Abstract
Objective
Placement of human placenta derived grafts during robotic-assisted radical prostatectomy (RARP) hastens the return of continence and potency. The long-term impact on the oncologic outcomes remains to be investigated. Our objective was to determine the oncologic outcomes of patients with dehydrated human amnion chorion membrane (dHACM) at RARP compared to a matched cohort.
Methods
In a referral centre, from August 2013 to October 2019, 599 patients used dHACM in bilateral nerve-sparing RARP. We excluded patients with less than 12 months follow-up, simple prostatectomy, and unilateral nerve-sparing. Patients with dHACM (amnio group) were 529, and were propensity score matched 1:1 to 2465 patients without dHACM (non-amnio group) and a minimum follow-up of 36 months. At the time of RARP, dHACM was placed around the neurovascular bundle in the amnio group. Continuous and categorical variables in matched groups was tested by two-sample Kolmogorov-Smirnov test and Fisher's exact test respectively. Outcomes measured were biochemical recurrence (BCR), adjuvant and salvage therapy rates.
Results
Propensity score matching resulted in two groups of 444 patients. Cumulative incidence functions for BCR did not show a difference between the groups (p=0.3). Patients in the non-amnio group required salvage therapy more frequently than the amnio group, particularly after partial nerve-sparing RARP (6.3% vs. 2.3%, p=0.001). Limitations are the absence of prospective randomization.
Conclusion
The data suggest that using dHACM does not have a negative impact on BCR in patients. Outcomes of cancer specific and overall survival will require follow-up study to increase our understanding of these grafts’ impact on prostate cancer biology.
Keywords: Prostatectomy, Prostate cancer, Robotic, Oncology, Outcome, Allograft, Biomaterial, Dehydrated human amnion chorion membrane
1. Introduction
Robotic-assisted radical prostatectomy (RARP) is a standard of care surgical option for the treatment of localized prostate cancer (PCa) [1]. The technique to nerve-sparing (NS) was introduced by Walsh and Donker in 1982 [2], which is a breakthrough in radical prostatectomy care due to the close anatomic relationship of the neurovascular bundle (NVB). The NVB plays a role in both recovery of potency and urinary continence [3]; the latter's recovery is also tied to preservation of supportive structures of the membranous urethra and pelvic floor. The innovative surgical step of robotic assistance has allowed a magnified view and minimal invasive dexterity to separate the NVB from the prostate. The period of potency recovery depended upon the intra-operative amount of NVB spared, while limiting traction and diathermy to them. In 2015, our institution investigated biological adjuncts and their role of nerve regeneration. Patel et al. [4] described the use of a dehydrated human amnion chorion membrane (dHACM) allograft wrap around the NVB, to improve the rate of return to continence and potency. To our knowledge, this was one of the first uses of this biological allograft from a high-volume oncologic center.
dHACM has proven utility in chronic wound management, particularly diabetic foot ulcers [5]. This allograft's numerous cytokines such as tissue inhibitor of matrix metalloproteinases-1, chemokine ligand 5, and Interleukin-8 have been proposed in previous studies to have an association in promoting tumor and biochemical recurrence (BCR) [[6], [7], [8]]. A subsequent follow-up study in 2018 from our institution revealed that dHACM allograft placement on the post-prostatectomy NVB did not increase the risk of BCR after RARP, but did improve the rate of return to potency compared to a matched group [9].
Alvim et al. [10] described an in vivo model of dHACM use in immunodeficient mice with flank injections of human PCa cell line (LNCaP) and human bladder cancer cell line (UM-UC-3). In their study, partial resection of the tumor resulted in faster tumor relapse and growth when the membrane was applied. Due to early clinical utilization of dHACM in urologic oncology, our objective was to determine the impact of this allograft placement on NVB in PCa treated by RARP, compared to a matched group.
2. Patients and methods
2.1. Study population and inclusion criteria
Full institutional review board approval was granted for the study (approval number 237998). From August 2013 to October 2019, 599 patients underwent NS RARP with dHACM allograft placement. In this group, 70 (11.7%) patients were excluded: 64 (10.7%) had less than 12 months of follow-up; 5 (0.8%) had simple prostatectomy; and 1 (0.2%) had unilateral NS. During the same time period, 2465 patients underwent NS RARP without dHACM allograft placement with no exclusion criteria for this group.
2.2. End point and assessment
The primary end point of this study was to evaluate whether patients undergoing NS RARP with dHACM allograft placement (amnio group) around the NVB experienced increased BCR compared to NS RARP without dHACM allograft placement (non-amnio group). As a secondary endpoint, a subgroup analysis was performed to compare patients with partial and full NS, and with age less than or equal to 55 years and greater than 55 years. The rates of adjuvant and salvage therapies between the two groups were also compared.
2.3. Surgical technique
All RARPs were performed by a single surgeon (Patel V), with a transperitoneal multi-port da Vinci Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Our technique is a retrograde athermal NS, with the capsular “landmark” artery as an anatomical reference for preserving the NVB [11,12]. A bladder neck reconstruction is our standard approach along with a modified posterior reconstruction [13]. The dHACM allograft was placed over each NVB after the posterior reconstruction, prior to the vesicourethral anastomosis. NS was performed in a partial or full manner according to tumor location, pre-operative prostate MRI, and the prostate biopsy core profile.
2.4. Definitions
Adjuvant treatment refers to initiation of therapeutics before a post-RARP prostate-specific antigen (PSA) threshold of 0.2 ng/mL; salvage treatment refers to initiation of therapeutics if BCR occurs. BCR is defined as a post-RARP PSA of more than 0.2 ng/mL.
2.5. Statistical analysis and propensity score (PS) matching
Continuous variables were reported as the median and interquartile range (IQR), and categorical as absolute and percentage frequency.
A PS was calculated using logistic regression analysis based on 10 preoperative variables: age, body mass index, PSA, Charlson Comorbidity Index, Sexual Health Inventory for Men score, American Urological Association symptom score, preoperative International Society of Urologic Pathology (ISUP) group, clinical stage, and degree of NS (full and partial). Matching was performed using the nearest-neighbor matching algorithm (caliper width 0.15 of the standard deviation of the logit score) with a 1:1 ratio without replacement. The amnio group was computer-matched to the non-amnio group with a minimum follow-up of 36 months, resulting in the analysis of two groups of 444 patients each. The degree of balance for the variables used in PS estimation was estimated using standardized differences [14]. Covariates with a standardized difference lower than 0.15 in absolute value were considered satisfactorily balanced. The hypothesis of equal distribution of continuous and categorical variables in the matched groups was tested using the two-sample Kolmogorov-Smirnov test and the Fisher's exact test, respectively.
Cumulative incidence function curves for BCR after surgery were estimated for up to 48 months of follow-up in amnio and non-amnio groups using the Kaplan-Meier method and their difference was tested using the log rank-test. Statistical significance was set at p<0.05 for all the two-tailed test. The statistical analyses were performed using Stata version 16 (Stata Corp., College Station, TX, USA) and R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Demographics and perioperative data
Table 1 illustrates the perioperative data of both groups of patients. The balance of covariates used in PS estimation was performed and no statistically significant differences were found.
Table 1.
Comparison of preoperative and NS variables in the study groups after 1:1 propensity score matching.
| Parameter | Amnioac (n=444) | Non-amniobc (n=444) | p-Value | Standardized difference |
|---|---|---|---|---|
| Age, year | 58 (53–62) | 58 (53–63) | 0.6 | 0.09 |
| PSA, ng/mL | 5.2 (3.9–7) | 5.3 (4.2–7.1) | 0.3 | 0.03 |
| Body mass index, kg/m2 | 27 (24.8–29.4) | 27 (24.8–29.7) | 0.8 | 0.01 |
| Preoperative SHIM score | 24 (21–25) | 24 (21–25) | 0.3 | 0.05 |
| Preoperative AUASS | 5.5 (3–12) | 7.96 (3–11) | 1 | 0.03 |
| Clinical stage | 0.1 | |||
| T1c | 396 (89.2) | 388 (87.4) | −0.06 | |
| T2a | 38 (8.6) | 53 (11.9) | 0.11 | |
| T2b | 7 (1.6) | 2 (0.5) | −0.11 | |
| T2c | 3 (0.7) | 1 (0.2) | −0.07 | |
| Charlson comorbidity index | 1 (1–2) | 1 (1–2) | 0.9 | 0.07 |
| Charlson comorbidity index | 0.9 | |||
| 0–1 | 229 (51.6) | 233 (52.5) | 0.02 | |
| 2–3 | 204 (45.9) | 199 (44.8) | −0.02 | |
| ≥4 | 11 (2.5) | 12 (2.7) | 0.01 | |
| Biopsy preoperative ISUP grade group | 0.8 | |||
| Grade group 1 | 197 (44.4) | 194 (43.7) | −0.01 | |
| Grade group 2 | 160 (36.0) | 159 (35.8) | −0.01 | |
| Grade group 3 | 56 (12.6) | 54 (12.2) | −0.01 | |
| Grade group 4 | 26 (5.9) | 34 (7.7) | 0.07 | |
| Grade group 5 | 5 (1.1) | 3 (0.7) | −0.05 | |
| Degree of NS | 0.9 | |||
| Bilateral partial | 119 (26.8) | 116 (26.1) | −0.01 | |
| Bilateral full | 325 (73.2) | 328 (73.9) | 0.01 | |
| No NS | 0 | 0 | 0 |
SHIM, Sexual Health Inventory for Men; AUASS, American Urological Association symptom score; ISUP, International Society of Urological Pathology; PSA, prostate-specific antigen; NS, nerve-sparing.
Patients undergoing NS robotic-assisted radical prostatectomy with dehydrated human amnion chorion membrane allograft placement.
Patients undergoing NS robotic-assisted radical prostatectomy without dehydrated human amnion chorion membrane allograft placement.
Values are presented as median (interquartile range) or n (%).
Regarding the degree of NS, in the amnio and non-amnio groups 73.2% and 73.9% underwent full NS, respectively.
3.2. Histopathology of prostate specimen
As shown in Table 2, following RARP, patients from amnio group had different pathological tumor stages compared to the non-amnio group for ≥T3a (24.8% vs. 38.7%, p<0.001) and for ISUP grade group 2 and higher (73.2% vs. 84.7%, p<0.001). There was no significant difference in positive surgical margins (PSMs) between the amnio and non-amnio groups, 15.5% and 18.5% respectively (p=0.3).
Table 2.
Comparison of histopathological prostate specimen outcomes per group.
| Outcome | Amnioa (n=444) | Non-amniob (n=444) | p-Value |
|---|---|---|---|
| Pathological stage, n (%) | <0.001 | ||
| ≤T2c | 334 (75.2) | 272 (61.3) | |
| T3a | 89 (20.0) | 135 (30.4) | |
| T3b | 19 (4.3) | 28 (6.3) | |
| T4 | 2 (0.5) | 9 (2.0) | |
| Prostate ISUP grade group, n (%) | <0.001 | ||
| Grade group 1 | 119 (26.8) | 68 (15.3) | |
| Grade group 2 | 207 (46.6) | 211 (47.5) | |
| Grade group 3 | 93 (20.9) | 108 (24.3) | |
| Grade group 4 | 6 (1.4) | 12 (2.7) | |
| Grade group 5 | 19 (4.3) | 45 (10.1) | |
| PSM, n (%) | 69 (15.5) | 82 (18.5) | 0.3 |
ISUP, International Society of Urological Pathology; PSM, positive surgical margin; NS, nerve-sparing.
Patients undergoing NS robotic-assisted radical prostatectomy with dehydrated human amnion chorion membrane allograft placement.
Patients undergoing NS robotic-assisted radical prostatectomy without dehydrated human amnion chorion membrane allograft placement.
3.3. BCR
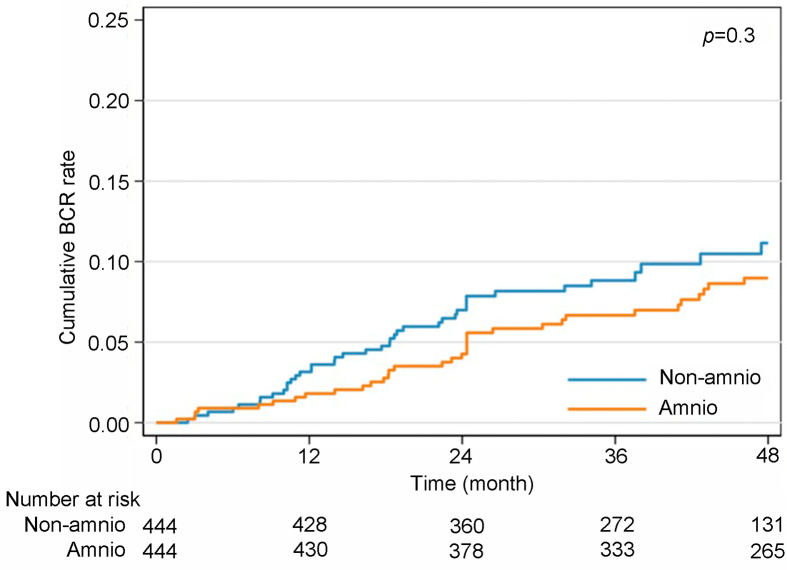
The comparison of the cumulative incidence functions for BCR did not show a statistically significant difference between the amnio and non-amnio groups (p=0.3) (Fig. 1). The hazard ratio of the amnio group compared to the non-amnio group for BCR was 0.8 (95% confidence interval 0.51–1.29).
Figure 1.
Comparison of cumulative incidence functions for BCR between amnio and non-amnio groups. BCR, biochemical recurrence. Amnio group means the group that patients undergoing nerve-sparing robotic-assisted radical prostatectomy with dehydrated human amnion chorion membrane allograft placement; non-amnio group means the group that patients undergoing nerve-sparing robotic-assisted radical prostatectomy without dehydrated human amnion chorion membrane allograft placement.
3.4. Salvage and adjuvant therapy
Table 3 reports oncological outcomes for BCR between groups according to PSM status, adjuvant or salvage therapy rates.
Table 3.
Comparison of oncologic outcomes between groups.
| Outcome | Amnioa | Non-amniob | p-Value |
|---|---|---|---|
| BCR (number of events) | 44 | 40 | 0.3 |
| In PSM | 11 | 4 | |
| In NSM | 33 | 36 | |
| Adjuvant therapy, n (%) | 7 (1.6) | 5 (1.1) | 0.8 |
| In full NS | 4 (0.9) | 3 (0.7) | 0.7 |
| In partial NS | 3 (0.7) | 2 (0.5) | 1 |
| In patients aged >55 years | 4 (0.9) | 2 (0.5) | 0.7 |
| In patients aged ≤55 years | 3 (0.7) | 3 (0.7) | 1 |
| Salvage therapy, n (%) | 28 (6.3) | 45 (10.1) | 0.05 |
| In full NS | 18 (4.1) | 17 (3.8) | 0.9 |
| In partial NS | 10 (2.3) | 28 (6.3) | 0.001 |
| In patients aged >55 years | 19 (4.3) | 30 (6.8) | 0.1 |
| In patients aged ≤55 years | 9 (2.0) | 15 (3.4) | 0.3 |
BCR, biochemical recurrence; NSM, negative surgical margin; NS, nerve-sparing; PSM, positive surgical margin.
Patients undergoing NS robotic-assisted radical prostatectomy with dehydrated human amnion chorion membrane allograft placement.
Patients undergoing NS robotic-assisted radical prostatectomy without dehydrated human amnion chorion membrane allograft placement.
3.4.1. Adjuvant therapy
There was no statistically significant difference in the number of patients that required adjuvant therapy overall between the two groups (p=0.8); neither was there a difference in adjuvant therapy between the two subgroups based on full NS (p=0.7) or partial NS (p=1), respectively (Table 3).
There was no statistically significant difference shown for amnio versus non-amnio patients receiving adjuvant therapy, as shown in subgroups of age more than 55 years (p=0.7), less than or equal to 55 years (p=1), PSM (p=1), or without PSM (p=0.8).
3.4.2. Salvage therapy
The number of patients that required salvage therapy was higher in the non-amnio group (10.1% vs. 6.3%, p=0.05). In the subgroup of patients who underwent partial NS, patients in the non-amnio group required salvage therapy more frequently than the amnio group (6.3% vs. 2.3%, p=0.001).
Patients undergoing full NS showed no significant difference in the rate of salvage therapy, with non-amnio patients at 3.8% and amnio patients at 4.1%. There was no statistically significant difference between the two groups receiving salvage therapy based on their age group: >55 years (p=0.1) and ≤55 years (p=0.3) (Table 3).
In patients without PSM, a significantly higher rate of salvage therapy was found in the non-amnio group compared to the amnio group (11.1% vs. 5.9%, p=0.012). In patients with PSM undergoing salvage therapy, there was no difference between the two groups. Each group had one mortality.
4. Discussion
The placement of a dHACM allograft around the NVB in RARP is a novel adjunct, in which the primary rationale for its use is in the potential of improved potency and continence recovery [4,9]. The aim of this study was to provide insight on the comparative oncological outcomes of patients that received this allograft compared to not received it. We did not analyze functional outcomes in this dataset, as it was described in a recent study [15]. The investigation into such biomaterials and their impact on outcomes after PCa surgery [16], is paramount due to their numerous growth factors and cytokines. These benefit tissue repair and nerve regeneration, but for their continued utilization, oncological results must be compared [10]. In this study, we investigated our experience of dHACM allograft for 529 eligible patients. It is important to note that many centers have utilized similarly dehydrated placental materials with positive impacts on post-RP potency recovery [17]. Porpiglia et al. [18] investigated covering the spared NVBs after RARP with a novel material known as chitosan, a chitin polysaccharide derivative from crustaceans. Their early results for potency recovery up to 2 months was favorable compared to a control population (52.2% vs. 39.2%; p=0.01).
The presence of BCR after RARP is a valuable predictor of outcomes in patients [19] and the only parameter in the trifecta for cancer control. The high volume of patients who have undergone RARP at our institution was used to perform a PS match, to compare patients with dHACM allograft placement around the NVB. Our single surgeon series controlled for variability among the study population, as to the surgical NS approach and overall PSM risk. At the time of RARP, the senior surgeon considered pre-operative ISUP grade, clinical and radiological T stage, tumor location (lateral edge of base, mid, or apical zones), and pre-operative Sexual Health Inventory for Men score when planning full or partial NS. However, the change from pre-planned full to partial NS may occur due to intra-operative findings of adherent surrounding tissue.
Our data demonstrated no difference in the rate of BCR between both groups in this study which is consistent with our previous short-term outcomes [9]. It must be noted that the matched non-amnio group had histopathology of advanced T stage (≥pT3) and higher ISUP group which is commonly associated with a higher risk of BCR, adjuvant or salvage therapy.
The hypothesis that amniotic membranes could lead to cancer recurrence after surgery, but importantly that it could lead to additional risk of recurrence with PSM was considered. The location or size of our PSM data was not analyzed, since a pre-clinical study with dHACM revealed rapid tumor relapse with partial tumor excision and an unmeasured PSM, in mice within 10 weeks [10]. Our study revealed the findings of no statistically significant difference in the timeline or overall comparative BCR risk, when analyzed from our data on patient PSM being present or not. The framework for tissue regeneration with dHACM is, in part, contributed by tumor growth factor beta and interleukin-10, which may impact RARP patients favorably as seen in other pathological studies [20]. Recent work on cell cycle progression scores in prostate specimens [21] may challenge the prognostic value of PSM overall, among other parameters. When cell cycle progression becomes validated, it would be a safety measure in the utility of adjuncts such as dHACM. Our data did not undergo an explorative analysis on BCR in relation to PSM per group, as the sample size was too small to draw clinically meaningful conclusions.
These amniotic allografts have consistently shown to improve wound healing in diabetic lower extremity ulcers, compared to control groups [5,25]. As mentioned above, the oncologic effect has been proposed by some groups to be negative [10,26] due to the chemokine and receptor interplay for tumor cell proliferation. There is also interplay with tumor promoter and suppressor function and the unknown impact of ethnicity, which will require further investigation of a dHACM in the PCa setting. The hypothesis that dHACM and other placenta derived products may influence prostate tumor biology should be regarded as exploratory at present. Further understanding of the inflammatory process behind prostate pathology, could highlight these allografts' role in regulating this response. Our clinical findings from dHACM allograft placement does not suggest an increased adjuvant or salvage therapy rates associated with its use. Further subanalysis of associations between outcomes, such as PSM to BCR, adjuvant and salvage therapy would add additional insight as an adequately powered randomized trial.
The analysis of single versus multiple site PSM, or PSM length in this study was not performed due to previous publications' work on predictive accuracy for BCR in these areas. The study by Stephenson et al. [22] included 7000 patients from two institutions, with a median follow-up of 38 months. It revealed that despite independent associations in PSM parameters with BCR, their validated nomogram had a similar C-index to a nomogram modeled on PSM being present or not. Thus, the impact of PSM on BCR will be a subject of further PCa research. Mortality has not been associated with BCR [15] and with only one event per group in our study, this outcome was not analyzed further.
Ogaya-Pinies et al. [9] discussed a sub-stratification of patients based on their ages and NS grades. Potency recovery significantly improved in patients receiving dHACM allograft and who were younger than 55 and underwent a full NS RARP respectively. This stratification based on our previous work on functional outcomes, formed the basis of our exploration for the rate of adjuvant and salvage therapy among these two groups. A statistically significant difference was seen in the rate of salvage therapy non-amnio patients received in partial NS (p=0.001) and negative surgical margin status (p=0.012). As mentioned earlier, the non-amnio group had high-risk disease on prostate pathology, and it is our practice to offer salvage radiotherapy in preference to adjuvant. A recent randomized control trial has shown this approach can avoid radiotherapy in half of cases, limiting associated genitourinary toxicity, and allows equal rates of freedom from BCR compared to adjuvant therapy [23]. Bilateral NS, specifically full NS that leaves a median of 0.5 mm nerve tissue on the prostate specimen [11], is known to increase the risk of PSM during RARP and subsequent BCR [24]. However, there was no difference in full NS patients receiving adjuvant or salvage therapy between the groups. Regarding post-RARP treatment, in our setting this includes radiotherapy and androgen deprivation therapy, based on a patient- and physician-shared decision at an oncology consultation. Androgen deprivation therapy protocols are not standardized in our study due to differing oncology practices managing the patient cohort, particularly those from out of state or international. PSA trend and doubling time were also impacted by the varied oncology approaches and timing of androgen deprivation therapy.
The limitations of this retrospective PS matched study are the absence of randomization and challenges of subanalysis for outcome associations, such as inability to interpret the BCR result in relation to PSM with a limited sample size. Additionally, we analyzed BCR and the need for salvage and adjuvant therapy over 48 months, which will need future follow-up to generate long-term outcome results. Although this is a suitable timeline to assess for recurrence, longer-term analysis with pooled data from other large series [27,28] will be informative. Similar institutions that have used placenta derived grafts, will add to the impact on PCa biology post-RARP. Multi-institution collaborative studies with median follow-up beyond 5 years should be a future focus for these allografts in RARP.
5. Conclusion
Our study suggests that using dHACM does not have an association of increased risk of BCR overall. This study reveals there is no rapid and harmful impact on cancer control from our experience, as compared to a partial tumor excision in an animal study. Longer-term outcomes of dHACM adjuncts in RARP and PCa, will need continued scrutiny in a follow-up study.
Author contributions
Study concept and design: Jonathan Noël, Vipul Patel.
Data acquisition: Jonathan Noël, Sunil Reddy, Seetharam Bhat, Ela Patel.
Data analysis: Marco Sandri, Sunil Reddy, Jonathan Noël.
Drafting of the manuscript: Daniel Stirt, Jonathan Noël, Anya Mascarenhas, Subuhee Ahmed.
Critical revision of the manuscript: Marcio Covas Moschovas, Abdel Rahman Jaber, Travis Rogers, Vipul Patel.
Conflicts of interest
Vipul Patel was previously a medical consultant for MiMedx. All remaining authors declare no conflict of interest. The study was partially funded by MiMedx, Georgia, USA to provide the grafts.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Ficarra V., Borghesi M., Suardi N., De Naeyer G., Novara G., Schatteman P., et al. Long-term evaluation of survival, continence and potency (SCP) outcomes after robot-assisted radical prostatectomy (RARP) BJU Int. 2013;112:338–345. doi: 10.1111/bju.12001. [DOI] [PubMed] [Google Scholar]
- 2.Walsh P.C., Donker P.J. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A., Chopra S., Pham A., Sooriakumaran P., Durand M., Chughtai B., et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2013;63:438–444. doi: 10.1016/j.eururo.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Patel V.R., Samavedi S., Bates A.S., Kumar A., Coelho R., Rocco B., et al. Dehydrated Human amnion/chorion membrane allograft nerve wrap around the prostatic neurovascular bundle accelerates early return to continence and potency following robot-assisted radical prostatectomy: propensity score-matched analysis. Eur Urol. 2015;67:977–980. doi: 10.1016/j.eururo.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Zelen C.M., Gould L., Serena T.E., Carter M.J., Keller J., Li W.W. A prospective, randomised, controlled, multi-center comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12:724–732. doi: 10.1111/iwj.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridge S.M., Bhattacharyya D., Dervan E., Naicker S.D., Burke A.J., Murphy J.M., et al. Secreted factors from metastatic prostate cancer cells stimulate mesenchymal stem cell transition to a pro-tumourigenic “activated” state that enhances prostate cancer cell migration. Int J Cancer. 2018;142:2056–2067. doi: 10.1002/ijc.31226. [DOI] [PubMed] [Google Scholar]
- 7.Corn P.G., Wang F., McKeehan W.L., Navone N. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res. 2013;19:5856–5866. doi: 10.1158/1078-0432.CCR-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R., Wang S., Wang N., Zheng Y., Zhou J., Yang B., et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234. doi: 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogaya-Pinies G., Palayapalam-Ganapathi H., Rogers T., Hernandez-Cardona E., Rocco B., Coelho R.F., et al. Can dehydrated human amnion/chorion membrane accelerate the return to potency after a nerve-sparing robotic-assisted radical prostatectomy? Propensity score-matched analysis. J Robot Surg. 2018;12:235–243. doi: 10.1007/s11701-017-0719-8. [DOI] [PubMed] [Google Scholar]
- 10.Alvim R.G., Hughes C., Somma A., Nagar K.K., Wong N.C., La Rosa S., et al. The potential risk of tumor progression after use of dehydrated human amnion/chorion membrane allograft in a positive margin resection model. Ther Adv Urol. 2019;11 doi: 10.1177/1756287219837771. 1756287219837771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatloff O., Chauhan S., Sivaraman A., Kameh D., Palmer K.J., Patel V.R. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012;61:796–802. doi: 10.1016/j.eururo.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Moschovas M.C., Patel V. Nerve-sparing robotic-assisted radical prostatectomy: how I do it after 15 000 cases. Int Braz J Urol. 2022;48:369–370. doi: 10.1590/S1677-5538.IBJU.2022.99.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coelho R.F., Chauhan S., Orvieto M.A., Sivaraman A., Palmer K.J., Coughlin G., et al. Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot-assisted radical prostatectomy. Eur Urol. 2011;59:72–80. doi: 10.1016/j.eururo.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noël J., Mascarenhas A., Patel E., Reddy S., Sandri M., Bhat S., et al. Nerve spare robot assisted laparoscopic prostatectomy with amniotic membranes: medium term outcomes. J Robot Surg. 2022;16:1219–1224. doi: 10.1007/s11701-022-01370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geuna S., Muratori L., Fregnan F., Manfredi M., Bertolo R., Porpiglia F. Strategies to improve nerve regeneration after radical prostatectomy: a narrative review. Minerva Urol Nefrol. 2018;70:546–558. doi: 10.23736/S0393-2249.18.03157-0. [DOI] [PubMed] [Google Scholar]
- 17.Krol B.C., Hemal A.K., Peak T., Liu S., Pathak R.A. Early return to continence and potency with use of dehydrated human umbilical cord graft at the time of robot-assisted radical prostatectomy: a case study and analysis of relevant literature. IJU Case Rep. 2021;4:151–153. doi: 10.1002/iju5.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porpiglia F., Manfredi M., Checcucci E., Garrou D., De Cillis S., Amparore D., et al. Use of chitosan membranes after nerve-sparing radical prostatectomy improves early recovery of sexual potency: results of a comparative study. BJU Int. 2019;123:465–473. doi: 10.1111/bju.14583. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson A.J., Eggener S.E., Hernandez A.V., Klein E.A., Kattan M.W., Wood D.P., Jr., et al. Do margins matter? The influence of positive surgical margins on prostate cancer-specific mortality. Eur Urol. 2014;65:675–680. doi: 10.1016/j.eururo.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Kusumowidagdo F. Dehydrated human amnion/chorion membrane (dHACM) closure in giant perianal condyloma acuminata (Buschke-Löwenstein tumor) J Pediatr Surg Case Rep. 2019;41:60–63. [Google Scholar]
- 21.Sommariva S., Tarricone R., Lazzeri M., Ricciardi W., Montorsi F. Prognostic value of the cell cycle progression score in patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69:107–115. doi: 10.1016/j.eururo.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson A.J., Wood D.P., Kattan M.W., Klein E.A., Scardino P.T., Eastham J.A., et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Kneebone A., Fraser-Browne C., Duchesne G.M., Fisher R., Frydenberg M., Herschtal A., et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020;21:1331–1340. doi: 10.1016/S1470-2045(20)30456-3. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda Y., Narita S., Okubo T., Mitsuzuka K., Hatakeyama S., Koizumi A., et al. Impact of nerve-sparing status on positive surgical margin location and biochemical recurrence in patients with prostate cancer post radical prostatectomy. Ann Surg Oncol. 2021;28:5341–5348. doi: 10.1245/s10434-021-10281-x. [DOI] [PubMed] [Google Scholar]
- 25.Tettelbach W., Cazzell S., Reyzelman A.M., Sigal F., Caporusso J.M., Agnew P.S. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16:19–29. doi: 10.1111/iwj.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rani A., Dasgupta P., Murphy J.J. Prostate cancer: the role of inflammation and chemokines. Am J Pathol. 2019;189:2119–2137. doi: 10.1016/j.ajpath.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Razdan S., Bajpai R.R., Razdan S., Sanchez M.A. A matched and controlled longitudinal cohort study of dehydrated human amniotic membrane allograft sheet used as a wraparound nerve bundle in robotic-assisted laparoscopic radical prostatectomy: a puissant adjunct for enhanced potency outcomes. J Robot Surg. 2019;13:475–481. doi: 10.1007/s11701-018-0873-7. [DOI] [PubMed] [Google Scholar]
- 28.Elliott P.A., Hsiang S., Narayanan R., Bierylo J., Chang S.C., Twardowski P., et al. Cryopreserved placental tissue allograft accelerates time to continence following robot-assisted radical prostatectomy. J Robot Surg. 2021;15:877–883. doi: 10.1007/s11701-020-01187-z. [DOI] [PubMed] [Google Scholar]