Abstract
Since its discovery more than 10 years ago, the atypical PKC (aPKC) subfamily has attracted great interest. A number of reports have shown that the kinases of this subfamily play critical roles in signaling pathways that control cell growth, differentiation and survival. Recently, several investigators have identified a number of aPKC-interacting proteins whose characterization is helping to unravel the mechanisms of action and functions of these kinases. These interactors include p62, Par-6, MEK5 and Par-4. The details of how these adapters serve to link the aPKCs to different receptor signaling pathways and substrates in response to specific stimuli are crucial not only for developing an understanding of the roles and functions of the aPKCs themselves, but also for more generally establishing a view of how specificity in signal transduction is achieved.
Introduction
The atypical protein kinase C (aPKC) subfamily of kinases is composed of two members, ζPKC and λ/ιPKC. These proteins are highly related, with a 72% overall amino acid identity (Nishizuka, 1995). The conservation is most striking in the catalytic domain, which is also conserved among the other PKC isotypes that belong to the classical and novel subfamilies. In contrast, the regulatory domain of the aPKCs is clearly different from those of the other members of the PKC superfamily; it has only one zinc finger whereas the others have two (Nishizuka, 1995). Like the novel PKCs, the aPKCs lack the characteristic C2 domain that is present in the classical isoforms. These important structural differences may explain why the aPKCs are insensitive to Ca2+, diacylglycerol and phorbol esters, which are potent activators of the other isoforms (Nishizuka, 1995). The exact mechanism of activation of the aPKCs is still largely unclear. However, there is abundant evidence that they play important roles in controlling cell growth and survival (Berra et al., 1993; Diaz-Meco et al., 1996a; Bjorkoy et al., 1997; Murray and Fields, 1997; Wooten, 1999), most likely through their regulation of critical signaling pathways including those that activate the AP-1 and NF-κB transcription factors (Diaz-Meco et al., 1993; Berra et al., 1995; Akimoto et al., 1996; Liao et al., 1997; Sontag et al., 1997; Takeda et al., 1999; Wooten, 1999). Although the participation of the aPKCs in these cascades was initially linked to Ras signaling (Berra et al., 1993; Diaz-Meco et al., 1994; Bjorkoy et al., 1997; Liao et al., 1997), further developments in this field have revealed a complex set of interactions with a number of proteins that are not known to participate in the Ras pathway, suggesting that sophisticated regulatory mechanisms are involved in aPKC signaling.
Of scaffolds and adapters
A critical issue concerning the involvement of the aPKCs in these pathways is what the connection between these kinases and the receptor signaling complexes is. In this regard, the protein p62 may provide some insight. p62 was isolated independently by two groups as a novel and selective aPKC-interacting protein, and it binds the aPKC V1 sequence, which comprises the first 126 amino acids upstream of the zinc finger domain (Puls et al., 1997; Sanchez et al., 1998). The site of p62 to which the aPCKs bind has been narrowed down to a short stretch of acidic amino acids (Figure 1) termed AID (for atypical PKC-interaction domain). p62 also has a novel cysteine-rich sequence that forms an atypical zinc finger termed the ZZ domain (Sanchez et al., 1998). Recent evidence strongly suggests that p62 provides a scaffold linking the aPKCs to the tumor necrosis factor α (TNFα) and interleukin-1 (IL-1) receptor signaling complexes through its interactions with RIP and TRAF6, respectively (Figure 2A and B). Both of these proteins are important mediators of the inflammatory response activated by the cytokines TNFα and IL-1 (Sanz et al., 1999, 2000). This implicates protein–protein interactions involving the aPKCs as critical events in the transduction of TNFα and IL-1 signaling.
Fig. 1. The AID sequences of p62 and MEK5 aligned with the corresponding putative region of Par-6. Identical and closely related residues are shown in gray.
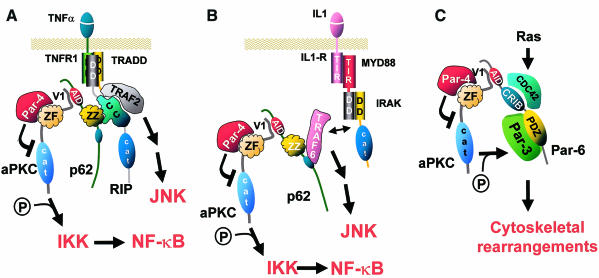
Fig. 2. The modulation of aPKC function by different protein complexes. (A) In response to TNFα receptor stimulation, the death domain (DD) of the corresponding receptor binds the adapter TRADD, which serves to recruit RIP. The coiled-coil (CC) region of RIP interacts with the atypical zinc finger (ZZ) of p62. The AID sequence of p62 interacts with the V1 domain of the aPKCs and serves to recruit them to the receptor signaling complex, and to activate them through an unknown mechanism. The inhibitory protein Par-4 can target the zinc finger (ZF) of the aPKCs, provoking their inhibition and the subsequent induction of apoptosis. (B) TRAF6 also interacts with p62, linking the aPKCs to the IL-1 signaling cascade. IRAK, after it is recruited to the IL-1 signaling complex, is hyperphosphorylated and released to interact with TRAF6. The recruitment of IRAK to the IL-1 receptor complex takes place through its interaction with the adapter MyD88, which binds the IL-1 receptor through the TIR domain. IKK can be recruited to these complexes through RIP or the TRAFs where its β subunit can be phosphorylated and activated by the aPKCs. (C) CDC42 or Rac may stimulate Par-6 (AID site), which forms a complex with the aPKCs (V1 site) in close proximity to Par-3, which interacts with the PDZ domain of Par-6. This results in the phosphorylation of Par-3 by the aPKCs, leading to downstream effects on cytoskeletal arrangement. The interaction between Par-6 and CDC42 may explain the role of the aPKCs in cytoskeletal remodeling during Ras transformation, since Ras is known to activate CDC42.
The main transducer of TNFα signaling is the TNFR1 molecule, a 55 kDa transmembrane protein with an intracellular death domain (DD) that serves to recruit the DD-containing protein TRADD. The simultaneous binding of TRADD to TRAF2 and RIP gives rise to an interconnected, activated complex in whose context RIP is necessary and sufficient for NF-κB activation (Goeddel, 1999). The interaction of p62–aPKC with RIP may offer a mechanistic explanation for the involvement of aPKCs in TNFα signaling downstream of RIP (Sanz et al., 1999). Exactly how the aPKCs are activated by their presence in these complexes is not yet understood. This could potentially involve conformational changes that make the aPKCs accessible to their substrates, or possibly the recruitment of an unidentified activator.
Interestingly, there is a certain parallelism between the TNFα and IL-1 signaling cascades. In the latter case, the intracellular domain of the IL-1 receptor interacts with MyD88, a functional analog of TRADD. MyD88 then recruits a kinase, IRAK, which interacts with TRAF6. In this case, both the kinase and the TRAF are required intermediaries for NF-κB activation (Lomaga et al., 1999; Thomas et al., 1999), with TRAF6 serving as the adapter that makes the connection with the p62–aPKC complex. aPKC activity is required for IL-1 and TRAF6 activation of NF-κB (Sanz et al., 2000). p62, then, appears to act as a point of convergence of the IL-1 and the TNFα signaling pathways. Its functional importance in NF-κB activation has been highlighted by the observation that its depletion severely abrogates NF-κB activation by both TNFα and IL-1 (Sanz et al., 2000). It is not yet clear whether p62 is also required for the activation of NF-κB by Ras or if, on the contrary, Ras can target the aPKCs through an independent pathway. In any event, an essential component of the NF-κB pathway is the IκB kinase (IKK) complex, which phosphorylates and triggers the degradation of IκB to release NF-κB from its cytosolic state, allowing its nuclear translocation (Karin, 1999). Recent results suggest that the aPKCs that are somehow activated by the complexes described above target the IKK β subunit, possibly through a direct interaction (Lallena et al., 1999; Wooten et al., 2000). Thus, p62 may serve to bring together RIP, TRAF6 (upstream components of the cytokine signaling pathways) and the aPKCs, an event that most likely serves to transmit a signal to the IKK complex.
Recently, another protein, the α isoform of the kinase MEK5, was identified as a second AID-containing molecule (M.T. Diaz-Meco and J. Moscat, unpublished observations). MEK5 is the upstream regulator of the kinase BMK1/ERK5, and both proteins are important in the control of cell growth (English et al., 1998; Kamakura et al., 1999). The aPKCs have been shown to interact with the AID site of MEK5 via their V1 domain. These interactions are mitogen inducible and important for the activation of the MEK5/ERK5 pathway. Therefore, it seems that p62 and MEK5 serve to direct the aPKCs into different signaling cascades using their respective AID sequences.
The V1 domains of the aPKCs also interact with Par-6 (partitioning-defective-6), a scaffold protein with yet another function: the control of cell polarity (Watts et al., 1996; Qiu et al., 2000). Par-6 has a CRIB-like domain that is responsible for its interactions with CDC42 and Rac (Qiu et al., 2000), and a PDZ domain that has been implicated in the interaction with Par-3. The mammalian Par-3, called ASIP, has been reported to interact with the catalytic domain of the aPKCs and to be a relatively good substrate (Izumi et al., 1998). Both the Caenorhabditis elegans aPKC homolog, PKC-3, and Par-3 are required for the proper control of embryonic polarity (Tabuse et al., 1998). In mammalian cells, ASIP appears to be involved in the establishment/maintenance of epithelial cell polarity (Izumi et al., 1998; Qiu et al., 2000). The Drosophila homolog of Par-3 is Bazooka and recent evidence demonstrates that the aPKC binds to Bazooka and is required for the control of polarity of epithelia and neuroblasts in this system (Wodarz et al., 2000). The aPKCs bind to a region of Par-6 that maps to residues 15–110 (Qiu et al., 2000). We have noted that this region contains a short amino acid sequence that displays significant homology with the AIDs of p62 and MEK5 (Figure 1). It is therefore very likely that Par-6 uses its putative AID site to interact with the V1 domain of the aPKCs. It seems then, that there is an outstanding parallelism between p62 and Par-6: p62 responds to cytokine signaling by linking the aPKCs to the NF-κB pathway and Par-6 seems to respond to CDC42 signaling by linking the aPKCs with the actin cytoskeletal structure (Figure 2). This provides a potential mechanistic explanation for the requirement of the aPKCs in both Ras- and CDC42-induced cell transformation (Bjorkoy et al., 1997; Qiu et al., 2000).
In summary, the V1 domain of the aPKCs is a region to which as many as three different adapters/effectors may bind. A particular interaction may result in the placement of an aPKC into a specific signaling complex where it interacts, either directly or indirectly, with the appropriate sets of both upstream and downstream effectors. Such interactions could thereby confer specificity to the actions of the aPKCs, allowing a single enzyme to serve different functional purposes in a context-dependent manner. This is an important issue, especially when, as in the case of PKCs, the kinase catalytic domains are highly similar and the cell has to devise mechanisms to impose specificity.
Par-4, a negative regulator
In addition to having distinct V1 site binding partners, the aPKCs are also reported to have two zinc finger domain binding partners: LIP (lambda-interacting protein) (Diaz-Meco et al., 1996b) and Par-4 (prostate androgen response-4) (Diaz-Meco et al., 1996a). In the cases of the novel and classical PKC isotypes, the zinc finger domains are targeted by lipid cofactors that influence kinase activity (Nishizuka, 1995). Not surprisingly, both LIP and Par-4 are able to modulate aPKC enzymatic activity, LIP being an activator (Diaz-Meco et al., 1996b) and Par-4 an inhibitor (Diaz-Meco et al., 1996a). Little is known about the precise mechanism of action of LIP or about its role in the cell. However, Par-4 emerges as an important molecule from a cell functional point of view (Diaz-Meco et al., 1996a), as it was previously identified as a gene induced in prostate cancer cells undergoing apoptosis (Sells et al., 1994). Subsequent studies have demonstrated that Par-4 levels increase during neuronal cell death (Guo et al., 1998), and the overexpression of Par-4 causes cells to undergo apoptosis in a manner that depends on its ability to block aPKC activity (Diaz-Meco et al., 1996a). Interestingly, the expression of Par-4 inhibits IKK and the ensuing activation of NF-κB by TNFα, making cells that are normally resistant to apoptosis susceptible to TNFα-induced death (Diaz-Meco et al., 1999). Par-4 levels are downregulated in Ras-transformed cells (Barradas et al., 1999), which has physiological implications for cancer. Indeed, restoration of Par-4 to normal parental levels makes these cells more sensitive to pro-apoptotic insults, including the action of chemotherapeutic agents (Barradas et al., 1999). Strikingly, experiments in vivo demonstrate that the progression of tumors derived from Ras-transformed cells expressing Par-4 is much more effectively reduced than those from Ras-transformed cells in which Par-4 levels are depleted (Barradas et al., 1999). This is reminiscent of what has been shown by others (Wang et al., 1999) and indicates that the blockage of NF-κB activity in Ras-transformed cells makes them more sensitive to the actions of chemotherapy. Altogether, these observations support an important role for the aPKCs in cell survival. Interestingly, recent evidence from Drosophila aPKC loss-of-function indicates that embryos die before cellularization is complete, showing premature cell death and increased TUNEL labeling (Wodarz et al., 2000).
Perspectives
The observations described above allow us to draw novel and exciting models that may begin to explain how the aPKCs contribute to the regulation of cell function. However, they also raise many questions that need to be addressed. For example, what determines which of the aPKCs interact with p62, Par-6 or MEK5 in a given situation? In this regard, the distinct cellular localization of the three adapters may contribute an additional level of specificity. Consistent with this notion, immunofluorescence analysis reveals that p62 displays punctate staining colocalizing with certain endosomal markers (Sanchez et al., 1998). Par-3, which constitutively associates with Par-6, localizes to the tight junctions in epithelial cells (Izumi et al., 1998). The localization of endogenous Par-6 has not been reported and is an important issue, as the ectopic expression of Par-6 disrupts tight junctions (Joberty et al., 2000); this finding seems to be inconsistent with its proposed role in maintaining cell polarity. What actually causes the activation of the aPKCs within the complexes? How do activated aPKCs phosphorylate the appropriate targets in reponse to a particular stimulus? From a broader perspective, what are the contributions of the aPKCs to NF-κB activation as compared with those of other putative IKK kinases? Is Par-3 the only transducer of the aPKC signals in the control of the cytoskeletal architecture? How does Par-6 orchestrate the signaling events that control cell polarity? What transcription factors are involved in the downregulation of Par-4 during oncogenic transformation?
Genetic models, including knock-out mice, need to be generated for the aPKCs and for their adapters. Analyses of these may provide a better understanding of the physiological implications of aPKC inactivation in a whole animal. Nevertheless, it is already apparent that, through their complex interactions with a variety of adapters, the aPKCs form part of distinct signaling complexes that endow promiscuous kinases with functional specificity.

Jorge Moscat and María Diaz-Meco
Acknowledgments
Acknowledgements
We thank Marie Wooten for critical reading of the manuscript.
References
- Akimoto K. et al. (1996) EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J., 15, 788–798. [PMC free article] [PubMed] [Google Scholar]
- Barradas M., Monjas, A., Diaz-Meco, M.T., Serrano, M. and Moscat, J. (1999) The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J., 18, 6362–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E., Diaz-Meco, M.T., Dominguez, I., Municio, M.M., Sanz, L., Lozano, J., Chapkin, R.S. and Moscat, J. (1993) Protein kinase Cζ isoform is critical for mitogenic signal transduction. Cell, 74, 555–563. [DOI] [PubMed] [Google Scholar]
- Berra E., Diaz-Meco, M.T., Lozano, J., Frutos, S., Municio, M.M., Sanchez, P., Sanz, L. and Moscat, J. (1995) Evidence for a role of MEK and MAPK during signal transduction by protein kinase Cζ. EMBO J., 14, 6157–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy G., Perander, M., Overvatn, A. and Johansen, T. (1997) Reversion of Ras- and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase Cλ is accompanied by the loss of constitutive nuclear mitogen-activated protein kinase/extracellular signal-regulated kinase activity. J. Biol. Chem., 272, 11557–11565. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M.T. et al. (1993) A dominant negative protein kinase Cζ subspecies blocks NF-κB activation. Mol. Cell. Biol., 13, 4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M.T., Lozano, J., Municio, M.M., Berra, E., Frutos, S., Sanz, L. and Moscat, J. (1994) Evidence for the in vitro and in vivo interaction of Ras with protein kinase Cζ. J. Biol. Chem., 269, 31706–31710. [PubMed] [Google Scholar]
- Diaz-Meco M.T., Municio, M.M., Frutos, S., Sanchez, P., Lozano, J., Sanz, L. and Moscat, J. (1996a) The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell, 86, 777–786. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M.T., Municio, M.M., Sanchez, P., Lozano, J. and Moscat, J. (1996b) λ-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol. Cell. Biol., 16, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M.T., Lallena, M.J., Monjas, A., Frutos, S. and Moscat, J. (1999) Inactivation of the inhibitory κB protein kinase/nuclear factor κB pathway by Par-4 expression potentiates tumor necrosis factor α-induced apoptosis. J. Biol. Chem., 274, 19606–19612. [DOI] [PubMed] [Google Scholar]
- English J.M., Pearson, G., Baer, R. and Cobb, M.H. (1998) Identification of substrates and regulators of the mitogen-activated protein kinase ERK5 using chimeric protein kinases. J. Biol. Chem., 273, 3854–3860. [DOI] [PubMed] [Google Scholar]
- Goeddel D.V. (1999) Signal transduction by tumor necrosis factor: the Parker B. Francis Lectureship. Chest, 116, 69S–73S. [DOI] [PubMed] [Google Scholar]
- Guo Q., Fu, W., Xie, J., Luo, H., Sells, S.F., Geddes, J.W., Bondada, V., Rangnekar, V.M. and Mattson, M.P. (1998) Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nature Med., 4, 957–962. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Hirose, T., Tamai, Y., Hirai, S., Nagashima, Y., Fujimoto, T., Tabuse, Y., Kemphues, K.J. and Ohno, S. (1998) An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol., 143, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Petersen, C., Gao, L.and Macara, I.G. (2000) The cell-polarity protein par6 links par3 and atypical protein kinase C to cdc42. Nature Cell Biol., 2, 531–539. [DOI] [PubMed] [Google Scholar]
- Kamakura S., Moriguchi, T. and Nishida, E. (1999) Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem., 274, 26563–26571. [DOI] [PubMed] [Google Scholar]
- Karin M. (1999) The beginning of the end: IκB kinase (IKK) and NF-κB activation. J. Biol. Chem., 274, 27339–27342. [DOI] [PubMed] [Google Scholar]
- Lallena M.J., Diaz-Meco, M.T., Bren, G., Pay, C.V. and Moscat, J. (1999) Activation of IκB kinase β by protein kinase C isoforms. Mol. Cell. Biol., 19, 2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D.F., Monia, B., Dean, N. and Berk, B.C. (1997) Protein kinase C-ζ mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J. Biol. Chem., 272, 6146–6150. [DOI] [PubMed] [Google Scholar]
- Lomaga M.A. et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev., 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N.R. and Fields, A.P. (1997) Atypical protein kinase Cι protects human leukemia cells against drug-induced apoptosis. J. Biol. Chem., 272, 27521–27524. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J., 9, 484–496. [PubMed] [Google Scholar]
- Puls A., Schmidt, S., Grawe, F. and Stabel, S. (1997) Interaction of protein kinase Cζ with ZIP, a novel protein kinase C-binding protein. Proc. Natl Acad. Sci. USA, 94, 6191–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R.G., Abo, A. and Steven Martin, G. (2000) A human homolog of the C. elegans polarity determinant par-6 links rac and cdc42 to PKCζ signaling and cell transformation. Curr. Biol., 10, 697–707. [DOI] [PubMed] [Google Scholar]
- Sanchez P., De Carcer, G., Sandoval, I.V., Moscat, J. and Diaz-Meco, M.T. (1998) Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol. Cell. Biol., 18, 3069–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L., Sanchez, P., Lallena, M.J., Diaz-Meco, M.T. and Moscat, J. (1999) The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J., 18, 3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L., Diaz-Meco, M.T., Nakano, H. and Moscat, J. (2000) The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1–TRAF6 pathway. EMBO J., 19, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells S.F., Wood, D.P., Jr, Joshi-Barve, S.S., Muthukumar, S., Jacob, R.J., Crist, S.A., Humphreys, S. and Rangnekar, V.M. (1994) Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ., 5, 457–466. [PubMed] [Google Scholar]
- Sontag E., Sontag, J.M. and Garcia, A. (1997) Protein phosphatase 2A is a critical regulator of protein kinase Cζ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J., 16, 5662–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y., Izumi, Y., Piano, F., Kemphues, K.J., Miwa, J. and Ohno, S. (1998) Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development, 125, 3607–3614. [DOI] [PubMed] [Google Scholar]
- Takeda H. et al. (1999) PI 3-kinase γ and protein kinase C-ζ mediate RAS-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J., 18, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.A., Allen, J.L., Tsen, M., Dubnicoff, T., Danao, J., Liao, X.C., Cao, Z. and Wasserman, S.A. (1999) Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol., 163, 978–984. [PubMed] [Google Scholar]
- Wang C.Y., Cusack, J.C., Jr, Liu, R. and Baldwin, A.S., Jr (1999) Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nature Med., 5, 412–417. [DOI] [PubMed] [Google Scholar]
- Watts J.L., Etemad-Moghadam, B., Guo, S., Boyd, L., Draper, B.W., Mello, C.C., Priess, J.R. and Kemphues, K.J. (1996) par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development, 122, 3133–3140. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath, A., Grimm, A. and Knust, E. (2000) Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol., 150, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten M.W. (1999) Function for NF-κB in neuronal survival: regulation by atypical protein kinase C. J. Neurosci. Res., 58, 607–611. [DOI] [PubMed] [Google Scholar]
- Wooten M.W., Seibenhener, M.L., Neidigh, K.B. and Vandenplas, M.L. (2000) Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells. Mol. Cell. Biol., 20, 4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]