Abstract
Objective
To evaluate the perioperative as well as early oncological outcomes of patients undergoing robotic retroperitoneal lymph node dissection for treatment of testicular cancer.
Methods
We conducted a prospective consecutive case series of patients undergoing robotic assisted retroperitoneal lymph node dissection for metastatic testicular cancer between May 2018 and July 2021 at our institution. Data were collected on patient and tumour characteristics, intraoperative and postoperative parameters, and functional and oncological outcomes. Descriptive statistics are presented.
Results
Nineteen patients were identified; 18 (94.7%) completed the procedure robotically and one was converted to open surgery; 78.9% of patients had stage ≥IIB and 12 (63.2%) patients had undergone prior chemotherapy. The median operative time was 300 (interquartile range [IQR] 240–315) min. Median blood loss was 100 (IQR 50–175) mL. Median length of stay was 2 (range 1–11) days. All robotically completed patients commenced diet and passed flatus on Day 1 and were discharged by Day 3. The median lymph node yield was 40.5 (IQR 38–51) nodes. All patients undergoing nerve-sparing procedures recovered antegrade ejaculatory function. One patient had a Clavien-Dindo III complication (chylous ascites requiring drainage). At a median follow-up of 22.3 (IQR 16.3–24.9) months, one patient developed retroperitoneal recurrence, which was successfully treated with second-line chemotherapy; no other patients have had recurrences.
Conclusion
Robotic retroperitoneal lymph node dissection is a safe and feasible alternative to open surgery in appropriately selected patients, offering low morbidity. Early oncological outcomes are promising. Larger cohorts and longer follow-ups are required to validate our institution's findings.
Keywords: Retroperitoneal lymph node dissection, Robotic surgery, Testicular cancer, Retroperitoneal node dissection
1. Introduction
Testicular cancer is the most common malignancy affecting young men, accounting for 1% of all cancers in men [1]. Approximately 25%–35% of patients will have metastatic disease on presentation, of which 90% will have disease confined to the retroperitoneum [2]. A variety of treatment options are available for patients with retroperitoneal disease, including retroperitoneal node dissection (RPLND), chemotherapy, and radiotherapy.
Prior to 1980s, primary RPLND was often favoured because the surgical morbidity was considered less severe than the toxicity associated with platinum-based chemotherapy [3]. Since then, there has been a precipitous fall in the toxicity of chemotherapy, with new supportive therapies such as novel anti-emetics and colony-stimulating growth factors decreasing treatment-related morbidity and discontinuation [4].
Although the advent of nerve-sparing techniques and modified templates reduced the sexual side effects of RPLND, the procedure itself continued to have significant morbidity and a long convalescence even at high-volume centres [5]. Both chemotherapy and radiotherapy, however, have long-term side effects, which have been shown to affect patients’ survival, most notably pulmonary [6] and cardiac toxicity [7], as well as increased risk of secondary malignancy [8,9]. Side effects such as hypogonadism, infertility, peripheral neuropathy, and ototoxicity have a significant effect on quality of life [10]. Given the young age of most testicular cancer patients, these survival and quality of life data should be weighed heavily in treatment decisions.
Minimally invasive surgery has emerged as an alternative to open surgery with the aim of reduction in surgical morbidity, and thus a potential alternative to primary chemotherapy or radiotherapy in early stage patients. Limited data suggest equivalent short- and medium-term oncological outcomes to open surgery in expert centres [11].
Herein, we present Australia's first case series of patients who have undergone robotic-RPLND (R-RPLND), the largest case series to date outside the United States and Europe.
2. Patients and methods
We conducted a prospective consecutive case series of patients undergoing R-RPLND at a single centre by a single surgeon (Ahmadi N) between May 2018 and July 2021. Ethical approval was granted by the Sydney Local Health District Ethics Review Committee (approval number X20-0194). All patients provided informed consents for surgery and participation in this series. The institution is one of the highest volume institutions for RPLND in Australia and a tertiary referral centre for testicular cancer. Demographic, intraoperative and postoperative data, complications, and functional outcomes were collected prospectively. Complications (90-day postoperative) were based on Clavien-Dindo classification.
R-RPLND was offered according to current guidelines and Australian practice patterns. All patients had stage II disease, either enlarged retroperitoneal nodes or post-chemotherapy residual masses. Patients were carefully selected based on tumour size, location, and respectability of nodal mass especially in relation to blood vessels. Patients with circumferential involvement of great vessels, great vessel invasion, and supra hilar nodal involvement were deemed unsuitable for this procedure. Three patients underwent open RPLND during the recruitment time period of this study, two of which required inferior vena cava resection and grafting.
All surgeries were performed using the Da Vinci Xi robotic surgery system (Intuitive Surgical Inc, Sunnyvale, CA, USA) with patient in supine position. Our approach for these cases were similar to that previously reported [12], using a transperitoneal technique with the patient in the Trendelenburg position at approximately 24-degree head down with 10-degree neck flexion.
A comprehensive anaesthetic assessment was performed including preoperative intraocular pressure measurement. Total intravenous anaesthesia was used in all cases. Anaesthetic considerations include meticulous pressure area care, appropriate fluid management, lower fraction oxygen for patients with previous bleomycin exposure, and opioid sparing to aid bowel recovery. Postoperative analgesia included regular paracetamol and a non-steroidal anti-inflammatory with a fast-release opioid for breakthrough pain.
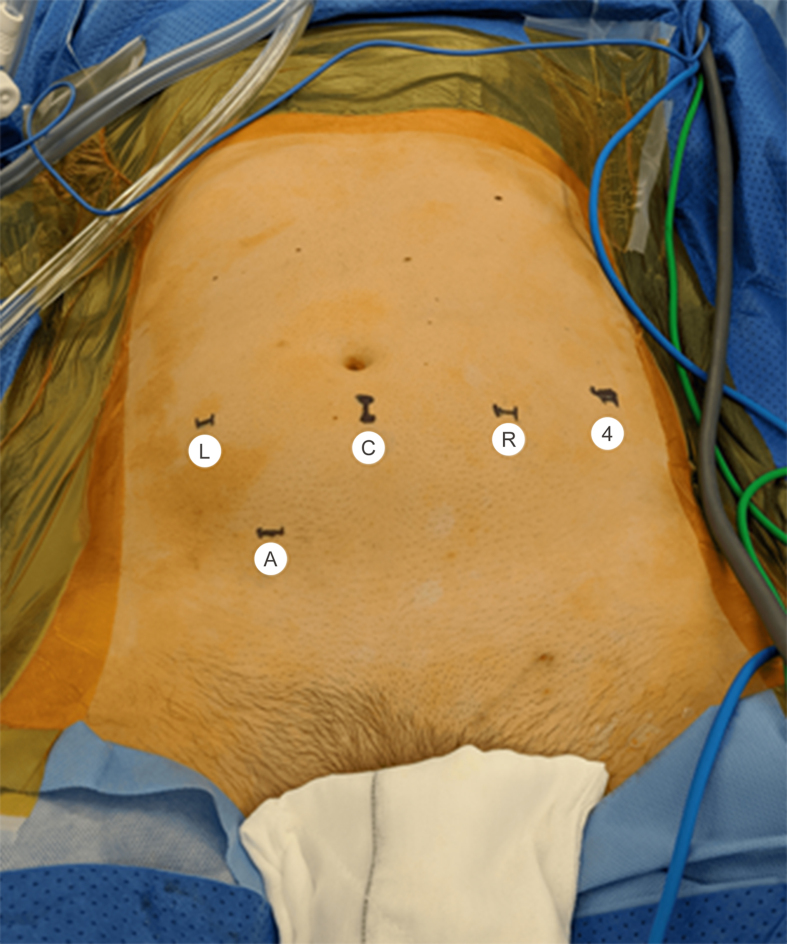
We implemented an enhanced recovery after surgery protocol, modified to include a preoperative ingestion of a fatty meal 6 h prior to the procedure to aid with identification of lymphatic channels [13]. Four robotic ports and one 12 mm AirSeal® port (ConMed, Utica, NY, USA) were used; port placement is demonstrated in Fig. 1. The pneumoperitoneum was maintained at 10–12 mmHg (1 mmHg=0.133 kPa) or 9–10 mmHg for paediatric or very thin patients. The root of the mesentery was mobilised via an oblique peritoneal incision from ileocecal junction to duodenojejunal junction and mesenteric peritoneal flaps were developed and suspended from the abdominal wall using traction sutures. Following exposure of retroperitoneal tissue, the dissection proceeded cephalad. The split and roll technique was used to ensure clearance of the retroaortic and retrocaval nodes. Lumbar arteries were ligated and divided if required to ensure full mobilisation of the great vessels. Dissection was based on Indiana University templates but extended if intraoperative findings or prior imaging indicated additional possible sites of the disease or at the discretion of the surgeon. For patients undergoing a nerve-sparing procedure, the post-ganglionic nerves were identified and dissected off the nodal packages. Care was taken to avoid excessive handling of the nodes and the nodal packages were kept en bloc with interaortocaval, retroaortic, and retrocaval nodes in one package and paraaortic, preaortic or paracaval, precaval nodes in the other package depending on the laterality of the dissection. Large lymphatic channels were ligated with Hem-o-Lok clips (Weck Closure Systems, NC, USA). Meticulous haemostasis and lymphostasis were confirmed. Lymph node packages were placed in endocatch bags and the neck of the bag was clipped to avoid spillage. Following completion of lymph node dissection, the robot was re-docked at 180-degree from the original orientation, the Trendelenburg reduced to 17-degree, and dissection of the cord completed.
Figure 1.
Port placement. The same port placement was used for left and right sided dissections. L, left arm; C, camera port; R, right arm; 4, 4th arm; A, assistant port.
Postoperative care included venous thromboembolism prophylaxis, early mobilization, resumption of (fat-free) diet following passage of flatus, and intravenous antibiotics for 24 h. Discharge from hospital was based on ability to tolerate diet, pain, and patient willingness to be discharged.
3. Results
Nineteen patients were identified. Baseline patient characteristics are shown in Table 1. All patients had pathological stage II disease (4 [21.1%] with stage IIA, 9 [47.4%] with stage IIB, and 6 [31.6%] with stage IIC); 12 (63.2%) patients had undergone prior primary chemotherapy. Primary RPLND patients all had stage II disease (1 [14.3%] with stage IIA, 4 [57.1%] with stage IIB, and 2 [28.6%] with stage IIC). Operative data are shown in Table 2. The median operative time was 300 (interquartile range [IQR] 240–315) min.
Table 1.
Summary of baseline patient and tumour characteristics.
| Demographic and tumour characteristic | Value |
|---|---|
| Agea at RPLND, year | 31 (25.5–37.5) |
| BMIa, kg/m2 | 27.5 (24.3–30.0) |
| ASA physical status classification systemb | |
| 2 | 8 (42.1) |
| 3 | 11 (57.9) |
| Primary tumour lateralityb | |
| Left | 12 (63.2) |
| Right | 6 (31.6) |
| Bilateral | 1 (5.3) |
| Histology on orchidectomyb,c | |
| Seminoma | 2 (10.5) |
| NSGCT/mixed GCT | 15 (78.9) |
| Embryonal rhabdomyosarcoma | 1 (5.3) |
| Necrosis/no viable tumour | 2 (10.5) |
| Primary chemotherapyb | |
| None | 7 (36.8) |
| One cycle (carboplatin) | 1 (5.3) |
| Three cycles BEP | 10 (52.6) |
| Four cycles BEP | 1 (5.3) |
| Pre-operative retroperitoneal tumour sizea, cm | 2.3 (0.9–12.0) |
| Pathological retroperitoneal tumour sizea, cm | 3.0 (1.3–7.0) |
| Pathological stageb | |
| IIA | 4 (21.1) |
| IIB | 9 (47.4) |
| IIC | 6 (31.6) |
RPLND, retroperitoneal node dissection; BMI, body mass index; ASA, American Society of Anaesthesiologists; NSGCT, non-seminomatous germ cell tumour; GCT, germ cell tumour; BEP, bleomycin, etoposide, and cisplatin.
Values are presented as median (interquartile range).
Values are presented as n (%).
Includes one patient with bilateral tumours, seminoma, and mixed GCT.
Table 2.
Summary of tumour and operative characteristics.
| Parameter | Value |
|---|---|
| Tumour locationa | |
| Paraaortic | 11 (57.9) |
| Interaortocaval | 3 (15.8) |
| Paraaortic and interaortocaval | 4 (21.1) |
| Precaval | 1 (5.3) |
| Templatea | |
| Modified left | 11 (57.9) |
| Modified right | 6 (31.6) |
| Bilateral | 2 (10.5) |
| Nerve sparea | |
| Yes | 16 (84.2) |
| No | 3 (15.8) |
| Conversion to open surgerya | 1 (5.3) |
| Operative timebc, min | 300 (240–315) |
| Robotic console timebc, min | 215 (183–255) |
| Estimated blood lossb, mL | 100 (50–175) |
| Transfusion requireda | 0 (0) |
Values are presented as n (%).
Values are presented as median (interquartile range).
Time included robotic pyeloplasty in one patient.
One (5.3%) patient required conversion to open surgery due to significant post-chemotherapy desmoplastic adherence to the aorta. At the time of conversion, the blood loss was 400 mL, primarily due to an inferior mesenteric artery injury, which was ligated after conversion. No patients required transfusion. The median blood loss was 100 (IQR 50–175) mL. Table 3 describes postoperative results. The median length of stay was 2 (range 1–11) days. All robotically completed patients commended were discharged by Day 3. All robotically completed patients commenced diet on Day 1, with median time to flatus of 1.0 (IQR 1.0–1.0) days. Median opiate use was 52.5 (IQR 26–126) mg of morphine equivalent [14], with 4 (21.1%) patients requiring no opiate analgesia in the postoperative period.
Table 3.
Postoperative morbidity and complications.
| Parameter | Value |
|---|---|
| Length of staya, day | |
| 1 | 3 (15.8) |
| 2 | 13 (68.4) |
| 3 | 2 (10.5) |
| 11 | 1 (5.3) |
| Opiate useb, mg of morphine equivalent | 52.5 (26.3–126.6) |
| Time to flatusb, day | 1 (1–1) |
| Readmission ratea | 0 (0) |
| Complicationa | |
| Clavien-Dindo Grade I | 0 (0) |
| Clavien-Dindo Grade II | |
| Open conversion with ileus | 1 (5.3) |
| Clavien-Dindo Grade III | |
| Chylous ascites requiring outpatient drainage | 1 (5.3) |
| Clavien-Dindo Grade IV or V | 0 (0) |
Values are presented as n (%).
Values are presented as median (interquartile range).
There was one low-grade complication (Clavien-Dindo Grade II), a prolonged ileus of 9 days in the patient who underwent conversion to open surgery. Additionally, one Clavien-Dindo Grade III complication was seen, chylous ascites at Week 4 post-procedure, requiring ultrasound-guided aspiration with no recurrence.
Oncological and functional outcomes are presented in Table 4. The lymph node packets were all completely embedded for histological examination and the median lymph node yield was 40.5 (IQR 38–51) nodes per procedure. Histology included necrosis only (21.1%), teratoma only (36.8%), viable germ cell tumour with or without teratoma (36.8%), and sarcoma (5.3%). Patients who underwent nerve-sparing procedures all recovered antegrade ejaculatory function, at a median of 2.5 (IQR 1.8–4.5) weeks postoperatively. All patients who underwent non-nerve sparing procedures had retrograde ejaculation. At a median follow-up of 22.3 (IQR 16.3–24.9) months, one post-chemotherapy patient developed recurrence of seminoma in the retroperitoneum, and underwent second-line chemotherapy followed by open approach for resection of residual lesions which confirmed no evidence of residual malignancy. No other patients have had recurrences to date.
Table 4.
Functional and oncological outcomes.
| Functional and oncological outcomes | Value |
|---|---|
| Antegrade ejaculation recovery time in nerve-spare patientsa, week | 2.5 (1.8–4.5) |
| Lymph node count, na | 40.5 (38–51) |
| Node pathology, n (%) | |
| Necrosis | 4 (21.1) |
| Teratoma only | 7 (36.8) |
| GCT with or without teratoma | 7 (36.8) |
| Embryonal rhabdomyosarcoma | 1 (5.3) |
| Follow-upa, month | 22.3 (16.3–24.9) |
| Recurrence, n (%) | |
| In field | 1 (5.3) |
| Out of field | 0 (0) |
GCT, germ cell tumour.
Values are presented as median (interquartile range).
4. Discussion
RPLND is a potential primary therapy for patients with metastatic testicular cancer confined to the retroperitoneum, which in select patients could avoid the need for chemotherapy or radiotherapy, and their potential long-term side effects. In the past, the morbidity of the procedure and its long convalescence time have been major sources of reservation when it comes to offering this modality as primary therapy. With advancements in minimally invasive platforms and skill sets, laparoscopic and R-RPLND have emerged as alternative options.
Multiple factors have inhibited the adoption of laparoscopic RPLND, most notably the advanced skill set required [15]. The complexity and length of the procedure was also a large barrier. Laparoscopic series routinely reported low lymph node counts which called into question the oncological equivalency of the procedure and it was criticised as a staging rather than therapeutic procedure as a result [16]. Robotic surgery significantly mitigates the downsides of laparoscopic surgery. Movement is precise and can be scaled; multiple arms make retracting and responding to bleeding vastly safer; and ergonomic issues are less of a concern for surgeons [17].
Since the first R-RPLND in 2006, multiple series have reported promising results. Initial series were performed in stage I disease; however, in 2015 Cheney et al. [18] demonstrated the feasibility of R-RPLND in the post-chemotherapy setting, including eight post-chemotherapy stage IIA or stage IIB patients in their series, of which 75% were completed robotically. Kamel et al. [19] then further expanded the scope of R-RPLND, including six stage III and three stage IIC patients, including multiple large masses. All series to date report no in-field recurrences, with lymph node counts ranging from three to 30 nodes [11,20].
This series demonstrates the applicability of R-RPLND to a wider proportion of patients with more advanced stages; 63.2% of patients were post-chemotherapy at the time of surgery, and 78.9% had stage IIB or greater. In our experience, post-chemotherapy non-seminomatous germ cell tumour patients in the poor risk category may pose a greater technical challenge due to severe desmoplastic reaction, evidenced by our patient requiring conversion to open surgery. Otherwise, a higher proportion stage IIB and stage IIC patients could be considered for R-RPLND, subject to local expertise and case feasibility.
Similar to prior publications, our series showed very low perioperative morbidity, with early return of bowel function, very low analgesic requirements, and no blood product transfusions. All patients completed robotically resumed regular diet on postoperative Day 1 and most passed flatus and bowel motion within the first day. The length of stay was 2 days in all but two robotically completed patients, both of which were due to discharge transport issues rather than clinical reasons. Analgesic requirements compare favourably with contemporary data for open RPLND [21]. Similarly, the length of stay, 2 days in this series, compares favourably with contemporary open series, which reported a mean length of stay in the order of 3–7 days [21].
This series continues the literature-wide trend of low rates of complications in R-RPLND series; however, these have been in highly selected cohorts, and comparison between open and robotic series requires attention to the complexity of cases, especially great vessel involvement. Many intraoperative complications, especially vascular complications, are primarily determined by the location and extent of the tumour, with a lesser contribution from the skill of the surgeon. Open surgery will remain the gold standard for complex cases with large tumours or the need for vascular reconstruction. Although major intraoperative complications are at times unavoidable in both open and robotic cohorts, we postulate that robotic surgery is likely to provide a significant reduction in wound complications, ileus, atelectasis, and thromboembolic events due to smaller wounds, lower analgesia requirements, and earlier mobility. In recent open series, 1%–3% of patients suffered a thrombosis-related complication and up to 4.7% a wound related complication [[22], [23], [24]]; however, reported complication rates vary widely, and highly specialised centres report lower rates [5]. None of this type of complication was seen in this series and although the literature so far is limited to case series, it is reasonable to expect that this lower rate of complications will continue in robotic series given data from other minimally invasive surgery [25].
Oncological outcomes remain the primary and most important objectives of RPLND. Lymph node yield is important both as a surrogate marker of dissection quality and a primary driver of outcomes [26]. Our lymph node yield is similar to that reported in open surgery series [26]. Intraoperatively, retroaortic and retrocaval nodal packets were removed completely by split and roll technique and templates were identical to open surgery [16]. Despite the relatively short follow-up, the results are encouraging as the majority of recurrences for germ cell tumours occur within first 2 years [27].
One patient had retroperitoneal recurrence. This patient initially presented with stage I classical seminoma and teratoma. He had a recurrence with 3.6 cm paraaortic lymphadenopathy at 1-year follow-up and underwent three cycles of bleomycin, etoposide, and cisplatin chemotherapy. The mass reduced to 1.5 cm and the patient underwent a R-RPLND using a modified left template. Fifty-six lymph nodes were removed, with necrosis found in seven but no residual germ cell tumour. Eighteen months later staging scans demonstrated a 22 mm suprarenal paraaortic mass. Fluorodeoxyglucose positron emission tomography (FDG-PET) showed high uptake in retroperitoneal nodes at the superior (supra-renal and retro-pancreatic) and inferior (iliac) extents of dissection as well as an in-field paraaortic mass and multiple small contralateral (paracaval) nodes. Masses above the renal arteries and inferior to the iliac vessels were biopsied demonstrating recurrent seminoma. The patient underwent second-line chemotherapy with radiological response but had a 1.5 cm residual mass at the supra hilar lesion. Full bilateral open RPLND was performed with excision of suprahilar nodes including coeliac axis and retro-pancreatic nodes with pathology indicating no residual viable germ cell tumour nor teratoma.
A small series reported by Calaway et al. [28] described five patients with unusual and diffuse metastases following R-RPLND. This may represent tumour spillage, especially on removal. Our technique is to remove the specimen in two en-block nodal packages which are immediately placed in endocatch bags clipped at the neck. We do not believe that tumour spillage had any role in our patient with recurrence as his pathology during R-RPLND showed only necrosis.
Disadvantages of R-RPLND include operative time and cost, though both are reducing as an issue. The operative time in this series is comparable to open and robotic series considering the high portion of post-chemotherapy and higher-stage disease.
The limitations of this study include its non-randomised nature and moderate duration of follow-up. Furthermore, it is a single-surgeon experience and its findings not broadly generalisable. We strongly advocate familiarity with oncological principles, expertise in minimally invasive surgery, and centralisation of this procedure, as it is technically demanding, and experience is likely to be a major predictor of patient outcomes. This series does, however, demonstrate the feasibility of performing the procedure outside very high-volume centres in high population countries.
A major strength of this study is it's generalisability—all patients had stage II disease; a high proportion were post-chemotherapy; and only three patients underwent open RPLND during the recruitment period, addressing limitations of recent series which have included high rates of stage IA patients [29] and very selective recruiting [30]. Which patients are most likely to benefit from a minimally invasive approach is currently not known; this series shows promise for its broader application to the metastatic testicular cancer population.
This series adds to the early evidence for the addition of R-RPLND to the armamentarium of treatments for testicular cancer for selected and suitable patients.
5. Conclusion
R-RPLND is a safe and feasible alternative to open surgery in appropriately selected patients, offering low morbidity. Early oncological outcomes are promising; however, larger cohorts and longer follow-ups are required to validate our institution's findings.
Author contributions
Study concept and design: George McClintock, Nariman Ahmadi.
Material support: Henry Woo.
Data acquisition: George McClintock, Nariman Ahmadi.
Data analysis: George McClintock.
Drafting of manuscript: George McClintock.
Critical revision of the manuscript: George McClintock, Ahmed S. Goolam, Don Perera, Ryan Downey, Scott Leslie, Peter Grimison, Henry Woo, Peter Ferguson, Nariman Ahmadi.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Albers P., Albrecht W., Algaba F., Bokemeyer C., Cohn-Cedermark G., Fizazi K., et al. Guidelines on testicular cancer: 2015 update. Eur Urol. 2015;68:1054–1068. doi: 10.1016/j.eururo.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson A.J., Sheinfeld J. The role of retroperitoneal lymph node dissection in the management of testicular cancer. Urol Oncol. 2004;22:225–233. doi: 10.1016/j.urolonc.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Beck S.D.W., Foster R.S., Bihrle R., Donohue J.P., Einhorn L.H. Is full bilateral retroperitoneal lymph node dissection always necessary for postchemotherapy residual tumor? Cancer. 2007;110:1235–1240. doi: 10.1002/cncr.22898. [DOI] [PubMed] [Google Scholar]
- 4.Hanna N., Einhorn L.H. Testicular cancer: a reflection on 50 years of discovery. J Clin Oncol. 2014;32:3085–3092. doi: 10.1200/JCO.2014.56.0896. [DOI] [PubMed] [Google Scholar]
- 5.Williams S.B., McDermott D.W., Winston D., Bahnson E., Berry A.M., Steele G.S., et al. Morbidity of open retroperitoneal lymph node dissection for testicular cancer: contemporary perioperative data. BJU Int. 2010;105:918–921. doi: 10.1111/j.1464-410X.2009.08888.x. [DOI] [PubMed] [Google Scholar]
- 6.Reinert T., da Rocha Baldotto C.S., Nunes F.A.P., de Souza Scheliga A.A. Bleomycin-induced lung injury. J Cancer Res. 2013;2013:1–9. [Google Scholar]
- 7.Zagars G.K., Ballo M.T., Lee A.K., Strom S.S. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22:640–647. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 8.Groot H.J., Leeuwen F.E., Lubberts S., Horenblas S., Wit R., Witjes J.A., et al. Platinum exposure and cause-specific mortality among patients with testicular cancer. Cancer. 2020;126:628–639. doi: 10.1002/cncr.32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel H.D., Srivastava A., Alam R., Joice G.A., Schwen Z.R., Semerjian A., et al. Radiotherapy for stage I and II testicular seminomas: secondary malignancies and survival. Urol Oncol. 2017;35:606.e1–606.e7. doi: 10.1016/j.urolonc.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Chovanec M., Zaid M.A., Hanna N., El-Kouri N., Einhorn L.H., Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol. 2017;28:2670–2679. doi: 10.1093/annonc/mdx360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray S., Pierorazio P.M., Allaf M.E. Primary and post-chemotherapy robotic retroperitoneal lymph node dissection for testicular cancer: a review. Transl Androl Urol. 2020;9:949–958. doi: 10.21037/tau.2020.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanian S., Patel M., Porter J. Robot-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer: evolution of the technique. Eur Urol. 2016;70:661–667. doi: 10.1016/j.eururo.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Kassis T., Yarlagadda S.C., Kohan A.B., Tso P., Breedveld V., Dixon J.B. Postprandial lymphatic pump function after a high-fat meal: a characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol. 2016;310:G776–G789. doi: 10.1152/ajpgi.00318.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statement regarding the use of opioid analgesics in patients with chronic non-cancer pain. 2021. https://www.anzca.edu.au/getattachment/6892fb13-47fc-446b-a7a2-11cdfe1c9902/PS01(PM)-(Appendix)-Opioid-Dose-Equivalence-Calculation-Table Appendix 1, Faculty of Pain Medicine, Australian and New Zealand College of Anaesthesia. [Google Scholar]
- 15.Schwen Z.R., Gupta M., Pierorazio P.M. A review of outcomes and technique for the robotic-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer. Adv Urology. 2018;2018 doi: 10.1155/2018/2146080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce S., Steinberg Z., Eggener S. Critical evaluation of modified templates and current trends in retroperitoneal lymph node dissection. Curr Urol Rep. 2013;14:511–517. doi: 10.1007/s11934-013-0366-1. [DOI] [PubMed] [Google Scholar]
- 17.Mittakanti H.R., Porter J.R. Robot-assisted laparoscopic retroperitoneal lymph node dissection: a minimally invasive surgical approach for testicular cancer. Transl Androl Urol. 2020;9:S66–S73. doi: 10.21037/tau.2019.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheney S.M., Andrews P.E., Leibovich B.C., Castle E.P. Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int. 2015;115:114–120. doi: 10.1111/bju.12804. [DOI] [PubMed] [Google Scholar]
- 19.Kamel M.H., Littlejohn N., Cox M., Eltahawy E.A., Davis R. Post-chemotherapy robotic retroperitoneal lymph node dissection: institutional experience. J Endourology Endourological Soc. 2016;30:510–519. doi: 10.1089/end.2015.0673. [DOI] [PubMed] [Google Scholar]
- 20.Werntz R.P., Pearce S.M., Eggener S.E. Indications, evolving technique, and early outcomes with robotic retroperitoneal lymph node dissection. Curr Opin Urol. 2018;28:461–468. doi: 10.1097/MOU.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 21.Calaway A.C., Foster R.S., Tong Y., Masterson T.A., Bihrle R., Cary C. Improving postoperative quality of care in germ cell tumor patients: does scheduled alvimopan, acetaminophen, and gabapentin improve short-term clinical outcomes after retroperitoneal lymph node dissection? Urol Oncol. 2020;38:305–312. doi: 10.1016/j.urolonc.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 22.King J., Kawakami J., Heng D., Gan C.L. Post-chemotherapy retroperitoneal lymph node dissection for non-seminomatous germ cell tumors: a single-surgeon, Canadian experience. Can Urol Assoc J. 2019;14:E407–E411. doi: 10.5489/cuaj.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruf C.G., Krampe S., Matthies C., Anheuser P., Nestler T., Simon J., et al. Major complications of post-chemotherapy retroperitoneal lymph node dissection in a contemporary cohort of patients with testicular cancer and a review of the literature. World J Surg Oncol. 2020;18:253. doi: 10.1186/s12957-020-02032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerdtsson A., Håkansson U., Törnblom M., Jancke G., Negaard H.F.S., Glimelius I., et al. Surgical complications in postchemotherapy retroperitoneal lymph node dissection for nonseminoma germ cell tumour: a population-based study from the Swedish Norwegian Testicular Cancer Group. Eur Urol Oncol. 2020;3:382–389. doi: 10.1016/j.euo.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Jaschinski T., Mosch C.G., Eikermann M., Neugebauer E.A., Sauerland S. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546. doi: 10.1002/14651858.cd001546.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhanvadia R.R., Rodriguez J., Bagrodia A., Eggener S.E. Lymph node count impacts survival following post-chemotherapy retroperitoneal lymphadenectomy for non-seminomatous testicular cancer: a population-based analysis. BJU Int. 2019;124:792–800. doi: 10.1111/bju.14798. [DOI] [PubMed] [Google Scholar]
- 27.Willis S.F., Winkler M., Savage P., Seckl M.J., Christmas T.J. Repeat retroperitoneal lymph-node dissection after chemotherapy for metastatic testicular germ cell tumour. BJU Int. 2007;100:809–812. doi: 10.1111/j.1464-410X.2007.07087.x. [DOI] [PubMed] [Google Scholar]
- 28.Calaway A.C., Einhorn L.H., Masterson T.A., Foster R.S., Cary C. Adverse surgical outcomes associated with robotic retroperitoneal lymph node dissection among patients with testicular cancer. Eur Urol. 2019;76:607–609. doi: 10.1016/j.eururo.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Supron A.D., Cheaib J.G., Biles M.J., Schwen Z., Allaf M., Pierorazio P.M. Primary robotic retroperitoneal lymph node dissection following orchiectomy for testicular germ cell tumors: a single-surgeon experience. J Robotic Surg. 2021;15:309–313. doi: 10.1007/s11701-020-01107-1. [DOI] [PubMed] [Google Scholar]
- 30.Hiester A., Nini A., Arsov C., Buddensieck C., Albers P. Robotic assisted retroperitoneal lymph node dissection for small volume metastatic testicular cancer. J Urol. 2020;204:1242–1248. doi: 10.1097/JU.0000000000001301. [DOI] [PubMed] [Google Scholar]