Abstract
Background
Rest‐activity and sleep‐wake cycles are controlled by the endogenous circadian rhythm generated by the suprachiasmatic nuclei (SCN) of the hypothalamus. Degenerative changes in the SCN appear to be a biological basis for circadian disturbances in people with dementia, and might be reversed by stimulation of the SCN by light.
Objectives
The review examines the effectiveness of light therapy in improving cognition, activities of daily living (ADLs), sleep, challenging behaviour, and psychiatric symptoms associated with dementia.
Search methods
ALOIS, the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG), The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS were searched on 20 January 2014 using the terms: "bright light*", "light box*", "light visor*", "dawn‐dusk*", phototherapy, "photo therapy", "light therapy" "light treatment", light* . The CDCIG Specialized Register contains records from all major healthcare databases (The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS) as well as from many trials databases and grey literature sources.
Selection criteria
All relevant, randomized controlled trials were included in which light therapy, at any intensity and duration, was compared with a control group for the effect of improving cognition, ADLs, sleep, challenging behaviour, and psychiatric symptoms associated with dementia (as well as institutionalization rates or cost of care). Included were people with dementia of any type and degree of severity.
Data collection and analysis
Two review authors independently assessed the retrieved articles for relevance, and four review authors independently assessed the selected studies for risk of bias and extracted the data. Statistically significant differences in outcomes between the treatment and control groups at the end of treatment and follow‐up were examined. Each study was summarized using a measure of effect (for example mean difference).
Main results
Eleven trials (13 articles) met the inclusion criteria. However, three of the studies could not be included in the analyses either because the reported data could not be used in the meta‐analysis or we were unable to retrieve the required data from the authors.
This updated review found no effect of light therapy on cognitive function, sleep, challenging behaviour (for example agitation), or psychiatric symptoms associated with dementia. Reduction in the development of ADL limitations was reported in one study, at three of five time points, and light therapy was found to have an effect after six weeks and two years but not after one year.
Authors' conclusions
There is insufficient evidence to justify the use of bright light therapy in dementia. Further research should concentrate on replicating the suggested effect on ADLs, and establishing the biological mechanism for how light therapy improves these important outcomes.
Keywords: Aged, Humans, Phototherapy, Affect, Cognition Disorders, Cognition Disorders/etiology, Cognition Disorders/therapy, Dementia, Dementia/complications, Depression, Depression/etiology, Depression/therapy, Psychomotor Agitation, Psychomotor Agitation/etiology, Psychomotor Agitation/therapy, Randomized Controlled Trials as Topic, Sleep Wake Disorders, Sleep Wake Disorders/etiology, Sleep Wake Disorders/therapy
Plain language summary
There is insufficent evidence to recommend the use of bright light therapy in dementia
This updated review examined whether light therapy is effective in improving cognition, ADLs, sleep, challenging behaviour, and psychiatric symptoms associated with dementia. Data from 11 trials were included in the analyses.
Rest‐activity and sleep‐wake cycles are controlled by the inborn daily rhythm generated by the suprachiasmatic nuclei (SCN) of the hypothalamus. Changes in the SCN appear to be the biological basis for changes in sleep patterns in people with dementia and might be reversed by stimulation of the SCN by light.
The light sources in the included studies were a light box placed approximately one metre away from the participants at a height within their visual fields; a light visor worn on their heads; ceiling mounted light fixtures; or dawn‐dusk simulation that mimics outdoor twilight transitions.
There was no effect of bright light therapy on cognitive function, sleep, agitation, or psychiatric symptoms associated with dementia. The results for a single outcome in a single study, which found a beneficial effect on ADLs, should be regarded with caution and need to be replicated before they could form the basis of a recommendation for the use of bright light therapy.
Background
Description of the condition
Dementia is a term for a syndrome that includes symptoms such as loss of memory, judgment and reasoning ability; psychiatric disturbances; and a variety of behavioural changes. Brain function is affected enough to interfere with a person's ability to function at work, in relationships, or in everyday activities (Alzheimer Society of Canada) (ASC 2013). The World Health Organization (WHO 2012) declared dementia a public health priority, citing the high global prevalence and economic impact on families, communities, and health service providers. As of 2010, more than 35.6 million people worldwide were living with dementia (World Alzheimer Report 2012). This number is expected to double every 20 years, to 65.7 million in 2030 and 115.4 million in 2050 (World Alzheimer Report 2012). Total healthcare costs for people with dementia amount to more than 1% of the global gross domestic product (GDP), or USD 604 billion in 2010 (World Alzheimer Report 2012).
Normal aging is associated with physiological changes to circadian rhythms. Compared to younger adults, people aged 65 years and over may experience changes in core body temperature, melatonin rhythm, and the circadian rest–activity cycle which may present as fragmented nocturnal sleep, multiple and prolonged awakenings in the second half of the night, and increased daytime napping (Campbell 1995; McCurry 2000). These abnormalities are more frequent and pronounced in older adults with dementia, and specifically those with Alzheimer's disease (AD) (McCurry 2000) when they may be associated with other related disturbances such as rest‐activity cycle disruptions and sundowning (Liu 2000). The neurobiological basis of these behavioural disorders is related to degenerative changes in the suprachiasmatic nucleus (SCN) of the hypothalamus that result in reduced expression of the vasopressin (AVP) gene (Liu 2000). Liu 2000 reported that the total amount of AVP‐mRNA expressed in the SCN was one‐third the amount in persons with AD than in age‐matched and time‐of‐death matched controls. In addition, the amount of AVP‐mRNA was three times higher during the daytime than at night in control adults aged 60 to 80 years whereas no clear diurnal rhythm was observed in persons with AD. Liu 2000 and colleagues emphasize that the reduction of AVP‐mRNA in the SCN does not necessarily only reflect neuronal death; neurons may still be present but inactive, and no longer able to express AVP‐mRNA. More recently, Harper 2008 revealed a circadian rhythm in patients with AD suggesting a more functional clock than had been previously supposed. Neurotensin neurons did not show a circadian rhythm when the count pattern was analysed, suggesting that neurotensin exerts its circadian effect through a different mechanism from AVP. These findings support the idea of a functional although perhaps not as robust SCN in AD.
The circadian pacemaker in the SCN is synchronized with the 24‐hour day by 'zeitgebers', or triggers, of which light is the most important. Light falling on the retina is transduced into neural activity that reaches the SCN through the retinohypothalamic and possibly the geniculohypothalamic tracts. Light leads to changes in the firing rates of specialized neurons in the SCN that in turn affect circadian rhythms (van Someren 1996). However, in older adults with dementia, most zeitgebers are reduced due to diminished social contacts, age‐related deficiencies in the eye (for example macular degeneration, cataracts, blindness), and less exposure to sufficient outdoor or bright light (Burns 2009; Gasio 2003; McCurry 2000). Reduced sensory input is likely to lower the 'general level of excitement' that is thought to play an important role in the entrainment of circadian rhythms (Burns 2009; van Someren 1996). Thus, an environment weak in phase prompts coupled with neuropathological damage causing poor sensitivity to such prompts can result in circadian rhythm disorders. A decreased ability to maintain a stable circadian pattern of daytime arousal and nocturnal quiescence may contribute to sleep disruptions (Ancoli‐Israel 2002; Burns 2009; McCurry 2000), cognitive dysfunction (Liu 2000; McCurry 2000), behavioural disturbances (for example agitation and sundowning) (Burns 2009; Haffmans 2001; McCurry 2000), functional impairment (McCurry 2000), and depression (Liu 2000; McCurry 2000) in persons with dementia. All of these symptoms reduce the quality of life of the individual with dementia, while sleep disruptions and behavioural disturbances also contribute to the burden on family caregivers. The stress that such disturbances place on family caregivers in particular is an important factor in the decision to institutionalize their family member with dementia.
Description of the intervention
Light therapy for persons with dementia may be delivered in a variety of ways, for example using a light box placed approximately one metre away from the participants at a height within their visual fields; a light visor worn on their heads; ceiling mounted light fixtures; or 'naturalistic' light therapy, known as dawn‐dusk simulation, that mimics outdoor twilight transitions. The light therapy may be administered for varying lengths of time and at different times of the day. The results from recent research have shown some consensus that the actual empirical peak wavelength for stimulation of melanopsin cells to shift circadian rhythm is probably in the short wavelength light range (approximately 450 to 500 nm), that is in the blue to green range of the light spectrum (Nowak 2011; Shirani 2009).
Compared to treatment with psychotropic drugs, such as sedative hypnotics, benzodiazepines, antipsychotics, and antidepressants, light therapy is a highly promising alternative with respect to adverse side effects (Nowak 2008; Paniagua 2008). When used to treat depression, seasonal affective disorder, or dementia, adverse effects of light therapy are typically reported to be mild and transient, and to occur less frequently than adverse effects of drug treatments (Nowak 2008).
How the intervention might work
As described above, persons with dementia often experience a reduction in general sensory input and less exposure to bright environmental light, as well as having reduced sensitivity to the effect of light on the SCN. Light therapy, by providing additional sources of light, may act through specialized neurons in the SCN to promote the synchronization of internal circadian rhythms with the environmental light‐dark cycles.
Why it is important to do this review
Several studies have examined the effectiveness of light therapy in managing disturbances of cognition, ADLs, sleep, behaviour, and psychiatric disturbances in individuals with dementia. There is preliminary evidence from some studies (for example Gasio 2003; Lyketsos 1999;Nowak 2008) that light therapy improves nocturnal sleep, while other studies (for example Dowling 2008; Skjerve 2004) demonstrated no improvement in people with dementia. The conflicting results may be due to heterogeneity of the studied population with respect to underlying diagnosis, stage of disease, visual impairment; methodological features such as the timing of light exposure with respect to core body temperature; and baseline light conditions affecting light sensitivity (light history) (Shirani 2009). Inconsistent results could also reflect bias in some of the studies. A systematic review of the evidence for light therapy is needed to determine whether or not light therapy is indeed effective in dementia and, if so, for which symptoms, and to explore the relationship between effectiveness and characteristics of treatment (for example light intensity, modality, time of administration, and duration).
Objectives
The objectives of the systematic review are:
to determine the effectiveness of light therapy in improving cognition, ADLs, sleep, challenging behaviour, and psychiatric disturbances associated with dementia.
Methods
Criteria for considering studies for this review
Types of studies
The review authors included all randomized controlled trials (RCTs) in which light therapy was compared with a control treatment. Studies should be at least single‐blind. Since the intervention consisted of bright light, blinding of participants may be difficult, although it is possible to compare bright light with dim light and not inform the participants about the true purpose of the study. The authors expected the outcome assessors to be blinded, however studies were included if this criterion was not met.
Types of participants
Participants had to have a diagnosis of dementia (Alzheimer's disease (AD), dementia with Lewy bodies, vascular dementia (VD), mixed dementia, or dementia due to another cause) according to accepted criteria such as those of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R, DSM‐IV) (APA 1995), the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) (McKhann 1984), or ICD‐10 (WHO 1992).
Types of interventions
The review authors included any intervention involving the use of bright light. Acceptable control interventions were usual care, possibly with dim red light or dim, low‐frequency blinking light at less than 300 lux.
Types of outcome measures
Included studies had to examine at least one objective outcome measure sensitive to the changes in cognition, ADLs, sleep, challenging behaviour, or psychiatric disturbances in dementia. These measures could be obtained at baseline, during the light therapy, immediately following the therapy, or at any interval of time after the treatment. The review authors accepted both dichotomous and continuous data.
Primary outcomes
Cognition (global or single domain, e.g. memory)
ADLs
Sleep‐wake disturbances
Challenging behaviour (e.g. agitation)
Psychiatric disturbances (e.g. depression)
Adverse effects
Secondary outcomes
Secondary outcomes of interest included:
rates of institutionalization;
cost of care.
Search methods for identification of studies
The Trials Search Co‐ordinator for the Cochrane Dementia and Cognitive Improvement Group searched ALOIS (www.medicine.ox.ac.uk/alois) on 20 January 2014. The search terms used were: light, phototherapy, "photo therapy".
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy adults. The studies are identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO, and LILACS.
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register; plus others).
Quarterly searches of the Central Register of Controlled Trials (CENTRAL) in The Cochrane Library.
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed in the 'methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
The librarian performed additional searches in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
The latest search (January 2014) retrieved a total of 276 results. After a first assessment and de‐duplication of these results the authors were left with three references to further review.
Data collection and analysis
Selection of studies
After merging search results and discarding duplicates, two authors (DF, ET) independently examined the titles and abstracts of citations. If a title or abstract appeared to represent our inclusion criteria, we retrieved the full article for further assessment. Two authors (DF, ET) then independently assessed the retrieved articles for inclusion in the review. The authors resolved disagreements by discussion or, if necessary, they referred to another author. The excluded articles and reasons for exclusion are listed in the ‘Characteristics of excluded studies’ table.
Data extraction and management
Four review authors (CB, PH, ET, SP) independently extracted data from the published articles including information regarding the publication date; authors; study setting; inclusion and exclusion criteria; participants’ diagnosis, gender, age; type, duration, intensity, frequency, and time of day of light therapy; control activity; outcome data; dropout rates and reasons, adherence, adverse events; randomization process, blinding, incomplete outcome data, selective reporting, and other bias. They recorded these in the ‘Characteristics of included studies’. The mean change from baseline to final measurements and the standard deviation (SD) of the change were often not reported in the published reports. Accordingly, the authors extracted the final mean measurements, the SD of the final mean, and the number of participants for each group at each assessment. The included trials did not report any dichotomous data of interest to this review. The review authors compared their extracted data and resolved disagreements by consensus or including another author.
Assessment of risk of bias in included studies
The authors based the criteria for judging risk of bias on the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, chapter 8 (Higgins 2011). Four authors (CB, PH, ET, SP) independently assessed and rated the trials according to the risk of bias criteria listed below. The authors used an assessment tool (see table 8.5.d, Higgins 2011) to determine if there was a low, high, or unclear risk of bias for each factor. The authors were not masked to the publication or author information. If the description of a process or outcome was unclear or missing, the authors contacted the original author of the trial in an attempt to retrieve the required information. Again, the authors resolved disagreements by discussion or, if necessary, by referral to a third author. They assessed the following criteria.
a. Selection bias, systematic differences between baseline characteristics of the groups that are compared
Random sequence generation
Allocation concealment
b. Performance bias, systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest
Blinding of participants and personnel
c. Detection bias, systematic differences between groups in how outcomes are determined
Blinding of outcome assessment
d. Attrition bias, systematic differences between groups in withdrawals from a study
Incomplete outcome data
e. Reporting bias, systematic differences between reported and unreported findings
Selective reporting
f. Other bias
Bias due to other problems
Measures of treatment effect
Each trial and each outcome of interest required summary statistics. For continuous data, the effect measure was the mean difference (MD) when the pooled trials used the same rating scale or test to assess an outcome. We used the standardized mean difference (SMD), which is the absolute mean difference divided by the SD, when the pooled trials used different rating scales or tests. The statistical method used in the meta‐analysis was inverse variance. We reported all outcomes using 95% confidence intervals. The included trials did not report any dichotomous data of interest to this review.
Unit of analysis issues
The participants in the included trials were the unit of analysis. Although the Riemersma 2008 trial included clusters of 12 facilities, the analyses performed with the MLwiN software (version 2.0, Institute of Education, London, England) accounted for the three‐level nested structure of the data set (that is a variable number of observations nested within participants and participants grouped in 12 facilities). Details are given in the online supplemental information (see http://www.jama.com). An estimate of the intra‐cluster correlation coefficient (ICC) was not provided to determine effective sample sizes. However, the sample sizes included in the meta‐analysis were not large (n = 74 to 87).
If a crossover design study was included, only the results prior to the crossover were considered for inclusion in our analysis. There were two crossover studies (Lyketsos 1999; Mishima 1998) but we were unable to obtain the first period data and the analyses reported had not used appropriate methods for paired data. Hence, we were unable to include any data from these studies.
If a trial included three or more arms, we gave consideration to the nature of the intervention and control arms. If appropriate, we considered combining the data from two treatment arms that were similar and had the same control group, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, section 16.5.4 (Higgins 2011). We combined the two treatment groups (morning and evening light therapy) in Dowling 2007/Dowling 2005 according to the Cochrane Handbook for Systematic Reviewds of Interventions (2011, 16.5.4).
Ancoli‐Israel 2003a/b reported only the combined findings of both the bright light therapy and dim red light groups because there were no significant differences between the groups. We requested group or individual data from the authors (29 October 2008) but these could not be obtained. Thus, the data from this study could not be included in the analysis.
Dealing with missing data
We found many types of information to be missing from the published articles, such as adequate descriptions of the process of randomization, blinding of outcome assessors, attrition and adherence to the light therapy, reasons for withdrawing, and required statistical data (that is means and standard deviations). We e‐mailed contact authors at least twice over a two‐month period and requested they provide the missing data. Some of these missing data are described in the risk of bias tables. The potential impact of the missing data on the results depended on the extent of the missing data, the pooled estimate of the treatment effect, and the variability of the outcomes. We also considered the variation in the degree of missing data as a potential source of heterogeneity. If available, we used intention‐to‐treat (ITT) data and if not available, we only used the reported completers’ data in the analyses.
Assessment of heterogeneity
We included trials that used a variety of light therapy approaches as the intervention and that examined similar outcomes to those in our meta‐analyses. However, when the intensity of the light therapy was very different (for example the dawn‐dusk simulation intervention compared with bright light) we conducted a separate analysis. We initially explored heterogeneity through a visual exploration of the forest plots. We then performed a test for statistical heterogeneity (a consequence of clinical or methodological diversity, or both, among trials) using the Chi2 test (P < 0.10) and I2 statistic. I2 is a useful statistic for quantifying inconsistency (I2 = [(Q ‐ df)/Q] x 100%, where Q is the Chi2 statistic and df is its degrees of freedom) (Higgins 2002; Higgins 2003). This describes the percentage of variability in effect estimates that was due to heterogeneity rather than sampling error (chance). If I2 equals 0% then there is no apparent heterogeneity, larger values (≥ 70%) indicate greater heterogeneity and caution should be used in interpreting the meta‐analysis). We considered a value greater than 50% to be substantial heterogeneity, and we attempted to explain this variation. We presented the overall estimate from a fixed‐effect model if the value was less than 30%. If, however, there was evidence of heterogeneity of the population or treatment effect, or both, between trials then we used a random‐effects model. In this case the confidence intervals were broader than those of a fixed‐effect model (Higgins 2011).
Assessment of reporting biases
There were too few studies included in any of the meta‐analyses to use funnel plots to examine possible publication bias. To investigate reporting biases within our included studies, we compared outcomes listed in the methods sections with reported results.
Data synthesis
We conducted the meta‐analysis using a fixed‐effect model except when the value of heterogeneity was greater than 30%. In these latter cases we used a random‐effects model in the analyses.
Subgroup analysis and investigation of heterogeneity
The authors decided a priori that the following subgroup analyses would be conducted to explore possible causes of heterogeneity, if there were sufficient data to permit these analyses.
Severity of dementia at baseline, assessed using the Mini‐Mental State Examination (MMSE) (Folstein 1975):
mild (MMSE > 17 to 26, or similar scale) (Hogan 2007);
moderate (MMSE 10 to 17, or similar scale) (Hogan 2007);
severe (MMSE < 10, or similar scale) (Feldman 2005).
Disease type:
Alzheimer's disease (AD);
vascular dementia;
mixed dementia;
unclassified or other dementia.
Type of bright light therapy:
ceiling mounted;
light box;
visor;
other.
Time of day light therapy was administered:
morning;
afternoon;
evening.
Duration of light therapy:
≤ 2 hours;
> 2 hours.
Strength of light therapy
≤ 2500 lux;
≥ 2500 lux.
Sensitivity analysis
We also considered sensitivity analyses a priori to explore possible causes of methodological heterogeneity such as including studies in the meta‐analysis that used a variety of measurement tools.
Results
Description of studies
Please see the ‘Characteristics of included studies’, ‘Characteristics of excluded studies’, and ‘Characteristics of ongoing studies’ tables.
Search results and included studies
Three new studies were included from the search in 2012 but the 2014 search revealed no new studies for inclusion.
From 1195 articles located through the 2012 database searches, 86 articles were screened for inclusion. Seventy‐nine were excluded after reading the abstracts and titles because they did not meet the relevance criteria. The remaining seven articles were retrieved and independently rated by two review authors. Two new articles (McCurry 2011; Nowak 2008) met the inclusion criteria and were added to the 11 articles (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Gasio 2003; Graf 2001; Lyketsos 1999; Mishima 1998; Riemersma 2008) included in the previous review (Forbes 2009), for a total of 13 articles. See Figure 1 for a flow chart.
1.
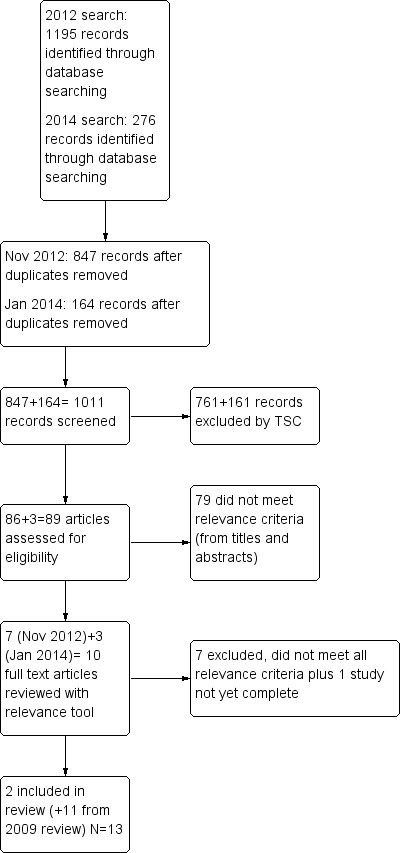
Study flow diagram from update search of November 2012.
The two Ancoli‐Israel papers (Ancoli‐Israel 2003a/b) reported on different outcomes from the same trial, as did Dowling 2007/Dowling 2005. Thus, although there were 13 articles there were 11 trials included in this review.
The included articles were published between 1998 and 2011. Six of the trials were conducted in the United States (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Dowling 2008; Lyketsos 1999; McCurry 2011; Nowak 2008), one in Japan (Mishima 1998), one in the United Kingdom (Burns 2009), one in the Netherlands (Riemersma 2008), one in Switzerland (Gasio 2003), and one in Austria (Graf 2001).
In 10 of the trials, participants were residents of long‐term care facilities of varying descriptions: assisted living (Riemersma 2008), nursing homes (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2008; Graf 2001; Nowak 2008), chronic care facilities (Dowling 2007/Dowling 2005; Lyketsos 1999), specialized wards (Mishima 1998), and nursing wings for residents with dementia (Gasio 2003). In one study (McCurry 2011) participants were living in the community with their caregivers.
The total number of participants in the included studies was 499. Of these participants, 398 to 399 completed the trial protocol (the range reflects the different outcomes measured in the same trial of Ancoli‐Israel 2003a/b).
Participants
The participants met the DSM‐IV or NINCDS‐ADRDA criteria for AD (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Mishima 1998; Nowak 2008; Riemersma 2008), VD (Burns 2009; Mishima 1998; Riemersma 2008); dementia with Lewy bodies (Burns 2009; Riemersma 2008), mixed dementia (Burns 2009; Riemersma 2008) or dementia (Lyketsos 1999). In another study (McCurry 2011) individuals were included if they had a clinical diagnosis of probable or possible AD according to medical records or confirmed in writing by participants’ primary care physicians. In one study (Graf 2001) individuals were included if they had a clinical diagnosis of AD or VD, depending on whether the progress of dementia was continuous suggesting AD, or was fluctuating suggesting VD; and evidence of focal neurologic deficits, essential hypertension, or vascular brain disease on a computerized tomographic scan indicating VD. These approaches were appropriate for ensuring that the participants have a diagnosis of dementia. Of the 499 participants in the included studies, 82% (n = 419) were diagnosed with probable AD. The remainder were diagnosed with either VD (n = 55, 11%) or another type of dementia (n = 35, 7%).
The mean MMSE scores of the participants in the included studies ranged from severe to moderate levels of dementia: 1.96 (SD 2.9) (Nowak 2008); 5.7 (SD 5.6) (Ancoli‐Israel 2003a/b); 5.9 (SD 5.5) (Burns 2009); 6.4 (SD 6.8) (Lyketsos 1999); 8.45 (range 3 to 17) (Mishima 1998); 9.3 (SD 7.9) Dowling 2008; 13.92 (SD 5.37) (Gasio 2003); 14.4 (SD 6.6) (Riemersma 2008); 15.9 (SD 5.90) (Graf 2001); and 17.9 (SD 7.0) (McCurry 2011).
In one study the participants were required to have a MMSE score below 24 (Graf 2001). In all trials the MMSE was used to measure the severity of dementia at baseline.
The exclusion criteria of the studies ensured that many of the potential confounders were eliminated. For example, residents who were blind or severely visually impaired, or who had severe motor symptoms or primary psychiatric disorders, were not included in the studies (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Gasio 2003; Graf 2001; Lyketsos 1999; McCurry 2011; Mishima 1998; Nowak 2008; Riemersma 2008).
Participants' medications were stabilized for various periods of time prior to initiating the trials: 12 weeks (Mishima 1998), one month (Graf 2001), and one week (Lyketsos 1999). In addition, Dowling 2007/Dowling 2005, Dowling 2008, Mishima 1998, and Nowak 2008 excluded participants who were taking melatonin, sedatives, hypnotics, or antipsychotics. Riemersma 2008 and Gasio 2003 kept the medications as constant as possible and listed each of the medications in a table. Burns 2009 reported that only one participant had her medication changed during the study. Ancoli‐Israel 2003a/b did not report if and how medication use was dealt with.
Light therapy approaches
In eight of the studies, light therapy was administered from either a SunRay light box (SunBox Company, Gaithersburg, MD) (McCurry 2011) or a Brite‐LiteTM box (Apollo Light Systems, Orem, Utah) (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Graf 2001; Lyketsos 1999; Mishima 1998) placed at approximately eye level one metre from the participant. The treatment groups received light therapy ranging from 2500 to 10,000 lux either in the morning or evening, for one to two hours, for 10 days to two months. The control groups received dim red light or dim, low‐frequency blinking light at less than 300 lux (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Graf 2001; Lyketsos 1999; Mishima 1998) or were offered nondirective dementia care support (McCurry 2011).
There were three exceptions. Gasio 2003 used a Dawn‐Dusk SimulatorTM which included a computer algorithm that drove an electronic controller connected to an overhead halogen lamp placed behind the participants' beds. Using the simulator, participants were exposed to a maximum of 400 lux or placebo dim red light (< 5 lux) for three weeks. Nowak 2008 used a 12,000 lux blue‐green light via a cap visor (Physician Engineered Products, Fryeburg, ME) or placebo dim red light for 30 minutes each morning for 14 days. In Riemersma 2008, residents were exposed to light by means of ceiling mounted fixtures with Plexiglass diffusers containing an equal number of Philips TLD840 and TLD940 florescent tubes, which were installed in the common living area. The lights were kept on between approximately 10.00 and 18.00 hours with the aim of an exposure of ± 1000 lux. Participants in the control group were exposed to dim (± 300 lux) light. The duration of participation of the facilities was a mean of 15 months (maximum period of 3.5 years).
Primary outcomes
Types of outcome measures
Objective outcome measures sensitive to changes in cognition, ADLs, sleep‐wake disturbances, challenging behaviour, or psychiatric disturbances were of interest to this review, as well as adverse events, institutionalization, and costs.
Cognition
Four of the included trials evaluated cognition by using the MMSE (Burns 2009; Gasio 2003; Graf 2001; Riemersma 2008).
ADLs
Riemersma 2008 used the Nurse‐Informant Activities of Daily Living measure to evaluate ADLs.
Sleep‐wake disturbances
Wrist actigraphy was used to evaluate sleep‐wake activity in eight of the included trials (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005, Dowling 2008; Gasio 2003; McCurry 2011; Mishima 1998; Nowak 2008; Riemersma 2008). A sleep log was used by Lyketsos 1999 to evaluate the sleep cycle.
Challenging behaviours
Two of the trials evaluated the agitated behaviour of participants by using the Cohen‐Mansfield Agitation Inventory (Burns 2009; Riemersma 2008), and one trial used the Agitated Behavior Rating Scale (Ancoli‐Israel 2003a/b). Lyketsos 1999 measured behaviour using the Behavioral Pathology in AD scale. Gasio 2003 used the short version of the Neuropsychiatric Inventory Nursing Home to evaluate behaviour, and Dowling 2007/Dowling 2005 used questions related to agitation and aggression from the Neuropsychiatric Inventory Nursing Home questionnaire.
Psychiatric symptoms
Two studies (Dowling 2007/Dowling 2005; Riemersma 2008) used the Neuropsychiatric Inventory (NPI), which comprises 10 domains: delusions, hallucinations, dysphoria, anxiety, agitation and aggression, euphoria, disinhibition, irritability and lability, apathy, and aberrant motor activity, to measure psychiatric disturbances. Five studies measured depression: Dowling 2007/Dowling 2005 used the depression or dysphoria domain of the NPI ‐ Nursing Home version (NPI‐NH), Gasio 2003 used the Geriatric Depression Scale (GDS), and Burns 2009, Lyketsos 1999, and Riemersma 2008 used the Cornell Scale for Depression in Dementia (CSDD).
Secondary outcomes
The one trial which included community‐dwelling participants at baseline did not report institutionalization. None of the included studies measured cost of care.
Risk of bias in included studies
Please see the table 'Characteristics of included studies'.
Random sequence generation (selection bias)
The process of randomization was assessed based on how the authors determined the allocation of participants to either a treatment or control group. Six of the authors (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Gasio 2003; Graf 2001; Mishima 1998; Nowak 2008) were contacted to determine the method of random sequence generation as the descriptions in the published articles were incomplete. A response was received from all authors except Mishima 1998.
In one study the risk of bias from random sequence generation was rated as high (Graf 2001) and in two studies it was rated as unclear (Lyketsos 1999; Mishima 1998).
The remaining trials had low risk of bias as sufficient evidence of random sequence generation was available (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Gasio 2003; McCurry 2011; Nowak 2008; Riemersma 2008). Randomization was conducted using various methods such as a randomization program on the internet (Burns 2009); a computer generated numbering scheme (Gasio 2003); the Microsoft Excel randomized number function (Riemersma 2008); and block stratified randomization (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Dowling 2008; Nowak 2008). In the Riemersma 2008 trial, 61 homes for the elderly were initially approached and 12 confirmed that they would be willing to participate. The facilities were randomly assigned to active or placebo light exposure using randomized sequence generation.
Allocation (selection bias)
The methods used to conceal allocations prior to group assignment were rated as unclear in three of the trials on the grounds of insufficient or absent information (Graf 2001; Lyketsos 1999; Mishima 1998). In the remaining trials, allocation concealment and overall selection bias were rated as at low risk. In the Ancoli‐Israel 2003a/b, Burns 2009, Dowling 2007/Dowling 2005, Dowling 2008, and Gasio 2003 articles participants were randomized by computer generated programs. In the Nowak 2008 study, a five‐block randomized block design was used. In the McCurry 2011 study, a research co‐ordinator assigned treatment conditions using sealed envelopes containing the random assignment. Participants were stratified according to their baseline sleep medication use. In the Riemersma 2008 study, facilities were randomly assigned to active or placebo light exposure using the Microsoft Excel random number function.
Blinding of participants and personnel (performance bias)
Six of the studies were rated low risk for performance bias. In Ancoli‐Israel 2003a/b, Graf 2001, and Lyketsos 1999 participants had a treatment light or a placebo light but were unaware of their assignment. In the Gasio 2003 study, residents and personnel were informed that both the white and red coloured light conditions were expected to show improvement and that the study was examining which colour was better. In the Riemersma 2008 study the participants were not aware of the treatment condition.
Two studies, Mishima 1998 and Nowak 2008, were rated as unclear because information on participant and personnel blinding was not specified.
The remaining five studies were rated as high risk. The Dowling 2007/Dowling 2005 and Dowling 2008 papers indicated that the nursing staff were potentially aware of the study group assignments of the participants. In Burns 2009, the nurse sat with the residents to ensure they remained by the light and also completed a number of the outcome records; and McCurry 2011 clearly identified that the participants, trainers, and caregivers were not blinded.
Blinding of outcome assessment (detection bias)
Seven studies reported that those who assessed the outcomes were blind to group allocation (Ancoli‐Israel 2003a/b; Gasio 2003; Graf 2001; Lyketsos 1999; McCurry 2011; Mishima 1998; Riemersma 2008). In the Gasio 2003 trial, the two raters were blinded and obtained their data from the sleep logs completed by the nursing staff, who were not aware of the type of intervention received by each group.
There was a high risk for outcome assessor bias in four of the studies (Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Nowak 2008). In the Burns 2009 study the nursing staff completed the sleep charts for all patients. In the Dowling studies (Dowling 2007/Dowling 2005; Dowling 2008) outcome assessors were potentially aware of group assignment. In Nowak 2008, the principal investigator administered the intervention, screened the participants in the chart review, and collected the qualitative data.
Incomplete outcome data (attrition bias)
Attrition rates varied from 0% to 47%. Seven of the studies were rated as low risk for incomplete outcome data (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2008; Graf 2001; McCurry 2011; Nowak 2008; Riemersma 2008). They reported their attrition rates, however two did not provide the reasons for the attrition (Ancoli‐Israel 2003a/b; Graf 2001). McCurry 2011 provided a flow diagram that clearly indicated the attrition of participants at specific points of time and from each of the groups. Nowak 2008 reported a 4% overall attrition rate and added that the light visor and the wrist actigraph were well tolerated by the participants. Two studies were rated as unclear risk. Dowling 2007/Dowling 2005 did not specify the attrition rate of their participants. Mishima 1998 did not refer to attrition in their study and did not reply to our correspondence.
Two studies were considered high risk. Gasio 2003 only had 13 patients who completed the study and seven dropped out due to non‐compliance with wearing the actimeter, fear of the light installation, or illness. Lyketsos 1999 also had a small sample of 15 residents, with only eight participants completing the trial. The researchers described at what point the participants left the trial and the reasons for their attrition.
Selective reporting (reporting bias)
Eight of the studies reported the results of all of their outcome measures at the specified points in time (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Dowling 2008; Graf 2001; McCurry 2011; Mishima 1998; Nowak 2008; Riemersma 2008). Three of the trials did not fully report all of their outcomes (Burns 2009; Gasio 2003; Lyketsos 1999). Burns 2009 did not report on the four‐ and eight‐week post‐intervention findings for the duration of sleep except to say that the findings were non‐significant. Gasio 2003 did not provide data for all of those outcomes for which they described non‐significant findings. Similarly, Lyketsos 1999 reported a non‐significant effect on depression but did not provide the data for this outcome.
Other potential sources of bias
Another potential source of bias was compliance with the light therapy or wearing the activity monitor, or both.
Four studies reported that participants received 65.7% (McCurry 2011), 77% (Ancoli‐Israel 2003a/b), 82% (SD 17%) (Dowling 2008), and 76% (Dowling 2007/Dowling 2005) of the light therapy. Burns 2009 reported that 90% of the participants tolerated a minimum of 90 minutes per day of light therapy. The cap visors were well tolerated in the Nowak 2008 study, averaging 414 minutes of wear out of a possible 420 minutes.
The range of compliance with wearing the activity monitors was 75% to 100% of the participants (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Gasio 2003; McCurry 2011; Riemersma 2008). Dowling 2008 reported that, of a total possible 108 hours of valid data, on average 105 (range 75 to 108) hours of baseline and 107 (range 90 to 108) hours of valid data at the end of the intervention were collected. Burns 2009, Mishima 1998, and Nowak 2008 did not report compliance with the actigraph.
Effects of interventions
Primary outcomes
Cognition
The only cognitive outcome measure in the included studies was the MMSE, which was used in four studies (Burns 2009; Gasio 2003; Graf 2001; Riemersma 2008). Morning bright light (10,000 lux) was compared with standard fluorescent tube light (100 lux) in Burns 2009, evening bright light (3000 lux) was compared with dim light (100 lux) in Graf 2001, all day bright light (1000 lux) was compared with dim light (300 lux) in Riemersma 2008, and dawn‐dusk simulation with light up to 400 lux was compared with dawn‐dusk simulation with dim red light (< 5 lux) in Gasio 2003. The data in the Burns 2009, Riemersma 2008, and Graf 2001 studies were combined because their light intensities were considered bright light. A fixed‐effect model was used as the I2 statistic for heterogeneity was 0%, indicating no apparent heterogeneity. The pooled data revealed no significant effect with 10 (Graf 2001), 14 (Burns 2009) and 42 days (Riemersma 2008) of treatment (MD 1.24, 95% CI ‐0.81 to 3.28, P = 0.24, n = 156) (Figure 2). The Riemersma 2008 data revealed similar results with one year of treatment (MD 1.70, 95% CI ‐1.03 to 4.43, P = 0.22, n = 55) and with two years of treatment (MD 3.60, 95% CI ‐1.05 to 8.25, P = 0.13, n = 26). Burns 2009 examined the results of bright light after four weeks of treatment but found no significant effect (MD 1.80, 95% CI ‐1.41 to 5.01, P = 0.27, n = 46). Similarly, Gasio 2003 reported no effect of their dawn‐dusk simulation intervention after three weeks of treatment (MD 0.46, 95% CI ‐14.14 to 15.06, P = 0.95, n = 13) or at follow up (three weeks after treatment) (MD ‐0.50, 95% CI ‐10.68 to 9.67, P = 0.92, n = 13).
2.
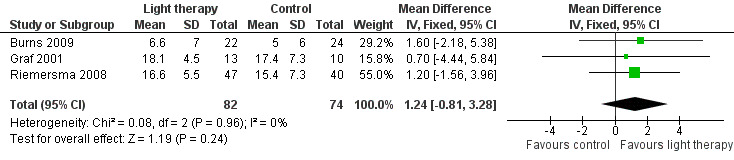
Forest plot of comparison: 1.1 Cognition following 10 to 42 days of treatment.
ADLs
One study (Riemersma 2008) measured ADLs using NI‐ADLs after six weeks, one year, and two years of treatment. After six weeks of treatment, light therapy had a positive effect in attenuating the decline in ADL performance (MD ‐5.00, 95% CI ‐9.87 to ‐0.13, P = 0.04, n = 87). After one year of treatment there was no significant effect (MD ‐5.00, 95% CI ‐11.16 to 1.16, P = 0.11, n = 55), however the ADL decline was significantly less after two years of light therapy (MD ‐16.00, 95% CI ‐26.21 to ‐5.79, P = 0.002, n = 26).
Sleep
Sleep latency, defined as the amount of time (in minutes) between reclining in bed and the onset of sleep (Davis 2001), was measured in Gasio 2003, Nowak 2008, and Riemersma 2008. However, only data from the Nowak 2008 and Riemersma 2008 studies were pooled due to the different light intensity used in the Gasio 2003 study. In the Nowak 2008 study a cap visor was worn for 30 minutes, and in the Riemersma 2008 study whole day ceiling bright light was received by the treatment group. Although these light sources were different, they are both considered bright light interventions. Two‐week data from Nowak 2008 were pooled with six‐week data from Riemersma 2008. Heterogeneity was low (I2 = 26%) and there was no significant improvement in sleep onset latency (MD ‐2.27, 95% CI ‐14.20 to 9.65, I2 = 26%, P = 0.71, n = 107) (Figure 3). The Riemersma 2008 study also reported no significant effect after one year of treatment (MD 5.00, 95% CI ‐24.79 to 34.79, P = 0.74, n = 55) or after two years of treatment (MD 10.00, 95% CI ‐11.33 to 31.33, P = 0.36, n = 26). Data from Gasio 2003 revealed that dawn‐dusk simulation did not significantly reduce sleep latency after three weeks of treatment (MD ‐79.00, 95% CI ‐327.17 to 169.17, P = 0.53, n = 13) and after three weeks of follow up (MD ‐62.00, 95% CI ‐216.55 to 92.55, P = 0.43, n = 13).
3.
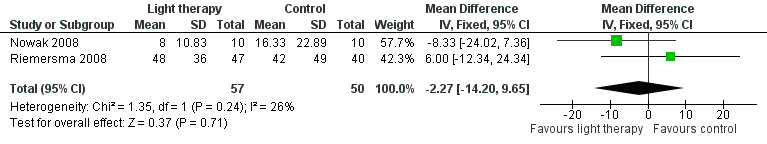
Forest plot of comparison: 1.2 Sleep onset latency following 2 to 6 weeks of treatment.
Nine studies measured total night sleep duration, following 10 days (Ancoli‐Israel 2003a/b), two weeks (Burns 2009; Nowak 2008), three weeks (Gasio 2003), four weeks (Lyketsos 1999), six weeks (Riemersma 2008), eight weeks (McCurry 2011), 10 weeks (Dowling 2007/Dowling 2005; Dowling 2008) of treatment. McCurry 2011 and Riemersma 2008 examined the effect after six months, and Riemersma 2008 continued the treatment for up to 3.5 years. Bright light therapy varied from ≥ 2500 to 10,000 lux, for 30 minutes to two hours in the morning (Ancoli‐Israel 2003a/b; Burns 2009; Dowling 2007/Dowling 2005; Dowling 2008; Lyketsos 1999; Nowak 2008) or evening (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; McCurry 2011) to all day bright light (1000 lux) (Riemersma 2008), or dawn‐dusk simulation (400 lux) morning and evening (Gasio 2003). The treatment groups were compared with the control groups who received dim light. The two treatment groups (morning and evening light therapy) in Dowling 2007/Dowling 2005 were combined. The combined treatment groups had a sample size of 53, a mean of 498.47 minutes, and a standard deviation of 108.23 minutes. Unfortunately Ancoli‐Israel 2003a/b reported only the combined findings of both the bright light therapy and dim red light groups because there were no significant differences between the groups. Group or individual data were requested from the authors (29 October 2008) but could not be obtained. Thus, the data from this study were not included in the analysis. In addition, the study by Lyketsos 1999, which was a crossover design, did not appear to have utilized analyses appropriate to a paired design. Group data prior to the crossover were requested (12 August 2003) but were not provided. Thus, the findings from Lyketsos 1999 also had to be excluded from the analyses.
Burns 2009, Dowling 2007/Dowling 2005, Dowling 2008, McCurry 2011, Nowak 2008, and Riemersma 2008 (six‐week data) were combined to reveal no effect of morning, evening, and all day bright light on total night sleep duration (MD ‐1.07, 95% CI ‐35.47 to 33.33, I2 = 61%, P = 0.95, n = 321) (Figure 4). However, there was substantial heterogeneity (61%). When the Nowak 2008 study was removed, there was 0% heterogeneity but the results remained non‐significant (MD 18.86, 95% CI ‐2.69 to 40.42, I2 = 0%, P = 0.09). The reason for this was difficult to explain. The heterogeneity may be due to the different light therapy approaches as Nowak 2008 used head visors and the remaining included studies used a Brite‐LiteTM box or ceiling mounted fixtures with Plexiglass diffusers. The heterogeneity may also be due to differences in severity of dementia as the Nowak 2008 participants had an average MMSE score of 1.96 (SD 2.9), which was much lower than the remaining participants, and only included female participants.
4.
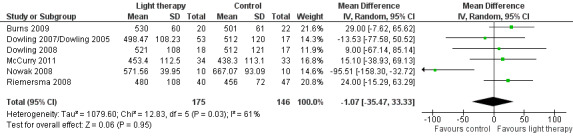
Forest plot of comparison: 1.3 Total sleep duration following 10 days to 10 weeks of treatment.
McCurry 2011 and Riemersma 2008 also examined the effect of bright light therapy on night sleep duration after six months of treatment (MD ‐7.78, 95% CI‐69.01 to 53.44, I2 = 65%, P = 0.80, n = 128) (Figure 5). A random‐effects model was used as the heterogeneity was 65%. This may be related to the differences in settings (community versus long‐term care) or sources of bright light (light box versus ceiling mounted light fixtures) used in the McCurry 2011 and Riemersma 2008 studies respectively. In addition, Riemersma 2008 reported that bright light had no effect on night sleep duration after one year (MD ‐36.00, 95% CI ‐84.21 to 12.21, P = 0.14, n = 55) and two years of treatment (MD ‐36.00, 95% CI ‐121.69 to 49.69, P = 0.41, n = 26).
5.
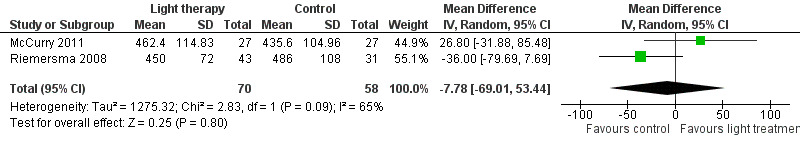
Forest plot of comparison: 1.4 Total sleep duration following 6 months of treatment.
Data from Gasio 2003 were analysed separately due to the lower intensity of treatment light. No effect was found after three weeks of treatment (MD 143.00, 95% CI ‐637.66 to 923.66, P = 0.72, n = 13) or at follow up (MD 110.00, 95% CI ‐77.22 to 297.22, P = 0.25, n = 13).
Four studies (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; McCurry 2011; Nowak 2008) measured sleep efficiency or the per cent of time asleep at night. Similar to the above analysis, the two treatment groups (morning and evening light therapy) in Dowling 2007/Dowling 2005 were combined. The combined treatment groups that examined sleep efficiency have a sample size of 53, a mean of 69.38, and SD of 14.97. For similar reasons to those cited above, the findings from Ancoli‐Israel 2003a/b could not be included in the analyses. Pooling data from Dowling 2007/Dowling 2005, McCurry 2011, and Nowak 2008 studies showed no effect on percentage of sleep time with morning and evening light therapy (MD 3.25, 95% CI ‐0.53 to 7.04, I2 = 4%, P = 0.09, n = 157) (Figure 6). A random‐effects model was used as the McCurry 2011 study was conducted in the community; the remaining trials were conducted in long‐term care facilities. The follow‐up periods of two weeks (MD 0.29, 95% CI ‐6.17 to 6.75, P = 0.93, n = 20), four weeks (MD 1.80, 95% CI ‐4.26 to 5.86, P = 0.76, n = 20), and six weeks (MD 1.10, 95% CI ‐5.17 to 7.37, P = 0.73, n = 20) did not reveal significant improvements in sleep efficiency (Nowak 2008).
6.
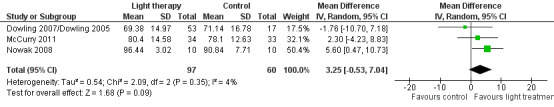
Forest plot of comparison: 1.5 Sleep efficiency following 2 to 10 weeks of treatment.
Four studies (Ancoli‐Israel 2003a/b; Dowling 2007/Dowling 2005; Gasio 2003; Mishima 1998) measured night time activity. The findings from Ancoli‐Israel 2003a/b could not be included in the analyses for reasons described above. In addition, the study by Mishima 1998, which was a crossover design, did not appear to utilize analyses appropriate to a paired design. Group data prior to the crossover were requested (13 August 2003) but were not provided. Thus, the findings from this study could not be included in the analyses. The findings from Dowling 2007/Dowling 2005 and Gasio 2003 could not be combined due to the differences in intensity of the light therapy. Dowling 2007/Dowling 2005 measured activity scores per night for both the morning and afternoon treatment groups compared with the control groups after 10 weeks of treatment. No effect on night time activity scores was found when bright light was administered in the morning (MD 855.78, 95% CI ‐867.84 to 2579.40, P = 0.33, n = 46) or afternoon (MD ‐78.60, 95% CI ‐627.17 to 469.97, P = 0.78, n = 41). These combined treatment groups had a sample size of 53, a mean of 67,171 activity counts, and SD of 37,054. No effect on night time activity counts was found when the two treatment groups (morning and evening light therapy) in Dowling 2007/Dowling 2005 were combined (MD 21,633, 95% CI ‐4770 to 48,036, P = 0.11, n = 70). In Gasio 2003, activity for each participant was averaged in one‐hour time periods and then over seven consecutive days of baseline, treatment, and follow‐up periods. No effect on night activity was found after three weeks of treatment (MD ‐20.60, 95% CI ‐46.52 to 5.32, P = 0.12, n = 13) or after three weeks of follow up (MD ‐24.70, 95% CI ‐52.70 to 3.30, P = 0.08, n = 13).
Dowling 2007/Dowling 2005, Dowling 2008, McCurry 2011, and Nowak 2008 measured the number of night time awakenings. Similar to the above analysis, the two treatment groups (morning and evening light therapy) in Dowling 2007/Dowling 2005 were combined. The combined treatment groups that examined night time awakenings had a sample size of 53, a mean of 37.81 night time awakenings, and SD of 26.70. Including all four studies resulted in a significant improvement in the number of night time awakenings (MD ‐2.17, 95% CI ‐3.84 to ‐0.49, I2 = 0%, P = 0.01, n = 192). However, when the Nowak 2008 study was removed the results were no longer significant (MD ‐1.55, 95% CI ‐5.43 to 2.33, I2 = 0%, P = 0.43, n = 172) (Figure 7). A random‐effects model was used as the McCurry 2011 study was conducted in the community; the remaining trials were conducted in long‐term care facilities. Including only the studies that incorporated morning bright light (Dowling 2008; Nowak 2008) resulted in a larger significant finding (MD ‐2.42, 95% CI ‐4.22 to ‐0.62, I2 = 0%, P = 0.008, n = 55). However, when the Nowak 2008 study was removed, the results were no longer significant (MD ‐4.00, 95% CI ‐11.06 to 3.06, P = 0.27, n = 35). In addition, the positive effects were not supported at two‐weeks follow up (MD ‐0.50, 95% CI ‐3.29 to 2.29, P = 0.73, n = 20), at four‐weeks follow up (MD ‐1.04, 95% CI ‐4.06 to 1.98, P = 0.50, n = 20), or at six‐weeks follow up (MD ‐1.56, 95% CI ‐4.90 to 1.78, P = 0.36, n = 20) (Nowak 2008). The inconsistencies in these results were difficult to explain. Perhaps the small sample size (n = 20) and the level of severity of dementia (MMSE average score of 1.96, SD 2.9) in the participants in the Nowak 2008 trial may have contributed to these inconsistencies. For these reasons, the Nowak 2008 was removed from the reported meta‐analysis in RevMan 5.2 (Figure 7).
7.
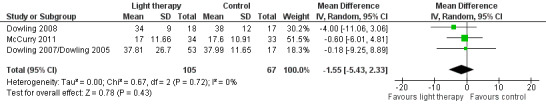
Forest plot of comparison: 1.6 Number of night time awakenings following 2 to 10 weeks of treatment.
Challenging behaviours
Six studies measured agitation: using the Agitated Behavior Rating Scale (ABRS) (Ancoli‐Israel 2003a/b), the subscale for Agitation and Aggression from the NPI‐NH (Dowling 2007/Dowling 2005; Gasio 2003), and the Cohen‐Mansfield Agitation Inventory (CMAI) (Burns 2009; Riemersma 2008, six week data). The findings from Lyketsos 1999 could not be included in the analyses as data prior to the crossover were requested on 12 August 2003 but were not provided. A random‐effects model and SMD were used to determine the effect of light therapy when different rating scales were used in the pooled studies.
Ancoli‐Israel 2003a/b and Dowling 2007/Dowling 2005 measured agitation in both morning light therapy and afternoon and evening light therapy groups. These two treatment groups were combined in each study. The combined Ancoli‐Israel 2003a/b groups measured in the evening had a sample size of 48, a mean agitation score of 0.30, with SD of 0.66. The combined groups in the Dowling 2007/Dowling 2005 had a sample size of 37, a mean agitation score of 5.17, with SD of 2.96. Light therapy administered during the morning, evening, or all day for between 10 days to 10 weeks had no effect on agitation (SMD ‐0.01, 95% CI ‐0.31 to 0.29, I2 = 16%, P = 0.95, n = 250) (Figure 8). Riemersma 2008 found no effect of daytime light therapy on agitation following treatment lasting one year (MD ‐2.00, 95% CI ‐11.71 to 7.71, P = 0.69, n = 55) or two years (MD ‐9.00, 95% CI ‐21.34 to 3.34, P = 0.15, n = 26). There was also no effect five days post‐treatment (MD 0.10, 95% CI ‐0.16 to 0.36, P = 0.46, n = 48) (Ancoli‐Israel 2003a/b) or after four weeks of follow up (MD 0.00, 95% CI ‐7.11 to 7.11, P = 1.00, n = 46) (Burns 2009).
8.
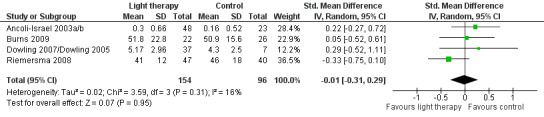
Forest plot of comparison: 1.7 Agitation following 10 days to 10 weeks of treatment.
Data provided by Gasio 2003 revealed no significant difference in agitation following three weeks of dawn‐dusk simulation or dim red light therapy (MD ‐3.19, 95%CI ‐9.83 to 3.45, P = 0.35) and after three weeks of follow up (MD ‐4.17, 95% CI ‐13.37 to 5.03, P = 0.37).
Psychiatric disturbances
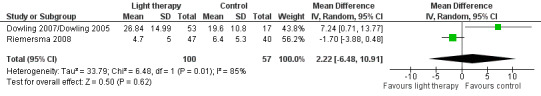
Dowling 2007/Dowling 2005 and Riemersma 2008 both examined 10 domains of psychiatric disturbances using the NPI‐NH. The morning and afternoon treatment groups in Dowling 2007/Dowling 2005 were combined. The combined groups had a sample size of 53, a mean NPI score of 26.84, with SD of 14.99. Data were pooled for these two studies, using the six‐week data from Riemersma 2008. There was considerable heterogeneity (I2 = 85%). No effect on the NPI score was observed after 6 to 10 weeks of treatment (MD 2.22, 95% CI ‐6.48 to 10.91, P = 0.62, n = 157) (Figure 9). Riemersma 2008 also found no effect after one year (MD ‐0.30, 95% CI ‐2.73 to 2.13, P = 0.81, n = 55) or after two years of light therapy (MD ‐3.30, 95% CI ‐7.03 to 0.43, P = 0.08, n = 26). Gasio 2003 used the NPI to examine psychiatric symptoms following three weeks of dawn‐dusk simulation or dim red light therapy. No effect was observed at the end of three weeks of treatment (MD ‐3.19, 95% CI ‐9.83 to 3.45, P = 0.35, n = 13) or three weeks later (MD ‐4.17, 95% CI ‐13.37 to 5.03, P = 0.37, n = 13) (Gasio 2003).
9.
Forest plot of comparison: 1.8 Psychiatric symptoms following 6 to 10 weeks of treatment.
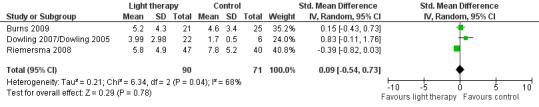
Five studies measured depression (Burns 2009; Dowling 2007/Dowling 2005; Gasio 2003; Lyketsos 1999; Riemersma 2008). Lyketsos 1999 reported that no significant differences in depression scores were found between groups at each time point. However, raw data were not reported and could not be retrieved as the data were archived (personal communication, Constantine Lyketsos, 31 May 2003). The morning and afternoon treatment groups in Dowling 2007/Dowling 2005 were combined. The combined groups had a sample size of 22, a mean NPI depression score of 3.99, with SD of 2.98. Because two different measuring scales were used in the pooled studies (Burns 2009; Dowling 2007/Dowling 2005; Riemersma 2008), a SMD was utilized. There was substantial heterogeneity (I2 = 68%). No effect on depression was seen following 2 to 10 weeks of light therapy (SMD 0.09, 95% CI ‐0.54 to 0.73, P = 0.78, n = 161) (Figure 10). In addition, the Riemersma 2008 data revealed no effect on depression after one year (MD ‐0.30, 95% CI ‐4.36 to 3.76, P = 0.88, n = 55) or after two years of treatment (MD ‐4.40, 95% CI ‐10.82 to 2.02, P = 0.18, n = 26). There was also no effect after four weeks of follow up (MD 0.50, 95% CI ‐1.15 to 2.15, P = 0.55, n = 45) (Burns 2009). Analysis of the data provided by Gasio 2003 revealed no effect on depression scores after three weeks of treatment (MD ‐0.82, 95% CI ‐4.33 to 2.69, P = 0.65, n = 13) or at follow up (MD ‐1.29, 95% Cl ‐3.99 to 1.41, P = 0.35, n = 13).
10.
Forest plot of comparison: 1.9 Depression following 2 to 10 weeks of treatment.
Secondary outcomes
None of the included trials reported on our secondary outcomes of rates of institutionalization and costs of care.
Adverse events
Only two trials (Lyketsos 1999; Nowak 2008) reported adverse events. Five participants in Lyketsos 1999 were removed by the study principal investigator due to a worsening of their agitation. In the Nowak 2008 trial one participant in the experimental group experienced an episode of forehead redness observed upon removal of the visor after 30 minutes of blue‐green light; this was minor and transient. Another participant in the control group, who had a long history of falling, experienced several falls during the light application phase and was removed from the study. No unexpected or serious adverse events attributed to the light therapy were reported by McCurry 2011 and Riemersma 2008. The remaining trials did not discuss adverse events in relation to the light therapy treatment.
Discussion
Summary of main results
This updated review included two new trials, resulting in a total of 11 trials (13 articles). The number of participants in the included trials was 499. Of these participants, 398 to 399 completed the protocol (the range reflects the different outcomes measured in the Ancoli‐Israel 2003a/b trial). Most participants were older persons with AD. The light therapy was most frequently administered through a light box. However, a dawn‐dusk simulator, cap visor, and ceiling mounted fixtures with Plexiglass diffusers containing florescent tubes were also light therapy sources.
Pooled data (Dowling 2007/Dowling 2005; Dowling 2008; McCurry 2011; Nowak 2008) resulted in a significant decrease in the number of night time awakenings at the endpoint of the treatment. Including only the studies that incorporated morning bright light (Dowling 2008; Nowak 2008) resulted in a larger significant finding. However, when the Nowak 2008 study was removed the results were no longer significant and no significant effect was revealed at two weeks, four weeks, and six weeks of follow up (Nowak 2008). One study in this review demonstrated that light therapy had a positive effect on one outcome of interest, ADLs. The Riemersma 2008 study revealed that light therapy had a positive effect on the treatment group in attenuating the increase in ADL limitations after six weeks and after two years of light therapy. The sample size was adequate at six weeks (n = 87) but by two years the sample size was reduced to 26 participants. No significant evidence was found that light therapy decreased the decline in cognition, shortened sleep latency time, increased sleep duration and efficacy, decreased night time activity counts, decreased challenging behaviours, or improved psychiatric symptoms including depression. Indeed, the four included trials that examined challenging behaviours (that is agitation) revealed that light therapy was not effective when administered in the morning, afternoon, evening, or all day at from 10 days to 10 weeks and with treatment lasting up to two years.
There were insufficient numbers of trials to be able to conduct subgroup analyses that would determine which modality of light therapy, at what time of day, intensity and duration, is most beneficial for specific types and severities of dementia. No RCTs were retrieved that measured the other outcomes of interest, namely changes in rates of institutionalization or impact on cost of care. Four trials (Lyketsos 1999; McCurry 2011; Nowak 2008; Riemersma 2008) examined adverse effects of light therapy. Only Lyketsos 1999 reported an increase in agitated behaviour in five participants. No other significant adverse effects were reported. The remaining studies did not report on adverse events.
Overall completeness and applicability of the results
Most included studies examined aspects of sleep (n = 8) and challenging behaviours (n = 6). The remaining primary outcomes of interest were examined by fewer studies (one to six studies). None of the retrieved studies examined our secondary outcomes, which were rates of institutionalization and cost of care.
Among the included studies there was great variability in the intensity of the light therapy. For example, Gasio 2003 used a Dawn‐Dusk SimulatorTM which exposed participants in the treatment group to a maximum of 400 lux, while the treatment groups in the remaining studies received light therapy ranging from 2500 to 10,000 lux. We did not consider it appropriate to combine the low exposure with the more intense exposures of light. This decision limited the number of included studies in each meta‐analysis.
While most of the participants in the included studies were diagnosed with AD (82%), the remaining participants had vascular or mixed dementia or the type of dementia was not diagnosed. Ancoli‐Israel 2003a/b, Dowling 2007/Dowling 2005,Dowling 2008, McCurry 2011, and Nowak 2008 included only participants with AD. This is an important design strategy as dementia should not be viewed as a single disease entity, and interventions such as light therapy may affect these conditions differently. Individuals with vascular dementia have heterogeneous brain pathology; their response to light therapy may depend on the areas in which ischaemic damage has occurred. The response to light therapy of individuals with scattered lesions of vascular dementia (Mishima 1998) or with frontotemporal degeneration (Harper 2001) may differ from that of people with AD, who commonly have damage to the hippocampi and medial temporal lobes of the brain. Indeed, Mishima 1998 reported that only the vascular dementia group showed a reduction in night time activity level, which may be explained by different origins of sleep and rhythm disturbances in persons with AD compared with those with vascular dementia (Mishima 1997). Investigators need to be sensitive to the importance of controlling for these differences in pathology when designing studies of light therapy. Differences in severity of dementia may also influence the results. Unfortunately, because of the small sample sizes and small number of trials that examined each outcome, subgroup analyses could not be conducted.
Only one study (McCurry 2011) was based in the community, all the remaining trials were conducted in an institutional setting. However, light therapy modalities implemented in residential facilities may not translate readily to a home setting as they may be impractical, unacceptable, or overly expensive for the family caregiver and person with dementia residing in the community (McCurry 2000). Most persons with dementia are cared for at home and most caregivers wish to keep their family member with dementia at home for as long as possible. Knowing how to support family caregivers and delay the symptoms of dementia will have profound benefits for all involved. In addition, enabling persons with dementia to remain in their homes for longer periods of time will lead to decreased healthcare costs. Further community‐based trials are needed that examine the effect of light therapy on multiple domains of the people with dementia and the impact on their family caregivers.
The non‐significant results may have been related to small sample sizes, which contribute to insufficient power to detect a difference, if one is present. Notable exceptions were the Ancoli‐Israel 2003a/b studies that included 92 participants, the McCurry 2011 trial that included 139 participants and their family caregivers, and the Riemersma 2008 study that included 94 participants. Clearly further research with larger sample sizes is required, and that examines all of the outcomes of interest.
Quality of the evidence
11.
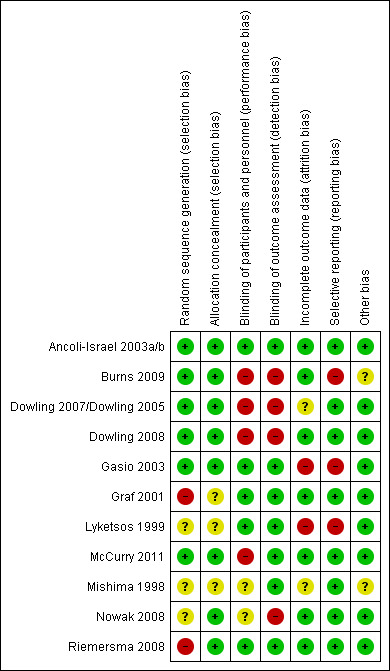
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
12.
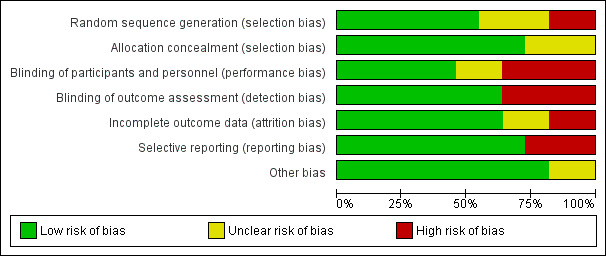
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
The quality of evidence may be impacted by the risk of bias associated with the random sequence generation and concealed allocation to groups. For example, it has been demonstrated that even with adequately concealed allocation sequence, trials with inadequate sequence generation yielded exaggerated estimates of intervention effects, on average, than trials with adequate sequence generation (Schulz 1995). In our review, it was difficult to determine these processes for most of the trials, and the authors were often requested to provide more detailed information. Some authors responded and others did not. Increasingly more researchers (Ancoli‐Israel 2003a/b; Burns 2009; Gasio 2003; McCurry 2011; Riemersma 2008) reported using a computerized random number generator. In another three trials randomization was unclear, and in one case random selection was rated as high risk. A computer random number generator is the recommended approach to generating the random selection and allocation concealment to groups. Inadequate concealment includes randomization by use of case record numbers, dates of birth, admission dates, day of the week, and any procedure transparent before allocation, such as an open list of random numbers (Wild 2003).
Several methodological studies have examined the effect of concealment of allocation sequence. A pooled analysis of seven methodological studies found that effect estimates from trials with inadequate concealment of allocation or unclear reporting of the technique used for concealment of allocation were on average 18% more ‘beneficial’ than effect estimates from trials with adequate concealment of allocation (95% CI 5 to 29) (Pildal 2007). Wood 2008 reported that the intervention effect estimates were exaggerated when there was inadequate allocation concealment in trials where a subjective outcome was analysed, but there was little evidence of bias in trials with objective outcomes.
Blinding of the participants and personnel was not an expectation of this review as the light therapy was often obvious to the participants and persons administering the light therapy. However, some researchers in the included trials were able to deceive the participants and personnel by informing them that the trial was examining the effectiveness of the colour of the light, and participants in both the light treatment and control groups sat in front of a light box.
We examined whether the outcome assessors were blinded as lack of blinding in trials has been shown to be associated with more exaggerated estimated intervention effects, by 9% on average, measured as odds ratio (Pildal 2007). These studies included both subjective and objective outcomes. The estimated effect has been observed to be more biased, on average, in trials with more subjective outcomes (Wood 2008). Although seven trials reported that the outcome assessors were blinded, in four trials the outcome assessors were not blinded.
While seven authors adequately reported on attrition rates, the remaining authors did not. McCurry 2011 and Riemersma 2008 provided excellent examples of reporting attrition through the use of a flow diagram that clearly described the reasons for attrition of participants in each group at specific points of time. Eight trial authors adequately described data for all of the outcomes of interest while three authors did not. This is apparently not unusual as missing data are common in medical journals and are often inadequately handled in the statistical analyses (Wood 2004). Higgins 2011 reported that several empirical studies found no clear evidence of bias associated with missing data (Balk 2002; Kjaergard 2001; Schulz 1995; Siersma 2007). Tierney 2005 observed a tendency for analyses conducted after trial authors excluded participants to favour the experimental intervention compared with analyses that included all participants. A review by Porta 2007 found more exaggerated effect estimates from ‘per protocol’ analyses compared with intention‐to‐treat analyses of the same trials. Thus, when there is missing data an intention‐to‐treat analysis is recommended.
Other potential sources of bias were related to the reporting of compliance with the light therapy or wearing the activity monitor. Only five studies reported on these.
Potential bias in the review process
This review was conducted as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), thus the introduction of bias during the review process was minimized. We are fairly confident that all relevant studies were identified as the literature searches were conducted by Anna Noel‐Storr of the Cochrane Dementia and Cognitive Improvement Group and updated at least every six months.
Three included studies did not provide useable data for inclusion in the meta‐analyses. For example, Lyketsos 1999 and Mishima 1998 used crossover designs and did not conduct analyses appropriate to a paired design, and Ancoli‐Israel 2003a/b only reported combined data from the treatment and control groups. This is unfortunate as the total number of trials that examined the effectiveness of light therapy in improving the symptoms of dementia is limited. It is important to include means and SDs for endpoint measures (before crossover) or change from baseline to final measurement scores for the treatment and control groups in published reports, or the authors should be willing to provide these data on request. Clearly, additional research is needed that examines these important outcomes (Weldemichael 2010) and provides the needed data for meta‐analysis.
Agreement and disagreement with other studies or reviews
Recent systematic reviews on this topic have summarized research literature similar to the studies included in this review (for example David 2010; Kong 2009; Padilla 2011). Salami 2011 synthesized the qualitative and quantitative evidence (n = 38 studies; included five RCTs) on non‐pharmacological and pharmacological treatments of sleep disturbance in persons with AD. Salami 2011 concluded that bright light therapy demonstrated the best results as pharmacological agents produce inconsistent results and their use is limited by their potential adverse effects.
Our non‐significant results related to challenging behaviours are supported by observational studies, for example Barrick 2010, which examined the effect of ambient bright light therapy on agitation among institutionalized persons with dementia. Four ambient lighting conditions were included, morning bright light, afternoon bright light, all day bright light, and standard light (control). Results revealed that for participants with mild to moderate dementia, agitation was higher in all of the treatment conditions compared with the control. For persons with severe dementia there was also a trend toward being more agitated during morning light than standard light (P = 0.053). Barrick 2010 concluded that ambient bright light is not effective in reducing agitation in dementia and may exacerbate this behavioural symptom.
Authors' conclusions
Implications for practice.
Only one study (Riemersma 2008) revealed that light therapy may have an effect in attenuating the increase in ADL limitations after six weeks (n = 87) and after two years (n = 26) but did not have an effect after one year (n = 55). It is thus premature to recommend the use of light therapy in practice. No significant adverse events related to the light therapy were reported except for a worsening of agitated behaviour (Lyketsos 1999).
Implications for research.
As there is limited evidence that light therapy may be effective in delaying deterioration in ADLs, further and better designed research is required. Research is needed to identify appropriate illumination intensity, frequency, interval, time of day (although trials that administered light therapy in the morning, afternoon, evening, and all day were included in this review), and length of intervention for individuals with different types and severities of dementia. Exploring different light therapy approaches (for example dawn‐dusk simulation, cap visor, ambient light) is also required to ensure that the light therapy is acceptable to persons with dementia. Unless they are comfortable with the light therapy, there will be low compliance. Outcomes that contribute to quality of life for persons with dementia and their caregivers should be examined as well as cost implications and potential adverse effects of light therapy.
Further research is also needed using outdoor light, as the importance of exposing persons with dementia to outdoor light has been demonstrated (Connell 2007; Martin 2007). For example, Connell 2007 revealed that a daily structured activity program offered outdoors, compared with indoors, over a two week period improved maximum sleep duration for persons with dementia who participated in the outdoor program. Persons residing in the community and those residing in long‐term care facilities with the assistance of healthcare aides or volunteers can greatly increase their daily light exposure by spending time outdoors. For example, the intensity of sunlight at midday measures over 100,000 lux (Shirani 2009) and on a cloudy day it ranges from 8000 to 10,000 lux; interior daytime exposure sitting near windows equals approximately 1000 lux (McCurry 2000). Since exposure to outdoor light has many potential benefits for the person with dementia, their family caregivers, and formal care providers, this is a recommended area of future research.
Researchers should also attempt to accurately diagnose and determine the severity of the dementia as it is possible that persons with mild to moderate AD with more intact SCNs and who are more receptive to other zeitgebers or triggers will have a greater response to light. Clearly further research is needed to be able to develop best practice guidelines that would be helpful to healthcare providers in advising persons with dementia living in institutional and community settings.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2014 | New citation required but conclusions have not changed | Conclusions unchanged |
| 20 January 2014 | New search has been performed | Two top up searches were performed for this update: one in November 2012 and one in January 2014. Three new studies were included from the 2012 search. There were no studies for inclusion from the 2014 search. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 3 December 2008 | New search has been performed | A new update search was performed on 4 March 2008. Some new studies were retrieved for inclusion or exclusion. Three new studies have been included in the updated review, and 4 new studies have been excluded. |
| 3 December 2008 | New citation required but conclusions have not changed | The title of this updated review has changed |
| 15 May 2006 | New search has been performed | New searches revealed one incomplete trial and two non‐RCTs. However, none met the inclusion criteria for this review. The Results and conclusions of the review remain unchanged. |
| 11 February 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Anna Noel‐Storr, Cochrane Dementia and Cognitive Improvement Group, for conducting the searches, and Sue Marcus, Cochrane Dementia and Cognitive Improvement Group, for her assistance throughout the review. We also wish to thank the following authors who contributed to our previous review: Ivan Culum, Andrea Lischka, Debra Morgan, Jennifer Forbes, and Sean Forbes.
Appendices
Appendix 1. Update searches: November 2012 and January 2014
|
Source |
Search strategy [date limits shown are for the first of the update searches; for the subsequenet top‐up search, the appropriate date limits were applied] | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Keyword search: light OR lig OR phototherapy OR "photo therapy" | Nov 2012: 48 (all dates) Jan 2014:6 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) [last searched 20 January 2014] | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. light*.ti,ab. 22. Phototherapy/ 23. phototherapy.ti,ab. 24. "photo therapy".ti,ab. 25. "dawn‐dusk*".ti,ab. 26. or/21‐25 27. randomized controlled trial.pt. 28. controlled clinical trial.pt. 29. randomi?ed.ab. 30. placebo.ab. 31. randomly.ab. 32. trial.ab. 33. groups.ab. 34. or/27‐33 35. (animals not (humans and animals)).sh. 36. 34 not 35 37. 20 and 26 and 36 38. (2008* or 2009* or 2010* or 2011* or 2012*).ed. 39. 37 and 38 |
Nov 2012: 107 Jan 2014: 25 |
| 3. EMBASE 1980‐2012 January 19 (OvidSP) [last searched 20 January 2014] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. light*.ti,ab. 25. phototherapy/ 26. phototherapy.ti,ab. 27. "photo therapy".ti,ab. 28. "dawn‐dusk*".ti,ab. 29. or/24‐28 30. 23 and 29 31. randomized controlled trial/ 32. controlled clinical trial/ 33. randomi?ed.ab. 34. placebo.ab. 35. randomly.ab. 36. trial.ab. 37. groups.ab. 38. ("double‐blind*" or "single‐blind*").ti,ab. 39. or/31‐38 40. 30 and 39 41. (2008* or 2009* or 2010* or 2011* or 2012*).em. 42. 40 and 41 |
Nov 2012: 360 Jan 2014: 118 |
| 4. PsycINFO 1806‐January week 3 2014 (OvidSP) [last searched 20 January 2014] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. light*.ti,ab. 26. "photo therapy".ti,ab. 27. phototherapy.ti,ab. 28. exp Phototherapy/ 29. "dawn‐dusk*".ti,ab. 30. or/25‐29 31. randomi?ed.ab. 32. randomly.ab. 33. exp Clinical Trials/ 34. placebo.ab. 35. ("double‐blind*" or "single‐blind*").ti,ab. 36. (RCT or CCT).ti,ab. 37. groups.ab. 38. "random* controlled trial".ti,ab. 39. or/31‐38 40. 24 and 30 and 39 41. (2008* or 2009* or 2010* or 2011* or 2012*).up. 42. 40 and 41 |
Nov 2012: 109 Jan 2014:15 |
| 5. CINAHL (EBSCOhost) [last searched 20 January 2014] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Phototherapy") S21 TX phototherapy S22 TX "photo therapy" S23 TX light* S24 TX "dawn‐dusk*" S25 S20 OR S21 OR S22 OR S23 OR S24 S26 S19 AND S25 S27 EM 2008 S28 EM 2009 S29 EM 2010 S30 EM 2011 S31 EM 2012 S32 S27 OR S28 OR S29 OR S30 OR S31 S33 S26 AND S32 S34 (MH "Randomized Controlled Trials") S35 AB randomly S36 AB groups OR "control group" S37 AB RCT OR CCT S38 AB "double‐blind*" OR "single‐blind*" S39 S34 OR S35 OR S36 OR S37 OR S38 S40 S33 AND S39 |
Nov 2012: 32 Jan 2014:4 |
| 6. ISI Web of Science (1945‐present) and conference proceedings [last searched 20 January 2014] |
Topic=(dementia OR alzheimer*) AND Topic=(light OR phototherapy OR "photo therapy" OR dawn OR dusk) AND Topic=(randomised OR randomized OR randomly or placebo or "double‐blind" or trial OR groups OR "controlled study" OR RCT OR "single‐blind*") AND Year Published=(2008‐2012) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. Lemmatization=On |
Nov 2012: 290 Jan 2014: 99 |
| 7. LILACS (BIREME) [last searched 20 January 2014] |
"bright light" or "light box*" or "light visor*" or "dawn‐dusk*" or phototherapy or "photo therapy" or "light therapy" or "light treatment" or light$ OR luz [Words] and Demências OR dementia OR dementias OR demência OR Alzheimer OR Alzheimers OR Alzheimer's OR cognitive OR cognitive OR cognitive OR cognition OR "déficit cognitive" OR cognición OR cognição OR Memória OR memory OR Memoria [Words] | Nov 2012: 186 Jan 2014:0 |
| 8. CENTRAL (The Cochrane Library) (Issue 1 of 12, 2014) [last searched 20 January 2014] |
#1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium] this term only #3 MeSH descriptor: [Wernicke Encephalopathy] this term only #4 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 light* #22 "photo therapy" #23 phototherapy #24 "dawn‐dusk*" #25 MeSH descriptor: [Phototherapy] explode all trees #26 #21 or #22 or #23 or #24 or #25 #27 #20 and #26 from 2008 to 2012, in Trials (Word variations have been searched) |
Nov 2012: 33 Jan 2014:4 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [last searched 20 January 2014] |
light OR phototherapy OR dusk OR dawn | Interventional Studies | dementia OR alzheimer OR alzheimers OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | received from 01/01/2008 to 11/27/2012 | Nov 2012: 13 Jan 2014:3 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [last searched 20 January 2014] |
light OR phototherapy OR dusk OR dawn | Interventional Studies | dementia OR alzheimer OR alzheimers OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | received from 01/01/2008 to 27/11/2012 | Nov 2012: 17 Jan 2014:2 |
| TOTAL before de‐duplication | Nov 2012: 1195 Jan 2014: |
|
| TOTAL after de‐duplication and first assessment | Nov 2012: 86 Jan2014: |
|
Data and analyses
Comparison 1. Bright light versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognition following 10‐42 days of treatment | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐0.81, 3.28] |
| 2 Sleep onset latency following 2‐6 weeks of treatment | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐14.20, 9.65] |
| 3 Total sleep duration following 10 days to 10 weeks of treatment | 6 | 321 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐35.47, 33.33] |
| 4 Total sleep duration following 6 months of treatment | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐7.78 [‐69.01, 53.44] |
| 5 Sleep efficiency following 2‐10 weeks of treatment | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 3.25 [‐0.53, 7.04] |
| 6 Number of night‐time awakenings following 2‐10 weeks of treatment | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐5.43, 2.33] |
| 7 Agitation following 10 days to 10 weeks of treatment | 4 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.31, 0.29] |
| 8 Psychiatric symptoms following 6 to 10 weeks of treatment | 2 | 157 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐6.48, 10.91] |
| 9 Depression following 2 to 10 weeks of treatment | 3 | 161 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.54, 0.73] |
1.1. Analysis.
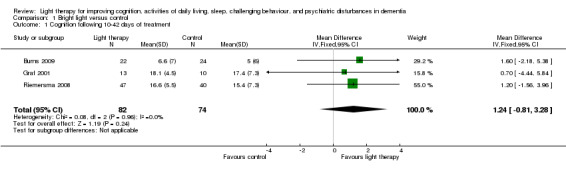
Comparison 1 Bright light versus control, Outcome 1 Cognition following 10‐42 days of treatment.
1.2. Analysis.
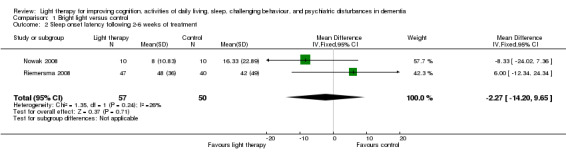
Comparison 1 Bright light versus control, Outcome 2 Sleep onset latency following 2‐6 weeks of treatment.
1.3. Analysis.
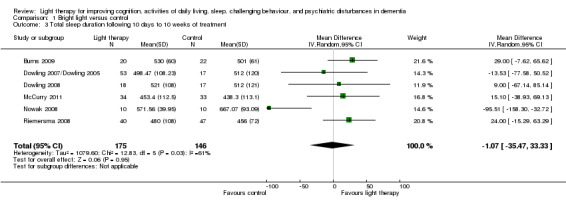
Comparison 1 Bright light versus control, Outcome 3 Total sleep duration following 10 days to 10 weeks of treatment.
1.4. Analysis.
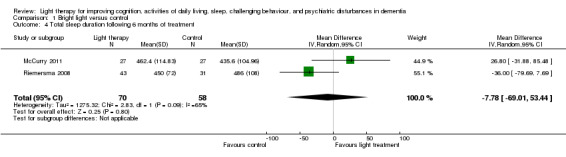
Comparison 1 Bright light versus control, Outcome 4 Total sleep duration following 6 months of treatment.
1.5. Analysis.
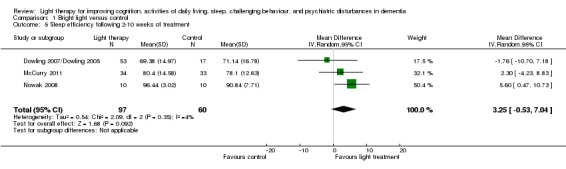
Comparison 1 Bright light versus control, Outcome 5 Sleep efficiency following 2‐10 weeks of treatment.
1.6. Analysis.
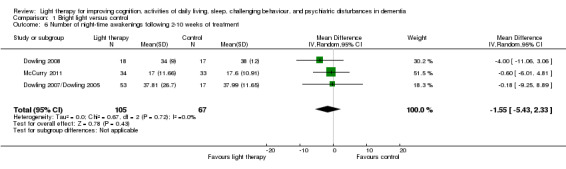
Comparison 1 Bright light versus control, Outcome 6 Number of night‐time awakenings following 2‐10 weeks of treatment.
1.7. Analysis.
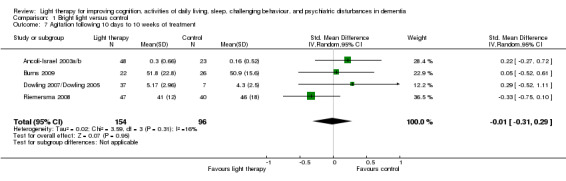
Comparison 1 Bright light versus control, Outcome 7 Agitation following 10 days to 10 weeks of treatment.
1.8. Analysis.
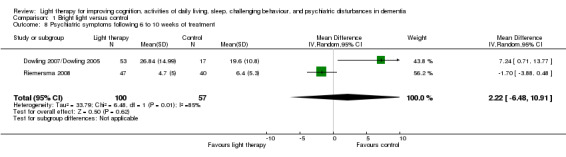
Comparison 1 Bright light versus control, Outcome 8 Psychiatric symptoms following 6 to 10 weeks of treatment.
1.9. Analysis.
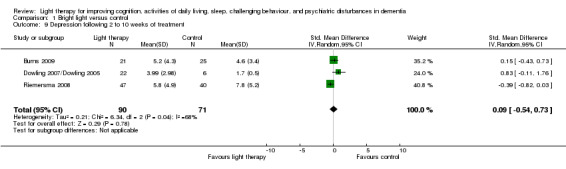
Comparison 1 Bright light versus control, Outcome 9 Depression following 2 to 10 weeks of treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ancoli‐Israel 2003a/b.
| Methods | Note: Ancoli‐Israel 2003a and Ancoli‐Israel 2003b articles report on the same trial. Ancoli‐Israel 2003a reports on the outcome of the effect of light on sleep, Ancoli‐Israel 2003b reports on the outcome of the effect of bright light therapy on agitated behaviour. Participants randomly assigned to one of three groups: 1) morning bright light (n=30), 2) evening bright light (n=31), or 3) morning dim red light (control) (n=31) Total = 92 Single blind (although nursing and research staff were told that both the white and red light conditions were expected to show improvement and the study was examining which colour light would be better) Residents were stratified by time of agitation |
|
| Participants | Country: USA 92 nursing home residents (63 women, 29 men); mean age 82.3 years (SD 7.6, range 61‐99); MMSE mean=5.7 (SD 5.6, range 0‐22) | |
| Interventions | Apollo "Brite‐Lite" box placed 1m from resident 1. Bright light > 2500 Lux: time of day 9.30‐11.30 or 17.30‐19.30 2. Dim, red light (control)< 300 Lux: time of day 9.30‐11.30 Received treatment daily Baseline data: three days Duration of treatment: 10 days Follow up: five days post‐treatment Control: The goal of group 2, morning dim red light, was to “act as control for placebo effects and for effects of staff‐patient interaction during treatment sessions” |
|
| Outcomes | Sleep: sleep duration, sleep efficiency, night‐time activity measured after 10 days of treatment Agitation: assessed using ABRS and CMAI measured after 10 days of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block stratified randomization using pre‐assignment by order of entry within the strata |
| Allocation concealment (selection bias) | Low risk | Participants were allocated via a random assignment computer generated by a statistician (e‐mail from author, March 26, 2013) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although nursing staff and research staff could not be kept blind to light treatment condition, they were told the study was examining which colour light was better |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although nursing staff and research staff could not be kept blind to light treatment condition, they were told the study was examining which colour light was better |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study a: 72 of 92 completed the study Study b: attrition from each group reported in Table 3 study (confirmed by author in March 26, 2013 e‐mail). Similar across the groups but reasons for attrition not specified. Reasons for attrition not specified |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. |
| Other bias | Low risk | None apparent. There were no significant differences in compliance across light treatment conditions. Treatment compliance: mean 92.1min. of bright light per 120‐min. bright light session. Actillumes worn by 91.3% of participants. |
Burns 2009.
| Methods | Participants randomly assigned to one of two groups: 1) standard light (control) (n=26), 2) bright light therapy (n=22). Total = 48 Randomization was conducted by a trial statistician using lists drawn up from www.randomization.com. Participants were stratified according to high (10‐30)/low (0‐9) sMMSE and high (> 8)/low (0‐7) CSDD scores Researchers who conducted the interviews were blinded and the standardized instruments were completed by the research nurse and an independent rater, blind to treatment condition |
|
| Participants | Country: UK
48 nursing home residents (32 women, 16 men); mean age: light therapy group 84.5 (SEM 1.7), placebo group 82.5 (SEM 1.5) Diagnosis: light therapy group: Alzheimer disease 10, vascular dementia 9, Dementia with Lewy bodies 3 , Mixed dementia 4; placebo group: Alzheimer disease 11, vascular dementia 7, Dementia with Lewy bodies 3, Mixed 4 dementia 1 MMSE mean: light therapy group 6.9 (SD 5.3), placebo group 5.1 (SD 5.6) Inclusion criteria: diagnosis of dementia, sleep disruptions at least two nights/week and presence of one or more agitated behaviours |
|
| Interventions | Brite‐Lite box placed in front of resident 1. Bright light 10,000 lux from 10.00 hrs ‐ noon 2. Control: Standard florescent tube light at 100 lux from 1000 hrs ‐ noon Received treatment daily for two weeks Baseline data: 1 week Duration of treatment: 14 days (weeks 2 and 3) Follow up: weeks 4 and 8 |
|
| Outcomes | Agitation assessed using the CMAI Cognition assessed using the MMSE Depression assessed using the CSDD Sleep duration: nusing staff recorded whether the participant was asleep or not in 30 minute blocks All outcomes were measured after two weeks of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out by the trial statistician using a randomization program on the internet that created lists, prior to the start of the study |
| Allocation concealment (selection bias) | Low risk | A randomization program was used by the statistician who generated the lists prior to the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Researchers who conducted the interviews were blind to which treatment the participants received Unclear if nursing staff in the homes were blinded because a nurse was present during each bright and standard light condition; ‐‐ e‐mail inquiries were not answered Research nurse was not blinded; sat with the resident in front of the light box during treatment An independent rater who was blind to the treatment condition completed the CMAI |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above; the research nurse completed a number of the instruments; the independent rater completed the CMAI; high for the secondary outcome measures; the nursing staff completed the sleep charts on all patients; lower risk for the primary outcome, which was the CMAI |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was hospitalized and one withdrew after three days of treatment but their data were included; two died between four and eight weeks; one resident in placebo group changed medication |
| Selective reporting (reporting bias) | High risk | Reported on four week and eight week post‐treatment primary and secondary outcome measures for all measures except no eight week information on effect of mood, amplitude of activity. No four and eight week post‐intervention information on mean duration of nocturnal sleep |
| Other bias | Unclear risk | Compliance with light therapy was reported but not compliance with actigraph |
Dowling 2007/Dowling 2005.
| Methods | Note: The Dowling 2005 and Dowling 2007 articles report on the same trial. Dowling 2005 reports on the effect of light therapy on reducing rest‐activity disruption, Dowling 2007 reports on the outcome of the effect of bright light therapy on disruptive behaviours. Participants randomly assigned to one of three groups: 1) morning bright light (n=29), 2) afternoon bright light (n=24), or 3) usual indoor light (control) (n=17) Total = 70 Single‐blind |
|
| Participants | Country: USA 70 nursing home residents (57 women, 13 men), mean age 84 (SD 10) ranging from 58 to 98, MMSE 0‐23 (mean=7, SD 7) |
|
| Interventions | Bright light exposure >2500 lux: Group 1: morning (9:30‐10:30am), Group 2: afternoon (3:30‐4:30pm) or supplemented using Apollo Brite Lite IV box placed at least 4 feet from resident Frequency: Daily, Monday through Friday Duration: 10 weeks Group 3: The control group received usual indoor light (150‐200 lux) and participated in their regular activities |
|
| Outcomes | Dowling 2005: Sleep efficiency, sleep duration, night time activity, nighttime awakenings Dowling 2007: Psychiatric disturbances, agitation, depression using the NPI‐NH scroes after 10 weeks of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants who met inclusion criteria and who agreed to participate (correspondence from Dowling April 14.2013) |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned using a permutated blocking procedure in which the number of participants allocated to each group was forced to be equal after an a priori defined “balancing” number of participants were enrolled in the study (correspondence from Dowling October 28, 2008) Participants were randomized by a computer generated program to the groups (correspondence from Dowling April 14 2013 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nursing staff were potentially aware of participants’ study group assignment (Dowling 2007, p966; April 14, 2013 confirmed) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were potentially aware of study group assignment (correspondence from Dowling April 14, 2013) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates and reasons for attrition for the two groups not specifically described but it was reported that there were no significant differences between groups. Reasons for drop out no longer available (correspondence from Dowling April 14, 2013) |
| Selective reporting (reporting bias) | Low risk | Both articles reported on outcomes identified in their objectives |
| Other bias | Low risk | Compliance with wearing the Actiwatch was 84% with no significant differences between the groups. The mean percentage of bright light therapy received was 76% (SD=17, range 28–100) and there was no significant difference in dose between the groups |
Dowling 2008.
| Methods | Participants randomly assigned to one of three groups: (1) morning bright light plus melatonin (n=15), (2) morning bright light plus drug placebo (n=18) or (3) usual indoor light (control) (n=17). Only groups (2) and (3) were included in this review (for a total of 35 particpants) since this review is only addressing the effects of light | |
| Participants | Country: USA 35 nursing home residents, subjects in the control group were significantly younger (82+10) than subjects in the light placebo group (89+7). The MMSE mean for all three groups was 9.3 (SD 7.9) and there was no significant differences between groups. All subjects diagnosed with Alzheimer's Disease |
|
| Interventions | Group 2: Bright light exposure >2,500 lux in gaze direction in the morning (9:30‐10:30am). Ambient light was supplemented using Apollo Brite Lite IV box placed 30‐34 inches from resident Frequency: Daily, Monday through Friday Duration: 10 weeks Group 3: The control group received usual indoor light (150‐200 lux) and participated in their regular activities |
|
| Outcomes | Sleep duration and number of nighttime awakenings following 10 weeks of treatment Rest–activity data were collected using the Actiwatch activity monitor (AW‐64, Mini Mitter Co., Inc., Bend, OR). Actiwatches are compact, battery‐operated activity monitors with physical characteristics similar to a small wristwatch. The devices use an ‘‘accelerometer’’ to monitor occurrence and degree of movement‐induced accelerations. Activity counts, representing movement, are stored in memory in the device in 1‐minute epochs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Same as Dowling 2007 (see above) |
| Allocation concealment (selection bias) | Low risk | Same as Dowling 2007 (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported for groups 2 and 3. Treatment group received a placebo pill while control group did not |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported for groups 2 and 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants did not complete the study; one because his doctor felt that if he was receiving the melatonin it may have contributed to incontinence and the other died due to inanition secondary to Alzheimer disease (correspondence from Dowling April 14, 2013) |
| Selective reporting (reporting bias) | Low risk | Article reported on outcomes identified in the objectives |
| Other bias | Low risk | On average, of the total possible 108 hours of light therapy there were 105 + 8 hours of valid data for baseline and 107 ± 3 hours of valid data at the end of intervention with no significant differences between the groups. The mean percentage of intervention received was 82 (± 17%), and there was no significant difference between light‐placebo and and light melatonin groups. The effect of seasonal variations, values for sunset, sunrise, day length, and rate of change in day length for both assessment weeks and averaged over the entire treatment periods were found to be non‐significant |
Gasio 2003.
| Methods | Participants randomly assigned to one of two groups: 1) dawn‐dusk simulation (DDS) light therapy (n=9) or 2) 'placebo' dim red light (DRL) (control group) (n=4). Total = 13 Single‐blind |
|
| Participants | Country: Switzerland
13 nursing home residents (12 women, 1 man) mean age 85.6 years Group 1: Dawn‐Dusk Simulation Age mean 86.8 (SD 4.5) MMSE mean 13.8 (SD 5.9) Probable AD (n=7) Probable Vascular (n=2) Group 2: Dim Red Light. control group Age mean 83.0 (SD 5.2) MMSE mean 14.3 (SD 4.1) Probable AD (n=3) Lewy body (n=1) |
|
| Interventions | Dawn‐Dusk Simulation using an overhead halogen lamp placed behind a diffusing membrane behind the resident's bed simulating a naturalistic form of light therapy Group 1: DDS max 400 Lux morning and evening Group 2 (control): used the same simulation parameters but replaced the white light with a 15W red light bulb. DRL < 5 Lux morning and evening Treatment time varied to mimic the duration and latitude of dawn and dusk Baseline data: 3 weeks Duration of the treatment: 3 weeks Follow up: 3 weeks post‐treatment |
|
| Outcomes | Cognition (using the MMSE); depression (using the GDS); sleep latency, sleep duration, and night time activity measured after 3 weeks of treatment and then 3 weeks of follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The researchers went through the case histories of residents with the physicians to determine who fit the study criteria and then used a computer‐generated assignment (e‐mail from author, March 26, 2013) |
| Allocation concealment (selection bias) | Low risk | A computer generated randomization to assign residents into the two groups (email from author, March 26, 2013) The group sizes were not balanced as a result of the randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurses and participants were blinded (e‐mail from author, March 26, 2013) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two independent raters estimated daily times of going to bed and getting up, with the help of the nurses’ logs |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very small sample size Thirteen participants completed the study and seven dropped out due to non‐compliance with wearing the actimeter, fear of the DDS installation, or illness. The attrition may be due to the intervention itself |
| Selective reporting (reporting bias) | High risk | Inconsistent descriptions; in some cases if results were not significant, no t‐scores or p‐values were mentioned, but in other cases of non‐significance they were mentioned |
| Other bias | Low risk | Compliance with wearing the activity monitor was reported |
Graf 2001.
| Methods | Participants randomly assigned (although rated as inadequate) to one of two groups: Group 1: bright light therapy (experimental) (n=13), or Group 2 (control): dim light therapy (n=10). Total = 23 Single‐blind |
|
| Participants | Country: Austria 23 nursing home residents, (proportions of male and female not stated), mean age 81.6 years (range 65‐94), diagnosed with AD (n=11) or vascular dementia (n=12), required to have a MMSE score below 24, participants MMSE mean 15.9 (SD 5.9) | |
| Interventions | Light placed 90 cm from resident Group 1: Bright light = 3000 Lux: time of day 1700‐1900 Group 2 (control): Dim, red dim light < 100 Lux, used the same simulation parameters, time of day 17.00‐19.00 Received treatment daily Baseline data: morning of initiation of study Duration of treatment: 10 days No follow up |
|
| Outcomes | Cognition (MMSE) measured after 10 days of treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants selected by being asked if they were willing to participate in the study (Email from author, March 26, 2013) Article states: using a “balanced, placebo‐controlled parallel‐group design” (p. 726); require further information in how this was achieved |
| Allocation concealment (selection bias) | Unclear risk | Treatment condition was randomly assigned as stated by the author (Email from author, March 26, 2013) No further elaboration was offered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo of dim light utilized for patients in the control group (p726) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The rater was blind with respect to treatment condition (p726) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | This is a study reporting on preliminary data and is done in the short‐term; what was collected (i.e., body temperature and MMSE scores) is reported on for each of the 23 participants |
| Selective reporting (reporting bias) | Low risk | Although this is a short article both outcomes measures were reported, even though body temperature had no statistically significant impact |
| Other bias | Low risk | None apparent |
Lyketsos 1999.
| Methods | Participants randomly assigned to one of two groups: 1) morning bright light therapy, or 2) control: dim light exposure. Total = 15 Single‐blind, crossover design |
|
| Participants | Country: USA 15 nursing home residents (14 women, 1 man) mean age 80.8 (SD 8.7) DSM‐IV criteria for AD (n=12) or Vascular Dementia (n=3) MMSE mean: 6.4 (SD 6.8) Behave‐AD: > 4 points | |
| Interventions | Light placed three feet from resident
Group 1: Bright light = 10,000 Lux: time of day morning Group 2: Dim light = Lux not specified: time of day morning The control condition was identical to the above except that a dim digital, low‐frequency blinking light positioned in the middle of the active bright light therapy was used. The 10,000 lux light bulb was off during the control condition treatments Both groups received treatment daily, mornings for one hour Baseline: one week Duration of treatment: four weeks Follow up: one week post‐treatment Then received other condition for four weeks |
|
| Outcomes | Agitation (Behave‐AD), depression (CSDD) and sleep duration measured following four weeks of treatment Other: adverse events (increased agitation) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state it was a randomized controlled crossover trial but do not state how randomized |
| Allocation concealment (selection bias) | Unclear risk | Authors do not indicate how the n=15 residents were allocated to the two groups. E‐mail inquiries were not answered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control group received dim light – it was blinking and so may be notably different than the BLT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | They state the outcome raters were blinded to the condition assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five participants were removed by the study principal investigator due to a worsening of their agitation One group treated as two: 15 participants pooled to create a dataset of 30, yet only eight completed the entire trial; analysis was completed with and without the last observation being carried forward and resulted in similar findings |
| Selective reporting (reporting bias) | High risk | Although graphs and further discussion takes place regarding two of the three outcomes measures, depression was only indicated as a non‐significant finding, with no other reporting or discussion |
| Other bias | Low risk | None apparent |
McCurry 2011.
| Methods | Participants randomly assigned to one of four groups (three active treatments, one control): (1) walking (n=32), (2) light (n=34), (3) combination walking, sleep, and guided sleep education (n=33), and (4) control contact (n=33) Only groups (2) and (4) were included in this review since this review is only addressing the effects of light Randomized controlled trial over two month period |
|
| Participants | Country: USA Setting: independent community living person with AD and their caregivers Diagnosis – type of dementia: AD Participants in groups 2 + 4 = 67 Group 2: 19 female, 15 male Group 4 (control): 17 female, 16 male Age (mean): 80.6 for group 2 (light group), 81.2 for group 4 (control) |
|
| Interventions | Frequency: Group 2. light ‐2500 lux for one hour/day in the evening Group 4. contact control –nondirective dementia care support Participants in both groups received three 1‐hour in‐home training visits and two brief telephone calls to reinforce caregiver use of the daily log. For the control group, at all three sessions trainers offered nondirective dementia care support but provided no training or homework related to changing sleep‐wake routines, implementing daily walking, increasing light exposure, or managing dementia related nocturnal behaviours |
|
| Outcomes | Sleep: sleep duration, sleep efficiency, night time awakenings measured following 8 weeks of treatment and at 6 months follow up. Sleep outcomes were measured using Micro‐Mini Motionlogger actigraphs (Ambulatory Monitoring, Inc., Ardsley, NY) worn on participants’ nondominant wrist. Data were collected in 1‐minute recoding epochs using the Proportional Integrating Measure (low sensitivity) operation mode. The Action4 software package (Ambulatory Monitoring, Inc.), which incorporates Cole and Kripke’s sleep scoring algorithm, was used to score sleep and wake Other: treatment adherence Adverse effects: No unexpected or serious adverse events were attributed to any intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation sequence was obtained from a computer program that blocked in groups of 12 participants |
| Allocation concealment (selection bias) | Low risk | A research coordinator assigned treatment conditions using sealed envelopes containing the random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were blinded to treatment assignment and conducted assessments at baseline, at 2 months, and at 6‐month follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no significant between‐group differences in attrition |
| Selective reporting (reporting bias) | Low risk | Authors have reported on all outcomes |
| Other bias | Low risk | None apparent. Results of adherence to actigraphy, walking, and light recommendations are outlined in detail |
Mishima 1998.
| Methods | Participants randomly assigned to one of two groups: (1) bright light and (2) control: dim light, total in two groups =22 Randomized (although process unclear), single‐blind, crossover design |
|
| Participants | Country: Japan 22 nursing home residents, (13 women, 9 men) mean age 79.6 years MRI, CT, and DSM‐IV criteria for AD (n=10; mean age: 78 years; MMSE: mean 9, range 3‐17) or Vascular Dementia (n=12; mean age: 81 years; MMSE: mean 8, range 3‐14) | |
| Interventions | Light light placed 90cm from resident Group 1: Bright light = 5000‐8000 Lux: time of day 9.00‐11.00 Group 2 (control): Dim light = 300 Lux: time of day 9.00‐11.00 Received treatment daily Baseline: one week Duration of treatment: two weeks Follow up: one week Interval between conditions: at least four weeks |
|
| Outcomes | Sleep: night time activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A randomized crossover design, although process of randomization unclear E‐mail inquiries were not answered |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment unclear. Authors do not indicate how the 15 residents were allocated to the two groups. E‐mail inquiries were not answered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants received both bright light and dim light therapy. E‐mail inquiries were not answered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An illuminometer was used to determine luminous intensity at the participant's eye position at 10‐minute intervals. Continuous R‐A monitoring was performed at 1 minute intervals throughout the study using an actigraph |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no significant differences in average luminous intensity between the VD and the DAT groups in both the bright light and dim light periods. Attrition rates not reported. Email inquiries were not answered |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Compliance with actigraph not reported |
Nowak 2008.
| Methods | Twenty participants randomly assigned to one of two groups: (1) blue green light (n=10) and (2) dim red light (n=10) Two‐factor experimental design with repeated measures |
|
| Participants | Country: USA Setting: Two Nursing Home Facilities (Assisted Living n=7, and Long‐term care n=13) N=20 Diagnosis: Alzheimer’s Disease (severe) based on participant’s history, as well as by examining the subsequent documentation by medical, geropsychiatric, and consulting staff Participants: Male: 0 Female: 100% n=20 (originally 21) n=10 (control) n=10 (intervention) Age: 85.9 (±6.24) years Mean MMSE: 1.96 (±2.86) |
|
| Interventions | Group 1: Blue‐green light, 12,000 lux via cap visor Group 2: Control Group: received dim red light as standardized control Both groups: Frequency: X 30 minutes/day between 06.00 – 07.00h Baseline: collected over seven consecutive days – global functioning, sleep, and daytime sleepiness Duration: X 14 consecutive days Follow up: phase five of study – days 26‐68; three follow‐up measurement periods occurred at two week intervals, data collected for five consecutive days Control: Dim red light was employed |
|
| Outcomes | Sleep: sleep latency, sleep efficiency, sleep duration, and night time awakenings Other: adverse effects: one study participant had an episode of forehead redness; another experienced an increase in falls (dropout) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random list generation not specified |
| Allocation concealment (selection bias) | Low risk | Participants were randomized to either experimental condition or control group utilizing a five‐block randomized block design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants in placebo group received red dim light therapy to control for placebo effects as well as for effects of research staff‐patient interactions during the treatment sessions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Principal investigator collected much of the data and there is no statement to indicate she was unaware of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rate and reason for attrition provided |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | Good adherence to light therapy but adherence to wearing actigraphs not reported |
Riemersma 2008.
| Methods | Participants randomly assigned to one of four groups (two active treatment, two control): (1) light only (n=49), (2) light plus melatonin (n=49), (3) inactive light control (n=45), and (4) melatonin only (n=46). Only groups (1) and (3) were included in this review since this review is only addressing the effects of light Double‐blind, placebo‐controlled randomized multi‐centre trial |
|
| Participants | Country: Netherlands Groups 1 + 3: n = 94 nursing home patients (85 women, 9 men), mean age 85 Diagnosis: AD, vascular dementia, other types of dementia or did not meet criteria for dementia |
|
| Interventions | Ceiling‐mounted light fixtures with Plexiglas diffusers Group 1: light exposure ±1000 lux from 9 am to 6 pm Group 3: control ‐ light exposure less than 400 lux from 9 am to 6 pm The control group had an equal number of fixtures installed but with half the tubes along with concealed band‐stop filters, and were installed at a greater distance from the eyes Duration of participation of the facilities was a mean of 15 months (maximum period of 3.5 years) |
|
| Outcomes | Cognition (MMSE) Sleep duration and sleep latency ADLs (NI‐ADL) Agitation (CMAI) Psychiatric symptoms (NPI‐NH) Depression (Cornell Scale) All outcomes were measured after 6 weeks of treatment and after one and two years of follow up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sixty‐one homes for older adults were approached to participate, 12 were willing to participate. Facilities were randomly assigned using the Microsoft Excel randomized number function |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by a research assistant not involved in the study and kept concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A long‐term, double‐blind, placebo‐controlled, 2 X 2 factorial randomized trials |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Codes were revealed to the researchers only after completion of the study and subsequent data reduction and processing steps |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed summary of participants lost to follow up and reasons were provided in Figure 1. All available data for participants that were lost to follow up at any stage were included in the mixed‐effect analyses |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent. None of the sponsors or funders had any involvement in the design or conduct of the study |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abegg 1993 | Not a randomized controlled trial design |
| Ancoli‐Israel 1997 | Not a randomized controlled trial design |
| Ancoli‐Israel 2002 | A more recent version of this study with a larger sample size is reported in Ancoli‐Israel 2003a |
| Barrick 2010 | Didn’t meet criteria – not randomized |
| Chong 2013 | Not an RCT. No control group |
| Colenda 1997 | Not a randomized controlled trial design |
| Connell 2007 | Didn’t meet criteria – another activity combined with bright light |
| Dawson 1999 | Not a randomized controlled trial design |
| Dowling 2005a | Preliminary results. Full study reported in Dowling 2005 |
| Fetveit 2003 | Not a randomized controlled trial design |
| Haffmans 2001 | Not a randomized controlled trial design |
| Hickman 2007 | Cross‐over trial not randomized |
| Hozumi 1990 | Not a randomized controlled trial design |
| Ito 1999 | Not a randomized controlled trial design |
| Ito 2001 | Not a randomized controlled trial design |
| Kobayashi 2001 | Not a randomized controlled trial design |
| Koyama 1999 | Not a randomized controlled trial design |
| Laborie 2010 | Article is a summary of Riemersma‐Van 2008 JAMA |
| Lovell 1995 | Not a randomized controlled trial design |
| McCurry 2005 | Light therapy not the only group difference |
| McCurry 2006 | Light therapy not the only group difference |
| Mishima 1994 | Not a randomized controlled trial design |
| Mishima 2000 | Not a randomized controlled trial design |
| NCT01816152 | Not an RCT; a before and after study |
| Okawa 1989 | Not a randomized controlled trial design |
| Okawa 1999a | Not a randomized controlled trial design |
| Okawa 1999b | Did not measure severity of behaviour |
| Okumoto 1998 | Not a randomized controlled trial design |
| Porter 2012 | Not an RCT. There were two control groups: healthy elderly (without dementia) and healthy young adults |
| Rheaume 1998 | Not a randomized controlled trial design |
| Riemersma 2001 | Not a randomized controlled trial design |
| Satlin 1992 | Not a randomized controlled trial design |
| Skjerve 2004 | Not a randomized controlled trial design |
| Sloane 2007 | Crossover not randomized |
| Thorpe 2000 | Not a randomized controlled trial design |
| Van Hoof 2009 | Didn’t meet criteria – not randomized |
| van Someren 1997 | Not a randomized controlled trial design |
| Yamadera 2000 | Not a randomized controlled trial design. All participants received light therapy |
Characteristics of ongoing studies [ordered by study ID]
Most 2010.
| Trial name or title | Prevention of depression and sleep disturbances in elderly with memory‐problems by activation of the biological clock with light‐‐a randomized clinical trial |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Study protocol |
Contributions of authors
DF: conceived, designed, and coordinated the review
CB: correspondence with included trial authors and review team members
DF and ET: critiqued and selected trials
DF: conducted meta‐analysis
DF, CB, PH, SP, ET: assessed risk of bias, extracted data, interpreted data analysis, drafted review versions, and reviewed all submissions
Sources of support
Internal sources
University of Alberta, Canada.
University of Western Ontario, Canada.
University of Saskatchewan, Canada.
Athabasca University, Canada.
External sources
Canadian Cochrane Centre, Canada.
Nova Scotia Cochrane Resource Centre, Canada.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ancoli‐Israel 2003a/b {published data only (unpublished sought but not used)}
- Ancoli‐Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey‐Bloom J, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's Disease patients. Behavioural Sleep Medicine 2003;1(1):22‐36. [DOI] [PubMed] [Google Scholar]
- Ancoli‐Israel S, Martin J, Gehrman P, Shochat T, Corey‐Bloom J, Marler M, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer Disease. American Journal of Geriatric Psychiatry 2003;11(2):194‐203. [PubMed] [Google Scholar]
Burns 2009 {published and unpublished data}
- Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: A randomized controlled trial. International Psychogeriatrics 2009;21(4):711‐21. [DOI] [PubMed] [Google Scholar]
Dowling 2007/Dowling 2005 {published data only}
- Dowling GA, Graf CL, Hubbard EM, Luxenberg JS. Light treatment for neuropsychiatric behaviors in Alzheimer’s Disease. Western Journal of Nursing Research 2007;29(8):961‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest‐activity disruption in institutionalized patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry 2005;20:738‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowling 2008 {published data only}
- Dowling GA, Burr RL, Someren EJW, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA. Melatonin and bright‐light treament for rest‐activity disruption in institutionalized patients with Alzheimer's Disease. Journal of the American Geriatrics Society 2008;56:239‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gasio 2003 {published and unpublished data}
- Gasio PF, Krauchi K, Cajochen C, Someren E, Amrhein I, Pache M, et al. Dawn‐dusk simulation light therapy of disturbed circadian rest‐activity cycles in demented elderly. Experimental Gerontology 2003;38(1‐2):207‐16. [DOI] [PubMed] [Google Scholar]
Graf 2001 {published data only}
- Graf A, Wallner C, Schubert V, Willeit M, Wlk W, Fischer P, et al. The effects of light therapy on Mini‐Mental State Examination scores in demented patients. Biological Psychiatry 2001;50(9):725‐7. [DOI] [PubMed] [Google Scholar]
Lyketsos 1999 {published data only}
- Lyketsos CG, Veiel LL, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long‐term care. International Journal of Geriatric Psychiatry 1999;14(7):520‐5. [PubMed] [Google Scholar]
McCurry 2011 {published data only}
- McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community‐dwelling persons with Alzheimer's disease: Results of a randomized, controlled trial. Journal of the American Geriatrics Society 2011;59(8):1393‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishima 1998 {published data only}
- Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest‐activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer's type. Chronobiology International 1998;15(6):647‐54. [DOI] [PubMed] [Google Scholar]
Nowak 2008 {published data only}
- Nowak L. The effect of timed blue‐green light on sleep‐wake patterns in women with Alzheimer's disease. Dissertation Abstracts International: Section B: The Sciences and Engineering 2008;69(6‐B):1‐154. [Google Scholar]
Riemersma 2008 {published data only}
- Riemersma‐van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA 2008;299(22):2642‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abegg 1993 {published data only}
- Abegg A, Wettstein A. Phototherapy of behavioral disorders as a sequela of disordered circadian rhythm in dementia in the elderly: Difficulties in practical applications [Lichttherapie von verhaltensstorungen als folge gestorter zirkadianer rhythmen bei dementiellen alterspatienten: Schwierigkeiten der praktisch‐klinischen anwendbarkeit]. Schweizer Archiv fur Neorologie und Psychiatrie Band 1993;144(1):63‐80. [PubMed] [Google Scholar]
Ancoli‐Israel 1997 {published data only}
- Ancoli‐Israel S, Klauber MR, Williams Jones D, Kripke DF, Martin J, Mason W, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing‐home patients. Sleep 1997;20(1):18‐23. [PubMed] [Google Scholar]
Ancoli‐Israel 2002 {published data only}
- Ancoli‐Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light therapy on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society 2002;50:282‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrick 2010 {published data only}
- Barrick AL, Sloane PD, Williams CS, Mitchell CM, Connell BR, et al. Impact of ambient bright light on agitation in dementia. International Journal of Geriatric Psychiatry 2010;25(10):1013‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2013 {published data only}
- Chong MS, Tan KT, Tay L, Wong YM, Ancoli‐Israel S. Bright light therapy as part of a multicomponent management program improves sleep and functional outcomes in delirious older hospitalized adults. Clinical Interventions in Aging 2013;8:565‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colenda 1997 {published data only}
- Colenda CC, Cohen W, McCall WV, Rosenquist PB. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: Results of a community‐based pilot study. Alzheimer Disease and Associated Disorders 1997;11:175‐8. [DOI] [PubMed] [Google Scholar]
Connell 2007 {published data only}
- Connell BR, Sanford JA, Lewis D. Therapeutic effects of an outdoor activity program on nursing home residents with dementia. Journal of Housing for the Elderly 2007;21(3‐4):195‐209. [Google Scholar]
Dawson 1999 {published data only}
- Dawson P. Bright light treatment for people with Alzheimer's disease. Perspectives 1999;23(1):25‐6. [PubMed] [Google Scholar]
Dowling 2005a {published data only}
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Someren EJW. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. International Psychogeriatrics 2005;17(2):221‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fetveit 2003 {published data only}
- Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly ‐ an open trial. International Journal of Geriatric Psychiatry 2003;18:520‐6. [DOI] [PubMed] [Google Scholar]
Haffmans 2001 {published data only}
- Haffmans PMJ, Sival RC, Lucius SAP, Cats Q, Gelder L. Bright light therapy and melatonin in motor restless behaviour in dementia: A placebo‐controlled study. International Journal of Geriatric Psychiatry 2001;16:106‐10. [DOI] [PubMed] [Google Scholar]
Hickman 2007 {published data only}
- Hickman SE, Barrick AL, Williams CS, Zimmerman S, Connell BR, Preisser JS, et al. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. Journal of the American Geriatrics Society 2007;55:1817‐24. [DOI] [PubMed] [Google Scholar]
Hozumi 1990 {published data only}
- Hozumi S, Okawa M, Mishima K, Hishikawa Y, Hori H, Takahashi K. Phototherapy for elderly patients with dementia and sleep‐wake rhythm disorders‐A comparison between morning and evening exposure. The Japanese Journal of Psychiatry and Neurology 1990;44(4):813‐4. [Google Scholar]
Ito 1999 {published data only}
- Ito T, Yamadera H, Ito R, Endo S. Effects of bright light on cognitive disturbances in Alzheimer‐type dementia. Nippon‐Ika_Daigaku‐Zusshi 1999;66(4):229‐38. [DOI] [PubMed] [Google Scholar]
Ito 2001 {published data only}
- Ito T, Yamadera H, Ito R, Suzuki H, Asayama K, Endo S. Effects of vitamin B12 on bright light on cognition and sleep‐wake rhythm in Alzheimer‐type dementia. Psychiatry Clinical Neuroscience 2001;55:281‐2. [DOI] [PubMed] [Google Scholar]
Kobayashi 2001 {published data only}
- Kobayashi R, Fukuda N, Kohsaka M, et al. Effects of bright light at lunchtime on sleep in a geriatric hospital. Psychiatry and Clinical Neurosciences 2001;55:287‐9. [DOI] [PubMed] [Google Scholar]
Koyama 1999 {published data only}
- Koyama E, Matsubara H, Nakano T. Bright light treatment for sleep‐wake disturbances in aged individuals with dementia. Psychiatry and Clinical Neuroscience 1999;53(2):227‐9. [DOI] [PubMed] [Google Scholar]
Laborie 2010 {published data only}
- Laborie S. Effect of bright light and melatonin on cognitive and non‐cognitive function. Cahiers de l'Annee Gerontologique 2010;2(3):194‐8. [Google Scholar]
Lovell 1995 {published data only}
- Lovell BB, Ancoli‐Israel S, Gevirtz R. Effect of bright light treatment on agitated behavior in institutionalized elderly subjects. Psychiatry Research 1995;57:7‐12. [DOI] [PubMed] [Google Scholar]
McCurry 2005 {published data only}
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s Disease: A randomized controlled trial. Journal of the American Geriatics Society 2005;53(5):793‐802. [DOI] [PubMed] [Google Scholar]
McCurry 2006 {published data only}
- McCurry SM, Logsdon RG, Gibbon LE, Vitiello MV, Teri L. Behavioral treatment for sleep disturbances in Alzheimer's Disease: The Nite‐AD study. Research and Practice in Alzheimer's Disease 2006;11:341‐6. [Google Scholar]
Mishima 1994 {published data only}
- Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatrica Scandinavica 1994;89:1‐7. [DOI] [PubMed] [Google Scholar]
Mishima 2000 {published data only}
- Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest‐activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiology International 2000;17(3):419‐32. [DOI] [PubMed] [Google Scholar]
NCT01816152 {published data only}
- NCT01816152. Methodology Issues in a Tailored Light Treatment for Persons With Dementia. ClinicalTrials.gov NCT01816152. [Google Scholar]
Okawa 1989 {published data only}
- Okawa M, Mishima K, Shimizu T, Iijima S, Hishikawa Y, Hozumi S, Hori H. Sleep‐waking rhythm disorders and their phototherapy in elderly patients with dementia. The Japanese Journal of Psychiatry and Neurology 1989;43(2):293‐5. [Google Scholar]
Okawa 1999a {published data only}
- Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H. Sleep disorder in elderly patients with dementia and trials of new treatments‐Enforcement of social interaction and bright light therapy. Unknown 1999;Unknown:128‐32. [Google Scholar]
Okawa 1999b {published data only}
- Okawa M, Mishima K, Hozumi S. Effects of environmental stimulation with bright light and transcranial electrostimulation on sleep and behavior disorders in elderly patients with dementia. Alzheimer's Disease and Related Disorders 1999;87:757‐62. [Google Scholar]
Okumoto 1998 {published data only}
- Okumoto Y, Koyama E, Matsubara H, Nakano T, Nakamura R. Aging and sleep: Sleep improvement by light in a demented aged individual. Psychiatry and Clinical Neurosciences 1998;52(2):194‐6. [DOI] [PubMed] [Google Scholar]
Porter 2012 {published data only}
- Porter G, Leonards U, Troscianko T, Haworth J, Bayer A, Tales A. Dealing with illumination in visual scenes: effects of ageing and Alzheimer's disease. PLoS ONE [Electronic Resource] 2012;7(9):e45104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rheaume 1998 {published data only}
- Rheame Y, Manning B, Harper D, Volcier L. Effect of light therapy upon disturbed behaviors in Alzheimer patients. American Journal of Alzheimer's Disease 1998;13(6):291‐5. [Google Scholar]
Riemersma 2001 {published data only}
- Riemersma R, Hutten R, Kalis A, Hoekstra R, Hoogendijk W, Scherder E, Swaab D, Someren E. Indirect bright light therapy decreases sleep fragmentation in institutionalized demented elderly. Sleep‐Wake Research in the Netherlands 2001;12:96‐8. [Google Scholar]
Satlin 1992 {published data only}
- Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's disease. American Journal of Psychiatry 1992;149:1028‐32. [DOI] [PubMed] [Google Scholar]
Skjerve 2004 {published data only}
- Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM. Improvement in behavioral symptoms and advance of activity acrophase after short‐term bright light treatment in severe dementia. Psychiatry and Clinical Neurosciences 2004;58:343‐7. [DOI] [PubMed] [Google Scholar]
Sloane 2007 {published data only}
- Sloane PD. High‐intensity environmental light in dementia: Effect on sleep and activity. Journal of the American Geriatrics Society 2007;55:1524‐33. [DOI] [PubMed] [Google Scholar]
Thorpe 2000 {published data only}
- Thorpe L, Middleton J, Russell G, Stewart N. Bright light therapy for demented nursing home patients with behavioral disturbance. American Journal of Alzheimer's Disease 2000;15(1):18‐26. [Google Scholar]
Van Hoof 2009 {published data only}
- Hoof J, Aarts MPJ, Rense CG, Schoutens AMC. Ambient bright light in dementia: effects on behaviour and circadian rhythmicity. Building and Environment 2009;44(1):146‐55. [Google Scholar]
van Someren 1997 {published data only}
- Someren EJW, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest‐activity rhythm disturbances in demented patients. Biological Psychiatry 1997;41:955‐63. [DOI] [PubMed] [Google Scholar]
Yamadera 2000 {published data only}
- Yamadera H, Ito T, Suzuki H, Asyama K, Ito R, Endo S. Effects of bright light on cognitive and sleep‐wake (circadian) rhythm disturbances in Alzheimer‐type dementia. Psychiatry and Clinical Neurosciences 2000;54:352‐3. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Most 2010 {published data only}
- Most EI, Scheltens P, Someren EJ. Prevention of depression and sleep disturbances in elderly with memory‐problems by activation of the biological clock with light‐‐a randomized clinical trial. Trials 2010;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
APA 1995
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1995. [Google Scholar]
ASC 2013
- Alzheimer Society of Canada. What is dementia?. http://www.alzheimer.ca/en/About‐dementia/Dementias/What‐is‐dementia accessed March 28 2013.
Balk 2002
- Balk EM, Bonis PAL, Moskowitz H, Schmid CH, Ioannidis JPA, Wang C, et al. Correlation of quality measures with estimates of treatment effect in meta‐analyses of randomized controlled trials. JAMA 2002;287:2973‐82. [DOI] [PubMed] [Google Scholar]
Campbell 1995
- Campbell SS, Terman M, Lewy AJ, et al. Light treatment for sleep disorders: Consensus report. V. Age‐related disturbances. Journal of Biological Rhythms 1995;10:151‐4. [DOI] [PubMed] [Google Scholar]
David 2010
- David R, Zeitzer J, Friedman L, Noda A, O'Hara R, Robert P, Yesavage JA. Non pharmacologic management of sleep disturbance in Alzheimer's disease. The Journal of Nutrition, Health & Aging 2010;14(3):203‐6. [DOI] [PubMed] [Google Scholar]
Davis 2001
- Davis FA. Taber's Cyclopedic Medical Dictionary. 19th Edition. Philadelphia: FA Davis, 2001. [Google Scholar]
Feldman 2005
- Feldman HH, Woodward M. The staging and assessment of moderate to severe Alzheimer disease. Neurology 2005;65 Suppl 3:S10‐7. [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Harper 2001
- Harper DG, Stopa EG, McKee AC, Satlin A, Harlan PC, Goldstein R, Volicer L. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Archives of General Psychiatry 2001;58:353‐60. [DOI] [PubMed] [Google Scholar]
Harper 2008
- Harper D, Stopa EG, Kuo‐Leblanc V, McKee AC, Asayama K, Volicer L, et al. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain 2008;131(P6):1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson, SG, Deeks, JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Hogan 2007
- Hogan DB, Bailey P, Carswell A, Clarke C, Forbes D, et al. Management of mild to moderate Alzheimer's disease and dementia. Alzheimer's & Dementia 2007;3(4):355‐84. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [DOI] [PubMed] [Google Scholar]
Kong 2009
- Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: A systematic review and meta‐analysis. Aging and Mental Health 2009;13(4):512‐20. [DOI] [PubMed] [Google Scholar]
Liu 2000
- Liu RY, Zhou JN, Hoogendijk WJG, Heerikhuize J, Kamphorst W, Unmehopa UA, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. Journal of Neuropathology and Experimental Neurology 2000;59(4):314‐22. [DOI] [PubMed] [Google Scholar]
Martin 2007
- Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residnets with disrupted sleep/wake patterns. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences 2007;62A(1):67‐72. [DOI] [PubMed] [Google Scholar]
McCurry 2000
- McCurry SM, Reynolds III CF, Ancoli‐Israel S, Teri L, Vitiello MV. Treatment of sleep disturbances in Alzheimer's disease. Sleep Medicine Reviews 2000;4(6):603‐28. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
Mishima 1997
- Mishima K, Okawa M, Satoh K, Shimizu T, Hozumi S, Hishikawa Y. Different manifestations of circadian rhythms in seniledementia of Alzheimer’s type and multi‐infarct dementia. Neurobiology of Aging 1997;18:105‐9. [DOI] [PubMed] [Google Scholar]
Nowak 2011
- Nowak L, Davis J. Qualitative analysis of therapeutic light effects on global function in Alzheimer’s Disease. Western Journal of Nursing Research 2011;33(7):933‐52 DOI: 10.1177/0193945910386248. [DOI] [PubMed] [Google Scholar]
Padilla 2011
- Padilla R. Effectiveness of environment‐based interventions for people with Alzheimer's disease and related dementias. American Journal of Occupational Therapy 2011;65(5):514‐22. [DOI] [PubMed] [Google Scholar]
Paniagua 2008
- Paniagua MA, Paniagua EW. The demented elder with insomnia. Clinics in Geriatric Medicine 2008;24:69‐81. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hrobjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36:847‐57. [DOI] [PubMed] [Google Scholar]
Porta 2007
- Porta N, Bonet C, Cobo E. Discordance between reported intention‐to‐treat and per protocol analyses. Journal of Clinical Epidemiology 2007;60:663‐9. [DOI] [PubMed] [Google Scholar]
Salami 2011
- Salami O, Lyketsos C, Rao V. Treatment of sleep disturbance in Alzheimer's dementia. International Journal of Geriatric Psychiatry 2011;26(8):771‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shirani 2009
- Shirani A, Louis EK. Illuminating rationale and uses for light therapy. Journal of Clinical Sleep Medicine 2009;5(2):155‐63. [PMC free article] [PubMed] [Google Scholar]
Siersma 2007
- Siersma V, Als‐Nielsen B, Chen W, Hilden J, Gluud LL, Gluud C. Multivariable modelling for meta‐epidemiological assessment of the association between trial quality and treatment effects estimated in randomized clinical trials. Statistics in Medicine 2007;26(14):2745‐58. [DOI] [PubMed] [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34:79‐87. [DOI] [PubMed] [Google Scholar]
van Someren 1996
- Someren EJW, Hagebeuk EEO, Lijzenga C, Scheltens P, Rooij SEJA, Jonker C, et al. Circadian rest‐activity rhythm disturbances in Alzheimer's Disease. Biological Psychiatry 1996;40:259‐70. [DOI] [PubMed] [Google Scholar]
Weldemichael 2010
- Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer's disease: A review. International Journal of Alzheimer's Disease 2010;2010:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, Division of Mental Health, 1992. [Google Scholar]
WHO 2012
- World Health Organization (WHO) and Alzheimer Disease International. Dementia: A Public Health Priority. http://whqlibdoc.who.int/publications/2012/9789241564458_eng.pdf 2012.
Wild 2003
- Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2004
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clinical Trials 2004;1:368‐76. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz K, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Alzheimer Report 2012
- Alzheimer Disease International. World Alzheimer Report 2012. Overcoming the stigma of dementia. http://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf September 2012.
References to other published versions of this review
Forbes 2004
- Forbes D, Morgan DG, Bangma J, Peacock S, Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Forbes 2009
- Forbes D, Culum I, Lischka AR, Morgan DG, Peacock S, Forbes J, Forbes S. Light therapy for managing cognitive, sleep, functional, behavioural, or mood disturbances in dementia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858C] [DOI] [PubMed] [Google Scholar]


