Abstract
Current evidence suggests that host defense in respiratory mycoplasmosis is dependent on both innate and humoral immunity. To further delineate the roles of innate and adaptive immunity in antimycoplasmal defenses, we intranasally infected C3H/HeSnJ-scid/scid (C3H-SCID), C3H/HeSnJ (C3H), C57BL/6J-scid/scid (C57-SCID), and C57BL/6N (C57BL) mice with Mycoplasma pulmonis and at 14 and 21 days postinfection performed quantitative cultures of lungs and spleens, quantification of lung lesions, and histopathologic assessments of all other major organs. We found that numbers of mycoplasmas in lungs were associated with genetic background (C3H susceptible, C57BL resistant) rather than functional state of adaptive immunity, indicating that innate immunity is the main contributor to antimycoplasmal defense of the lungs. Extrapulmonary dissemination of mycoplasmas with colonization of spleens and histologic lesions in multiple organs was a common occurrence in all mice. The absence of adaptive immune responses in severe combined immunodeficient (SCID) mice resulted in increased mycoplasmal colonization of spleens and lesions in extrapulmonary sites, particularly spleens, hearts, and joints, and also reduced lung lesion severity. The transfer of anti-M. pulmonis serum to infected C3H-SCID mice prevented extrapulmonary infection and disease, while the severity of lung lesions was restored by transfer of naive spleen cells to infected C3H-SCID mice. Collectively, our results strongly support the conclusions that innate immunity provides antimycoplasmal defense of the lungs and humoral immunity has the major role in defense against systemic dissemination of mycoplasmal infection, but cellular immune responses may be important in exacerbation of mycoplasmal lung disease.
Mycoplasma pneumoniae causes up to 30% of all pneumonias in the general population (33) and frequently exacerbates other respiratory diseases, including asthma (24, 53) and chronic obstructive pulmonary disease (37, 38). The mechanisms of host defense in respiratory mycoplasmosis remain poorly understood, but recent evidence from human and animal studies suggests that innate immunity associated with alveolar macrophages (AMs) and humoral immunity are the major contributors (13, 18, 21, 25, 26).
Cell-mediated immunity appears to be of limited importance in defense against respiratory mycoplasmosis, as pneumonia due to M. pneumoniae is not increased in severity in patients with T-cell deficiencies (21, 35), and T-cell-deficient mice are not more susceptible to infection than immunocompetent controls following intranasal (i.n.) inoculation of M. pulmonis (9, 16, 32). Patients with humoral immunodeficiencies also have no more severe lung disease than immunocompetent patients during early stages of M. pneumoniae infection, but they eventually develop chronic pneumonia and disseminated infections, especially arthritis (21). Following i.n. infection with M. pulmonis, severe combined immunodeficient (SCID) mice develop arthritis and have less severe lung disease than immunocompetent controls (18).
Direct evidence that innate immunity is important in respiratory mycoplasmosis comes mostly from studies of M. pulmonis infection in resistant C57BL mice and susceptible C3H mice. Within 72 h postinfection (p.i.), the numbers of mycoplasmas in the lungs of C57BL mice decrease by more than 83% whereas the numbers in C3H mice increase by 18,000% (15). There is strong evidence that innate immunity associated with AMs is responsible for this antimycoplasmal resistance of C57BL mice: (i) significant mycoplasmacidal activity occurs within 4 h p.i., long before recruitment of additional cells into the lungs or the appearance of specific antibody in serum (4, 13, 15, 41); (ii) intrapulmonary killing is abrogated by impairment of AMs following exposure to nitrogen dioxide (13) or depletion of AM numbers by administration of toxic liposomes (26); and (iii) surfactant protein A has been shown to mediate the killing of mycoplasmas by AMs in vitro through a nitric oxide-dependent mechanism (25).
The purpose of this study was to further delineate the roles of innate and adaptive immunity in pulmonary and extrapulmonary antimycoplasmal defenses, using SCID mice. We intranasally infected C3H/HeSnJ-scid/scid (C3H-SCID), C3H/HeSnJ (C3H), C57BL/6J-scid/scid (C57-SCID), and C57BL/6N (C57BL) mice with M. pulmonis and performed quantitative cultures on lungs and spleens, subjective lesion scoring on lungs, and pathologic evaluations on all other major organs. The results showed that numbers of mycoplasmas in lungs were related to strain background (C3H susceptible, C57BL resistant) rather than functional state of adaptive immunity, demonstrating the importance of innate immunity in antimycoplasmal defense of the lungs. Lack of adaptive immune responses in SCID mice (1) was associated with reduced lung lesion severity and with increased mycoplasmal colonization and disease in extrapulmonary sites. The transfer of naive spleen cells from immunocompetent mice to M. pulmonis-infected SCID mice restored the severity of the lung lesions, while the transfer of convalescent anti-M. pulmonis serum from immunocompetent mice to M. pulmonis-infected SCID mice did not restore lung lesion severity but prevented arthritis. These findings provide additional strong evidence for the importance of innate immunity in defense of the lungs and humoral immunity in defense against systemic spread of infection during respiratory mycoplasmosis and support the possibility that cellular immune responses may be important in exacerbation of mycoplasmal lung disease.
MATERIALS AND METHODS
Animals.
Equal numbers of male and female C3H-SCID, C3H, C57-SCID, and C57BL mice were used in experiments at 8 to 10 weeks of age. All mice were bred in our colony at the University of Alabama at Birmingham (UAB) and monitored for the presence of murine pathogens by bacterial cultures, virus serologies, parasite examinations, and histopathology of all major organs, with consistently negative results. All uninfected control mice were negative by enzyme-linked immunosorbent assay (ELISA) for serum antibodies to M. pulmonis. Mice were maintained in sterile Microisolator cages (Lab Products, Maywood, N.J.). All cages were provided with sterile hardwood chip bedding (PJ Murphy Forest Products, Rochelle Park, N.J.); sterile food (Agway, Inc., Syracuse, N.Y.) and water were available ad libitum. For inoculation with mycoplasmas and collection of tissues and blood, mice were anesthetized with a combination of ketamine HCl (8.7 mg/100 g of body weight; Aveco, Fort Dodge, IA) and xylazine (1.3 mg/100 g of body weight; Haver, Shawnee, Kans.) given intramuscularly.
Mycoplasmas.
The UAB CT strain of M. pulmonis was used in all experiments (12). Stock cultures were grown in mycoplasma broth A and frozen in 1-ml aliquots at −70°C as previously described (12). For animal inoculations, thawed ampoules contained an average of 2 × 107 CFU/ml and were diluted in broth A to the appropriate concentration for inoculations. Each inoculum was quantitatively cultured at the time of inoculation and contained the desired number of organisms (104/50 μl). Inoculations were given i.n. in 50-μl volumes. Control mice were given the same volume of broth A alone. To assay serum for antimycoplasmal antibody, M. pulmonis cell lysate was prepared as described previously (4) and used as antigen in the mycoplasmal ELISA (28).
Quantitative mycoplasmal cultures.
Lungs and spleens were quantitatively cultured as described previously (12, 48). Briefly, whole lungs were removed aseptically, individually minced, and sonicated for 30 s in broth A. Tenfold serial dilutions were made in broth A; color-changing units (CCU) were determined after incubation at 37°C for 7 days in room air with 95% relative humidity, and CFU were determined after plating the 10-fold dilutions onto mycoplasmal agar and similar incubation. Spleens were processed in the same manner except that one-half of each spleen was used for quantitative mycoplasma culture and one-half was used for histopathologic evaluation.
Quantification of mycoplasmal lung lesions.
Lungs were removed, fixed in 95% ethanol, embedded in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin and eosin for light microscopy. Subjective scoring of lesion severity (scale of 0 to 4) was performed by two observers using randomly coded sections to quantify each of the four characteristic lung lesions of murine respiratory mycoplasmosis: (i) airway exudate, (ii) airway epithelial hyperplasia, (iii) lymphoid infiltrate, and (iv) alveolar exudate, with calculation of lesion indices as described previously (3, 4, 43).
Assessment of extrapulmonary mycoplasmal disease.
Classification of disease as clinical arthritis was based on visual detection of enlargement of limb joints; classification as microscopic arthritis was based on histopathologic evaluation of at least one representative longitudinal section through each joint. Incidences of clinical and microscopic arthritis were defined as the fractions of animals affected in each experimental group.
Representative tissues of all major organs (nasal passages, middle ears, larynx, lungs, heart, brain, eyes, liver, pancreas, stomach, intestine, kidney, adrenal glands, ovary, uterus, testes, spleen, thymus, skeletal muscle, and selected joints [shoulder, elbow, wrist, knee, and ankle]) were processed for light microscopy as described above and evaluated for histopathologic lesions. Thus, there were five pairs of limb joints (10 joints) examined for lesions in each animal. Bones were demineralized (S/P Decalcifying Solution; Baxter Healthcare) and sectioned longitudinally.
Passive transfer of serum or cells.
Convalescent sera were collected during a previous study (4) from C3H mice 14, 21, 28, 42, and 63 days following infection with doses of M. pulmonis ranging from 103 to 106 CFU. These sera were pooled, and 0.25 ml was injected intraperitoneally into C3H-SCID mice 7 days after i.n. inoculation with 104 CFU of M. pulmonis. Spleen cells were collected from naive C3H mice and injected intraperitoneally into C3H-SCID mice at a concentration of 2 × 107 cells per mouse 7 days before i.n. inoculation with 104 CFU of M. pulmonis.
Statistical analysis.
Lesion score, CFU, and CCU data were analyzed by using the Kruskal-Wallis one-way analysis of variance and the Mann-Whitney U test for statistical significance. The V-squared test was used to analyze for significant differences in incidence data. Probabilities (P) of 0.05 or less were considered significant.
RESULTS
Effects of SCID on mycoplasma numbers and disease severity in lungs.
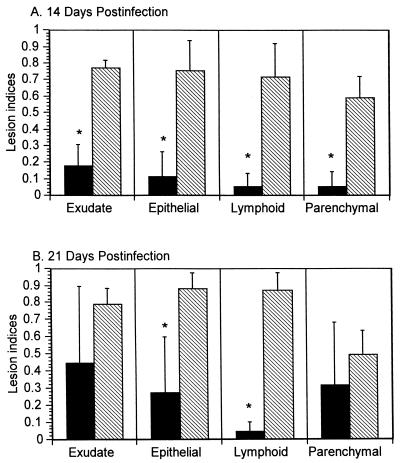
To investigate the effects of SCID on lung infection and disease, C3H-SCID, C3H, C57-SCID, and C57BL mice were infected i.n. with 104 CFU of M. pulmonis. At 14 and 21 days p.i., we determined the numbers of mycoplasmas in their lungs and quantified lung disease severity. The numbers of organisms recovered at 14 days p.i. from the lungs of infected C3H-SCID mice (8.4 ± 0.8 log10) did not differ significantly from the numbers recovered from C3H mice (8.1 ± 0.53 log10), and similar numbers of organisms were obtained from the lungs of both mouse strains at 21 days p.i. However, at 14 days p.i., each of the lung lesions in C3H-SCID mice was significantly (P < 0.05) less severe than in C3H mice (Fig. 1A). At 21 days p.i., C3H-SCID mice still had less severe epithelial hyperplasia and lymphoid hyperplasia than C3H mice (Fig. 1B). In contrast, relatively few mycoplasmas and only minimal lung disease (data not shown) were present in C57-SCID or C57BL mice at either 14 or 21 days p.i. There was no significant difference in the numbers of mycoplasmas recovered from the lungs of C57-SCID (1.9 ± 2.7 log10) and C57BL (2.9 ± 3.5 log10) mice at 14 days p.i. (similar numbers of mycoplasmas were obtained from both mouse strains at 21 days p.i.). Thus, C3H-SCID and C3H mice had 5.9 to 6.5 log10 more organisms (P < 0.05) than C57-SCID and C57BL mice. Serum antibodies to M. pulmonis were not detected by ELISA in either C3H-SCID or C57-SCID mice at 14 days p.i. (not tested at 21 days p.i.).
FIG. 1.
Lung lesion indices (±standard errors) for C3H-SCID (solid bars; n = 6) and C3H (hatched bars; n = 4) mice 14 (A) days and 21 (B) days after i.n. inoculation with 104 CFU of M. pulmonis. Asterisks denote significant differences between mouse strains (P < 0.05).
Effects of SCID on extrapulmonary dissemination of mycoplasmas and disease.
During the experiment described above, we noted that some of the C3H-SCID mice had splenitis and arthritis. To evaluate the effects of SCID on dissemination of infection, mice of the four strains were i.n. infected with 104 CFU M. pulmonis. At 14 and 21 days p.i., the numbers of M. pulmonis in spleens were determined, and all major organs were evaluated histopathologically. At 14 and 21 days p.i., most (100 and 85%, respectively) C3H mice had microscopic pulmonary lesions characteristic of murine respiratory mycoplasmosis (Table 1). Both C3H-SCID and C57-SCID mice had significantly (P < 0.05) higher rates of positive spleen cultures and greater numbers of mycoplasmas in their spleens than were found in the spleens of their immunocompetent controls. The spleens of C3H-SCID and C57-SCID mice had 1 to 2 log10 more organisms at 14 days p.i., and 4 to 5 log10 more organisms at 21 days p.i., than C3H and C57BL mice. Control mice lacked microscopic lung lesions and had negative spleen cultures for mycoplasmas.
TABLE 1.
Incidence of mycoplasmal pneumonia, positive spleen cultures and extrapulmonary disease 14 and 21 days after M. pulmonis inoculationa
| Days p.i. | Mouse strain | Pneumonia, microscopic lung lesions (% animals positive) | Spleen culture
|
Extrapulmonary disease (% animals positive)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Animals positive | Mean CCU/positive culture | Splenitis | Pericarditis | Myocarditis | Arthritis | Mesenteric lymphadenitis | Miscellaneous | |||
| 14 | C3H-SCID | 83 (5/6) | 83 (5/6) | 2 × 102 | 83 (5/6) | 83 (5/6) | 0 (0/6) | 50 (3/6) | 17 (1/6) | 0 (0/6) |
| C3H | 100 (5/5) | 0 (0/5)b | 0b | 0 (0/5) | 0 (0/5) | 0 (0/5) | 0 (0/5) | 0 (0/5) | 0 (0/5) | |
| C57-SCID | 0 (0/8) | 75 (6/8) | 102 | 25 (2/8) | 0 (0/8) | 0 (0/8) | 25 (2/8) | 25 (2/8) | 0 (0/8) | |
| C57BL | 0 (0/9) | 11 (1/9)c | 101c | 0 (0/9) | 0 (0/9) | 0 (0/9) | 0 (0/9) | 0 (0/9) | 0 (0/9) | |
| 21 | C3H-SCID | 100 (9/9) | 100 (9/9) | 105 | 78 (7/9) | 78 (7/9) | 78 (7/9)d | 100 (9/9) | 0 (0/9) | 0 (0/9) |
| C3H | 85 (11/13) | 14 (1/7)b | 101b | 0 (0/13)b | 0 (0/13)b | 0 (0/13)b | 8 (1/13)b | 0 (0/13) | 0 (0/13) | |
| C57-SCID | 25 (4/16) | 94 (15/16) | 105 | 0 (0/16) | 38 (6/16) | 0 (0/16) | 81 (13/16) | 0 (0/16) | 38 (6/16)e | |
| C57BL | 8 (1/13) | 0 (0/13)c | 0c | 0 (0/13) | 0 (0/13) | 0 (0/13) | 31 (4/13)c | 0 (0/13) | 8 (1/13)f | |
| Controlsg | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | 0 (0/12) | |
Mice were inoculated with 104 CFU of M. pulmonis, and incidences of microscopic pneumonia, positive spleen cultures, and clinical and microscopic arthritis were determined. CCU were determined by the number of organisms cultured from one-half of spleen/animal with positive spleen culture. Numbers in parentheses are numbers of affected animals/total number of animals.
Significant difference (P < 0.05) between C3H-SCID and C3H mice.
Significant difference (P < 0.05) between C57BL-SCID and C57BL mice.
Some also had mitral valvulitis.
Perioophoritis.
Meningitis.
Three mice from each strain inoculated with broth only.
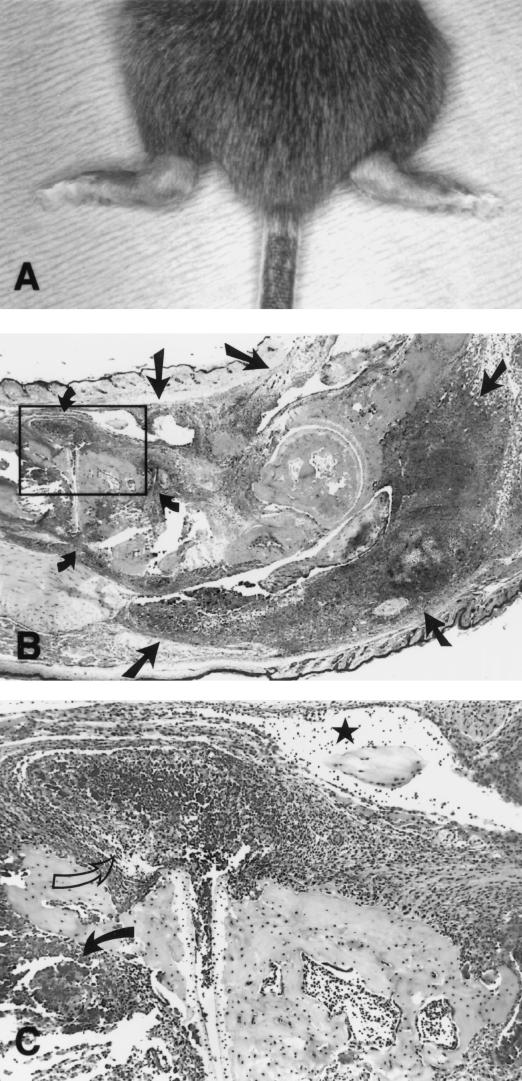
The rates of arthritis in the four strains of infected mice followed the same general pattern as rates of positive spleen cultures except that the rates of arthritis were lower (Table 1). Both strains of SCID mice had higher rates of arthritis than their immunocompetent controls. Although clinical arthritis (Fig. 2A) was not apparent at 14 days p.i., 50% of C3H-SCID mice and 25% of C57-SCID mice had histologic arthritis. Affected joints had severe neutrophilic inflammation of joint capsules, joint spaces, and tendon sheaths (Fig. 2B and C). Based on total joints evaluated, the rates of affected joints in C3H-SCID mice was significantly (P < 0.05) higher than in C3H mice, and this difference was even greater 21 days p.i. Similarly, C57-SCID mice had significantly (P < 0.05) more microscopic arthritis than C57BL mice at 21 days p.i. That the arthritis was due to mycoplasmas was confirmed by the finding that three affected joints evaluated by quantitative culture at 21 days p.i. each contained >106 CCU of M. pulmonis. None of the control mice had arthritis.
FIG. 2.
C3H-SCID mice 21 days after infection with 104 CFU of M. pulmonis. (A) Mouse with bilateral swelling of ankle joints due to arthritis (magnification, ×1.5). (B) Sagittal section through ankle joint, showing severe neutrophilic inflammation involving the joint capsules (small arrows) and tendon sheaths (large arrows) (×15). (C) Area boxed in panel B, showing neutrophilic inflammation in joint capsule (open arrow), tendon sheath (star), and bone marrow (solid arrow) (×60).
Histopathologic evaluation of other organs from the four strains of infected mice also revealed widely disseminated disease. At 14 and 21 days p.i., all strains had respiratory tract lesions typical of murine respiratory mycoplasmosis, but airway inflammatory responses were greatest in C3H-SCID and C3H mice, and peribronchial and perivascular lymphoid infiltrates were much more severe in C3H and C57BL mice. At 14 days p.i., 83% of C3H-SCID mice had suppurative splenitis and pericarditis and 17% had suppurative mesenteric lymphadenitis, while only 25% of C57-SCID mice had suppurative splenitis and mesenteric lymphadenitis (Table 1). At 21 days p.i., 78% of C3H-SCID mice had suppurative splenitis, mitral valvulitis, myocarditis, and pericarditis, compared to 38% of C57-SCID mice with either suppurative pericarditis or perioophoritis; 62% of C3H mice had lymphoid hyperplasia in the spleen; 23% of C57BL mice had lymphoid hyperplasia in the spleen, and 8% had mild lymphocytic meningitis. Extrapulmonary lesions were not found in major organs from control mice.
Passive transfer of antimycoplasmal antibodies to infected C3H-SCID mice prevented arthritis but did not restore lung disease severity.
To evaluate the role of antibody in respiratory mycoplasmosis and mycoplasmal arthritis, 25 C3H-SCID mice were infected i.n. with 104 CFU of M. pulmonis UAB CT. At 7 days p.i., 11 of the infected mice were given intraperitoneally 0.25 ml of convalescent anti-M. pulmonis serum (1:50 dilution of sera with 1:1,000 relative anti-M. pulmonis titer [4]) and 14 mice were not given serum. At 21 days p.i., all mice were evaluated for clinical arthritis and histologic lung disease. There was no significant difference between the groups in the severity of airway exudate, airway epithelial hyperplasia, lymphoid infiltrate, or alveolar exudate (data not shown), the four histologic lung lesions characteristic of murine respiratory mycoplasmosis (3, 4, 34, 43). However, 79% (11 of 14) of the C3H-SCID mice which did not receive serum developed clinical arthritis by 21 days p.i. None of the 11 mice given serum had detectable clinical arthritis.
Reconstitution of C3H-SCID mice with naive spleen cells restored lung disease severity.
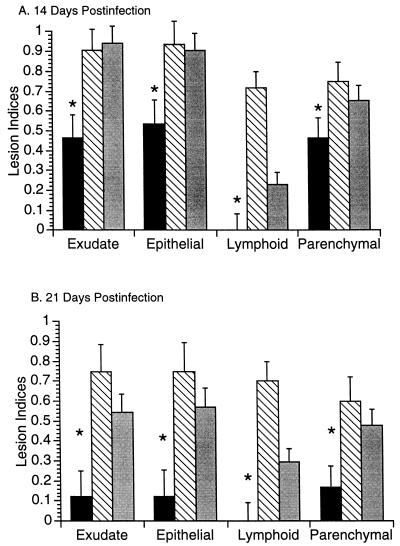
Studies were done to evaluate the role of lymphoid cells in the development or prevention of mycoplasmal lung and joint disease. C3H-SCID mice reconstituted with 2 × 107 spleen cells (from C3H mice of same gender), unreconstituted C3H-SCID mice (given medium only), and C3H mice were infected with 104 CFU of M. pulmonis and monitored for disease by clinical and histopathological observation at 21 days p.i. C3H mice and reconstituted C3H-SCID mice had more severe lung lesions than did unreconstituted C3H-SCID mice at both 14 and 21 days p.i. (Fig. 3). Reconstituted C3H-SCID mice had significantly less (P < 0.05) lymphoid hyperplasia than C3H mice. Only two mice, both unreconstituted C3H-SCID, developed clinical arthritis by 21 days p.i.
FIG. 3.
Lung lesion indices (±standard errors) in C3H-SCID (solid bars), C3H (hatched bars), and C3H-SCID mice given 2 × 107 spleen cells intraperitoneally 7 days prior to infection with 104 CFU of M. pulmonis (shaded bars) at 14 (A) and 21 (B) days p.i. (n = 6 to 10 mice/group).
DISCUSSION
Previous studies of M. pulmonis infection in C3H and C57BL mice had demonstrated larger numbers of mycoplasmas in lungs and much higher responses in all classes of antibody in susceptible C3H mice and that the mechanisms of adaptive immunity are unable to eliminate mycoplasmas from the lungs of either mouse strain by 63 days p.i. (4). In the present study, the numbers of organisms recovered from the lungs of C3H-SCID, C3H, C57-SCID, and C57BL mice after infection with 104 CFU M. pulmonis differed according to strain background, as infected C3H-SCID and C3H mice had 5.9 to 6.5 log10 more mycoplasmas in their lungs than C57-SCID and C57BL mice. That there was no difference in organism numbers between SCID and immunocompetent mice of each genetic background, at either 14 or 21 days p.i., demonstrated that pulmonary host defenses independent of adaptive immunity defends against mycoplasmas in the lungs, at least out to 21 days p.i. These data are consistent with other recent results from this laboratory. Our in vivo study in which AMs were depleted with toxic liposomes also gave strong evidence that the early resistance of C57BL mice to M. pulmonis infection is due to innate defense mechanism(s) associated with AMs and conversely, the susceptibility of C3H mice is due to impairment of these mechanisms (26). Furthermore, we have shown in vitro that the resistance of C57BL mice probably is, at least in part, due to surfactant protein A-mediated killing of mycoplasmas by AMs through a nitric oxide-dependent mechanism (25). Our results, however, appear incongruent with the report of Sandstedt et al. (47) showing that male X-linked immunodeficient (xid/Y) CBA mice infected with M. pulmonis developed less lung disease than infected male immunocompetent (+/Y) CBA mice. Inasmuch as both strains of their mice had the CBA genetic background, we would have expected similar lung responses to mycoplasmal infection because of identical innate pulmonary defenses.
The numbers of organisms and incidences of lesions that we found at extrapulmonary sites in the four strains of mice given 104 CFU of M. pulmonis also followed a distinct pattern (Table 1). Infected C3H-SCID and C57-SCID mice had very high (75% or more) rates of positive spleen cultures compared to immunocompetent mice (11% or less) at 14 days p.i., and the rates for SCID mice, but not immunocompetent mice, increased further by 21 days p.i. C3H-SCID mice consistently had higher rates of positive spleen cultures than C57-SCID mice. Similarly, SCID mice consistently had much higher rates of arthritis (50% of C3H-SCID and 25% of C57-SCID) than immunocompetent mice (0% of C3H and C57BL) at 14 days p.i., and the rates of arthritis in SCID mice increased further to very high levels (100% of C3H-SCID and 81% of C57-SCID) compared to immunocompetent mice (8% of C3H and 31% of C57BL) by 21 days p.i. C3H-SCID mice consistently had more extrapulmonary disease than C57-SCID mice.
The high rates of positive spleen cultures, splenitis, arthritis, pericarditis, and myocarditis in SCID mice at 14 and 21 days p.i. were to some extent predictable because of the disseminated mycoplasmal diseases due to M. pneumoniae and other mycoplasmas found in hypogammaglobulinemic patients (21–23, 30, 36, 49) and in SCID mice infected with M. pulmonis (18). However, the findings of positive spleen cultures in 11% of C57BL mice at 14 days p.i. and of positive spleen cultures in 14% of C3H mice, arthritis in 11% of C3H and 31% of C57BL mice, and meningitis in 8% of C57BL mice at 21 days p.i. were not expected. These data give strong support to the proposal that systemic dissemination with direct invasion of tissues by mycoplasmas accounts for many of the extrapulmonary complications (neurologic, cardiac, dermatologic, arthritic, etc.) associated with M. pneumoniae infection in immunocompetent patients (2, 5, 7, 44).
Despite the difficulties of sampling and culturing extrapulmonary sites for mycoplasmas in human patients, some data supporting direct invasion have been obtained. Narita et al. (39) studied 32 patients with M. pneumoniae-associated meningoencephalitis, the most common neurologic complication of this infection, and found that cerebrospinal fluids from 16 (50%) were positive for M. pneumoniae by PCR. The organism also has been isolated from brain tissue at autopsy (7). Myocarditis, pericarditis, perimyocarditis, complete heart block, and hypertrophic cardiomyopathy have been reported as complications of M. pneumoniae infection in human patients (2, 7). Presence of the organism in tissues and exudates from many of the patients with pericarditis has been demonstrated by cultural methods (7, 19, 31, 46). M. pneumoniae has been isolated from skin of patients with Stevens-Johnson syndrome (5, 7) and is increasingly being demonstrated in synovial fluid from patients with arthritis (5, 7, 44). Also, it is interesting that concordance has been demonstrated between the annual incidence of M. pneumoniae infection in children and the incidence of juvenile rheumatoid arthritis in one community (40).
Our results also support the possibility that subclinical mycoplasmal diseases such as arthritis and meningitis may be much more common than previously thought in humans following respiratory mycoplasmosis. The fact that the arthritis was not seen in our immunocompetent mice until 21 days p.i. suggests that mycoplasmal arthritis develops slowly. If the same is true of arthritis and other extrapulmonary lesions in humans, their association with previous respiratory infection due to M. pneumoniae would not be recognized (by patients or physicians). The fact that C3H mice, which are more susceptible to M. pulmonis pneumonia, did not have more arthritis than C57BL mice suggests that the mechanisms of pathogenesis may be different in pneumonia and arthritis, a possibility supported by the reduced severity of pneumonia versus greater severity of arthritis in SCID mice.
As expected, M. pulmonis-specific antibody was not detectable by ELISA in serum samples from mycoplasma-infected SCID mice in the present study. The finding that C3H-SCID mice generally had higher rates of positive spleen cultures and extrapulmonary lesions than C57-SCID mice appeared to reflect the higher numbers of organisms in their lungs (4, 15, 41) due to impaired intrapulmonary innate antimycoplasmal defenses associated with the C3H genetic background (26). In contrast, the low rates of positive spleen cultures and extrapulmonary lesions found in C3H and C57BL mice implicate adaptive immunity in preventing extrapulmonary colonization, even in C3H mice with the larger numbers of organisms in their lungs. Our study in which antimycoplasmal antibody was given to C3H-SCID mice infected with M. pulmonis provided strong evidence that specific antibody defends against dissemination of mycoplasmal infection to extrapulmonary sites. Our findings are in agreement with human studies incriminating hypogammaglobulinemia as the cause of heightened susceptibility to disseminated disease (e.g., arthritis, osteomyelitis, and meningitis) due to M. pneumoniae (7, 21, 22, 29). The lack of arthritis in xid/Y CBA mice infected with M. pulmonis by Sandstedt et al. (47) might be explained by the fact these mice have only a relatively mild B-cell deficiency that primarily affects levels of immunoglobulins G3 and M.
We did not find any difference in severity of lung disease between the infected C3H-SCID mice given antimycoplasmal antibody and those infected C3H-SCID which were not given antibody, in agreement with our previous study showing that once infection is established in the lungs, adaptive immune responses are ineffective in eliminating the mycoplasma infection (4). Although antibody-mediated opsonophagocytosis by macrophages has been proposed as an effective mechanism of defense against mycoplasmas, including M. pneumoniae (6, 11, 17, 45) and M. pulmonis (14, 27, 50, 52), its importance relative to innate mechanisms in antimycoplasmal defense of the respiratory tract remains questionable (4). The supporting evidence is that giving anti-M. pulmonis serum i.n. prior to (8) or simultaneously with (51) i.n. infection of M. pulmonis, or giving anti-M. pulmonis serum intravenously prior to i.n. infection of M. pulmonis (50), has been reported to afford complete protection against pneumonia but not infection of the upper respiratory tract.
We found that mycoplasmal lung disease was much less severe in C3H-SCID mice than C3H mice at 14 days p.i., although they had equivalent burdens of organisms in their lungs. The modest lymphocyte infiltrate in the lungs of mycoplasma-infected SCID mice was in keeping with the lack of functional lymphoid cells in SCID mice (1). However, the dramatic reductions in all other characteristic lung lesions (i.e., airway exudate, airway epithelial hyperplasia, and alveolar exudate) of murine respiratory mycoplasmosis were unexpected but possibly reflected a reduction in cytokine production due to lack of lymphoid cells. The passive transfer of naive spleen cells from immunocompetent mice to infected SCID mice resulted in mycoplasmal lung disease in which all characteristic lesions except lymphoid infiltration were equivalent to those in infected immunocompetent mice. The lower levels of lymphoid infiltration that we observed probably resulted from incomplete reconstitution of the SCID mice. These results suggest that lymphocyte (most likely T-cell) responses in mycoplasmal infection, through various effector mechanisms such as cytokine production (20, 42), can have the deleterious effect of exacerbating lung lesions, a concept also proposed recently to explain fulminant M. pneumoniae infections in humans (10). Further studies of the potential roles (beneficial and deleterious) cell-mediated immunity has in respiratory mycoplasmosis are warranted. However, the data reported here strongly support the view that innate and humoral mechanisms are the more important direct participants in antimycoplasmal defense in respiratory mycoplasmosis and prevention of organism dissemination, respectively. Further research to understand these mechanisms should prove valuable in prevention and/or treatment of respiratory mycoplasmosis and its many extrapulmonary complications.
ACKNOWLEDGMENTS
We gratefully acknowledge the excellent technical assistance of Sandy Williams, Marilyn Shackelford, Jane Hosmer, and Mark Phillips.
This work was supported in part by Public Health Service grants RR11105 (J.R.L.), 2T32 HL07553 (G.H.C.), and NIAD-DMID AI 15128 (J.W.S.) and by funds from the Veterans Administration Research Service (J.R.L.).
REFERENCES
- 1.Bosma M J, Carroll A M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 2.Broughton R A. Infections due to Mycoplasma pneumoniae in childhood. Pediatr Infect Dis. 1986;5:71–85. doi: 10.1097/00006454-198601000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Cartner S C, Simecka J W, Briles D E, Cassell G H, Lindsey J R. Resistance to mycoplasmal lung disease in mice is a complex genetic trait. Infect Immun. 1996;64:5326–5331. doi: 10.1128/iai.64.12.5326-5331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartner S C, Simecka J W, Lindsey J R, Cassell G H, Davis J K. Chronic respiratory mycoplasmosis in C3H/HeN and C57BL/6N mice: lesion severity and antibody response. Infect Immun. 1995;63:4138–4142. doi: 10.1128/iai.63.10.4138-4142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassell G H. Severe mycoplasma disease—rare or underdiagnosed? West J Med. 1995;162:172–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell G H, Clyde W A, Davis J K. Mycoplasmal respiratory infections. In: Razine S, Barille M F, editors. The mycoplasmas. IV. Mycoplasma pathogenicity. New York, N.Y: Academic Press; 1985. pp. 65–106. [Google Scholar]
- 7.Cassell G H, Gray G C, Waites K B. Mycoplasmal infections. In: Isselbacher K J, Braunwald E, Wilson J D, Martin J, Fauci A S, Kasper D L, editors. Harrison’s principles of internal medicine. New York, N.Y: McGraw-Hill Inc.; 1998. pp. 1052–1055. [Google Scholar]
- 8.Cassell G H, Lindsey J R, Overcash R G, Baker H J. Murine mycoplasma respiratory disease. Ann NY Acad Sci. 1973;225:395–412. [Google Scholar]
- 9.Cassell G H, McGhee J R. Pathologic and immunologic response of nude mice following intranasal inoculation of Mycoplasma pulmonis. J Reticuloendothel Soc. 1975;18:342. [Google Scholar]
- 10.Chan E D, Welsh C H. Fulminant Mycoplasma pneumoniae pneumonia. West J Med. 1995;162:133–142. [PMC free article] [PubMed] [Google Scholar]
- 11.Clyde W A., Jr . Mycoplasma pneumoniae infections of man. In: Tully J G, Whitcomb R F, editors. The mycoplasmas. II. New York, N.Y: Academic Press; 1979. pp. 275–306. [Google Scholar]
- 12.Davidson M K, Lindsey J R, Parker R F, Tully J G, Cassell G H. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect Immun. 1988;56:2156–2162. doi: 10.1128/iai.56.8.2156-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis J K, Davidson M K, Schoeb T R, Lindsey J R. Decreased intrapulmonary killing of Mycoplasma pulmonis after short term exposure to NO2 is associated with damaged alveolar macrophages. Am Rev Respir Dis. 1992;145:406–411. doi: 10.1164/ajrccm/145.2_Pt_1.406. [DOI] [PubMed] [Google Scholar]
- 14.Davis J K, Delozier K M, Asa D K, Minion F C, Cassell G H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980;29:590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J K, Parker R F, White H, Dziedsic D, Taylor G, Davidson M K, Cox N R, Cassell G H. Strain differences in susceptibility to murine respiratory mycoplasmosis in C57BL/6N and C3H/HeN mice. Infect Immun. 1985;50:647–654. doi: 10.1128/iai.50.3.647-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denny F W, Taylor-Robinson D, Allison A C. The role of thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J Med Microbiol. 1972;5:327–336. doi: 10.1099/00222615-5-3-327. [DOI] [PubMed] [Google Scholar]
- 17.Erb P, Bredt W. Interaction of Mycoplasma pneumoniae with alveolar macrophages: viability of adherent and ingested mycoplasmas. Infect Immun. 1979;25:11–15. doi: 10.1128/iai.25.1.11-15.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evengard B, Sandstedt K, Bolske G, Feinstein R, Riesenfelt-Orn I, Smith C I E. Intranasal inoculation of Mycoplasma pulmonis in mice with severe combined immunodeficiency (SCID) causes a wasting disease with grave arthritis. Clin Exp Immunol. 1995;98:388–394. doi: 10.1111/j.1365-2249.1994.tb05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farraj R S, McCully R B, Oh J K, Smith T F. Mycoplasma-associated pericarditis. Mayo Clin Proc. 1997;72:33–36. doi: 10.4065/72.1.33. [DOI] [PubMed] [Google Scholar]
- 20.Faulkner C B, Simecka J W, Davidson M K, Davis J K, Schoeb T R, Lindsey J R, Everson M P. Gene expression and production of tumor necrosis factor alpha, interleukin 1, interleukin 6, and gamma interferon in C3H/HeN and C57BL/6N mice in acute Mycoplasma pulmonis disease. Infect Immun. 1995;63:4084–4090. doi: 10.1128/iai.63.10.4084-4090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foy, H. M. 1993. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin. Infect. Dis. 17(Suppl. 1):S37–S46. [DOI] [PubMed]
- 22.Furr P M, Taylor-Robinson D, Webster A B D. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinemia and their role in arthritis: microbiological observations over twenty years. Ann Rheum Dis. 1994;53:183–187. doi: 10.1136/ard.53.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelfand, E. W. 1993. Unique susceptibility of patients with antibody deficiency to mycoplasma infection. Clin. Infect. Dis. 17(Suppl. 1):S250–S253. [PubMed]
- 24.Gil J C, Cedillo R L, Mayagoitia B G, Paz M D. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–25. [PubMed] [Google Scholar]
- 25.Hickman-Davis J M, Lindsey J R, Zhu S, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages. Am J Physiol. 1998;274:270–277. doi: 10.1152/ajplung.1998.274.2.L270. [DOI] [PubMed] [Google Scholar]
- 26.Hickman-Davis J M, Michalek S M, Gibbs-Erwin J, Lindsey J R. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not in mycoplasma-susceptible C3H mice. Infect Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard C J, Taylor G. Interaction of mycoplasmas and phagocytes. Yale J Biol Med. 1983;56:643–648. [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz S A, Cassell G H. Detection of antibodies to Mycoplasma pulmonis by enzyme-linked immunosorbent assay. Infect Immun. 1978;22:161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston C L W, Webster A D B, Taylor-Robinson D, Rapaport G, Hughes G R V. Primary late-onset hypogammaglobulinaemia associated with inflammatory polyarthritis and septic arthritis due to Mycoplasma pneumoniae. Ann Rheum Dis. 1983;42:108–110. doi: 10.1136/ard.42.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorup-Ronstrom C, Ahl T, Hammarstrom H, Smith C I E, Rylander M, Hallander H. Septic osteomyelitis and polyarthritis with Ureaplasma in hypogammaglobulinemia. Infection. 1989;17:301–303. doi: 10.1007/BF01650712. [DOI] [PubMed] [Google Scholar]
- 31.Kenney, R. T., J. S. Li, W. A. Clyde, Jr., T. C. Wall, C. M. O’Connor, P. T. Campbell, P. Van Tright, and G. R. Corey. 1993. Mycoplasmal pericarditis: evidence of invasive disease. Clin. Infect. Dis. 17(Suppl. 1):S58–S62. [DOI] [PubMed]
- 32.Keystone E C, Taylor-Robinson D, Osborn M F, Ling L, Pope C, Fornasier V. Effect of T-cell deficiency on the chronicity of arthritis induced in mice by Mycoplasma pulmonis. Infect Immun. 1980;27:192–196. doi: 10.1128/iai.27.1.192-196.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause D C, Taylor-Robinson D. Mycoplasmas which infect humans. In: Maniloff J, editor. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 417–444. [Google Scholar]
- 34.Lindsey J R, Cassell G H, Davidson M K. Mycoplasmal and other bacterial diseases of the respiratory system. In: Foster H L, Small J D, Fox J G, editors. The mouse in biomedical research. II. New York, N.Y: Academic Press; 1982. pp. 21–41. [Google Scholar]
- 35.Lo S-C. Mycoplasmas and AIDS. In: Maniloff J, editor. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 525–545. [Google Scholar]
- 36.Madoff S, Hooper D. Nongenitourinary infections caused by Mycoplasma hominis in adults. Rev Infect Dis. 1988;10:602–613. doi: 10.1093/clinids/10.3.602. [DOI] [PubMed] [Google Scholar]
- 37.Mansel J K, Rosenow E C, Martin J W., Jr Mycoplasma pneumoniae pneumonia. Chest. 1989;95:639–646. doi: 10.1378/chest.95.3.639. [DOI] [PubMed] [Google Scholar]
- 38.Melbye H, Kongerud J, Vorland L. Reversible airflow limitation in adults with respiratory infection. Eur Respir J. 1994;7:1239–1245. doi: 10.1183/09031936.94.07071239. [DOI] [PubMed] [Google Scholar]
- 39.Narita M, Itakura O, Matsuzono Y, Togashi T. Analysis of mycoplasmal central nervous system involvement by polymerase chain reaction. Pediatr Infect Dis J. 1995;14:236–237. doi: 10.1097/00006454-199503000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Oen K, Fast M, Post B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975–92: cycles in incidence. J Rheumatol. 1995;22:745–750. [PubMed] [Google Scholar]
- 41.Parker R F, Davis J K, Blalock D K, Thorp R B, Simecka J W, Cassell G H. Pulmonary clearance of Mycoplasma pulmonis in C57BL/6N and C3H/HeN mice. Infect Immun. 1987;55:2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietsch K, Ehlers S, Jacobs E. Cytokine gene expression in the lungs of BALB/c mice during primary and secondary intranasal infection with Mycoplasma pneumoniae. Microbiology. 1994;140:2043–2048. doi: 10.1099/13500872-140-8-2043. [DOI] [PubMed] [Google Scholar]
- 43.Pinson D M, Schoeb T R, Lindsey J R, Davis J K. Evaluation by scoring and computerized morphometry of lesions of early Mycoplasma pulmonis infection and ammonia exposure in F344/N rats. Vet Pathol. 1986;23:550–555. doi: 10.1177/030098588602300502. [DOI] [PubMed] [Google Scholar]
- 44.Ponka A. Arthritis associated with Mycoplasma pneumoniae infection. Scand J Rheumatol. 1979;8:27–32. [PubMed] [Google Scholar]
- 45.Powell D A, Clyde W A., Jr Opsonin-reversible resistance if Mycoplasma pneumoniae to in vitro phagocytosis by alveolar macrophages. Infect Immun. 1975;11:540–550. doi: 10.1128/iai.11.3.540-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sands M J, Satz J E, Turner W E, Jr, Soloff L A. Pericarditis and perimyocarditis associated with active Mycoplasma pneumoniae infection. Ann Intern Med. 1977;86:544–548. doi: 10.7326/0003-4819-86-5-544. [DOI] [PubMed] [Google Scholar]
- 47.Sandstedt K, Berglof A, Feinstein R, Bolske G, Evengard B, Smith C I E. Differential susceptibility of Mycoplasma pulmonis intranasal infection in X-linked immunodeficient (xid), severe combined immunodeficient (scid), and immunocompetent mice. Clin Exp Immunol. 1997;108:490–496. doi: 10.1046/j.1365-2249.1997.3981294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoeb T R, Davidson M K, Lindsey J R. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tracts of rats. Infect Immun. 1982;38:212–217. doi: 10.1128/iai.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.So A, Furr P, Taylor-Robinson D. Arthritis caused by Mycoplasma salivarium in hypogammaglobulinemia. Br Med J. 1983;286:762–763. doi: 10.1136/bmj.286.6367.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor G, Howard C J. Protection of mice against Mycoplasma pulmonis infection using purified mouse immunoglobulins: comparison between protective effect and biological properties of immunoglobulin classes. Immunology. 1981;43:519–525. [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor G, Taylor-Robinson D. Effects of active and passive immunization on Mycoplasma pulmonis-induced pneumonia in mice. Immunology. 1976;30:611–618. [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor G D. Immunity to mycoplasma infections of the respiratory tract: a review. J R Soc Med. 1979;72:520–526. [PMC free article] [PubMed] [Google Scholar]
- 53.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]