Abstract
The immunosuppressive effects of cyclosporin A (CsA) and FK506 are mediated through binding to immunophilins. Here we show that FK506–FKBP complex suppresses the activation of JNK and p38 pathways at a level upstream of mitogen-activated protein kinase (MAPK) kinase kinase (MAPKK-K) besides the calcineurin–NFAT pathway. A238L, a viral gene product that binds to immunophilin, also blocks activation of both pathways. In contrast, direct inhibitors of calcineurin, Cabin 1 and FR901725, suppress the activation of NFAT but not the JNK or p38 pathway. We further demonstrate that co-expression of a constitutively active NFAT and a constitutively active MEKK1 renders the interleukin-2 promoter in Jurkat T lymphocytes resistant to CsA and FK506, whereas Jurkat cells expressing a constitutively active NFAT alone are still sensitive to CsA or FK506. Therefore, CsA and FK506 exert their immunosuppressive effects through targeting both the calcineurin-dependent NFAT pathway and calcineurin-independent activation pathway for JNK and p38.
INTRODUCTION
NFAT family members play a key role in the transcriptional activation of cytokine genes, including interleukin (IL)-2, IL-4 and tumor necrosis factor (TNF)-α, upon T-cell activation led by stimulation through the T-cell receptor (TCR) complex in the presence of appropriate co-stimulatory signals such as CD28 engagement (Rao et al., 1997; Crabtree, 1999). The importance of a Ca2+-dependent serine/threonine phosphatase calcineurin in NFAT activation has been highlighted by studies on the immunosuppressive drugs cyclosporin A (CsA) and FK506 (Schreiber, 1992). CsA and FK506, with their cognate binding proteins cyclophilin (CyP) and FKBP (collectively termed immunophilins), respectively, bind to and inactivate calcineurin, and hence impair NFAT-dependent gene expression. Thus, inhibition of calcineurin has been considered to be a basis of the immunosuppressive nature of these compounds.
The mitogen-activated protein kinase (MAPK) pathway is a conserved eukaryotic signaling cascade that mediates the effects of extracellular stimuli on a wide array of biological processes (Nishida and Gotoh, 1993; Schaeffer and Weber, 1999). In T lymphocytes, JNK and p38 are synergistically activated by co-stimulation of the TCR and CD28 receptors or by combined treatment with a phorbol ester (such as TPA) and a Ca2+ ionophore (such as A23187), whereas ERK can be fully activated by either engagement of TCR or treatment with phorbol ester alone (Su et al., 1994; Matsuda et al., 1998). The MAPK family is thought to be involved in inducing IL-2 gene expression through activation of AP-1, which is a heterodimer consisting of Jun and either Fos or ATF family members (Karin, 1995). In addition to its binding to AP-1 recognition sites, AP-1 assists stable binding of NFAT family members to the composite NFAT recognition elements in the IL-2 promoter (Rao et al., 1997).
Recent studies have revealed that CsA suppresses JNK and p38 activation in T cells stimulated by engagement of both TCR and CD28 (Su et al., 1994; Matsuda et al., 1998), although mechanisms of inhibition remain obscure. Here we demonstrate that immunophilin–ligand complexes block activation of JNK and p38 pathways induced during T-cell activation, but not by cellular stresses. In contrast, direct inhibitors of calcineurin fail to suppress JNK and p38 activation, suggesting that inhibition of JNK and p38 activation by CsA and FK506 is mediated through a calcineurin-independent mechanism(s).
RESULTS AND DISCUSSION
CsA and FK506 block JNK and p38 pathways during T-cell activation at a level upstream of MAPKK-K
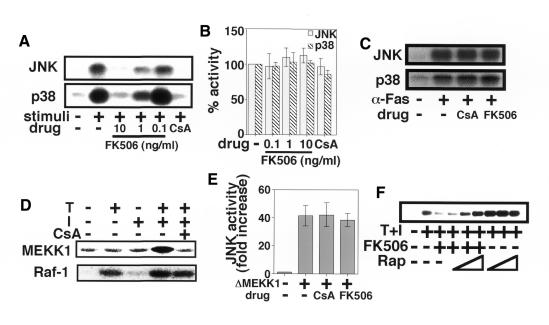
Both FK506 and CsA block the activation of JNK and p38 pathways but not the ERK pathway during T-cell activation (Figure 1A), while rapamycin, a derivative of FK506, has little effect on either pathway (Figure 1F and data not shown). None of the above reagents inhibited the activation of JNK and p38 in response to hyper-osmolar media (Figure 1B), anisomycin (data not shown) or anti-Fas monoclonal antibody (mAb) (CH-11) treatment (Figure 1C). Furthermore, JNK and p38 activation pathways activated by a combination of TPA and Ca2+ ionophore were not perturbed in the presence of FK506 or CsA in non-lymphoid cells such as Cos7 and KB cells (Su et al., 1994; our unpublished observation). These results collectively indicate that the inhibitory effect of CsA and FK506 is specific to the signaling pathway(s) involved in T-cell activation. T-cell stimulation signals also induced activation of an endogenous mitogen-activated protein kinase (MAPK) kinase kinase (MAPKK-K), MEKK1, in Jurkat cells in a CsA-sensitive manner, whereas CsA had no effect on the activation of Raf-1, a MAPKK-K for ERK (Figure 1D). These results suggest that CsA and FK506 specifically inhibit JNK and p38 signaling pathways at a level upstream of MAPKK-K. Accordingly, CsA and FK506 failed to block the activation of the JNK signaling pathway driven by a constitutively active mutant of MEKK1 termed ΔMEKK1 (Figure 1E). ΔMEKK1 is capable of activating the ERK and JNK pathways, but not the p38 pathway in Jurkat cells (Lange-Carter et al., 1993; Minden et al., 1994). CsA and FK506 bind to CyP and FKBP, respectively, to exert their function (Schreiber, 1992). Since rapamycin competes with FK506 for binding to FKBP, it is able to cancel the biological actions of the FK506–FKBP complex such as the blockade of nuclear translocation of NFAT (Dumont et al., 1990). As shown in Figure 1F, rapamycin restored the inhibitory effect of FK506 on the JNK and p38 pathways in a dose-dependent manner, indicating that the FK506–FKBP complex is responsible for the inhibition of JNK and p38 signaling pathways.
Fig. 1. CsA and FK506 inhibit T-cell specific JNK and p38 activation pathways. (A) Jurkat cells were pre-treated with the indicated concentrations of FK506 or CsA, and then stimulated with TPA and A23187 and assayed for JNK and p38 activities. This experiment is a representative of five. (B) Jurkat cells were pre-treated with FK506 or CsA followed by exposure to 0.7 M NaCl for 40 min, and then assayed for JNK and p38 activities. Three independent experiments were performed, and data are expressed as per cent means ± SD of the activity without drug. (C) Jurkat cells were pre-treated with FK506 or CsA followed by treatment with anti-Fas mAb (CH-11, 500 ng/ml) for 3 h, and then assayed for JNK and p38 activities. This experiment is a representative of two. (D) Jurkat cells were pre-treated with CsA, and then stimulated with TPA (T) and/or A23187 (I) and assayed for MEKK1 and Raf-1 activities. This experiment is a representative of two. (E) Jurkat cells were transfected with HA-JNK in combination with or without ΔMEKK1. The cells were then treated with vehicle, CsA or FK506 for 40 h, and assayed for JNK activity. Three independent experiments were performed and data are presented as means ± SD. (F) Jurkat cells were pre-treated with 10, 100 or 1000 ng/ml rapamycin (Rap) with or without 10 ng/ml FK506, followed by stimulation with TPA and A23187. The cells were then assayed for p38 activity. This experiment is a representative of three. Essentially the same result was obtained for JNK activity.
Calcineurin-independent inhibition of the JNK and p38 activation during T-cell activation
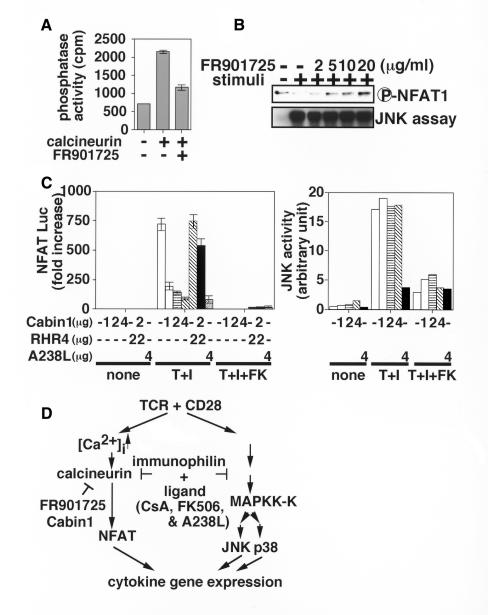
We further examined the involvement of calcineurin in the JNK and p38 activation pathways using various calcineurin inhibitors. FR901725 (also called violaceol II; Table I) serves as a specific inhibitor of calcineurin without interacting with immunophilins (H. Mori, F. Abe, M. Hashimoto, S. Takase, M. Hino and S. Hashimoto, in preparation). Accordingly, FR901725 blocked calcineurin activation (Figure 2A) and subsequent dephosphorylation of NFAT1 (Figure 2B, upper panel) in a dose-dependent manner. In marked contrast, FR901725 had no effect on the JNK (Figure 2B, lower panel) or p38 activities (data not shown), suggesting that calcineurin is dispensable for the JNK and p38 activation. Cabin1, another inhibitor, directly binds to and inactivates calcineurin (Lai et al., 1998; Sun et al., 1998) (Table I). As shown in Figure 2C, expression of Cabin1 in Jurkat cells blocked the transcriptional activation of the NFAT-Luc containing a luciferase gene under the control of three copies of the distal NFAT element of the human IL-2 promoter (Northrop et al., 1993) in a dose-dependent manner. Co-expression of RHR4, a constitutively active mutant of NFAT4 (see below), with Cabin1 restored the transcriptional activation (Figure 2C, left panel), confirming that Cabin1 targets the calcineurin–NFAT pathway alone. Consistent with this observation, the JNK activation pathway was not influenced by Cabin1 under the condition where FK506 blocked the JNK activation (Figure 2C, right panel). These data clearly indicate that the JNK pathway is activated in a calcineurin-independent manner during T-cell activation. Although not shown, overexpression of either dominant-negative or constitutively active forms of calcineurin had little effect on JNK and p38 activation pathways, further supporting the conclusion. In contrast to FR901725 and Cabin1, a viral protein A238L binds to both CyP and calcineurin (Miskin et al., 1998) (Table I). When overexpressed, A238L, like CsA and FK506, inhibited JNK activation (Figure 2C, right panel) as well as the activation of NFAT-Luc (Figure 2C, left panel). These results collectively indicate that the immunophilin–ligand complexes, such as CsA–CyP, FK506–FKBP and A238L–CyP, target not only calcineurin, but also another unidentified molecule(s) involved in the JNK and p38 activation pathways (Figure 2D). Although identification of the alternative target(s) is under way, the molecular mechanisms for the inhibition of JNK and p38 activation pathways remain to be elucidated.
Table I. Properties of inhibitors affecting MAPK family members and calcineurin.
| Inhibition of |
Binding to immunophilin | References | |||||
|---|---|---|---|---|---|---|---|
| ERK | JNK | p38 | Cn a | NFAT | |||
| CsA | no | yes | yes | yes | yes | yes | Schreiber (1992) |
| Su et al. (1994) | |||||||
| Matsuda et al. (1998) | |||||||
| FK506 | nob | yes | yes | yes | yes | yes | Schreiber (1992) |
| A238L | no | yes | yes | yes | yes | yes | Miskin et al. (1998) |
| FR901725c | no | no | no | yes | yes | no | |
| Cabin1 | no | no | no | yes | yes | no | Lai et al. (1998) |
| Sun et al. (1998) | |||||||
| LL-Z1640-2 | no | yes | yes | no | no | no | Takehana et al. (1999) |
| SB203580 | no | no | yes | no | no | no | Lee et al. (1994) |
aCalcineurin.
bUnderlined results were obtained in this work.
cThis reagent does not affect other phosphatases such as PP2A (H. Mori, F. Abe, M. Hashimoto, S. Takase, M. Hino and S. Hashimoto, in preparation).
Fig. 2. Calcineurin is not involved in the inhibition of JNK and p38 pathways. (A) PKA-phosphorylated [32P]RII protein was incubated with 200 nM calcineurin in the presence of 500 nM calmodulin with or without 1 µM FR901725. Released 32P was measured as described in Methods. (B) Jurkat cells were pre-treated with the indicated concentrations of FR901725 for 1 h, followed by stimulation with TPA and A23187. The cells were then assayed for phosphorylation status of NFAT1 as well as JNK activity. This experiment is a representative of two. (C) Jurkat cells were transfected with pNFAT-Luc (left panel) or HA-JNK (right panel) along with the indicated amount of Cabin1, RHR4 and A238L expression vectors, followed by incubation for 40 h. The cells were stimulated with TPA and A23187 (T+I) in the presence (+FK) or absence of FK506 for 8 h and assayed for luciferase activities (left panel), or stimulated for 15 min and then assayed for JNK activity (right panel). This experiment is a representative of three. (D) Schematic diagram showing the effects of various inhibitors on the calcineurin–NFAT pathway and the JNK and p38 activation pathways.
JNK and p38 signaling pathways as well as the nuclear translocation of NFAT are the physiological targets for immunosuppression
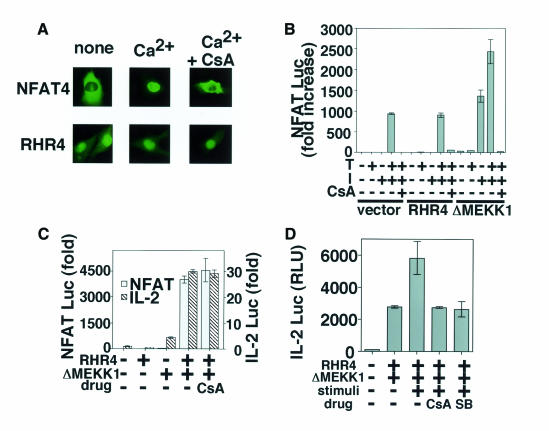
We next examined the effects of CsA and FK506 on the NFAT pathway and the JNK and p38 pathways separately. To this end, we generated a constitutively active mutant of NFAT. RHR4, a CsA-insensitive mutant of NFAT4, consists of the Rel homology region without the N-terminal regulatory domain (Hoey et al., 1995; Wolfe et al., 1997). While the wild-type NFAT4 shows Ca2+-dependent and CsA-sensitive nuclear localization, the RHR4 mutant was constitutively localized in the nucleus even in the presence of CsA (Figure 3A). RHR4 was transfected into Jurkat cells along with a reporter plasmid NFAT-Luc. Note that the transcriptional activity at the distal NFAT element is regulated through co-operation between NFAT transcription factors and AP-1 (Rao et al., 1997). As shown in Figure 3B, the activation-induced luciferase activity in the RHR4-transfected cells was suppressed to a nearly basal level by CsA, indicating the requirement of a CsA-sensitive signaling pathway(s) other than nuclear transport of NFAT, presumably the JNK and p38 pathways. Essentially the same results were obtained with FK506 (data not shown). Although transfection of a constitutively active form of MEKK1, ΔMEKK1 (see Figure 1E), alone induced no transcriptional activation of NFAT-Luc, addition of Ca2+ ionophore induced luciferase activity and its effect was completely inhibited by CsA (Figure 3B) or FK506 (data not shown), confirming the essential role of the CsA-sensitive nuclear transport of NFAT in the transcriptional activation from the distal NFAT element. It is thus likely that both nuclear transport of NFAT and the activation pathways of JNK and p38 are the physiological targets of CsA and FK506. Accordingly, co-expression of RHR4 and ΔMEKK1 resulted in constitutive transcriptional activation of NFAT-Luc in a CsA-insensitive manner (Figure 3C). We also found that co-expression of RHR4 and ΔMEKK1 is sufficient for inducing transcriptional activation of the IL-2 promoter (Figure 3C). We have previously shown that the p38 pathway functions in a manner additive to the JNK pathway in IL-2 gene activation (Matsuda et al., 1998). In fact, the luciferase activity induced by co-transfection of RHR4 and ΔMEKK1 was further enhanced by the treatment with TPA and Ca2+ ionophore (Figure 3D, stimuli +), which induces activation of endogenous p38. Pre-treatment of the cells with either CsA or SB203580 reduced the transcriptional activity to the level achieved by a combination of RHR4 and ΔMEKK1 in the absence of TPA and A23187 (Table I; Figure 3D). SB203580 is a highly specific inhibitor of p38 (Lee et al., 1994), indicating that the enhancement of transcriptional activity was mediated by activation of the p38 pathway. These results collectively indicate that the JNK and p38 signaling pathways as well as the NFAT pathway are targets of CsA and FK506.
Fig. 3. NFAT and MAPK family pathways are independent targets of CsA and FK506. (A) BHK cells transfected with NFAT4 or RHR4 were stimulated with (Ca2+) or without (none) A23187 in the presence (+CsA) or absence of CsA. After 20 min, NFAT4 and RHR4, both of which are GFP fusion proteins, were observed under a fluorescence microscope. (B) A human T-cell line, Jurkat, was transfected with reporter constructs and expression vectors as indicated, followed by incubation for 48 h, and assayed for luciferase activities. After 40 h incubation, the cells were stimulated with TPA (T) and/or A23187 (I) with or without CsA for a further 8 h. This experiment is a representative of five. (C) Jurkat cells were transfected with reporter constructs and expression vectors as indicated followed by incubation for 48 h, and assayed for luciferase activities. The cells were incubated for 48 h with vehicle or CsA. This experiment is a representative of five. (D) Jurkat cells were transfected with ΔMEKK1 and RHR4. The cells were incubated with vehicle, CsA, or SB203580 (SB) for 40 h and either left untreated or stimulated with TPA and A23187 for a further 8 h. This experiment is a representative of three. Luciferase activities are expressed as fold increases (B and C) or RLU (relative luciferase unit) (D) according to Berthold.
JNK and p38 signaling pathways are novel targets for immunotherapeutic application
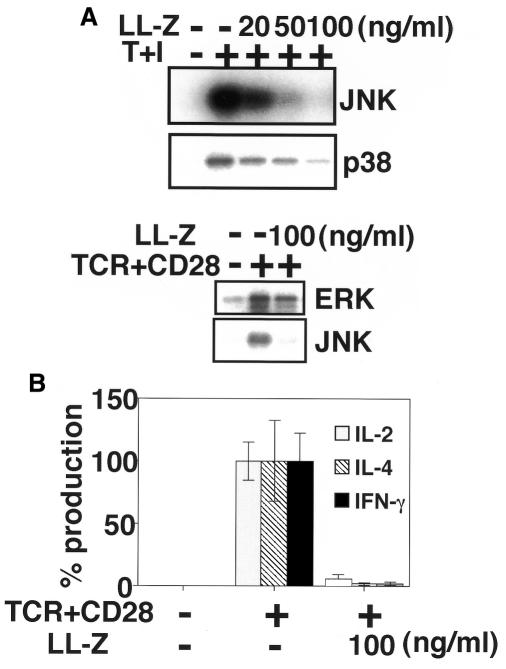
The above results collectively raise a possibility that T-cell specific JNK and p38 activation pathways provide us with novel targets for immunotherapeutic applications. Since JNK and p38 pathways constitute redundant signaling pathways (Matsuda et al., 1998) (Figure 3D), blockade of both pathways is likely to be required for the inhibition of T-cell activation. T-cell activation was indeed blocked by a radicicol-related macrocyclic nonaketide, LL-Z1640-2, known to inhibit both JNK and p38 pathways (Takehana et al., 1999) (Figure 4). While LL-Z1640-2 inhibited activation of both JNK and p38, it had little effect on ERK, NF-κB or Ca2+ pathways during T-cell activation (Figure 4A and data not shown). Consequently, LL-Z1640-2 markedly reduced the production of IL-2, IL-4 and interferon (IFN)-γ from mouse splenocytes stimulated with a combination of antibodies against TCR and CD28 (Figure 4B). These results clearly indicate that the JNK and p38 activation pathways are essential for cytokine production resulting from T-cell activation, and that a specific inhibitor for JNK and p38 pathways is a latent immunosuppressant.
Fig. 4. Blockade of JNK and p38 pathways suppresses T-cell activation. (A) Jurkat cells were pre-treated with the indicated concentrations of LL-Z1640-2 (LL-Z) for 1 h, followed by stimulation with TPA and A23187 (T+I; upper panel) or antibodies against TCR and CD28 (TCR+CD28; lower panel). The cells were then assayed for kinase activities as indicated. These experiments are a representative of three. (B) Splenocytes prepared from 8-week-old BALB/c mice were stimulated with antibodies against TCR and CD28 (TCR+CD28) in the presence or absence of LL-Z1640-2 (LL-Z) for 48 h. Levels of cytokines in culture supernatants were measured by ELISA. Three independent experiments were performed, and results are expressed as percentage means ± SD of the activity without drug (IL-2, 12.5 ± 1.9 ng/ml; IL-4, 407.7 ± 130.5 pg/ml; IFN-γ, 34.7 ± 7.6 ng/ml).
In summary, we have demonstrated that CsA and FK506 have two distinct mechanisms of action. One is inhibition of the protein phosphatase activity of calcineurin, leading to the blockade of the nuclear transport of NFAT transcription factors. The other is the calcineurin-independent inhibition of JNK and p38 activation pathways. It is likely that the presence of two distinct targets in T-cell activation makes CsA and FK506 highly potent immunosuppressive drugs. Although CsA and FK506 have made human organ transplantation nearly a routine practice, they have some toxic effects and sometimes cause serious complications such as renal dysfunction, hypertension, neurological toxicity, insulin-dependent diabetes and severe gastrointestinal disturbances (Sigal and Dumont, 1992). Future studies undertaken with the understanding that these drugs have two distinct mechanisms of action may reveal the causes of the above side-effects and facilitate the development of less toxic, novel immunosuppressive drugs.
METHODS
Cells and reagents. The cell lines Jurkat and SV40 large T-antigen transfected Jurkat (TAg-Jurkat) provided by G.R. Crabtree were cultured in RPMI1640/10% fetal calf serum (FCS). BHK cells were cultured in Dulbecco’s modified Eagle’s medium/10% FCS. Anti-ERK2, anti-JNK1 and anti-p38 antibodies were purchased from Santa Cruz. Anti-Fas mAb (clone CH-11) was purchased from MBL. An antibody specific for phosphorylated NFAT1 was purchased from Transduction Laboratories. Anti-Raf-1 and anti-MEKK1 antibodies were characterized previously (Matsuda et al., 1995). Jurkat cells were stimulated with 5 ng/ml TPA, 1 µg/ml A23187, or a combination of both. After 15 min incubation, lysates were prepared and subjected to immune complex kinase assays as described below. Alternatively, the cells were exposed to 0.7 M NaCl for 40 min or were treated with 500 ng/ml anti-Fas mAb for 3 h, and then assayed for JNK and p38 activities. Calcineurin and calmodulin were purchased from Sigma. Inhibitors included: 100 ng/ml CsA, 10 ng/ml FK506, 10 ng/ml rapamycin, 50 µM PD98059 and 5 µM SB203580 unless indicated otherwise.
Plasmid constructs. The mammalian expression vector for green fluorescent protein (GFP)–NFAT4 (pcDNA3-Myc-GFP-NFAT4) has been described previously (Shibasaki et al., 1996). The expression vector for GFP–RHR4 (pEGFP-RHR4) was constructed by PCR amplification of the Rel homology region of NFAT4 (residues 407–590) from pcDNA3-Myc-GFP-NFAT4, using oligonucleotides NFAT4-CT5′ (GCTCGAGCTGGCCACACCCCTATATTTCGC) and NFAT4-RHR3′ (CCTAAGCAGACCGCTGGGAGCACTC). After cleavage by XhoI and SalI, the PCR product was cloned into the pEGFP-C1 vector (Clontech). Expression of either GFP–NFAT4 or GFP–RHR4 in the transfected cells was visualized under a fluorescein isothiocyanate filter on a Zeiss Axiovert microscope equipped with a CCD camera containing a Kodak KAF1400 chip. The A238L fragment (Miskin et al., 1998) was excised from pcDNA3-A238L provided by L.K. Dixon and inserted into pEB6CAG (Niwa et al., 1991; Tanaka et al., 1999) to construct pEB6CAG-A238L. To express Cabin1 (Lai et al., 1998; Sun et al., 1998), the BamHI–NotI fragment of KIAA0330 provided by T. Nagase was cloned into the pEGFP-C2 vector (Clontech). Expression vectors for HA-JNK and HA-p38 under the control of the SRα promoter have been described previously (Moriguchi et al., 1997). The expression vector for ΔMEKK1 (pFC-MEKK1) was purchased from Stratagene.
Immune complex kinase assays. Immunoprecipitation was performed as described previously (Matsuda et al., 1995; Moriguchi et al., 1997). Immunoprecipitates were incubated with 3 µg of substrate (myelin basic protein for ERK, His-tagged c-Jun for JNK and His-tagged ATF2 for p38) for 20 min at 30°C in a final volume of 15 µl containing 20 mM Tris–HCl pH 7.5, 2 mM EGTA, 12.5 mM β-glycerophosphate, 2 mM dithiothreitol (DTT), 10 mM MgCl2 and 100 µM [γ-32P]ATP (74 kBq). Reactions were terminated by the addition of Laemmli’s sample buffer solution and boiling. Substrate phosphorylation was detected and quantified by autoradiography and image analyzer (Fujix BAS2000). To assay for Raf-1 activity, the immunoprecipitate was incubated with 1 µg of glutathione S-transferase (GST)–MAPKK and 3 µg of His-tagged kinase-negative MAPK for 40 min at 30°C in a final volume of 15 µl containing 20 mM Tris–HCl pH 7.5, 2 mM EGTA, 12.5 mM β-glycerophosphate, 2 mM DTT, 10 mM MgCl2 and 100 µM [γ-32P]ATP (74 kBq). Phospharylated kinase-negative MAPK was resolved by SDS–PAGE and quantified on an image analyzer (Fujix BAS2000). To assay for MEKK1 activity, the immunoprecipitate was incubated with 0.2 µg of GST–SEK1/MKK4 and 3 µg of His-tagged kinase-negative JNK for 60 min at 30°C in a final volume of 15 µl containing 20 mM Tris–HCl pH 7.5, 2 mM EGTA, 12.5 mM β-glycerophosphate, 2 mM DTT, 10 mM MgCl2 and 100 µM [γ-32P]ATP (185 kBq). Phosphorylated kinase-negative JNK was resolved by SDS–PAGE and quantified on an image analyzer (Fujix BAS2000).
Dephosphorylation of RII protein. GST-fused RII peptide (Blumenthal et al., 1986), referred to here as RII protein, immobilized on glutathione–Sepharose was phosphorylated by PKA catalytic subunit (Sigma) in the presence of [γ-32P]ATP. 32P-labeled RII protein was incubated with 200 nM calcineurin and 500 nM calmodulin for 30 min at 30°C in 50 µl of 20 mM HEPES pH 7.4, 2 mM CaCl2, 1 mM MnCl2 and 2 mM DTT. Where indicated, calcineurin/calmodulin was pre-incubated with 1 µM FR901725 on ice for 30 min. Released 32P was quantified in the supernatant after the addition of 500 µl of 10% trichloroacetic acid and 50 µl of 10 mg/ml bovine serum albumin.
Luciferase assays. TAg-Jurkat cells were transiently transfected with an IL-2 luciferase reporter (pIL-2-Luc; provided by M. Iwashima) or an NFAT Luciferase reporter [pNFAT-Luc (Northrop et al., 1993)], in combination with pRL-SV40 (Promega) for normalization. Transfections were performed using SuperFect (Qiagen) according to the manufacturer’s instructions. Luciferase activities in cell lysates were measured in duplicate on a luminometer (LB9507; Berthold), using the Dual-Luc assay system (Promega).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs T. Taniguchi, K. Matsumoto, T. Akiyama and L.K. Clayton for valuable discussion, and Drs G.R. Crabtree, L.K. Dixon, M. Iwashima, Y. Miwa, J. Miyazaki and T. Nagase for materials. This work was supported by grants-in-aid from the Ministry of Education, Science, Culture of Japan (to S.M. and S.K.), Keio University Grant-in-Aid for Encouragement of Young Medical Scientist (to S.M.), Keio Gijuku Academic Development Funds (to S.M.), The Ryoichi Naito Foundation for Medical Research (to S.K.) and the Toray Science Foundation (to S.K.).
REFERENCES
- Blumenthal D.K., Takio, K., Hansen, R.S. and Krebs, E.G. (1986) Dephosphorylation of cAMP-dependent protein kinase regulatory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J. Biol. Chem., 261, 8140–8145. [PubMed] [Google Scholar]
- Crabtree G.R. (1999) Generic signals and specific outcomes: Signaling through Ca2+, calcineurin and NF-AT. Cell, 96, 611–614. [DOI] [PubMed] [Google Scholar]
- Dumont F.J., Melino, M.R., Staruch, M.J., Koprak, S.L., Fischer, P.A. and Sigal, N.H. (1990) The immunosuppressive macrolides FK506 and rapamycin act as reciprocal antagonists in murine T cells. J. Immunol., 144, 1418–1424. [PubMed] [Google Scholar]
- Hoey T., Sun, Y.L., Williamson, K. and Xu, X. (1995) Isolation of two new members of the NF-AT proteins. Immunity, 2, 461–472. [DOI] [PubMed] [Google Scholar]
- Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem., 270, 16483–16486. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Burnett, P.E., Wolosker, H., Blackshaw, S. and Snyder, S.H. (1998) Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem., 273, 18325–18331. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C.A., Pleiman, C.M., Gardner, A.M., Blumer, K.J. and Johnson, G.L. (1993) A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science, 260, 315–319. [DOI] [PubMed] [Google Scholar]
- Lee J.C. et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature, 372, 739–746. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Kawasaki, H., Moriguchi, T., Gotoh, Y. and Nishida, E. (1995) Activation of protein kinase cascades by osmotic shock. J. Biol. Chem., 270, 12781–12786. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Moriguchi, T., Koyasu, S. and Nishida, E. (1998) T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J. Biol. Chem., 273, 12378–12382. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin, A., McMahon, M., Lange-Carter, C., Derijard, B., Davis, R.J., Johnson, G.L. and Karin, M. (1994) Diferential activation of ERK and JNK mitogen activated protein kinases by Raf-1 and MEKK. Science, 266, 1719–1723. [DOI] [PubMed] [Google Scholar]
- Miskin J.E., Abrams, C.C., Goatley, L.C. and Dixon, L.K. (1998) A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science, 281, 562–565. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Toyoshima, F., Masuyama, N., Hanafusa, H., Gotoh, Y. and Nishida, E. (1997) A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J., 16, 7045–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E. and Gotoh, Y. (1993) The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci., 18, 128–131. [DOI] [PubMed] [Google Scholar]
- Niwa H., Yamamura, K. and Miyazaki, J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 108, 193–200. [DOI] [PubMed] [Google Scholar]
- Northrop J.P., Ullman, K.S. and Crabtree, G.R. (1993) Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem., 268, 2917–2923. [PubMed] [Google Scholar]
- Rao A., Luo, C. and Hogan, P.G. (1997) Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol., 15, 707–748. [DOI] [PubMed] [Google Scholar]
- Schaeffer H.J. and Weber, M.J. (1999) Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol. Cell. Biol., 19, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S.L. (1992) Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell, 70, 365–368. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Price, E.R., Milan, D. and McKeon, F. (1996) Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature, 382, 370–373. [DOI] [PubMed] [Google Scholar]
- Sigal N.H. and Dumont, F.J. (1992) Cyclosporin A, FK-506 and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu. Rev. Immunol., 10, 519–560. [DOI] [PubMed] [Google Scholar]
- Su B., Jacinto, E., Hibi, M., Kallunki, T., Karin, M. and Ben-Neriah, Y. (1994) JNK is involved in signal integration during costimulation of T lymphocytes. Cell, 77, 727–736. [DOI] [PubMed] [Google Scholar]
- Sun L., Youn, H.-D., Loh, C., Stolow, M., He, W. and Liu, J.O. (1998) Cabin1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity, 8, 703–711. [DOI] [PubMed] [Google Scholar]
- Takehana K., Sato, S.-i., Kobayasi, T. and Maeda, T. (1999) A radicicol-related macrocyclic nonaketide compound, antibiotic LL-Z1640-2, inhibits the JNK/p38 pathways in signal-specific manner. Biochem. Biophys. Res. Commun., 257, 19–23. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Miwa, Y., Miyoshi, K., Ueno, A. and Inoue, H. (1999) Construction of Epstein–Barr virus-based expression vector containing mini-OriP. Biochem. Biophys. Res. Commun., 264, 938–943. [DOI] [PubMed] [Google Scholar]
- Wolfe S.A., Zhou, P., Dotsch, V., Chen, L., You, A., Ho, S.N., Crabtree, G.R., Wagner, G. and Verdine, G.L. (1997) Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature, 385, 172–176. [DOI] [PubMed] [Google Scholar]