Abstract
Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT) were found to inhibit intracellular antigen processing. Processing was not inhibited by mutant LT with attenuated ADP-ribosyltransferase activity, CT B or LT B subunit, which enhanced presentation of preexisting cell surface peptide-class II major histocompatibility complex complexes. Inhibition of antigen processing correlated with A subunit ADP-ribosyltransferase activity.
Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT) are related ADP-ribosylating toxins with five identical B subunits that bind to cell surface ganglioside receptors and an enzymatically active A subunit that enters the cell and catalyzes the ADP-ribosylation of guanine nucleotide binding proteins of the adenylate cyclase complex, causing constitutive activation of adenylate cyclase and increased intracellular cyclic AMP (cAMP).
LT and CT are potent mucosal adjuvants (7, 8, 12, 20, 22, 23, 29, 31–33). Some degree of A subunit enzymatic activity is required for oral adjuvant function (20, 23, 32, 33). While ADP-ribosyltransferase activity enhances adjuvanticity, it also confers toxicity. For an optimal adjuvant, reduced toxicity would be desirable, and mutant LT (6, 9–11, 15, 17, 21, 26, 34) and CT (5, 35) molecules have been constructed with altered A subunits, reduced ADP ribosylation activity, and reduced toxicity, yet with maintained adjuvant function (9–11, 13, 25, 26, 35). Mutation studies with LT revealed that residues at positions 7, 110, and 112 of LT A subunit (LTA) are important for ADP-ribosyltransferase activity (6, 21, 28), with Glu-112 providing a catalytic role. A conservative mutation (Asp to Glu) at position 112 produced a mutant toxin, rLT-E112D, with substantially reduced (<2% of wild type) but detectable ADP-ribosyltransferase activity (6).
CT and LT affect many components of immune responses, including antigen presentation (3, 4, 18), with inhibitory as well as enhancing effects. We previously showed that CT enhances macrophage presentation of cell surface peptide-class II major histocompatibility complex (MHC-II) complexes to T cells but inhibits intracellular antigen processing (24). However, the effects of LT have not been similarly investigated. Furthermore, mutant LT molecules provide tools to determine the role of A subunit enzymatic activity in immunomodulation and toxicity.
The present study was designed to investigate the effects of LT and mutant LT molecules on antigen processing and presentation by macrophages. In particular, we examined the effects of LT on the processing and presentation of a model antigen expressed in bacteria (a system to which LT has natural relevance) by using Escherichia coli strain HB101 expressing the Crl-HEL fusion protein (HB101.Crl-HEL) (27), which contains the HEL(48-61) epitope. LT, the mutant toxin rLT-E112D, and recombinant LTB (rLTB) (Table 1) were prepared as described previously (6, 15). rLTB was produced by using a vector encoding LTB and the A2 fragment of LT (LTA2), but subsequent chromatographic purification produced isolated rLTB, as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. Trypsin-cleaved LT was produced as described previously (15) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Highly purified CT was purchased from List Biologicals (Campbell, Calif.). Recombinant CTB (rCTB) was a gift from Jan Holmgren (University of Gøteborg, Gøteborg, Sweden) and was prepared as described previously (30).
TABLE 1.
Toxin composition and enzymatic activity
| Toxin | Composition | Enzymatic activitya |
|---|---|---|
| LT | E. coli heat-labile enterotoxin (holotoxin) | Wild-type LT |
| Trypsin-cleaved LT | Holotoxin with nicked, activated LTA | >Wild-type LT |
| rLT-E112D | LT holotoxin with mutated A subunit | <2% of wild-type LT (6) |
| rLTB | B subunit of LT | None |
| CT | Cholera toxin (holotoxin) | Wild-type CT |
| rCTB | B subunit of CT | None |
Enzymatic activity is ADP-ribosyltransferase activity.
LT inhibits macrophage processing of HB101.Crl-HEL but not presentation of preexisting peptide–MHC-II complexes.
To determine the impact of LT on antigen processing, activated Listeria-elicited macrophages were obtained from CBA/J mice (H-2k) (16), plated at 2 × 105 cells/well in 96-well microtiter plates, washed to remove nonadherent cells, and incubated overnight with LT. The cells were then washed, incubated with viable E. coli HB101.Crl-HEL for 2 h to allow antigen processing, fixed in 1% paraformaldehyde, washed, and then incubated with 3A9 T hybridoma cells, as previously described (24). LT inhibited the processing of HB101.Crl-HEL for presentation to 3A9 cells at doses of 1 to 10 μg of LT per ml (data not shown; see below). Although cleavage of LTA into the A1 and A2 fragments may be required for LT enzymatic activity (14), we observed that trypsin-cleaved LT and intact LT had similar effects on antigen processing (although trypsin cleavage slightly enhanced the magnitude of inhibition). LT may be cleaved by cell-derived proteases during uptake into cells, making prior in vitro cleavage unnecessary (19). Subsequent studies were done with uncleaved LT at 1 μg/ml.
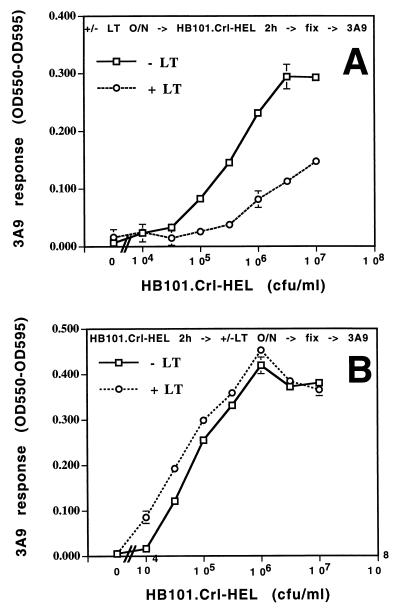
In order to assess the stage of antigen processing and presentation that was affected by LT, macrophages were sequentially exposed to LT and antigen in various orders. In the first protocol, macrophages were incubated with LT prior to incubation with viable HB101.Crl-HEL. In the second protocol, macrophages were first incubated with HB101.Crl-HEL to allow unaltered bacterial antigen processing, production of peptide–MHC-II complexes, and expression of these complexes on the cell surface. The macrophages were then washed and incubated with or without LT. The inhibitory effects observed when the antigen incubation followed LT exposure (Fig. 1A) were not observed when macrophages were first incubated with antigen and then exposed to LT (Fig. 1B). These results indicate that LT inhibited an intracellular stage of bacterial antigen processing, prior to expression of peptide–MHC-II complexes on the cell surface, since the presentation of complexes that were previously expressed on the cell surface was not altered by LT.
FIG. 1.
Overnight treatment of macrophages with LT inhibits intracellular processing of HB101.Crl-HEL but does not inhibit the presentation of preexisting surface peptide–MHC-II complexes. Macrophages were incubated with viable HB101.Crl-HEL for 2 h either after (A) or before (B) overnight treatment with LT (1 μg/ml). (A) Macrophages were treated with or without LT overnight, washed, incubated with HB101.Crl-HEL for 2 h at 37°C, fixed with 1% paraformaldehyde, and washed extensively. (B) Macrophages were incubated with HB101.Crl-HEL for 2 h at 37°C, washed, treated with or without LT overnight, fixed, and washed extensively. Antigen presentation was determined by incubation with HEL-specific 3A9 T hybridoma cells (105/well) for 20 to 24 h at 37°C, followed by a bioassay for interleukin 2 production (16). Interleukin 2-dependent CTLL-2 cells were incubated for 24 h at 37°C with supernatants collected from antigen presentation assays. The cells were then pulsed for 18 to 24 h with Alamar blue. Both reduced and oxidized forms of Alamar blue have high absorbance near 570 nm, whereas only the oxidized form has high absorbance near 600 nm. Production of the reduced form (a measure of cell growth and metabolic activity) can be measured by subtracting the optical density at 600 nm (OD600) from OD570 (2) or subtracting OD595 from OD550. All data points are presented as mean (OD550 − OD595) ± standard deviation for triplicate points.
In addition, two other observations suggest that the inhibitory mechanism involved changes in intracellular antigen processing, as opposed to changes in the overall expression of MHC-II molecules or the ability of T cells to recognize peptide–MHC-II complexes that were expressed by the macrophages. First, treatment of macrophages with LT did not alter the expression of I-Ak at the cell surface, as determined by flow cytometry (data not shown). In addition, when macrophages were first treated with LT and then incubated with HEL(48-61) peptide, which does not require intracellular processing, presentation to 3A9 cells was not inhibited (data not shown). Thus, LT inhibited an intracellular stage of antigen processing and (within this time frame) did not affect the MHC-II expression or the presentation of peptide–MHC-II complexes on the surface of the cell.
Inhibition of HB101.Crl-HEL processing by LT is not due to inhibition of antigen catabolism.
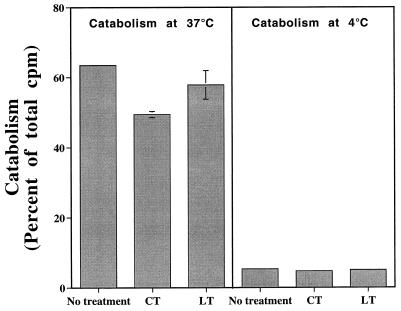
Additional studies assessed whether the inhibition of antigen processing by LT was due to a decrease in the ability of macrophages to internalize and catabolize bacteria and their antigens. Macrophages were incubated overnight with or without LT or CT. The ability of the macrophages to internalize and degrade 125I-labeled HB101.Crl-HEL was then assessed (Fig. 2). LT produced no consistent change in bacterial uptake and catabolism (minimal decreases were observed in some experiments), and CT produced only slight decreases in bacterial uptake and catabolism (Fig. 2). Thus, LT had little or no effect on bacterial uptake and catabolism, indicating that other aspects of the antigen-processing pathway were affected by LT.
FIG. 2.
LT and CT do not inhibit macrophage catabolism of HB101.Crl-HEL. Macrophages (2 × 106 cells/well in 24-well plates) were incubated overnight with or without LT or CT (1 μg/ml). 125I-labeled HB101.Crl-HEL was centrifuged onto the macrophages at 2,500 × g for 10 min at 4°C. The plates were then incubated at either 4°C (negative control) or 37°C for 20 min, washed to remove extracellular bacteria, and then incubated for 2 h at either 4 or 37°C to allow for processing and catabolism of intracellular bacteria. High-molecular-weight proteins were precipitated from both the media and cell lysates (cells solubilized in 1% Triton X-100 in phosphate-buffered saline) with 10% trichloroacetic acid at 4°C. Bacterial catabolism was reflected by trichloroacetic acid-soluble radioactivity in the medium, shown here as a mean percentage of the total counts per minute in the well plus or minus the standard deviation of duplicate samples.
LTA activity is necessary for inhibition of intracellular antigen processing, whereas LTB enhances the presentation of cell surface peptide–MHC-II complexes.
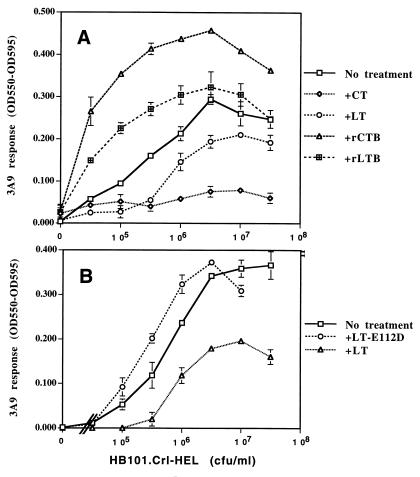
Macrophages that were treated overnight with rLTB or rCTB showed enhanced presentation of antigens that were subsequently added, in contrast to the inhibition seen with LT or CT holotoxins (Fig. 3A). rLTB produced less enhancement than rCTB. In addition, rLTB and rCTB enhanced the presentation of synthetic HEL(48-61) peptide (data not shown), indicating that the enhancement involved increased recognition of peptide–MHC-II complexes present at the cell surface, as opposed to increased intracellular processing. The mechanism for this is unclear, but it does not involve increased MHC-II expression, which remained unchanged as determined by flow cytometry analysis (data not shown), and it may be caused by changes in cell surface adhesion or costimulator molecules (1). We conclude that the A subunit of LT is required for inhibition of antigen processing but not for enhancement of surface complex presentation.
FIG. 3.
Ribosyltransferase activity of the A subunit is necessary for inhibition of antigen processing, whereas antigen presentation is enhanced by toxin preparations that lack A subunit enzymatic activity. Macrophages were treated overnight with or without the toxin preparations (1 μg/ml), washed, incubated with viable HB101.Crl-HEL for 2 h at 37°C, and fixed. Antigen presentation was determined by incubation with 3A9 T hybridoma cells for 20 to 24 h at 37°C.
ADP-ribosyltransferase activity is necessary for the inhibition of antigen processing mediated by LT.
rLT-E112D, an LT holotoxin containing a point mutation in LTA, was previously shown to have <2% of wild-type ADP-ribosyltransferase activity (6). In contrast to wild-type LT, rLT-E112D did not inhibit antigen processing (Fig. 3B). Thus, inhibition of antigen processing by LT requires significant levels of ADP-ribosylation activity. In fact, rLT-E112D produced a slight enhancement of antigen processing (Fig. 3B), possibly due to the effects of the B subunit of this recombinant toxin in the absence of sufficient A subunit activity to produce inhibition.
Effect of LT and mutant LT on intracellular cAMP levels.
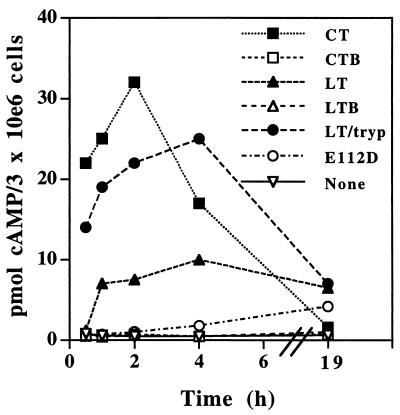
Since the result of toxin-mediated ADP-ribosylation of Gs proteins is the accumulation of intracellular cAMP, the ability of LT and related molecules to elevate cAMP levels in macrophages was determined. CT caused transient increases of cAMP to high levels, with initial rises occurring within 0.5 h, a peak in cAMP levels at 1 to 2 h, and return of cAMP levels to normal levels by 19 h (Fig. 4). LT also increased intracellular cAMP but to lower levels and with slower kinetics of both onset (after 1 h) and decay (cAMP levels were still elevated at 19 h). Trypsin-cleaved LT increased cAMP more rapidly and to higher levels than LT, with levels approaching but not equaling those seen with CT within 0 to 2 h. This suggests that lack of prior cleavage of the A subunit may be important in the delayed kinetics and lower magnitude of the LT effect. Trypsin-cleaved LT, like LT, produced a longer-lasting elevation of cAMP than that seen with CT, with elevation persisting at 19 h. rLT-E112D produced little or no elevation of cAMP before 4 h but consistently produced low-level cAMP elevation at 19 h. As predicted, rLTB and rCTB produced no significant elevation of cAMP.
FIG. 4.
Elevation of intracellular cAMP levels in macrophages after treatment with toxin preparations. Macrophages were treated overnight with the indicated toxin preparations (1 μg/ml), the cells were lysed in 66% ethanol, and cAMP levels were determined by using a TiterZyme dual-range cAMP enzyme immunoassay kit from PerSeptive Diagnostics (Cambridge, Mass.).
In summary, the experiments reported here show that LT, like CT, inhibits intracellular processing of bacterial antigens for presentation by macrophages, although the extent of inhibition was less with LT than CT. In contrast, rLTB and rCTB had enhancing effects on antigen presentation. Furthermore, different recombinant and mutant LT molecules were used to explore the molecular mechanisms of these effects, particularly with regard to the role of LTA ribosyltransferase activity.
Compared with CT, LT produced increases in cAMP characterized by lower magnitude, slower onset, and slower decay (Fig. 4), and LT may have lower specific ADP-ribosyltransferase activity than CT. This suggests that the ability of the toxins to inhibit antigen processing correlates with ribosyltransferase activity and their ability to induce cAMP, with greater inhibition of antigen processing being associated with either faster induction or higher levels of cAMP. The results with recombinant toxin molecules support this hypothesis, since rCTB and rLTB both failed to increase cAMP levels and did not inhibit antigen processing. Furthermore, rLT-E112D produced only a low elevation of cAMP levels and only at late time points, and this correlated with its inability to inhibit antigen processing. Trypsin-cleavage of LT produced accelerated and higher cAMP induction, which was accompanied by a slight enhancement of its ability to inhibit antigen processing (data not shown). Thus, ribosyltransferase-deficient molecules failed to inhibit antigen processing, and the inhibitory capacity of toxins generally correlated with their ability to induce cAMP. However, cAMP may not be the only signalling mechanism involved in the inhibition, since the toxins may have other mechanisms to transduce signals or mediate effects that act simultaneously with increases in cAMP. Thus, the increases in cAMP alone may not be sufficient to explain or cause inhibition of antigen processing.
LT and CT inhibited an intracellular stage of antigen processing and did not inhibit the presentation of previously processed antigen or exogenous preprocessed synthetic peptide. Antigen uptake and catabolism were not inhibited by the toxins (Fig. 2), indicating that the effect occurred at a subsequent step in the pathway, possibly concerned with the supply of peptide-receptive MHC-II. The cell surface level of I-Ak molecules, as measured by flow cytometry, was not altered by overnight incubation of macrophages with LT, rLTB, rLT-E112D, CT, or rCTB (data not shown). However, it is still possible that LT caused a decrease in MHC-II synthesis that had not yet affected the overall plasma membrane expression level. Such a decrease in synthesis could decrease the availability of peptide-receptive molecules in intracellular compartments. Alternatively, LT may have altered intracellular trafficking of MHC-II, or H-2DM expression, localization, or function, in a manner to cause inhibition of antigen processing. Thus, the exact inhibitory mechanism remains to be elucidated, but it appears to involve decreased binding of antigen-derived peptides to intracellular MHC-II molecules.
Although LT and CT have inhibitory effects on intracellular antigen-processing mechanisms, the overall net effect of in vivo administration of either toxin together with another antigen is enhancement of the immune response. One consideration is that the inhibitory effects are manifested only after long periods of incubation (e.g., 18 h), allowing significant processing of coadministered antigen to occur before the inhibitory phase. After production of peptide-MHC complexes on the plasma membrane, the ability of both LT and CT to enhance the presentation of surface complexes may contribute to the adjuvant effect. Thus, the inhibition of antigen processing by both CT and LT may be overcome by kinetic considerations and other strong enhancing effects in vivo. Furthermore, the use of mutant LT or CT molecules with diminished ADP-ribosyltransferase activity may decrease inhibitory and toxic effects, providing optimized adjuvant function.
Acknowledgments
This work was supported in part by NIH grants AI34343, AI35726, and CA70149 to C.H. and AI40701 to J.N. Milita Matousek was supported by NIH training grant AI-07427.
REFERENCES
- 1.Ågren L C, Ekman L, Löwenadler B, Lycke N Y. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997;158:3936–3946. [PubMed] [Google Scholar]
- 2.Ahmed S A, Gogal R M, Jr, Walsh J E. A new rapid, and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to H3-thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–2914. [PubMed] [Google Scholar]
- 4.Bromander A K, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnette W N, Mar V L, Platler B W, Schlotterbeck J D, McGinley M D, Stoney K S, Rohde M F, Kaslow H R. Site-specific mutagenesis of the catalytic subunit of cholera toxin: substituting lysine for arginine 7 causes loss of activity. Infect Immun. 1991;59:4266–4270. doi: 10.1128/iai.59.11.4266-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cieplak W, Jr, Mead D J, Messer R J, Grant C C R. Site-directed mutagenic alteration of potential active site residues of the A subunit of Escherichia coli heat-labile enterotoxin. J Biol Chem. 1995;270:1–6. doi: 10.1074/jbc.270.51.30545. [DOI] [PubMed] [Google Scholar]
- 7.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–275. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 8.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as non-toxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2741. [PubMed] [Google Scholar]
- 13.Ghiara P, Rossi M, Marchetti M, Di Tommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, De Magistris M T, Pizza M, Rappuoli R, Del Giudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon V M, Leppla S H. Proteolytic activation of bacterial toxins: role of bacterial and host cell proteases. Infect Immun. 1994;62:333–340. doi: 10.1128/iai.62.2.333-340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant C C R, Messer R J, Cieplak W., Jr Role of trypsin-like cleavage at arginine 192 in the enzymatic and cytotonic activities of Escherichia coli heat-labile enterotoxin. Infect Immun. 1994;62:4270–4278. doi: 10.1128/iai.62.10.4270-4278.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding C V. Techniques for studying phagocytic processing of bacteria for class I or II MHC-restricted antigen recognition by T lymphocytes. Methods Cell Biol. 1994;45:307–320. doi: 10.1016/s0091-679x(08)61859-2. [DOI] [PubMed] [Google Scholar]
- 17.Harford S, Dykes C W, Hobdin A N, Read M J, Halliday I J. Inactivation of the Escherichia coli heat-labile enterotoxin by in vitro mutagenesis of the A-subunit gene. Eur J Immunol. 1989;183:311–316. doi: 10.1111/j.1432-1033.1989.tb14930.x. [DOI] [PubMed] [Google Scholar]
- 18.Hörnquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993;23:2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- 19.Lencer W I, Constable C, Moe S, Rufo P A, Wolf A, Jobling M G, Ruston S P, Madara J L, Holmes R K, Hirst T R. Proteolytic activation of cholera toxin and Escherichia coli labile toxin by entry into host epithelial cells. Signal transduction by a protease-resistant toxin variant. J Biol Chem. 1997;272:15562–15568. doi: 10.1074/jbc.272.24.15562. [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Lamm M E, Nedrud J G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988;141:1495–1501. [PubMed] [Google Scholar]
- 21.Lobet Y, Cluff C W, Cieplak W., Jr Effect of site-directed mutagenic alterations on ADP-ribosyltransferase activity of the A subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1991;59:2870–2879. doi: 10.1128/iai.59.9.2870-2879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and E. coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 24.Matousek M P, Nedrud J G, Harding C V. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J Immunol. 1996;156:4137–4145. [PubMed] [Google Scholar]
- 25.Nedrud, J. G., S. J. Czinn, and W. Cieplak, Jr. 1997. Mutant E. coli heat labile toxin molecules with reduced ADP-ribosylation activity act as oral mucosal adjuvants and can promote protective immune responses versus Helicobacter felis. Immunol. Cell Biol. 75(Suppl.1):A91.
- 26.Partidos C D, Pizza M, Rappuoli R, Steward M W. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89:483–487. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer J, Wick M J, Russell D, Normark S, Harding C V. Recombinant Escherichia coli express a defined, cytoplasmic epitope that is efficiently processed in macrophage phagolysosomes for class II MHC presentation to T lymphocytes. J Immunol. 1992;149:2576–2584. [PubMed] [Google Scholar]
- 28.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 29.Rollwagen F M, Pacheco N D, Clements J D, Pavlovskis O, Rollins D M, Walker R I. Killed Campylobacter elicits immune response and protection when administered with an oral adjuvant. Vaccine. 1993;11:1316–1320. doi: 10.1016/0264-410x(93)90101-3. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 32.Tamura S-I, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 33.Tamura S-I, Yamanaka A, Shimohara M, Tomita T, Komase K, Tsuda Y, Suzuki Y, Nagamine T, Kawahara K, Danbara H, Aizawa C, Oya A, Kurata T. Synergistic action of cholera toxin B subunit (and Escherichia coli heat-labile toxin B subunit) and a trace amount of cholera whole toxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:419–426. doi: 10.1016/0264-410x(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji T, Inoue T, Miyama A, Okamoto K, Honda T, Miwatani T. A single amino acid substitution in the A subunit of Escherichia coli enterotoxin results in a loss of its toxicity. J Biol Chem. 1990;265:22520–22525. [PubMed] [Google Scholar]
- 35.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]