brüning R., Markowitsch H.J., Kalla R., Darlington C., Smith P., Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain : J. Neurol. 2005;128(Pt 11):2732–2741. doi: 10.1093/BRAIN/AWH617. [DOI] [PubMed] [Google Scholar]
- Brandt T., Schautzer F., Hamilton D.A., Brüning R., Markowitsch H.J., Kalla R., Darlington C., Smith P., Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128(11):2732–2741. doi: 10.1093/BRAIN/AWH617. [DOI] [PubMed] [Google Scholar]
- Bundy A.C., Fisher A.G., Freeman M., Lieberg G.K., Izraelevitz T.E. Concurrent validity of equilibrium tests in boys with learning disabilities with and without vestibular dysfunction. Am. J. Occup. Ther. : Official Publication of the American Occupational Therapy Association. 1987;41(1):28–34. doi: 10.5014/AJOT.41.1.28. [DOI] [PubMed] [Google Scholar]
- Chari D.A., Liu Y.H., Chung J.J., Rauch S.D. Subjective cognitive symptoms and dizziness handicap inventory (DHI) performance in patients with vestibular migraine and menière’s disease. Otol. Neurotol. : Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2021;42(6):883–889. doi: 10.1097/MAO.0000000000003081. [DOI] [PubMed] [Google Scholar]
- Collip D., Habets P., Marcelis M., Gronenschild E., Lataster T., Lardinois M., Nicolson N.A., Myin-Germeys I. Hippocampal volume as marker of daily life stress sensitivity in psychosis. Psychol. Med. 2013;43(7):1377–1387. doi: 10.1017/S003329171200219X. [DOI] [PubMed] [Google Scholar]
- Creavin S.T., Gallacher J., Bayer A., Fish M., Ebrahim S., Ben-Shlomo Y. Metabolic syndrome, diabetes, poor cognition, and dementia in the caerphilly prospective study. J. Alzheim. Dis. 2012;28(4):931–939. doi: 10.3233/JAD-2011-111550. [DOI] [PubMed] [Google Scholar]
- Dawes P., Emsley R., Cruickshanks K.J., Moore D.R., Fortnum H., Edmondson-Jones M., McCormack A., Munro K.J. Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS One. 2015;10(3) doi: 10.1371/JOURNAL.PONE.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Gruenewald T.L., Kemeny M.E. When the social self is threatened: shame, physiology, and health. J. Pers. 2004;72(6):1191–1216. doi: 10.1111/J.1467-6494.2004.00295.X. [DOI] [PubMed] [Google Scholar]
- Dobbels B., Mertens G., Gilles A., Claes A., Moyaert J., Van De Berg R., Van De Heyning P., Vanderveken O., Van Rompaey V. Cognitive function in acquired bilateral vestibulopathy: a cross-sectional study on cognition, hearing, and vestibular loss. Front. Neurosci. 2019;13 doi: 10.3389/FNINS.2019.00340. APR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy P.M., Leon J., Cummings J.L., Marin D., Neumann P.J. Prevalence and impact of medical comorbidity in alzheimer's disease. J. Gerontol.: Series A. 2002;57(3):M173–M177. doi: 10.1093/GERONA/57.3.M173. [DOI] [PubMed] [Google Scholar]
- Gacek R.R. Pathology of benign paroxysmal positional vertigo revisited. Ann. Otol. Rhinol. Laryngol. 2003;112(7):574–582. doi: 10.1177/000348940311200702. [DOI] [PubMed] [Google Scholar]
- Gispen W.H., Biessels G.J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23(11):542–549. doi: 10.1016/S0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Granick S., Kleban M.H., Weiss A.D. Relationships between hearing loss and cognition in normally hearing aged persons. J. Gerontol. 1976;31(4):434–440. doi: 10.1093/GERONJ/31.4.434. [DOI] [PubMed] [Google Scholar]
- Hajjar I., Quach L., Yang F., Chaves P.H.M., Newman A.B., Mukamal K., Longstreth W., Inzitari M., Lipsitz L.A. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation. 2011;123(8):858–865. doi: 10.1161/CIRCULATIONAHA.110.978114/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013;29(4):737–752. doi: 10.1016/J.CGER.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Yoo D.M., Min C., Choi H.G. Increased risk of neurodegenerative dementia after benign paroxysmal positional vertigo. Int. J. Environ. Res. Publ. Health. 2021;18(19) doi: 10.3390/IJERPH181910553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S., Wersching H., Lohmann H., Berger K., Ringelstein E.B. How much does hypertension affect cognition?: explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J. Neurol. Sci. 2009;283(1–2):149–152. doi: 10.1016/J.JNS.2009.02.362. [DOI] [PubMed] [Google Scholar]
- Kumar R., Looi J.C.L., Raphael B. Type 2 diabetes mellitus, cognition and brain in aging: a brief review. Indian J. Psychiatr. 2009;51(1) S35./pmc/articles/PMC3038537/ [PMC free article] [PubMed] [Google Scholar]
- Lawrence B.J., Jayakody D.M.P., Henshaw H., Ferguson M.A., Eikelboom R.H., Loftus A.M., Friedland P.L. Auditory and cognitive training for cognition in adults with hearing loss: a systematic review and meta-analysis. Trends in Hearing. 2018;22 doi: 10.1177/2331216518792096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.H., Yu H., Ha S.S., Son G.M., Park K.J., Lee J.J., Kim D.K. Association between late-onset ménière’s disease and the risk of incident all-cause dementia. J. Personalized Med. 2022;12(1):19. doi: 10.3390/JPM12010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.R. Hearing loss and cognition among older adults in the United States. J. Gerontol.: Series A. 2011;66A(10):1131–1136. doi: 10.1093/GERONA/GLR115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.F., Locklear T.D., Sharon J.D., Lacroix E., Nguyen S.A., Rizk H.G. Quantification of cognitive dysfunction in dizzy patients using the neuropsychological vertigo inventory. Otol. Neurotol. 2019;40(7):E723–E731. doi: 10.1097/MAO.0000000000002311. [DOI] [PubMed] [Google Scholar]
- Luethke L.E., Krubitzer L.A., Kaas J.H. Cortical connections of electrophysiologically and architectonically defined subdivisions of auditory cortex in squirrels. J. Comp. Neurol. 1988;268(2):181–203. doi: 10.1002/CNE.902680205. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., De Leon M., De Santi S., Convit A., Tarshish C., Nair N.P.V., Thakur M., McEwen B.S., Hauger R.L., Meaney M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1(1):69–73. doi: 10.1038/271. 1998 1:1. [DOI] [PubMed] [Google Scholar]
- Lyketsos C.G., Toone L., Tschanz J.A., Rabins P.V., Steinberg M., Onyike C.U., Corcoran C., Norton M., Zandi P., Breitner J.C.S., Welsh-Bohmer K. Population-based study of medical comorbidity in early dementia and “cognitive impairment, No dementia (CIND)”: association with functional and cognitive impairment: the cache county study. Am. J. Geriatr. Psychiatr. 2005;13(8):656–664. doi: 10.1097/00019442-200508000-00004. [DOI] [PubMed] [Google Scholar]
- Lyness S.A., Zarow C., Chui H.C. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: a meta-analysis. Neurobiol. Aging. 2003;24(1):1–23. doi: 10.1016/S0197-4580(02)00057-X. [DOI] [PubMed] [Google Scholar]
- Nandi R., Luxon L.M. Development and assessment of the vestibular system. 2009;47(9):566–577. doi: 10.1080/14992020802324540. [DOI] [PubMed] [Google Scholar]
- Peracino A. Hearing loss and dementia in the aging population. Audiol. Neuro. Otol. 2014;19(1):6–9. doi: 10.1159/000371595. [DOI] [PubMed] [Google Scholar]
- Power M.C., Tchetgen E.J.T., Sparrow D., Schwartz J., Weisskopf M.G. Blood pressure and cognition:Factors that may account for theirinconsistent association. Epidemiology. 2013;24(6):886–893. doi: 10.1097/EDE.0B013E3182A7121C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rise I.V., Haro J.M., Gjervan B. Clinical features, comorbidity, and cognitive impairment in elderly bipolar patients. Neuropsychiatric Dis. Treat. 2016;12:1203. doi: 10.2147/NDT.S100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis C., Biessels G.J., Gorter K.J., Van Den Donk M., Kappelle L.J., Rutten G.E.H.M. Cognition in the early stage of type 2 diabetes. Diabetes Care. 2009;32(7):1261–1265. doi: 10.2337/DC08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeppi U., Krinke G., FitzGerald R.E., Classen W. Impaired tunnel-maze behavior in rats with sensory lesions: vestibular and auditory systems. Neurotoxicology. 1991;12(3):445–454. https://europepmc.org/article/med/1745435 [PubMed] [Google Scholar]
- Seo Y.J., Kim J., Kim S.H. The change of hippocampal volume and its relevance with inner ear function in Meniere's disease patients. Auris Nasus Larynx. 2016;43(6):620–625. doi: 10.1016/J.ANL.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Skoog I. Hypertension and cognition. Int. Psychogeriatr. 2003;15(S1):139–146. doi: 10.1017/S1041610203009104. [DOI] [PubMed] [Google Scholar]
- Smith P.F., Zheng Y. From ear to uncertainty: vestibular contributions to cognitive function. Front. Integr. Neurosci. 2013;7(NOV):84. doi: 10.3389/FNINT.2013.00084/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitfeld B.D. The fiber connections of the temporal lobe with emphasis on the rhesus monkey. Int. J. Neurosci. 1980;11(1):51–71. doi: 10.3109/00207458009147579. [DOI] [PubMed] [Google Scholar]
- Van Cruijsen N., Hiemstra W.M., Meiners L.C., Wit H.P., Albers F.W.J. Hippocampal volume measurement in patients with Ménière’s disease: a pilot study. 2009;127(10):1018–1023. doi: 10.1080/00016480601127000. [DOI] [PubMed] [Google Scholar]
- Wei E.X., Oh E.S., Harun A., Ehrenburg M., Agrawal Y. Vestibular loss predicts poorer spatial cognition in patients with alzheimer's disease. J. Alzheim. Dis. 2018;61(3):995–1003. doi: 10.3233/JAD-170751. [DOI] [PubMed] [Google Scholar]
- Xie Y., Bigelow R.T., Frankenthaler S.F., Studenski S.A., Moffat S.D., Agrawal Y. Vestibular loss in older adults is associated with impaired spatial navigation: data from the triangle completion task. Front. Neurol. 2017;8(APR):173. doi: 10.3389/FNEUR.2017.00173/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.S., Kim S.H., Lee J.D., Lee W.S. Clinical significance of vestibular evoked myogenic potentials in benign paroxysmal positional vertigo. Otol. Neurotol. 2008;29(8):1162–1166. doi: 10.1097/MAO.0B013E31818A0881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and materials used in this study are available upon request.