Abstract
To dissect the effects of corticosteroids mediated by the mineralocorticoid (MR) and the glucocorticoid receptor (GR) in the central nervous system, we compared MR–/– mice, whose salt loss syndrome was corrected by exogenous NaCl administration, with GR–/– mice having a brain-specific disruption of the GR gene generated by the Cre/loxP-recombination system. Neuropathological analyses revealed a decreased density of granule cells in the hippocampus of adult MR–/– mice but not in mice with disruption of GR. Furthermore, adult MR–/– mice exhibited a significant reduction of granule cell neurogenesis to 65% of control levels, possibly mediated by GR due to elevated corticosterone plasma levels. Neurogenesis was unaltered in adult mice with disruption of GR. Thus, we could attribute long-term trophic effects of adrenal steroids on dentate granule cells to MR. These MR-related alterations may participate in the pathogenesis of hippocampal changes observed in ageing, chronic stress and affective disorders.
INTRODUCTION
Adrenal steroids affect neuronal birth and death in the hippocampus, a brain structure important for specific components of learning and memory (Gould and McEwen, 1993). Chronic elevation of plasma corticosteroid levels leads to decreased neurogenesis and neurodegeneration, and has been associated with stress, ageing and affective disorders in humans (de Kloet et al., 1998; McEwen, 1999). In contrast, removal of adrenal steroids by adrenalectomy results in both increased production and increased degeneration of dentate granule cells (Sloviter et al., 1989, 1993; Woolley et al., 1991; Gould and McEwen, 1993). Corticosteroids act via intracellular receptors that recognize specific palindromic DNA sequences in the promoter region of target genes and thereby modulate transcription (Beato et al., 1995). Molecular cloning has revealed two receptor subtypes that are effective in the brain: type 1 or mineralocorticoid receptor (MR), and type 2 or glucocorticoid receptor (GR) (Beato et al., 1995). To define the role of MR and GR in neurogenesis and neurodegeneration, we have developed genetic mouse models to allow the distinction between MR- and GR-mediated signalling in the hippocampus. We demonstrate here that the disruption of the MR gene, but not of the GR gene in mice results in granule cell degeneration in the hippocampus. Furthermore, adult MR-, but not GR-mutant mice show significantly reduced granule cell neurogenesis.
RESULTS AND DISCUSSION
MR–/– mice with disruption of the MR gene were generated by homologous recombination and analysed in an F4 backcross generation to the C57Bl/6 background as described (Berger et al., 1998). When untreated, MR–/– mice develop pseudohypoaldosteronism after birth and die between postnatal days 8 and 13 due to severe renal loss of sodium and water (Berger et al., 1998). MR–/– animals, however, can be rescued by exogenous NaCl administration and subsequently studied during adulthood (Bleich et al., 1999). Since most mice with overall disruption of the GR gene die perinatally due to respiratory failure (Cole et al., 1995), the role of GR was studied in GRNesCre mice (Tronche et al., 1999). GRNesCre mice have a brain-specific disruption of the GR gene using the Cre/loxP-recombination system, with the Cre recombinase under the control of the rat nestin promoter, which inactivates the GR gene early during development in neuronal and glial cell precursors (Tronche et al., 1999).
Neuropathological analyses revealed changes in the hippocampus of adult NaCl-rescued MR–/– mice but not in GRNesCre mice. Decreased density of granule cells was observed in Nissl-stained sections in the dentate gyrus, mainly in the upper blade (Figure 1). In areas with granule cell reduction, reactive activation of astrocytes, which indicated a degenerative process, could be demonstrated by MAP-kinase (MAPK) immunocytochemistry (Figure 1) (Mandell and VandenBerg, 1999). Direct evidence for apoptosis could not be obtained, since TUNEL staining for apoptotic neurons revealed only very low numbers of single positive nuclei in both wild-type and MR–/– mice, suggesting a slow rate of degeneration in MR–/– mice with no significant difference from control (data not shown). Furthermore, an increase in the density of hippocampal hilus neurons was observed by Nissl staining (Figure 1, indicated by an asterisk). Increased hilar cell density was also demonstrated by using an antibody against calretinin, which is a marker for a subset of GABAergic hilus neurons (Figure 1). To analyse the projection of granule cell axons to the CA3 field of the hippocampus, a Timm reaction was performed to label the Zn2+-containing mossy fiber terminals. Compared with wild-type controls, MR–/– mice showed strongly extended mossy fiber intra/infrapyramidal projection fields that extended up to the distal end of CA3 (Figure 2). All these neuropathological changes described were exclusively observed in mice with the MR gene knockout. Mice with a brain-specific mutation of the GR gene demonstrated regular cell density of granule and hilus neurons (Figure 1), regular extension of mossy fiber projections to CA3 (data not shown) and a lack of activated astrocytes (Figure 1). Outside the hippocampus, neither MR–/– nor GRNesCre mice exhibited any neuropathological changes. At postnatal day 8, an age when formation of the hippocampus is still in process, histological examination of the central nervous system (CNS) of MR–/– mice did not reveal any structural anomalies in the CNS (data not shown). In particular, no pathology such as neuronal death or reactive gliosis was observed in the hippocampus, indicating that corticosteroid signalling via MR is necessary, not for the formation but for the maintenance of cellular and structural integrity of the hippocampus.
Fig. 1. Hippocampal granule cell degeneration in adult MR–/– mice. In Nissl-stained sections, adult MR–/– mice, but not GRNesCre mice, show a significant reduction of granule cells in the hippocampus, most pronounced in the upper blade of the dentate gyrus (arrowheads). In the hilus of the dentate gyrus (asterisk), an increase in the number of neurons is seen in MR–/– mice. In the same areas of dentate gyrus in MR–/– mice where the granule cell number is reduced, an increase of activated astrocytes is found by immunostaining for activated MAP-kinase (MAPK). Increased cell density in the hilus is also demonstrated by immunostaining for calretinin, a marker for a subset of GABAergic hilus neurons.
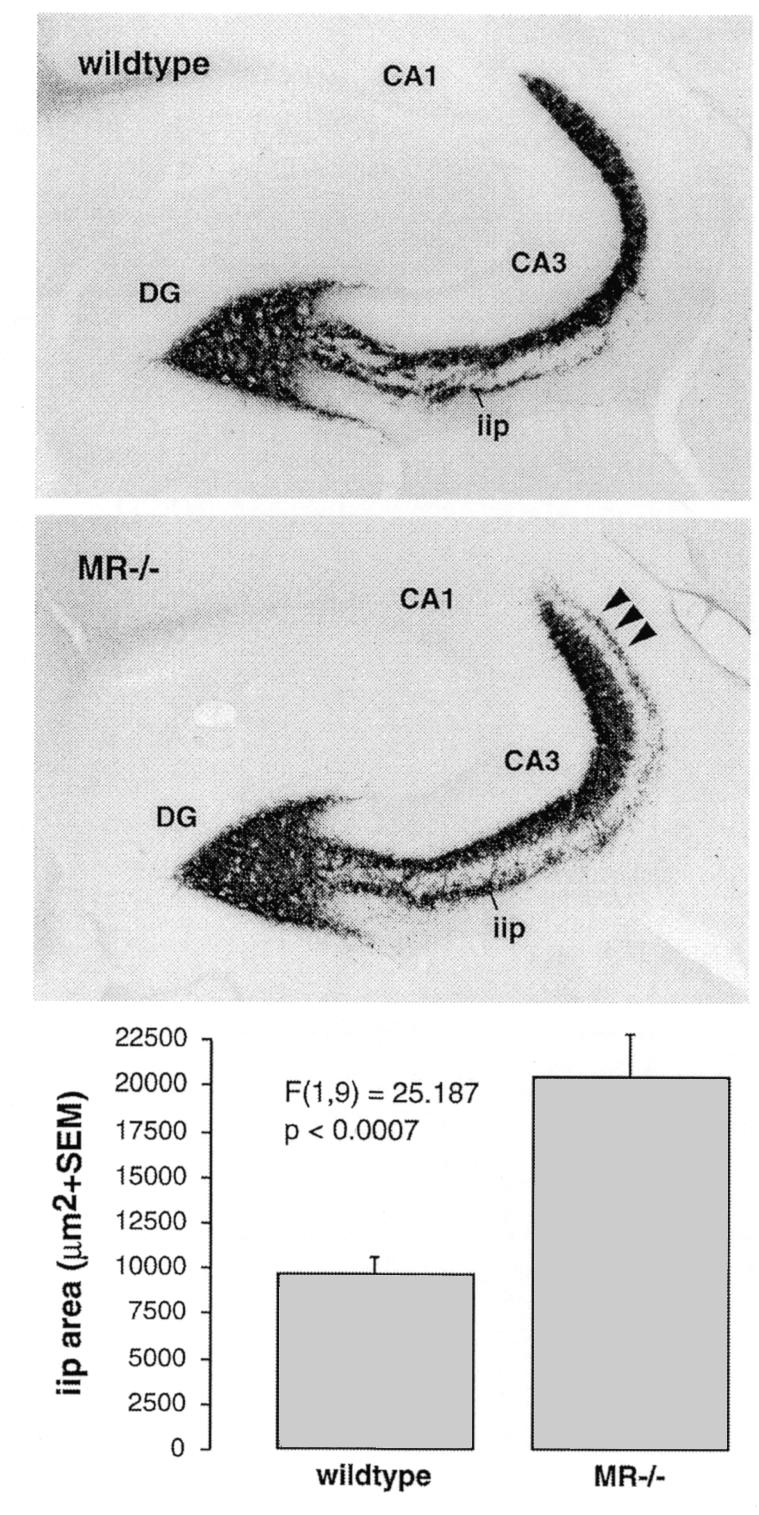
Fig. 2. Mossy fiber sprouting in the hippocampus of adult MR–/– mice. In comparison to wild-type mice with regular distribution of Timm-stained mossy fiber terminals, MR–/– mice demonstrate significantly prolonged intra/infrapyramidal projection fields (iip) that extend up to the distal end of CA3 (arrowheads). Quantitative morphometrical analysis confirmed that the intra/infrapyramidal projection field was on average twice as large in MR–/– mice, whereas the suprapyramidal and hilar projection fields were not significantly enlarged.
Adult NaCl-rescued MR–/– mice show a loss of hippocampal granule cells with activation of astrocytes, as has been described after adrenalectomy (Sloviter et al., 1989, 1993). Since lack of GR does not result in neuropathological alterations, our genetic mouse models demonstrate that long-term survival of at least a subset of granule cells depends on MR-mediated signalling. Our study also extends short-term pharmacological experiments, showing that, in rats, adrenalectomy-induced granule cell degeneration could be prevented by administration of an MR agonist (Woolley et al., 1991). Granule cell loss in the dentate gyrus of MR-deficient mice was not as pronounced as shown for adrenalectomy, which leads to unoccupied MR and GR (Sloviter et al., 1989). The less pronounced loss of granule cells of MR–/– mice compared with that reported for adrenalectomy may be due to persistent expression of GR and moderately elevated corticosterone levels in MR–/– mice (2.7-fold at 8 a.m., p <0.001, data not shown). Other pharmacological studies demonstrated that treatment of rats with the synthetic GR agonist, dexamethasone, exacerbates apoptosis in the dentate gyrus, most likely due to downregulation of plasma corticosterone levels and consequent hypoactivity of MR (Hassan et al., 1996; Sousa et al., 1999; Almeida et al., 2000). Two observations underline this interpretation: (i) increased rates of apoptosis could be prevented by aldosterone or corticosterone in doses leading to selective MR activation; and (ii) treatment with a supraphysiological dose of corticosterone sufficient to occupy MR and GR did not result in elevated rates of apoptosis (Hassan et al., 1996; Sousa et al., 1999).
Granule cell neurogenesis is known to be influenced by adrenal steroids during development and adulthood (Gould et al., 1991; Gould and Tanapat, 1999; McEwen, 1999). Therefore, we studied granule cell neurogenesis in NaCl-rescued MR–/– mice immunocytochemically with the proliferation marker Ki67 (Starborg et al., 1996). Adult MR-deficient mice demonstrate a significant reduction of granule cell neurogenesis to 65% of wild-type littermates (Figure 3). Thus, not only neuronal degeneration but also decreased neurogenesis may contribute to a reduction of granule cells in adult MR–/– mice. Interestingly, at postnatal day 8 no difference in granule cell proliferation could be demonstrated between MR–/– mice and wild-type littermates (data not shown). Neurogenesis was undisturbed in adult GRNesCre mice (Figure 3), indicating that the basal rate of granule cell proliferation does not depend on corticosterone-evoked signalling mediated by GR. However, granule cell proliferation has been reported to increase following adrenalectomy, i.e. in a state when MR and GR are unoccupied (Gould et al., 1992; Cameron and Gould, 1994, 1996; Cameron and McKay, 1999). Furthermore, treatment with high doses of corticosterone, which usually signifies action via GR rather than MR, diminishes the number of proliferating cells in the dentate gyrus (Cameron and Gould, 1994; Cameron et al., 1998). These findings suggest that the reduced neurogenesis in MR–/– mice may be an indirect effect of GR activation due to increased corticosterone plasma levels. Therefore, we tried to investigate adrenalectomized MR–/– mice, which failed due to almost 100% post-operative lethality of the animals.
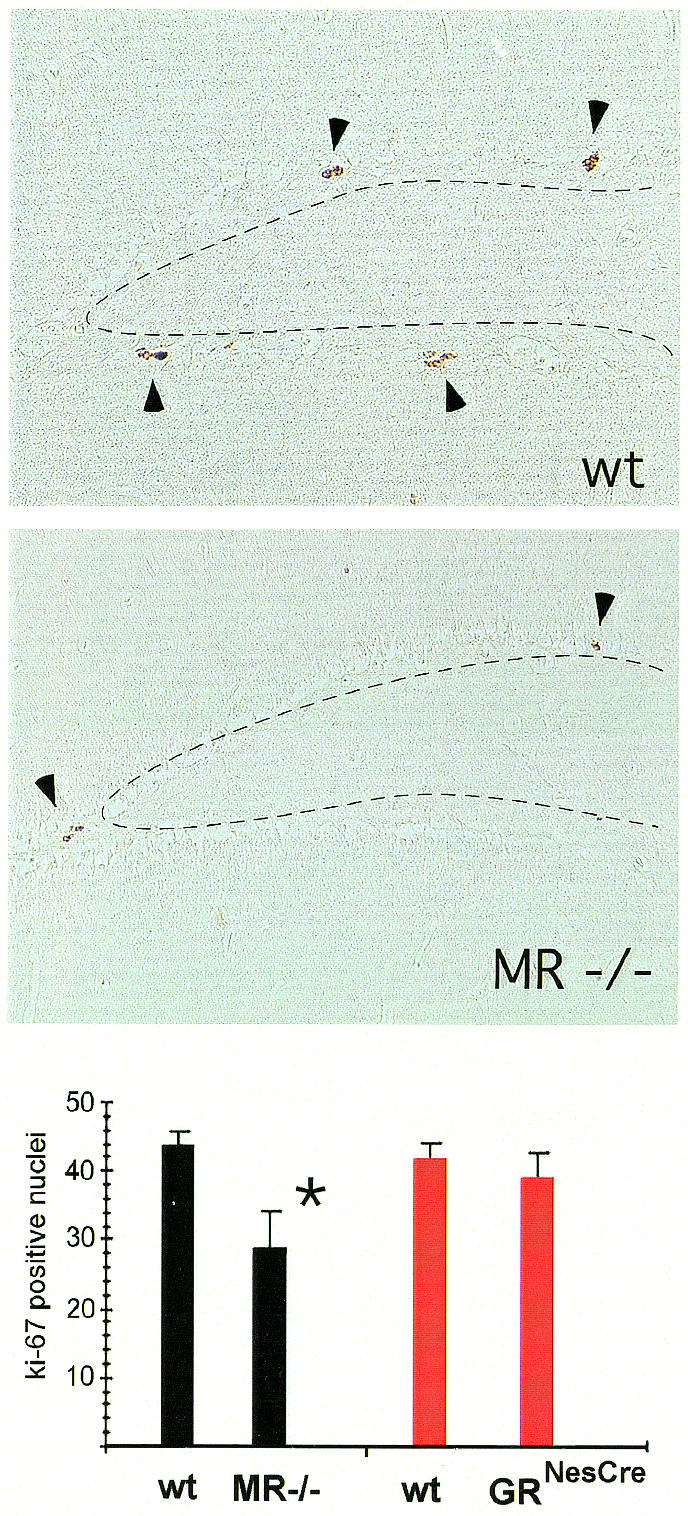
Fig. 3. Reduced neurogenesis in the hippocampus of adult MR–/– mice. Proliferating hippocampal granule cells were labelled with the monoclonal antibody Ki67 directed against the proliferation marker Mib-1. Three representatively selected hippocampal sections at the level of the dorsal hippocampus were analysed in six mutant and six wild-type animals. On average, adult MR–/– mice exhibit only 29 labelled nuclei in the three sections analysed, while MR wild-type littermate mice show 44 Ki67 positive nuclei (P = 0.025, Student’s t-test). In contrast to MR–/– mice, GRNesCre mice demonstrate unaltered numbers of Ki67 positive nuclei when compared with their respective control littermates (GRNesCre exhibit 39, GRNesCre wild-type littermates 42 Ki67 labelled nuclei, respectively).
In summary, the present study showed that inactivating the MR in mice produces a reduction of dentate granule cells similar to that observed after adrenalectomy. By using genetic mouse models with disruption of MR and GR, respectively, we can selectively attribute the long-term trophic effect of adrenal steroids on dentate granule cells in adult mice to the MR, and can thus confirm by genetic means the current concept based on pharmacological evidence (Woolley et al., 1991; Hassan et al., 1996; Sousa et al., 1999). Furthermore, we have demonstrated reduced neurogenesis in adult MR-deficient mice (but not GRNesCre mice), possibly reflecting elevated corticosterone levels. These MR-related alterations may participate in the pathogenesis of hippocampal changes observed in ageing, chronic stress and affective disorders, and should help focus attention on this path of neuronal corticosteroid signalling (de Kloet et al., 1998; Eriksson et al., 1998; Cameron and McKay, 1999; McEwen, 1999).
METHODS
Animals. Mice with a targeted disruption of the MR gene were generated from an F4 backcross in the C57Bl/6 background (Berger et al., 1998). When untreated, MR knockout mice develop pseudohypoaldosteronism after birth and die of renal failure between postnatal days 8 and 13 (Berger et al., 1998). However, when salt wasting is compensated by s.c. injection of physiological saline, animals can be rescued and survive to adult age (Bleich et al., 1999). According to a recently established protocol, we rescued MR knockout animals by saline injections during the first 3 weeks of life, followed by NaCl replacement in food pellets and drinking water thereafter (Bleich et al., 1999). To avoid effects of handling and injection on the outcome of experiments, wild-type littermates were treated accordingly. GRNesCre mice were generated by crossing a mouse strain with the Cre recombinase under the control of the rat nestin promoter with a mouse strain in which exon 3 of the GR gene was flanked by loxP sites (Tronche et al., 1999). GRNesCre mice show a brain-specific disruption of the GR gene early during development in neuronal and glial cell precursors (Tronche et al., 1999).
Histological and immunocytochemical analyses. CNS structures of mice were analysed at postnatal day 8 as well as in adult animals at 5–6 months. For histological and immunocytochemical investigations, animals were killed under deep pentobarbital anaesthesia by transcardiac perfusion with 4% (w/v) paraformaldehyde. Histological examination of CNS structures was performed on routine Nissl-, and Haematoxylin & Eosin-stained paraffin sections from coronal serial sections throughout the CNS of MR–/– animals and wild-type littermates (n = 5, respectively).
Immunocytochemical analyses were performed on serial coronal free-floating 50 µm vibratome sections of MR–/– and GRNesCre mice, as well as wild-type littermates (n = 5, respectively). Antibodies were used in the following dilutions: rabbit antiserum against glucocorticoid receptor 1:2000 (Santa Cruz Biotechnology, Santa Cruz, CA); monoclonal mouse anti-calretinin 1:2000, anti-calbindin-D28K 1:5000 and anti-parvalbumin 1:5000 (SWANT, Bellinzona, Switzerland); rabbit antiserum against phosphorylated MAP-kinase 1:1000 (Boehringer Mannheim, Germany); polyclonal mouse anti-Ki67 1:200 (Dianova, Hamburg, Germany). Immunoreactivity was visualized by the avidin biotin complex method (Vectastain, Vector Laboratories).
Hippocampal mossy fiber synaptic fields were visualized by Timm staining in adult mice (MR–/–, n = 6; wild-types, n = 4) as described elsewhere (Schwegler and Lipp, 1983). In brief, animals were perfused transcardially with phosphate buffered 1% sodium sulfide and 3% glutaraldehyde. After postfixation and cryoprotection, 40 µm horizontal cryosections were obtained and developed in a solution containing Arabic gum, hydroquinone, citric acid and silver nitrate. Quantitative analysis was carried out on the dorsal-most section not hitting the septal pole of the hippocampal formation, and on additional sections taken 120, 240, 360, 480 and 1080 µm more ventrally. Grey level images (1024 × 1276 pixel) were obtained of left and right hippocampi with a Kodak Megaplus™ 1400 CCD camera, and morphometry was performed with the public domain image processing software NIH Image 1.61 (Schwegler and Lipp, 1983).
Acknowledgments
ACKNOWLEDGEMENTS
The authors wish to acknowledge the excellent technical assistance of Heike Glaser, Ralf Klären, Rosmarie Lang, Stefanie Ridder and Christiane Zacher. This work was supported by grants from the Deutsche Forschungsgemeinschaft (427/4-1 to P.G.; KR1984/1-1 to O.K., Un34/19-3 to G.S.) and EC grants (BI04-98-0297 to D.P.W., BI04CT80297/BBW98.0125 to H.P.L. and G.S., PL 960179 to G.S.).
REFERENCES
- Almeida O.F.X., Conde, G.L., Crochemore, C., Demeneix, B.A., Fischer, D., Hassan, A.H.S., Meyer, M., Holsboer, F. and Michaelidis, T.M. (2000) Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J., 14, 779–790. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich, P. and Schütz, G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Berger S. et al. (1998) Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc. Natl Acad. Sci. USA, 95, 9424–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich M. et al. (1999) Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch., 438, 245–254. [DOI] [PubMed] [Google Scholar]
- Cameron H.A. and Gould, E. (1994) Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience, 61, 203–209. [DOI] [PubMed] [Google Scholar]
- Cameron H.A. and Gould, E. (1996) Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J. Comp. Neurol., 369, 56–63. [DOI] [PubMed] [Google Scholar]
- Cameron H.A. and McKay, R.D.G. (1999) Restoring production of hippocampal neurons in old age. Nature Neurosci., 2, 894–897. [DOI] [PubMed] [Google Scholar]
- Cameron H.A.; Tanapat, P. and Gould, E. (1998) Adrenal steroids and N-methyl-d-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience, 82, 349–354. [DOI] [PubMed] [Google Scholar]
- Cole T.J. et al. (1995) Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev., 9, 1608–1621. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Vreugdenhil, E., Oitzl, M.S. and Joels, M. (1998) Brain corticosteroid receptor balance in health and disease. Endocr. Rev., 19, 269–301. [DOI] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva, E., Björk-Eriksson, T., Alborn, A.M., Nordborg, C., Peterson, D.A. and Gage, F.H. (1998) Neurogenesis in the adult human hippocampus. Nature Med., 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Gould E. and McEwen, B.S. (1993) Neuronal birth and death. Curr. Opin. Neurobiol., 3, 676–682. [DOI] [PubMed] [Google Scholar]
- Gould E. and Tanapat, P. (1999) Stress and hippocampal neurogenesis. Biol. Psychiatry, 46, 1472–1479. [DOI] [PubMed] [Google Scholar]
- Gould E., Woolley, C., Cameron, H.A., Daniels, D.C. and McEwen, B.S. (1991) Adrenal steroids regulate postnatal development of the adult rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J. Comp. Neurol., 313, 486–493. [DOI] [PubMed] [Google Scholar]
- Gould E., Cameron, H.A., Daniels, D.C., Woolley, C. and McEwen, B.S. (1992) Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci., 12, 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.H.S., von Rosenstiel, P., Patchev, V.K., Holsboer, F. and Almeida, O.F.X. (1996) Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp. Neurol., 140, 43–52. [DOI] [PubMed] [Google Scholar]
- Mandell J.W. and VandenBerg, S.R. (1999) ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport, 10, 3567–3572. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. (1999) Stress and hippocampal plasticity. Ann. Rev. Neurosci., 22, 105–122. [DOI] [PubMed] [Google Scholar]
- Schwegler H. and Lipp, H.P. (1983) Hereditary covariations of neuronal circuitry and behaviour: correlations between the proportions of hippocampal synaptic fields in the regio inferior and two-way avoidance in mice and rats. Behav. Brain Res., 7, 1–38. [DOI] [PubMed] [Google Scholar]
- Sloviter R.S., Valiquette, G., Abrams, G.M., Ronk, E.C., Sollas, A.L., Paul, L.A. and Neubort, S. (1989) Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science, 243, 535–538. [DOI] [PubMed] [Google Scholar]
- Sloviter R., Sollas, A.L., Dean, E. and Neubort, S. (1993) Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: characterization of an in vivo model of controlled neuronal death. J. Comp. Neurol., 330, 324–336. [DOI] [PubMed] [Google Scholar]
- Sousa N., Paula-Barbosa, M.M. and Almeida, O.F.X. (1999) Ligand and subfield specificity of corticoid-induced neuronal loss in the rat hippocampal formation. Neuroscience, 89, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Starborg M., Gell, K., Brundell, E. and Hoog, C. (1996) The murine Ki67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J. Cell Sci., 109, 143–153. [DOI] [PubMed] [Google Scholar]
- Tronche F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P., Bock, R., Klein, R. and Schütz, G. (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genet., 23, 99–103. [DOI] [PubMed] [Google Scholar]
- Woolley C.S., Gould, E., Sakai, R.R., Spencer, R.L. and McEwen, B.S. (1991) Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res., 554, 312–315. [DOI] [PubMed] [Google Scholar]



