Abstract
Recent studies have reported new mechanisms that mediate the transcriptional synergy of strong tissue-specific enhancers, involving the cooperative assembly of higher-order nucleoprotein complexes called enhanceosomes. Here we show that the HPV18 enhancer, which controls the epithelial-specific transcription of the E6 and E7 transforming genes, exhibits characteristic features of these structures. We used deletion experiments to show that a core enhancer element cooperates, in a specific helical phasing, with distant essential factors binding to the ends of the enhancer. This core sequence, binding a Jun B/Fra-2 heterodimer, cooperatively recruits the architectural protein HMG-I(Y) in a nucleoprotein complex, where they interact with each other. Therefore, in HeLa cells, HPV18 transcription seems to depend upon the assembly of an enhanceosome containing multiple cellular factors recruited by a core sequence interacting with AP1 and HMG-I(Y).
INTRODUCTION
Papillomavirus infection induces benign dysplastic lesions of the epidermis, such as skin warts. Cervical lesions containing high-risk human papillomaviruses (HPV), such as types 16 and 18, are prone to progress to malignancy. These viruses are found in 80% of cervical carcinoma lesions, suggesting that they are the etiological agents of these tumours. HPV18, which is associated with more advanced neoplasia (Arends et al., 1993), expresses two oncogenes, E6 and E7, that neutralize the products of cellular tumour suppressor genes, p53 and pRb, respectively (Dyson et al., 1989; Scheffner et al., 1990).
In carcinoma cells, HPV18 E6 and E7 oncogenes are transcribed from a single promoter P105, which is activated by a cell-specific enhancer of 230 bp (Garcia-Carranca et al., 1988). The HPV18 enhancer exhibits a cellular specificity that restricts its activity to epithelial cells, such as keratinocytes and cervical carcinoma cell lines (Bouallaga and Thierry, 1999). The molecular basis of this specificity is still unresolved. Putative binding sites for various cellular transcription factors can be predicted from the sequences of these enhancers including AP1, Oct-1 and NF-1 (O’Connor et al., 1995). However, with the exception of the AP1 binding site, directed mutagenesis of the predicted binding sites had little effect on the enhancer activity (Thierry et al., 1992; Butz and Hoppe-Seyler, 1993).
Recent studies on the transcription regulatory region of the interferon-β (IFNβ) gene have revealed that the synergy and tissue specificity of enhancers can be conferred by the assembly of higher-order nucleoprotein complexes called enhanceosomes (Thanos and Maniatis, 1995). Enhanceosomes are characterized by the cooperative formation of a three-dimensional structure depending on architectural factors that bend DNA, such as HMG-I(Y) (Falvo et al., 1995), and on the stereo-alignment of transcription factors interacting with the enhancer sequences (Thanos and Maniatis, 1995). These structures create a specific activating surface that recruits transcriptional activators like CBP/p300 (Merika et al., 1998) and the basal transcriptional complex (Kim et al., 1998).
In this study, we used both functional and biochemical approaches to decipher the high transcriptional activity of the HPV18 enhancer in HeLa cells. Deletion mapping experiments showed that a minimal core enhancer sequence of 109 bp, including an AP1 binding site, cooperates in a specific helical phasing with essential factors situated at the 5′ and 3′ ends of the enhancer. This minimal core could cooperatively recruit a nucleoprotein complex, containing the Jun B/Fra-2 heterodimer and the HMG-I(Y) architectural protein. Moreover, GST pull-down experiments indicated that these proteins interact with each other. Added to the strong tissue specificity of this enhancer, these features are characteristic of enhanceosomes.
RESULTS
Deletion analysis of the HPV18 enhancer
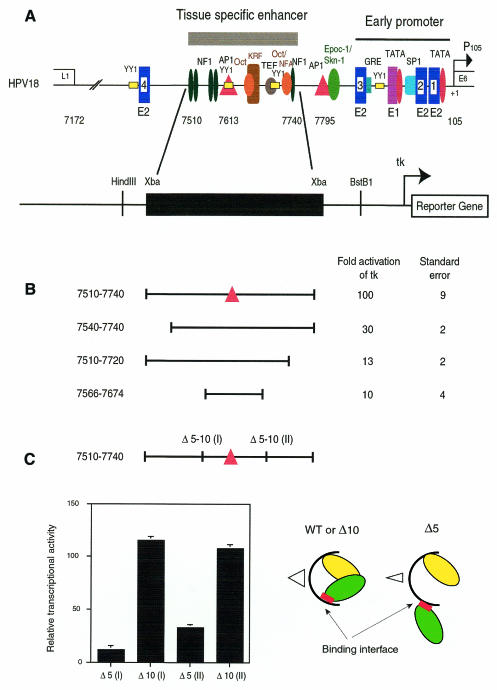
The HPV18 enhancer sequence has been previously defined as a 230 bp fragment whose activity is dependent on a central AP1 binding site (Figure 1A). Footprinting experiments performed with the 230 bp fragment indicated that it was almost completely protected on both strands by HeLa nuclear extracts, including two large footprints at its 5′ and 3′ ends (data not shown). To appreciate the functional importance of these regions, we deleted 30 nucleotides at the 5′ end of the enhancer or 40 nucleotides at its 3′ end. In HeLa cells, these deleted sequences lost 70% or more of the transcriptional activity of the entire enhancer, indicating that at least two essential factors were binding to both ends of the 230 bp enhancer fragment (Figure 1B).
Fig. 1. Deletion analysis of the HPV18 enhancer. (A) Schematic representation of the HPV18 long control region (LCR). Predicted binding sites for cellular and viral proteins were represented along the LCR of HPV18, which is composed of three functionally distinct regions: a proximal region, containing the early promoter P105 that is devoid of intrinsic transcriptional activity; an upstream tissue-specific enhancer of 230 nucleotides between nucleotides 7510 and 7740; and a 5′ region dispensable for the P105 transcriptional activation (Garcia-Carranca et al., 1988). The enhancer was cloned upstream of the heterologous minimal tk promoter in front of the CAT reporter gene, as well as 3′ or 5′ deletion mutants (B) and stereo-alignment mutants (C). Internal deletions of half a turn (five nucleotides) or a complete turn (10 nucleotides) of the DNA helix were made either upstream, between nucleotides 7594 and 7598 for Δ5(I), or 7589 and 7598 for Δ10(I), or downstream, between nucleotides 7677 and 7681 for Δ5(II), or 7672 and 7681 for Δ10(II), of the 109 bp core fragment. Structural effects of these deletions were schematized on the right, underlining the loss of synergy. CAT activities are the mean of at least three independent transfection experiments.
Then we searched for the smallest fragment that could still activate the basal promoter, by combining 5′ and 3′ deletions around the AP1 binding site. A fragment of 109 bp (nucleotides 7566–7674) showed a residual activity of ∼10% of the full-length enhancer, corresponding to a 10-fold activation of the tk promoter (Figure 1B). Further deletion of this fragment led to a total loss of enhancer activity (not shown). Therefore, this fragment was identified as the core of the enhancer.
To pinpoint possible interactions between the core structure and the factors binding to the ends of the enhancer, internal deletions of half a turn of the DNA helix (five nucleotides) that disturb a potential stereo-alignment of DNA bound proteins, or a complete turn of the DNA helix (10 nucleotides) that re-established the stereo-alignment, were introduced upstream and downstream of the core DNA sequences (Figure 1C), in sequences that were only partially protected in Dnase I footprinting experiments (data not shown). Internal deletions of five nucleotides either upstream or downstream of the AP1 binding site resulted in a 90 or 70% decrease in enhancer activity. In contrast, overlapping deletions of 10 nucleotides restored the full enhancer activity (Figure 1C).
The smallest transcriptionally active fragment of the HPV18 enhancer cooperatively recruits a complex containing AP1 and the architectural protein HMG-I(Y)
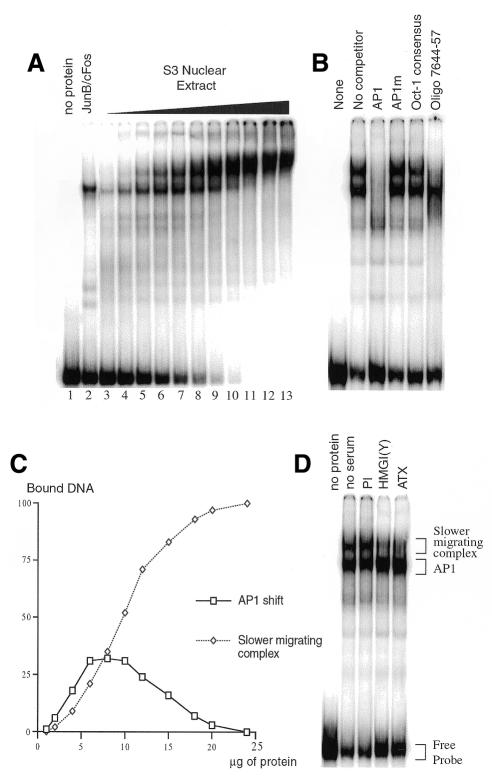
To study the core enhancer components, we used the 109 bp enhancer fragment (nucleotides 7566–7674) in gel shift assays with various concentrations of HeLa nuclear extracts. When small amounts of nuclear extract, up to 4 µg, were incubated with the 109 bp probe, we observed a band-shift at a position similar to that obtained with an in vitro-translated AP1 heterodimer (Figure 2A, lanes 3–5 compared with lane 2). A slower migrating complex appeared when the amount of nuclear extract was increased (Figure 2A, lanes 6–13). Both shifted bands were specifically competed by excess AP1 oligonucleotide, indicating that they both contain an AP1 factor (Figure 2B). Besides the essential AP1, a short sequence at position 7644–57 containing a non-consensus Oct binding site (GCTTGCAT) was required for the formation of the slower migrating complex, as shown by competition experiments, while the consensus Oct (ATTTGCAT) did not affect its formation (Figure 2B).
Fig. 2. Cooperative formation of a higher-order complex containing AP1 and HMG-I(Y). (A) The 109 bp fragment was labelled by Klenow filling and incubated with increasing concentrations of HeLa nuclear extract (1, 2, 4, 6, 8, 10, 12, 15, 18, 20 and 24 µg, respectively, in lanes 3–13; no extract in lane 1), or with 1 µl of an in vitro-translated AP1 heterodimer (Jun B/c-Fos), before loading onto a 5% native polyacrylamide gel. (B) Competition experiments were performed with 10 µg of nuclear extract in the presence of an 80-fold excess of oligonucleotides containing either the wild-type or mutated AP1 binding site, and either a consensus or non-consensus (Oligo 7644-57) Oct binding site. (C) The graph presents quantification of the radioactivity found in the two bound complexes relative to the total radioactivity of the probe, plotted against the amount of nuclear proteins used in the assays shown in (A). (D) Presence of HMG-I(Y) in the slower migrating complex was assessed by use of two specific antibodies prepared either against the full-length HMG-I(Y) protein, or its DNA binding domain (ATX).
Values of the PhosphorImager quantification of the retarded complexes in the gel shift electrophoresis were plotted against concentrations of protein extract (Figure 2C). The AP1 shift increased to reach 28% of bound DNA with ∼8 µg of nuclear extract. At higher protein concentrations, the slower migrating complex accumulated at the expense of AP1, until reaching 100% of the bound probe with ∼20 µg of nuclear extract. Formation of the slower migrating complex is sigmoidal, indicating a clear cooperativity in the recruitment of additional factor(s). Therefore, the 109 bp fragment can recruit a complex whose formation is cooperative and absolutely dependent on AP1 binding.
The HMG-I(Y) protein is an architectural protein involved in enhanceosome structures (Yie et al., 1999), binding to A–T rich sequences including Oct like binding sites (Eckner and Birnstiel, 1989). It was also shown to interact with AP1 factors like c-Jun and ATF-2 in the IFNβ enhanceosome (Du et al., 1993). Therefore, we tested the presence of the HMG-I(Y) protein in the slower migrating complex by using two antibodies directed against the full-length protein (αHMG) or its DNA binding domain (αATX). Addition of these antibodies specifically removed the slower migrating complex and increased the AP1 binding, indicating that HMG-I(Y) is a component of the higher-order nucleoprotein complex (Figure 2D). The binding site for HMG-I(Y) may be the non-consensus Oct binding site required for the formation of the slower migrating complex.
Model of the HPV18 enhanceosome
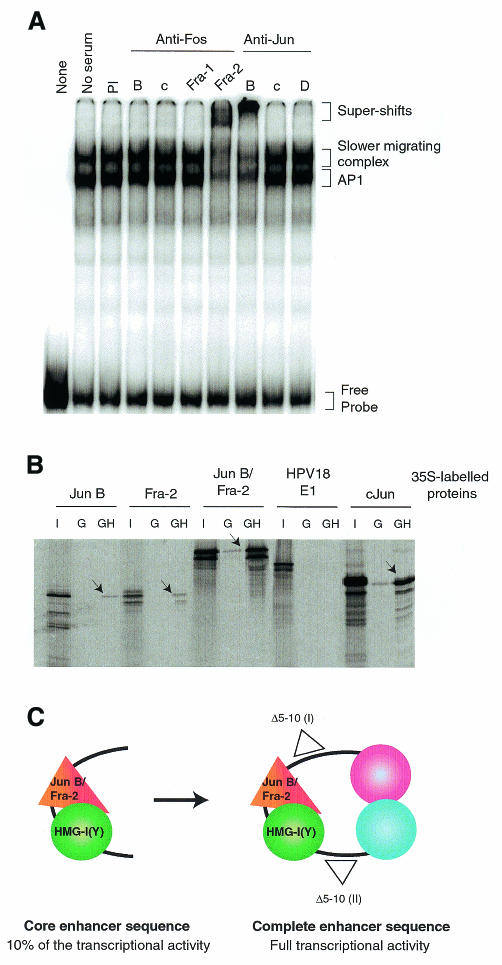
To precisely identify the protein–protein interactions within the enhancer structure, we explored the composition of AP1 in the retarded complexes in gel shift assays using specific antibodies prepared against every member of the Jun and Fos families (Lallemand et al., 1997). We verified that, in HeLa cells as in primary keratinocytes, the predominant Jun member was Jun B and that this factor belongs to the nucleoprotein complex (Figure 3A). We also found that the Fos member involved was Fra-2, whereas the other AP1 members were excluded from the complexes (Figure 3A).
Fig. 3. A model for the structure of the HPV18 enhanceosome. (A) The AP1 factor binding the HPV18 enhancer is a Jun B–Fra-2 heterodimer. Eight micrograms of nuclear extract were incubated with the 109 bp fragment in the absence, or in the presence, of pre-immune serum (PI) or of specific antibodies against Fos B, c-Fos, Fra-1, Fra-2, Jun B, c-Jun or Jun D, as indicated. (B) HMG-I(Y) interacts specifically with Jun B and Fra-2. The GST–HMG-I(Y) protein was incubated with the 35S-labelled in vitro-translated proteins JunB, Fra-2 or the Jun B–Fra-2 fusion protein, the HPV18 E1 protein as a negative control, or cJun as a positive control. One tenth of the input proteins were loaded (I) as well as reactions with the GST (G) alone and the GST–HMG-I(Y) fusion (GH) proteins. (C) The core enhancer sequence containing nucleotides 7566–7674 binds the Jun B/Fra-2 heterodimer and the architectural factor HMG-I(Y), forming a cooperative complex that may include other factor(s). This core sequence cooperates in a stereo-specific manner with at least two distal factors.
GST pull-down experiments indicated that HMG-I(Y) could interact weakly with Jun B, and with Fra-2, but not with the HPV18 E1 protein (Figure 3B). In contrast, when using a fusion protein of Jun B and Fra-2 (L. Bakiri, personal communication), this interaction was greatly enhanced, revealing another level of cooperativity.
We propose a model in which the Jun B/Fra-2 heterodimer and HMG-I(Y) interact with the DNA and with each other, to form a core enhancer (Figure 3C). This core cooperates, in a stereo-specific manner, with transcription factors that bind to the ends of the 230 bp enhancer, to form a structure that can synergistically activate transcription of the minimal tk promoter >100-fold in HeLa cells.
DISCUSSION
Transcriptional enhancers are not constituted solely of the linear alignment of multiple factors on the DNA. Transcriptional activation requires the establishment of functional interfaces from multiple protein–DNA and protein–protein interactions of regulatory factors. Since HPV transcriptional control could not be explained by binding of crucial specific transcription factors (O’Connor et al., 1995), we explored the possible existence of a more complex structure, and we showed that the HPV18 enhancer displays several features of enhanceosomes: (i) the activity of the enhancer is synergistic and tissue specific; (ii) stereo-alignment of factor binding sites within the enhancer is crucial for full transcriptional activity; (iii) the core enhancer sequence cooperatively forms a higher-order nucleoprotein complex containing AP1; (iv) the architectural protein HMG-I(Y) is required for the formation of this nucleoprotein complex; and (v) this protein interacts cooperatively with the Jun B/Fra-2 heterodimer. Therefore, the HPV18 enhancer activates transcription via a three-dimensional structure resembling the previously described prototypic enhanceosomes of IFNβ (Thanos and Maniatis, 1995) and TCRα (Giese et al., 1995).
Architectural proteins are essential elements of enhanceosome structures. They interact via an HMG binding motif with the minor groove of the DNA helix and, by altering the structure of the DNA, allow cooperative recruitment of activators. HMG-I(Y) can interact, at the protein–protein level, with an unusually diverse array of structurally unrelated proteins including NFκB, ATF2–c-Jun, Elf-1 and Tst-1–Oct proteins (Du and Maniatis, 1994; John et al., 1995; Leger et al., 1995; Abdulkadir et al., 1998; Yie et al., 1999), and JunB/Fra-2 as shown in the present paper. However, other factor(s) may be involved in the HPV18 core enhancer complex since reconstitution in vitro with purified JunB/Fra-2 and HMGI(Y) proteins was not possible (data not shown).
An interesting question concerns the relationship between enhanceosome and chromatin structure, since the HPV16 and HPV18 regulatory regions have been shown to contain nucleosomes (Stunkel and Bernard, 1999). It has been reported that the TCRα enhanceosome assembles specifically on chromatin templates (Mayall et al., 1997). Regarding the HPV18 enhancer, involvement of a nucleosomal structure is further supported by our previous finding that hbrm, a member of the SWI/SNF remodelling complex, activates transcription of the HPV18 enhancer (Medina-Martinez et al., 1996). On the other hand, enhanceosomes have been shown to recruit the CBP/p300 histone acetyl transferase (Carey, 1998). Therefore, these various elements strongly suggest that both structures are co-existing in vivo.
One remarkable feature of the enhanceosomes so far described is that organization of promiscuous transcription factors in a three-dimensional structure constitutes a basis for their cell specificity (Carey, 1998). Indeed, the very tight transcriptional regulation of HPV18 E6 and E7 transforming genes restricts their expression in keratinocytes and cervical carcinoma cells. Whether or not some of the factors interacting with the HPV18 enhancer are involved in its cell specificity remains to be demonstrated. An alternative mechanism could be that a specific factor would interact with the new interface created by the higher-order nucleoprotein rather than with a specific DNA sequence. This would be reminiscent of MHC class II transcriptional regulation by the CIITA coactivator (Mach, 1999; Masternak et al., 2000). Such specific coactivators have not yet been described for keratinocyte-specific transcription, but would represent putative targets for therapeutic antiviral approaches.
In conclusion, our results show that the high HPV18 early transcription in HeLa cells depends on a structure that may constitute a model of epitheliospecific enhanceosome.
METHODS
Plasmids and transfections. A tk-CAT reporter plasmid was used to clone the enhancer fragments upstream of the minimal tk promoter (Thierry et al., 1990). The deleted enhancer fragments were obtained by a PCR-based technique and were inserted in a XbaI restriction site located upstream of tk. Transfection experiments of HeLa cells were carried out with 4 µg of plasmids, by the calcium phosphate coprecipitation method, in 6 cm Petri dishes. HeLa cells were harvested 40 h after transfection. All transfections were done in the presence of the reference plasmid pSVE β-Gal to standardize the transfection efficiencies.
Gel shift assays. The DNA probes used were prepared by PCR amplification, followed by quantification using the ‘DNA Quant’ kit (Promega). Fragments (3.3 pmoles) were labelled by Klenow filling in the presence of 10 µCi of [α-32P]dATP. Nuclear extracts, prepared according to Dignam (Dignam et al., 1983) from HeLa cells grown in suspension, were pre-incubated with 1 µg of poly(dI–dC) for 30 min before addition of 0.04 pmoles of the labelled DNA probe. After 5 min of incubation, samples were loaded on a 5% native polyacrylamide gel.
When indicated, an 80-fold excess of unlabelled competitors was added during pre-incubation. These probes were obtained by hybridization of the two oligonucleotides: 5′-GATCCTATTAGTCATTG-3′ and 5′-GATCCAATGACTAATAG-3′ for wild-type AP1; 5′-CTAGAATATAAGTAAGCT-3′ and 5′-CTAGAGCTTACTTATATT-3′ for mutated AP1; 5′-TGTCGAATGCAAATCACT-3′ and 5′-TCTAGTGATTTGCATTCG-3′ for the consensus Oct binding site; and 5′-GATCCGCTTGCATAACTAT-3′ and 5′-GATCCATAGTTATGCAAGC-3′ for Oligo 7644-57.
In supershift assays, nuclear extracts were pre-incubated at room temperature for 30 min before addition of the labelled probe, with 1 µl of preimmune antiserum, or specific antiserum against members of the Jun and Fos families (Lallemand et al., 1997), or with antibodies against the full-length HMG-I(Y) protein (αHMG) or its DNA binding domain (αATX) (Amirand et al., 1998).
GST pull-down experiments. The GST-HMG-I(Y) fusion vector was constructed by inserting a PCR fragment in the pGEX 4T-1 plasmid (Pharmacia Biotech). GST fusion proteins on beads (12 µl) were incubated overnight with 20 µl of 35S-labelled in vitro-translated proteins in 300 µl of A250 buffer (25 mM Tris pH 7.5, 15 mM MgCl2, 15 mM EGTA, 10% glycerol, 0.3% Triton, 250 mM NaCl, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 0.1% bovine serum albumin). The beads were washed four times with the A250 and proteins were resolved by SDS–PAGE.
Acknowledgments
ACKNOWLEDGEMENTS
We thank P. Debey, M. Chebrout and E. Käs for the kind gift of the HMG-I(Y) antibodies; T. Maniatis for HMG-I(Y) expression vectors; L. Bakiri for the AP1 expression vectors; D. Lallemand and Y. Spyrou for the Jun/Fos specific antibodies. We are very grateful to C. Demeret, C. Desaintes and J. Weitzman for critical reading of the manuscript. This work was supported by the ‘Association pour la recherche contre le cancer’ (ARC) and the ‘Ministère de l’éducation et de la recherche’ (MESR).
REFERENCES
- Abdulkadir S.A., Casolaro, V., Tai, A.K., Thanos, D. and Ono, S.J. (1998) High mobility group I/Y protein functions as a specific cofactor for Oct-2A: mapping of interaction domains. J. Leukoc. Biol., 64, 681–691. [DOI] [PubMed] [Google Scholar]
- Amirand C., Viari, A., Ballini, J.P., Rezaei, H., Beaujean, N., Jullien, D., Kas, E. and Debey, P. (1998) Three distinct sub-nuclear populations of HMG-I protein of different properties revealed by co-localization image analysis. J. Cell Sci., 111, 3551–3561. [DOI] [PubMed] [Google Scholar]
- Arends M.J., Donaldson, Y.K., Duvall, E., Wyllie, A.H. and Bird, C.C. (1993) Human papillomavirus type 18 associates with more advanced cervical neoplasia than human papillomavirus type 16. Hum. Pathol., 24, 432–437. [DOI] [PubMed] [Google Scholar]
- Bouallaga I. and Thierry, F. (1999) Control of Human Papillomavirus type 18 transcription: role in carcinogenesis. Recent Res. Devel. Virol., 1, 369–383. [Google Scholar]
- Butz K. and Hoppe-Seyler, F. (1993) Transcriptional control of human papillomavirus (HPV) oncogene expression: composition of the HPV type 18 upstream regulatory region. J. Virol., 67, 6476–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. (1998) The enhanceosome and transcriptional synergy. Cell, 92, 5–8. [DOI] [PubMed] [Google Scholar]
- Dignam J.D., Lebovitz, R.M. and Roeder, R.G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W. and Maniatis, T. (1994) The high mobility group protein HMG I(Y) can stimulate or inhibit DNA binding of distinct transcription factor ATF-2 isoforms. Proc. Natl Acad. Sci. USA, 91, 11318–11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Thanos, D. and Maniatis, T. (1993) Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell, 74, 887–898. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley, P.M., Münger, K. and Harlow, E. (1989) The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science, 243, 934–937. [DOI] [PubMed] [Google Scholar]
- Eckner R. and Birnstiel, M.L. (1989) Cloning of cDNAs coding for human HMG I and HMG Y proteins: both are capable of binding to the octamer sequence motif. Nucleic Acids Res., 17, 5947–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo J.V., Thanos, D. and Maniatis, T. (1995) Reversal of intrinsic DNA bends in the IFN β gene enhancer by transcription factors and the architectural protein HMG I(Y). Cell, 83, 1101–1111. [DOI] [PubMed] [Google Scholar]
- Garcia-Carranca A., Thierry, F. and Yaniv, M. (1988) Interplay of viral and cellular proteins along the long control region of human papillomavirus type 18. J. Virol., 62, 4321–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K., Kingsley, C., Kirshner, J.R. and Grosschedl, R. (1995) Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein–protein interactions. Genes Dev., 9, 995–1008. [DOI] [PubMed] [Google Scholar]
- John S., Reeves, R.B., Lin, J.X., Child, R., Leiden, J.M., Thompson, C.B. and Leonard, W.J. (1995) Regulation of cell-type-specific interleukin-2 receptor α-chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-κB family proteins. Mol. Cell. Biol., 15, 1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.K., Kim, T.H. and Maniatis, T. (1998) Efficient recruitment of TFIIB and CBP-RNA polymerase II holoenzyme by an interferon-β enhanceosome in vitro. Proc. Natl Acad. Sci. USA, 95, 12191–12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D., Spyrou, G., Yaniv, M. and Pfarr, C.M. (1997) Variations in Jun and Fos protein expression and AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene, 14, 819–830. [DOI] [PubMed] [Google Scholar]
- Leger H., Sock, E., Renner, K., Grummt, F. and Wegner, M. (1995) Functional interaction between the POU domain protein Tst-1/Oct-6 and the high-mobility-group protein HMG-I/Y. Mol. Cell. Biol., 15, 3738–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B. (1999) Perspectives: immunology. Regulating the regulator. Science, 285, 1367. [DOI] [PubMed] [Google Scholar]
- Masternak K., Muhlethaler-Mottet, A., Villard, J., Zufferey, M., Steimle, V. and Reith, W. (2000) CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev., 14, 1156–1166. [PMC free article] [PubMed] [Google Scholar]
- Mayall T.P., Sheridan, P.L., Montminy, M.R. and Jones, K.A. (1997) Distinct roles for P-CREB and LEF-1 in TCR α enhancer assembly and activation on chromatin templates in vitro. Genes Dev., 11, 887–899. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O., Morales-Peza, N., Yaniv, M., Garcia-Carranca, A. and Thierry, F. (1996) A single element mediates glucocorticoid hormone response of HPV18 with no functional interactions with AP1 or hbrm. Virology, 217, 392–396. [DOI] [PubMed] [Google Scholar]
- Merika M., Williams, A.J., Chen, G., Collins, T. and Thanos, D. (1998) Recruitment of CBP/p300 by the IFN-β enhanceosome is required for synergistic activation of transcription. Mol. Cell, 1, 277–287. [DOI] [PubMed] [Google Scholar]
- O’Connor M., Chan, S. and Bernard, H.U. (1995) Transcription factor binding sites in the long control region of genital HPVs. In Myers, G., Bernard, H.U., Delius, H., Baker, K., Icenogle, J., Halpern, A. and Wheeler, C. (eds), Human Papillomaviruses, 1995 Compendium, Part III. Los Alamos National Laboratory, Los Alamos, NM, pp. 21–40.
- Scheffner M., Werness, B.A., Huibregtse, J.M., Levine, A.J. and Howley, P.M. (1990) The E6 oncoprotein encoded by human papillomaviruses types 16 and 18 promotes the degradation of p53. Cell, 63, 1129–1136. [DOI] [PubMed] [Google Scholar]
- Stunkel W. and Bernard, H.U. (1999) The chromatin structure of the long control region of human papillomavirus type 16 represses viral oncoprotein expression. J. Virol., 73, 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D. and Maniatis, T. (1995) Virus induction of human IFNβ gene expression requires the assembly of an enhanceosome. Cell, 83, 1091–1100. [DOI] [PubMed] [Google Scholar]
- Thierry F., Dostatni, N., Arnos, F. and Yaniv, M. (1990) Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol. Cell. Biol., 10, 4431–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Spyrou, G., Yaniv, M. and Howley, P.M. (1992) Two AP1 sites binding JunB are essential for HPV18 transcription in keratinocytes. J. Virol., 66, 3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yie J., Merika, M., Munshi, N., Chen, G. and Thanos, D. (1999) The role of HMG I(Y) in the assembly and function of the IFN-β enhanceosome. EMBO J., 18, 3074–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]