This case-control study compares the genome expression at birth and associated outcome in neonates with hypoxic-ischemic encephalopathy in a high-income country (Italy) and their counterparts in low- to middle-income countries (India, Sri Lanka, and Bangladesh).
Key Points
Question
Do genome expression profiles at birth in neonates with hypoxic-ischemic encephalopathy (HIE) in a high-income country (HIC) differ from those of their counterparts in South Asia, and can this difference explain the lack of hypothermic neuroprotection?
Findings
In this case-control study of 134 neonates with HIE from an HIC or South Asia and 14 healthy controls from the HIC, neonates in the HIC cohort who had an adverse outcome after HIE had a different host genome expression profile at birth compared with neonates in the South Asia cohort and displayed opposite regulation of the significant genes in common.
Meaning
Findings of this study suggest that differences in the nature and timing of cerebral hypoxia ischemia explain the lack of hypothermic neuroprotection in South Asia.
Abstract
Importance
Induced hypothermia, the standard treatment for hypoxic-ischemic encephalopathy (HIE) in high-income countries (HICs), is less effective in the low-income populations in South Asia, who have the highest disease burden.
Objective
To investigate the differences in blood genome expression profiles of neonates with HIE from an HIC vs neonates with HIE from South Asia.
Design, Setting, and Participants
This case-control study analyzed data from (1) a prospective observational study involving neonates with moderate or severe HIE who underwent whole-body hypothermia between January 2017 and June 2019 and age-matched term healthy controls in Italy and (2) a randomized clinical trial involving neonates with moderate or severe HIE in India, Sri Lanka, and Bangladesh recruited between August 2015 and February 2019. Data were analyzed between October 2020 and August 2023.
Exposure
Whole-blood RNA that underwent next-generation sequencing.
Main Outcome and Measures
The primary outcomes were whole-blood genome expression profile at birth associated with adverse outcome (death or disability at 18 months) after HIE in the HIC and South Asia cohorts and changes in whole-genome expression profile during the first 72 hours after birth in neonates with HIE and healthy controls from the HIC cohort. Blood samples for RNA extraction were collected before whole-body hypothermia at 4 time points (6, 24, 48, and 72 hours after birth) for the HIC cohort. Only 1 blood sample was drawn within 6 hours after birth for the South Asia cohort.
Results
The HIC cohort was composed of 35 neonates (21 females [60.0%]) with a median (IQR) birth weight of 3.3 (3.0-3.6) kg and gestational age of 40.0 (39.0-40.6) weeks. The South Asia cohort consisted of 99 neonates (57 males [57.6%]) with a median (IQR) birth weight of 2.9 (2.7-3.3) kg and gestational age of 39.0 (38.0-40.0) weeks. Healthy controls included 14 neonates (9 females [64.3%]) with a median (IQR) birth weight of 3.4 (3.2-3.7) kg and gestational age of 39.2 (38.9-40.4) weeks. A total of 1793 significant genes in the HIC cohort and 99 significant genes in the South Asia cohort were associated with adverse outcome (false discovery rate <0.05). Only 11 of these genes were in common, and all had opposite direction in fold change. The most significant pathways associated with adverse outcome were downregulation of eukaryotic translation initiation factor 2 signaling in the HIC cohort (z score = −4.56; P < .001) and aldosterone signaling in epithelial cells in the South Asia cohort (z score = null; P < .001). The genome expression profile of neonates with HIE (n = 35) at birth, 24 hours, 48 hours, and 72 hours remained significantly different from that of age-matched healthy controls in the HIC cohort (n = 14).
Conclusions and Relevance
This case-control study found that disease mechanisms underlying HIE were primarily associated with acute hypoxia in the HIC cohort and nonacute hypoxia in the South Asia cohort. This finding might explain the lack of hypothermic neuroprotection.
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a leading cause of death and disability among neonates born at full term. Worldwide, approximately 1 million neonates die or survive with a major disability every year from HIE.1 South Asia, particularly India, shoulders the highest disease burden, accounting for 60% of all HIE-related deaths in the world.2,3 Between 2010 and 2019, although neonatal mortality decreased by 32% in India, HIE-specific mortality remained unchanged, resulting in an economic loss of US $60 to $135 billion.3
Whole-body hypothermia has been associated with reduced death or disability after moderate or severe HIE4 in high-income countries (HICs) and is the standard treatment. However, the Hypothermia for Encephalopathy in Low-Income and Middle-Income Countries (HELIX) trial,5 the largest clinical trial of hypothermia in the world that was conducted in South Asia, reported that hypothermia did not reduce brain injury but increased mortality. Whole-body hypothermia was administered using servocontrolled devices in the HELIX trial, and attainment of the target temperature was earlier than in hypothermia trials in HICs.6 Nonetheless, hypothermia was not neuroprotective, regardless of the duration of birth depression or birth acidosis7 and place of birth.6 It is not known whether the differential response to whole-body hypothermia between HICs and South Asia is associated with the underlying disease mechanisms. Whole-blood gene expression profile is increasingly used to characterize host responses in various infectious diseases,8,9,10,11 including neonatal cytomegalovirus infections,12 and in HIE wherein the gene profiles are different for healthy neonates vs those with sepsis.13,14
In this study, we investigated the differences in blood genome expression profiles of neonates with HIE from an HIC vs neonates with HIE from South Asia, to understand why one population may benefit from hypothermic neuroprotection and another may not. In addition, we assessed the temporal changes in genome expression profile over the first 72 hours after birth in neonates with HIE compared with matched healthy controls in an HIC.
Methods
Study Design
This case-control study analyzed data collected from (1) a prospective observational study involving consecutive neonates with moderate or severe HIE who underwent whole-body hypothermia at the University Hospital of Campania in Italy between January 2017 and June 2019 (hereafter, the HIC cohort) and contemporary age-matched term healthy controls and (2) the HELIX randomized clinical trial, which recruited 408 neonates with moderate or severe HIE from 7 tertiary public sector neonatal intensive care units in India, Sri Lanka, and Bangladesh between August 2015 and February 2019 (hereafter, the South Asia cohort).5 The Imperial College London Research Ethics Committee and the local research ethics committees at all sites approved both previous studies and the present case-control study. Informed consent was obtained from parents prior to recruitment. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
In the HIC cohort, blood samples for RNA extraction were collected before initiation of whole-body hypothermia (within 6 hours after birth) and then again after 24, 48, and 72 hours. Age-matched term healthy controls were recruited from postnatal wards and had normal neonatal examination (eMethods in Supplement 1). In the South Asia cohort, there was only 1 blood sample drawn within 6 hours after birth, before initiation of hypothermia. Healthy controls could not be recruited in South Asia.
Sample Collection and Processing
Peripheral venous or arterial blood samples were collected using customized blood RNA tubes (PAXgene; PreAnalytiX),14 frozen, and then later extracted. The RNA from samples underwent next-generation sequencing using sequencers (Illumina). Details of the sequencing method, quality control, and analysis are provided in the eMethods, eTable 1, and eFigures 1-3 in Supplement 1.
Clinical Assessments
Within 6 hours after birth, all neonates with HIE were examined by certified examiners using the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network hypothermia trial encephalopathy staging (modified Sarnat staging),15 and the stage of encephalopathy was classified. Detailed neurodevelopmental examination between 18 to 24 months of age using the Bayley Scales of Infant Development III was performed by a certified examiner. Adverse outcome was defined as death or moderate or severe disability15 (eMethods in Supplement 1).
Statistical Analysis
We used R, version 4.1.0 (R Project for Statistical Computing), for data analysis. Differential expression analysis to identify the genome expression profile of neonates with adverse neurological outcome was carried out with DESeq2, version 1.32.0 (Bioconductor) first unadjusted and then adjusted for birth weight, sex, and gestational age and treatment received (whole-body hypothermia vs usual care in the South Asia cohort). The R package maSigPro was used to identify genes that were differentially expressed over time, which were then clustered according to their pattern of expression. We corrected all of the P values for multiple testing with the Benjamini-Hochberg false discovery rate (FDR) method to control for type I error (5% FDR). Pathway analyses were conducted by using the differentially expressed genes (DEG; FDR <0.05 and absolute log2 fold change >1), which were associated with biological functions in the Ingenuity Pathways Knowledge Base (Ingenuity Systems)16 (eMethods in Supplement 1).
Statistical analyses for clinical variables were performed with SPSS Statistics, version 24 (IBM and SPSS Inc). Differences in continuous variables between groups were assessed with Mann-Whitney or unpaired, 2-tailed t test, as appropriate. Two-sided P < .01 was considered to be statistically significant for clinical variables. Data were analyzed between October 2020 and August 2023.
Results
Patient Characteristics
The HIC cohort was composed of 35 neonates (21 females [60.0%], 14 males [40.0%]) with a median (IQR) birth weight of 3.3 (3.0-3.6) kg and gestational age of 40.0 (39.0-40.6) weeks. The South Asia cohort consisted of 99 neonates (42 females [42.4%], 57 males [57.6%]) with a median (IQR) birth weight of 2.9 (2.7-3.3) kg and gestational age of 39.0 (38.0-40.0) weeks. Healthy controls included 14 neonates (9 females [64.3%], 5 males [35.7%]) with a median (IQR) birth weight of 3.4 (3.2-3.7) kg and gestational age of 39.2 (38.9-40.4) weeks (Table; eTable 2 in Supplement 1).
Table. Clinical Characteristics of Neonates With Hypoxic-Ischemic Encephalopathy (HIE) From a High-Income Country (HIC) or South Asia.
| Characteristic | Neonates with HIE, No. (%) | P value (HIC vs South Asia)a | Healthy controls in HIC cohort, No. (%) (n = 14) | P value (with HIE vs healthy controls)a | |
|---|---|---|---|---|---|
| HIC cohort (n = 35) | South Asia cohort (n = 99) | ||||
| Maternal | |||||
| Hypertension | 3 (8.6) | 7 (7.0) | .40 | 0 | .02 |
| Diabetes | 1 (2.8) | 2 (2.0) | .48 | 0 | .04 |
| Hypothyroidism | 2 (5.7) | 1 (1.0) | .16 | 0 | .05 |
| Intrapartum sentinel events | 12 (34.3) | 16 (16.2) | .02 | 0 | .29 |
| Neonatal | |||||
| Emergency cesarean delivery | 16 (45.7) | 27 (27.3) | .05 | 0 | .002 |
| Sex | |||||
| Female | 21 (60.0) | 42 (42.4) | .08 | 9 (64.3) | >.99 |
| Male | 14 (40.0) | 57 (57.6) | .08 | 5 (35.7) | >.99 |
| Birth weight, median (IQR), kg | 3.3 (3.0-3.6) | 2.9 (2.7-3.3) | <.001 | 3.4 (3.2-3.7) | .52 |
| Gestational age, median (IQR), wk | 40.0 (39.0-40.6) | 39.0 (38.0-40.0) | .008 | 39.2 (38.9-40.4) | .92 |
| Umbilical cord pH, median (IQR) | 7.0 (6.9-7.0) | 7.1 (6.8-7.3) | <.001 | NA | NA |
| 5-min Apgar score, median (IQR) | 6.5 (5.0-8.0) | 5.0 (4.0-6.0) | <.001 | 10.0 (9.5-10.0) | <.001 |
| Intubation at birth | 14 (40.0) | 50 (50.5) | .32 | 0 | .01 |
| Clinical course | |||||
| Moderate encephalopathy | 31 (88.6) | 76 (76.8) | .15 | 0 | NA |
| Severe encephalopathy | 4 (11.4) | 23 (23.2) | .15 | 0 | NA |
| Induced hypothermia (sample collection within 6 h after birth) | 35 (100.0) | 44 (44.4) | NA | 0 | NA |
| Seizuresb | 10 (28.6) | 87 (87.9) | <.001 | 0 | NA |
| Age of onset of seizures, median (IQR), h | 18.0 (13.2-25.3) | 3.0 (1.0-5.0) | .002 | NA | NA |
| Early-onset culture-positive sepsis | 0 | 3 (3.0) | .56 | 0 | NA |
| MRI | |||||
| MRI available | 32 (91.4) | 62 (62.6) | <.001 | 0 | NA |
| Basal ganglia or thalamic injury | 8 (25.0) | 16 (25.8) | >.99 | NA | NA |
| White-matter injury | 10 (31.3) | 44 (71.0) | <.001 | NA | NA |
| Cortical injury | 4 (12.5) | 15 (24.2) | .27 | NA | NA |
| Outcomes at 18-22 mo | |||||
| Death | 4 (11.4) | 40 (40.4) | .002 | NA | NA |
| Moderate or severe disability | 7 (20.0) | 11 (11.1) | .24 | NA | NA |
| Death and moderate or severe disability | 11 (31.4) | 51 (51.5) | .04 | NA | NA |
Abbreviations: MRI, magnetic resonance imaging; NA, not applicable.
P < .01 was statistically significant.
Seizures were electrographic and/or clinical in an HIC, whereas seizures were only clinical and electroencephalographic data were not obtained in South Asia.
Pregnancy-related illnesses were similar in mothers of neonates with HIE in the HIC and South Asia cohorts. The median (IQR) umbilical cord pH (7.0 [6.9-7.0] vs 7.1 [6.8-7.3]; P < .001) was lower and the 5-minute Apgar score (6.5 [5.0-8.0] vs 5.0 [4.0-6.0]; P < .001) was higher in the HIC vs the South Asia cohort. The median (IQR) onset of seizures was earlier (3.0 [1.0-5.0] hours vs 18.0 [13.2-25.3] hours; P = .002) and more neonates had seizures within 6 hours after birth (87 of 99 [87.9%] vs 10 of 35 [28.6%]; P < .001) in the South Asia cohort compared with the HIC cohort. The neonates from South Asia had more white-matter injury on magnetic resonance imaging (44 of 62 [71.0%] vs 10 of 32 [31.3%]; P < .001) and higher mortality (40 of 99 [40.4%] vs 4 of 35 [11.4%]; P = .002) at 18 months. Healthy controls did not have any maternal morbidities or intrapartum sentinel events.
Comparison of Genome Expression Profile at Birth Associated With Adverse Outcome
This analysis included 35 neonates with HIE (28 with adequate quality samples taken within 6 hours after birth) from the HIC cohort and 99 from the South Asia cohort. Although the neonates were normothermic at the blood sample draw within 6 hours after birth, all 35 neonates in the HIC cohort and 44 (44.4%) in the South Asia cohort subsequently received whole-body hypothermia. The clinical characteristics of South Asia neonates with (n = 99) vs without (n = 309) genome expression data were not different (eTable 3 in Supplement 1).
The genome expression data of the neonates who developed adverse outcome (death or disability at 18 months) (51 in South Asia cohort; 9 in HIC cohort) were compared with the genome expression data of the neonates with good outcome (48 in South Asia cohort; 19 in HIC cohort). A total of 2143 genes in the HIC cohort and 83 genes in the HIC cohort were differentially expressed (FDR <0.05) between adverse outcome and good outcome groups (eFigure 4 in Supplement 1; eTables 4 and 5 in Supplement 2). The top DEGs in the HIC cohort were CD163L1 (FDR <0.001; log2 fold change, 1.89), RCVRN (FDR <0.001; log2 fold change, 2.84), and LZTS2 (FDR = 0.001; log2 fold change, −0.96). In the South Asia cohort, the top DEGs were HSPD1 (FDR <0.001; log2 fold change, 1.17), FKBP4 (FDR = 0.002; log2 fold change, 1.18), and SERPINH1 (FDR = 0.002; log2 fold change, 1.36) (Figure 1). The most significant pathways associated with adverse outcome were eukaryotic translation initiation factor 2 signaling in the HIC cohort (P < .001; z score = −4.56) and aldosterone signaling in epithelial cells in the South Asia cohort (P < .001; z score = null). A list of the significant pathways is provided in eTables 6 and 7 in Supplement 2.
Figure 1. Comparison of Neonates With Hypoxic-Ischemic Encephalopathy With Adverse and Good Outcomes.
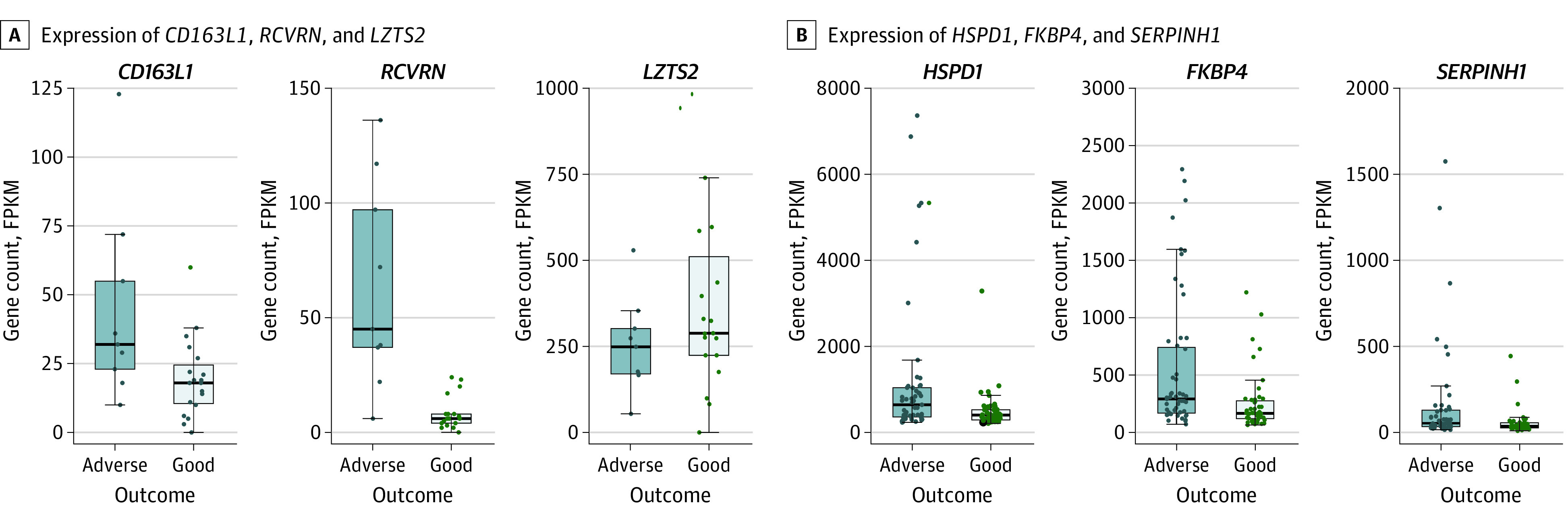
Gene count values are expressed as fragments per kilobase of transcript per million mapped reads (FPKM). Each box plot constitutes upper and lower bounds representing the first and third quartiles and a horizontal line representing the median. Boundaries of the whiskers are based on the 1.5 IQR value; all data points outside the boundary are outliers. Each dot represents a patient’s gene count. A, CD163L1, RCVRN, and LZTS2 were the 3 most significant genes after comparison of neonates with HIE with adverse and good outcomes in a high-income country (HIC). B, HSPD1, FKBP4, and SERPINH1 were the 3 most significant genes after comparison of neonates with HIE with adverse and good outcomes in South Asia. All 3 genes displayed a higher expression in the adverse vs the good outcome group.
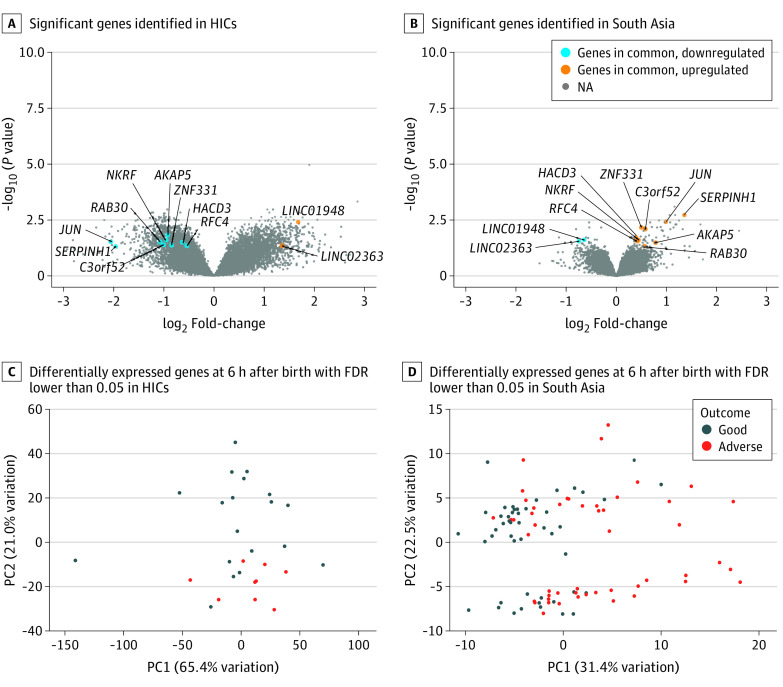
We then repeated the analysis by using only the genes with nonzero counts and that were in common between the South Asia and HIC cohorts (n = 30 121). A total of 1793 genes in the HIC cohort and 99 genes in the South Asia cohort were significantly differentially expressed (FDR <0.05). Only 11 significant genes were in common (eTables 8-10 in Supplement 2). None of the 11 genes had concordance in the direction of expression in both datasets compared with good outcome. JUN, SERPINH1, RAB30, C3orf52, AKAP5, NKRF, ZNF331, HACD3, and RFC4 were downregulated in the neonates from the HIC but upregulated in the neonates from South Asia. LINC01948 and LINC02363 were upregulated in the HIC and downregulated in South Asia (Figure 2A and B). Principal component analysis plots based on the DEG (1793 in the HIC cohort; 99 in the South Asia cohort) displayed a separation of the neonates with HIE with adverse outcome from neonates with good outcome, although this separation was clearer in the HIC than in South Asia cohort (Figure 2C and D).
Figure 2. Genome Expression Profile After Birth in Neonates With Hypoxic-Ischemic Encephalopathy (HIE) With Adverse Outcomes.
FDR indicates false discovery rate; HIC, high-income country; PC1, first principal component; PC2, second principal component.
Longitudinal Genome Expression of Neonates Over First 72 Hours
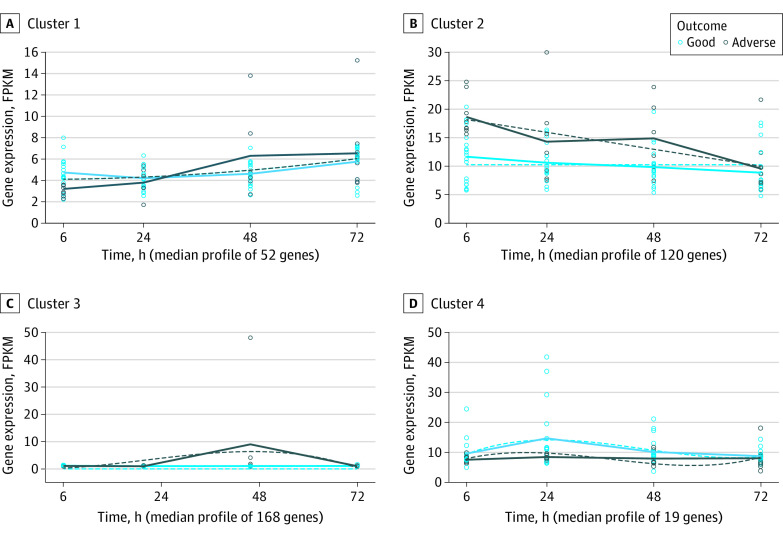
We evaluated how the genome expression profile changed over time in neonates with HIE in the HIC cohort who later developed adverse neurological outcomes and in neonates with HIE compared with healthy controls. A total of 104 samples collected at 4 different time points (6 hours, 24 hours, 48 hours, and 72 hours) from neonates with HIE in the HIC were included in this analysis. Differential expression identified 1604 significant DEGs between neonates with HIE with adverse outcomes vs their counterparts with good outcomes over time. A total of 359 genes fulfilled the R2 predefined cutoff value (eTable 11 in Supplement 2). These genes were clustered according to their similarities in expression patterns over time. A total of 4 clusters were identified (Figure 3). The most significant genes were SERPINE1 (FDR <0.001, cluster 3), FN1 (FDR <0.001, cluster 3), and COL4A1 (FDR <0.001, cluster 3).
Figure 3. Temporal Evolution of Genome Expression of Neonates With Hypoxic-Ischemic Encephalopathy and Adverse Outcome.
Solid lines represent the actual mean values of gene expression at each time point. Fitted curves are shown as dashed lines. FPKM indicates fragments per kilobase of transcript per million mapped reads.
In all 4 clusters, the difference between adverse and good outcome groups decreased after 72 hours with a similar expression profile (Figure 3). The top pathway of cluster 1 was enriched for cell cycle control of chromosomal replication. Cluster 2 was enriched in leukocyte extravasation signaling. Cluster 3 was enriched in pulmonary fibrosis idiopathic signaling pathway. Cluster 4 was enriched in cell cycle control of chromosomal replication. The list of significant pathways is provided in eTable 12 in Supplement 2.
A total of 150 samples (104 from neonates with HIE; 46 from healthy controls) collected at the 4 time points were included in this analysis. Differential expression over time identified 10 785 significant DEGs between neonates with HIE and controls. Significant genes were then clustered according to their similarities in expression pattern over time. A total of 2306 genes with the R2 predefined cutoff value were identified in 9 clusters (eTable 13 in Supplement 2; Figure 4; eFigure 5 in Supplement 1). The most significant genes were CALHM6 (FDR <0.001, cluster 7), TLR7 (FDR <0.001, cluster 7), and PLEKHO1 (FDR <0.001, cluster 3) (eTable 13 in Supplement 2).
Figure 4. Temporal Evolution of Genome Expression of Neonates With Hypoxic-Ischemic Encephalopathy (HIE) vs Healthy Controls.
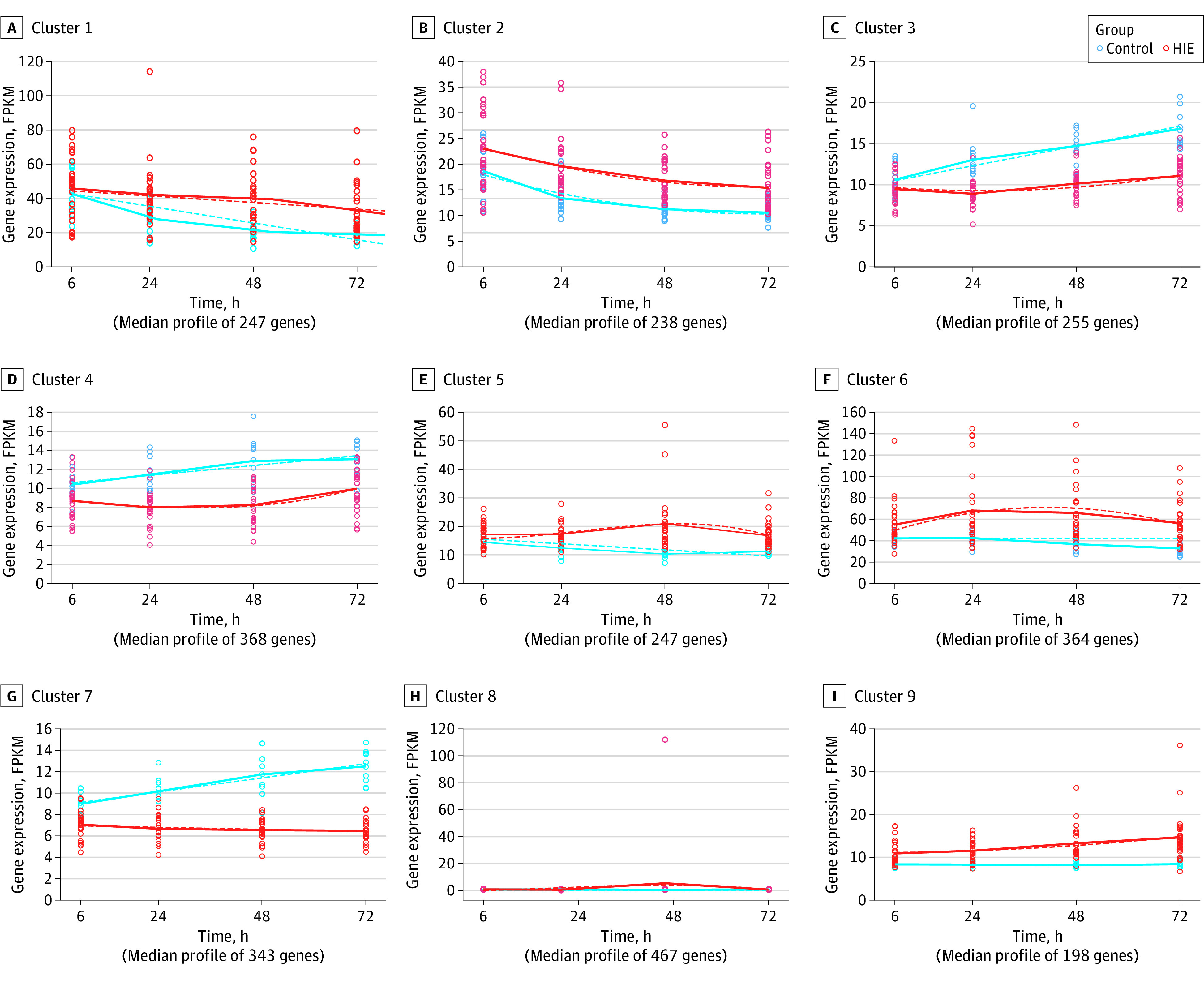
Solid lines represent the actual mean values of gene expression at each time point. Fitted curves are shown as dashed lines. FPKM indicates fragments per kilobase of transcript per million mapped reads.
The top pathway of cluster 1 was enriched for p38 MAPK signaling (eTable 14 in Supplement 2). Cluster 2 was enriched for JAK family kinases in interleukin 6–type cytokine signaling. Cluster 3 was enriched for type 2 diabetes signaling. Cluster 4 was enriched for TH1 pathway. Cluster 5 was enriched for glycolysis I. Cluster 6 was enriched for ferroptosis signaling pathway. Cluster 7 was enriched for antigen presentation pathway. Cluster 8 was enriched for pulmonary fibrosis idiopathic signaling pathway. Cluster 9 was enriched for cell cycle control of chromosomal replication. See eTable 14 in Supplement 2 for these clusters.
Discussion
We found that the whole-blood host genome expression profiles soon after birth associated with adverse outcomes in neonates with HIE from an HIC and neonates with HIE from South Asia were substantially different. Furthermore, the few common genes that were differentially expressed in both cohorts showed opposite associations with outcome. Downregulation of eIF2 at birth was associated with adverse outcome in the HIC cohort, whereas aldosterone signaling was associated with adverse outcome in the South Asia cohort. We also showed that neonates with HIE had a specific genome expression profile over the first 3 days after birth compared with healthy controls.
To our knowledge, this case-control study has been the largest study to explore genome expression profiles in HIE. The lack of concordance between the HIC and South Asia cohorts may suggest a different timing in the underlying mechanisms of intrapartum hypoxia ischemia between these neonates.
In the HIC cohort, the most significant genes associated with adverse outcomes within 6 hours after birth were CD163L1, RCVRN, and LZTS2. These genes are associated with the hypoxia-inducible factor-1α (HIF-1α) signaling, the master switch responsible for orchestrating the cellular response to acute hypoxia.17,18 CD163L1 is a marker of the anti-inflammatory phenotype of macrophages and is mediated by interleukin 10, which is induced by HIF-1α.19 RCVRN forms part of the photoreceptors of the retina20 associated with hypoxia-induced retinogenesis.21 LZTS2 interacts with the Wnt/β-catenin pathway to inhibit its transcriptional activity. It is upregulated in multiple forms of cancer-induced hypoxia through inhibition of the β-catenin activity, which is a pathway known to cross talk with HIF-1α.22 These patterns suggest an acute hypoxia ischemia in the HIC cohort.
In contrast, the most significant genes associated with adverse outcomes in South Asia were HSPD1, FKBP4, and SERPINH1. These genes are mainly associated with oxidative stress and intermittent hypoxia resembling reperfusion injury, production of reactive oxygen species, and inflammation.23,24 HSPD1, a heat shock factor, is upregulated within 8 hours after short-term chronic hypoxia.25 HSPD1 is also increased in patients with temporal lobe epilepsy in response to seizures.26 FKBPs are chaperone molecules and promote protein folding. Abnormal expression of SERPINH1 is considered a prognostic marker for cancer, and it is associated with immunoregulators and immune infiltration.27
The biological function of the DEG was also different between neonates with HIE from an HIC and neonates with HIE from South Asia. In the HIC cohort, the eIF2 signaling pathway was initially downregulated. In preclinical models, eIF2 phosphorylation occurs rapidly after neonatal hypoxia ischemia,28 leading to the inhibition of transcription and translation and to repression of protein synthesis shortly after the hypoxic-ischemic insult29 (downregulation). This phosphorylation reverses after reoxygenation, resulting in the resumption of protein synthesis (upregulation).29 On the other hand, aldosterone signaling was the most significant pathway identified in the South Asia cohort. This pathway has been implicated in chronic intermittent hypoxia settings, such as in obstructive sleep apnea, a chronic condition leading to intermittent hypoxia and subsequent activation of the renin-angiotensin-aldosterone system.30 Thus, this gene expression pattern suggests a nonacute nature of intrapartum hypoxia ischemia in South Asia. These findings agree with the changes to the genome expression previously reported in HIE.13,14 In a small cohort of 12 neonates with HIE in an HIC, the main biological pathways involved soon after birth indicated an acute oxygen deprivation as shown by the olfactory receptor response.13 Similarly, when the genome expression profile was examined in 47 infants with encephalopathy from South Asia, overrepresentation of genes involved in neuroinflammation was found.14 Each of these phases reflect a different timing of the disease mechanism starting with the acute hypoxic-ischemic insult; followed by a decrease of energy phosphates, oxidative stress, and apoptosis; and finally persistent inflammation and gliosis.
Subtle differences in the clinical phenotype, such as lower birth weight, lower incidence of intrapartum sentinel events, lesser birth acidosis, early onset of seizures, and higher white-matter injury on magnetic resonance imaging in neonates in South Asia vs an HIC, are consistent with the differences found on host genome expression profile. These data provide a mechanistic explanation for why whole-body hypothermia, the standard treatment for HIE in an HIC, was not neuroprotective but increased mortality among low-income populations in South Asia with the highest disease burden.
These data provide biological confirmation of our hypothesis about the compromised fetus in the womb, which explains that there is a high occurrence of HIE among low-income populations in South Asia and a lack of hypothermic neuroproection.31 Thus, the fetus is already compromised in the womb and is unable to cope with the normal hypoxic process of labor, especially if it is medically augmented.32 This clinical scenario, therefore, represents a nonacute hypoxia, as shown by preclinical models of growth-restricted animals and intermittent umbilical cord occlusion, where the seizure onset is earlier.33
This study also showed that the genome expression trajectory in neonates with HIE from an HIC who later develop adverse long-term neurological outcomes is different from those with favorable outcomes. In particular, increasing severity of outcomes was associated with upregulation of immunological and hypoxia-related pathways. In addition, the most significant genes (SERPINE1, FN1, and COL4A1) over time in neonates with adverse outcome are all HIF1 gene targets, and their expression is substantially increased after oxidative stress.34 These data highlight that oxidative stress cascade is crucial in the extent of brain injury in neonates with HIE in an HIC, as it amplifies the cellular damage.
These data have implications for both HICs and South Asia. In an HIC cohort, adverse outcomes were still seen in 30% of the neonates with HIE despite whole-body hypothermia,35 highlighting that a one-size-fits-all strategy is not effective in HIE and that there is room for improvement in patient stratification, treatment, and management (ie, personalized diagnosis or treatments). In contrast, whole-body hypothermia was not neuroprotective and was possibly harmful in South Asia, and these neonates required different neuroprotective approaches that did not involve whole-body hypothermia.36 Host gene expression profiles may reflect the underlying etiological mechanisms and offer a unique approach for disease stratification. In particular, the trajectory of genome expression profile in neonates with HIE may help a prompt identification of those neonates at higher risk of adverse outcomes later. The role of gene expression as a diagnostic and stratification tool has been demonstrated in a range of diseases that are otherwise difficult to diagnose.8,9,11,37,38
Limitations
Although, to our knowledge, this study is the first-ever detailed examination of genome expression profiles using in-depth next-generation sequencing in well characterized HIE cohorts from different continents, there are some limitations. First, the South Asian and HIC blood samples were sequenced in different laboratories and with different sequencers due to regulatory issues that prohibited the transport of blood samples out of India. Therefore, we could not analyze the 2 datasets together; instead, we performed an indirect comparison of the differential expression analyses. However, the same protocol was followed for both blood sample collection and RNA extraction. Second, the healthy controls were only from the HIC, again due to logistical and ethical challenges of collecting blood samples from healthy controls in South Asia. The challenge of recruiting healthy control infants led to similar limitations in previous studies with consistent results.12,39 Third, half of the neonates in South Asia underwent whole-body hypothermia as part of the HELIX trial. However, all the blood samples were collected before whole-body hypothermia was initiated and the differential expression analysis was adjusted for treatment. Hence, induced hypothermia is unlikely to have altered the preintervention genome expression data, although there may be an association with the final clinical outcome and allocation of each patient to the adverse outcome analysis group. Fourth, the HELIX trial was conducted in 3 South Asian countries, and its results may not be generalizable to other low- and middle-income countries. Fifth, although we collected blood samples on all 408 neonates recruited to the HELIX trial, only 99 had adequate-quality RNA suitable for next-generation sequencing. Many RNA samples were of poor quality due to breakdowns and temperature fluctuations of the deep freezers in South Asia and challenges of blood sample transport in dry ice. Nevertheless, there were no systematic differences between the neonates with RNA sequencing and those without.
Conclusions
In this case-control study, unique differences in whole-blood genome expression at birth were found between neonates with HIE in an HIC and neonates with HIE in South Asia. The underlying mechanisms were associated with acute hypoxia in the HIC cohort and nonacute hypoxia in the South Asia cohort, which may explain the lack of hypothermic neuroprotection in latter settings. Moreover, the genome expression profile of neonates with HIE over the first 3 days after birth remained significantly different from the genome expression profile of healthy controls in an HIC. Whole-blood genome expression profile may be useful for rapid disease stratification for personalized neuroprotection in HIE and for monitoring therapeutic response.
eMethods.
eTable 1. Clinical Variables of HIE Infants in Low-and-Middle-Income Countries in Batches 1 and 2
eTable 2. Mean (SD) Age and Mean (SD) Body Temperature at Blood Sample Collection
eTable 3. Clinical Characteristics of Babies From the HELIX Trial (LMIC) With Gene Expression Data and That Were Included in the Analysis, and Those Without Gene Expression Data
eFigure 1. Batch Effect Correction
eFigure 2. Identification of Any Sources of Variance in the High-Income Countries Dataset
eFigure 3. Identification of Any Sources of Variance in the Low-and-Middle-Income Countries Dataset
eFigure 4. Genome Expression Profile After Birth in Neonates With HIE With Adverse Outcome
eFigure 5. Temporal Evolution of Genome Expression of Neonates With HIE as Opposed to Healthy Controls
eTable 4. Differentially expressed genes associated with adverse outcome in neonates with HIE in LMIC (FDR < 0.05)
eTable 5. Differentially expressed genes associated with adverse outcome in neonates with HIE in HIC (FDR < 0.05)
eTable 6. Reported are the results of the Ingenuity Pathway Analysis for the differentially expressed genes associated with adverse outcome at T0 (HIC) – adjusted
eTable 7. Reported are the results of the Ingenuity Pathway Analysis for the differentially expressed genes associated with adverse outcome at T0 (LMIC) – adjusted
eTable 8. Differentially expressed genes associated with adverse outcome in neonates with HIE in HIC (FDR < 0.05)
eTable 9. Differentially expressed genes associated with adverse outcome in neonates with HIE in LMIC (FDR < 0.05)
eTable 10. Differentially expressed genes associated with adverse outcome and in common between LMIC and HIC in neonates with HIE (FDR < 0.05)
eTable 11. Differentially expressed genes between HIE neonates with adverse versus good outcome over time
eTable 12. Cluster 1. Reported are the results of the Ingenuity Pathway Analysis for cluster 1 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 2. Reported are the results of the Ingenuity Pathway Analysis for cluster 2 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 3. Reported are the results of the Ingenuity Pathway Analysis for cluster 3 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 4. Reported are the results of the Ingenuity Pathway Analysis for cluster 4 identified over the time when comparing adverse and good outcome (HIC)
eTable 13. Differentially expressed genes between HIE neonates versus controls over time
eTable 14. Cluster 1. Reported are the results of the Ingenuity Pathway Analysis for cluster 1 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 2. Reported are the results of the Ingenuity Pathway Analysis for cluster 2 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 3. Reported are the results of the Ingenuity Pathway Analysis for cluster 3 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 4. Reported are the results of the Ingenuity Pathway Analysis for cluster 4 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 5. Reported are the results of the Ingenuity Pathway Analysis for cluster 5 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 6. Reported are the results of the Ingenuity Pathway Analysis for cluster 6 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 7. Reported are the results of the Ingenuity Pathway Analysis for cluster 7 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 8. Reported are the results of the Ingenuity Pathway Analysis for cluster 8 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 9. Reported are the results of the Ingenuity Pathway Analysis for cluster 9 identified over the time when comparing HIE neonates and healthy controls (HIC)
Data Sharing Statement
References
- 1.Lawn JE, Cousens S, Zupan J; Lancet Neonatal Survival Steering Team . 4 million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891-900. doi: 10.1016/S0140-6736(05)71048-5 [DOI] [PubMed] [Google Scholar]
- 2.Global Research on Developmental Disabilities Collaborators . Accelerating progress on early childhood development for children under 5 years with disabilities by 2030. Lancet Glob Health. 2022;10(3):e438-e444. doi: 10.1016/S2214-109X(21)00488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.More K, Vidavalur R. Valuing disease burden due to neonatal encephalopathy and birth trauma: a health economic evaluation. SSRN. Preprint posted online June 27, 2022. 10.2139/ssrn.4147535 [DOI]
- 4.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013(1):CD003311. doi: 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayyil S, Pant S, Montaldo P, et al. ; HELIX consortium. Hypothermia for moderate or severe neonatal encephalopathy in low and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9(9):e1273-e1285. doi: 10.1016/S2214-109X(21)00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thayyil S, Montaldo P, Krishnan V, et al. Whole-body hypothermia, cerebral magnetic resonance biomarkers, and outcomes in neonates with moderate or severe hypoxic-ischemic encephalopathy born at tertiary care centers vs other facilities: a nested study within a randomized clinical trial. JAMA Netw Open. 2023;6(5):e2312152. doi: 10.1001/jamanetworkopen.2023.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgod C, Mazlan M, Pant S, et al. Duration of birth depression and neurodevelopmental outcomes after whole-body hypothermia for hypoxic ischemic encephalopathy in India, Sri Lanka and Bangladesh—an exploratory analysis of the HELIX trial. Lancet Reg Health Southeast Asia. Published online October 4, 2023. doi: 10.1016/j.lansea.2023.100284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herberg JA, Kaforou M, Wright VJ, et al. ; IRIS Consortium . Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA. 2016;316(8):835-845. doi: 10.1001/jama.2016.11236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med. 2014;370(18):1712-1723. doi: 10.1056/NEJMoa1303657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li HK, Kaforou M, Rodriguez-Manzano J, et al. Discovery and validation of a three-gene signature to distinguish COVID-19 and other viral infections in emergency infectious disease presentations: a case-control and observational cohort study. Lancet Microbe. 2021;2(11):e594-e603. doi: 10.1016/S2666-5247(21)00145-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259-269. doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouellette CP, Sánchez PJ, Xu Z, et al. Blood genome expression profiles in infants with congenital cytomegalovirus infection. Nat Commun. 2020;11(1):3548. doi: 10.1038/s41467-020-17178-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montaldo P, Kaforou M, Pollara G, et al. Whole blood gene expression reveals specific transcriptome changes in neonatal encephalopathy. Neonatology. 2019;115(1):68-76. doi: 10.1159/000492420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montaldo P, Cunnington A, Oliveira V, et al. Transcriptomic profile of adverse neurodevelopmental outcomes after neonatal encephalopathy. Sci Rep. 2020;10(1):13100. doi: 10.1038/s41598-020-70131-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran S, Laptook AR, Pappas A, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318(1):57-67. doi: 10.1001/jama.2017.7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523-530. doi: 10.1093/bioinformatics/btt703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh SJ, Koh MY, Powis G. The hypoxic inducible stress response as a target for cancer drug discovery. Semin Oncol. 2006;33(4):486-497. doi: 10.1053/j.seminoncol.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience. 2008;155(3):937-947. doi: 10.1016/j.neuroscience.2008.06.038 [DOI] [PubMed] [Google Scholar]
- 19.González-Domínguez É, Samaniego R, Flores-Sevilla JL, et al. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukoc Biol. 2015;98(4):453-466. doi: 10.1189/jlb.3HI1114-531R [DOI] [PubMed] [Google Scholar]
- 20.Zang J, Neuhauss SCF. The binding properties and physiological functions of Recoverin. Front Mol Neurosci. 2018;11:473. doi: 10.3389/fnmol.2018.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garita-Hernández M, Diaz-Corrales F, Lukovic D, et al. Hypoxia increases the yield of photoreceptors differentiating from mouse embryonic stem cells and improves the modeling of retinogenesis in vitro. Stem Cells. 2013;31(5):966-978. doi: 10.1002/stem.1339 [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. doi: 10.1038/s41392-020-0110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visniauskas B, Perry JC, Gomes GN, et al. Intermittent hypoxia changes the interaction of the kinin-VEGF system and impairs myocardial angiogenesis in the hypertrophic heart. Physiol Rep. 2021;9(9):e14863. doi: 10.14814/phy2.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyugovskaya L, Berger S, Polyakov A, Lavie P, Lavie L. Intermittent hypoxia affects the spontaneous differentiation in vitro of human neutrophils into long-lived giant phagocytes. Oxid Med Cell Longev. 2016;2016:9636937. doi: 10.1155/2016/9636937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning W, Chu TJ, Li CJ, Choi AM, Peters DG. Genome-wide analysis of the endothelial transcriptome under short-term chronic hypoxia. Physiol Genomics. 2004;18(1):70-78. doi: 10.1152/physiolgenomics.00221.2003 [DOI] [PubMed] [Google Scholar]
- 26.Marino Gammazza A, Colangeli R, Orban G, et al. Hsp60 response in experimental and human temporal lobe epilepsy. Sci Rep. 2015;5:9434. doi: 10.1038/srep09434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Gu W, Wen W, Zhang X. SERPINH1 is a potential prognostic biomarker and correlated with immune infiltration: a pan-cancer analysis. Front Genet. 2022;12:756094. doi: 10.3389/fgene.2021.756094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia-ischemia: role of protein synthesis and autophagic pathways. Exp Neurol. 2014;255:103-112. doi: 10.1016/j.expneurol.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 29.Koumenis C, Naczki C, Koritzinsky M, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22(21):7405-7416. doi: 10.1128/MCB.22.21.7405-7416.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol (1985). 2002;92(2):627-633. doi: 10.1152/japplphysiol.00152.2001 [DOI] [PubMed] [Google Scholar]
- 31.Thayyil S, Montaldo P, Krishnan V, et al. Whole-body hypothermia, cerebral magnetic resonance biomarkers, and outcomes in neonates with moderate or severe hypoxic-ischemic encephalopathy born at tertiary care centers vs other facilities: a nested study within a randomized clinical trial. JAMA Netw Open. 2023;6(5):e2312152. doi: 10.1001/jamanetworkopen.2023.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgod C, Pant S, Morales MM, et al. Effect of intra-partum oxytocin on neonatal encephalopathy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21(1):736. doi: 10.1186/s12884-021-04216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wassink G, Bennet L, Davidson JO, Westgate JA, Gunn AJ. Pre-existing hypoxia is associated with greater EEG suppression and early onset of evolving seizure activity during brief repeated asphyxia in near-term fetal sheep. PLoS One. 2013;8(8):e73895. doi: 10.1371/journal.pone.0073895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR. Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep. 2017;7(1):15140. doi: 10.1038/s41598-017-15474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu YW, Comstock BA, Gonzalez FF, et al. ; HEAL Consortium . Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N Engl J Med. 2022;387(2):148-159. doi: 10.1056/NEJMoa2119660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montaldo P, Pauliah SS, Lally PJ, Olson L, Thayyil S. Cooling in a low-resource environment: lost in translation. Semin Fetal Neonatal Med. 2015;20(2):72-79. doi: 10.1016/j.siny.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 37.Pennisi I, Rodriguez-Manzano J, Moniri A, et al. Translation of a host blood RNA signature distinguishing bacterial from viral infection into a platform suitable for development as a point-of-care test. JAMA Pediatr. 2021;175(4):417-419. doi: 10.1001/jamapediatrics.2020.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright VJ, Herberg JA, Kaforou M, et al. ; Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium and the Pediatric Emergency Medicine Kawasaki Disease Research Group (PEMKDRG) . Diagnosis of Kawasaki disease using a minimal whole-blood gene expression signature. JAMA Pediatr. 2018;172(10):e182293. doi: 10.1001/jamapediatrics.2018.2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahajan P, Kuppermann N, Mejias A, et al. ; Pediatric Emergency Care Applied Research Network (PECARN) . Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846-857. doi: 10.1001/jama.2016.9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Clinical Variables of HIE Infants in Low-and-Middle-Income Countries in Batches 1 and 2
eTable 2. Mean (SD) Age and Mean (SD) Body Temperature at Blood Sample Collection
eTable 3. Clinical Characteristics of Babies From the HELIX Trial (LMIC) With Gene Expression Data and That Were Included in the Analysis, and Those Without Gene Expression Data
eFigure 1. Batch Effect Correction
eFigure 2. Identification of Any Sources of Variance in the High-Income Countries Dataset
eFigure 3. Identification of Any Sources of Variance in the Low-and-Middle-Income Countries Dataset
eFigure 4. Genome Expression Profile After Birth in Neonates With HIE With Adverse Outcome
eFigure 5. Temporal Evolution of Genome Expression of Neonates With HIE as Opposed to Healthy Controls
eTable 4. Differentially expressed genes associated with adverse outcome in neonates with HIE in LMIC (FDR < 0.05)
eTable 5. Differentially expressed genes associated with adverse outcome in neonates with HIE in HIC (FDR < 0.05)
eTable 6. Reported are the results of the Ingenuity Pathway Analysis for the differentially expressed genes associated with adverse outcome at T0 (HIC) – adjusted
eTable 7. Reported are the results of the Ingenuity Pathway Analysis for the differentially expressed genes associated with adverse outcome at T0 (LMIC) – adjusted
eTable 8. Differentially expressed genes associated with adverse outcome in neonates with HIE in HIC (FDR < 0.05)
eTable 9. Differentially expressed genes associated with adverse outcome in neonates with HIE in LMIC (FDR < 0.05)
eTable 10. Differentially expressed genes associated with adverse outcome and in common between LMIC and HIC in neonates with HIE (FDR < 0.05)
eTable 11. Differentially expressed genes between HIE neonates with adverse versus good outcome over time
eTable 12. Cluster 1. Reported are the results of the Ingenuity Pathway Analysis for cluster 1 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 2. Reported are the results of the Ingenuity Pathway Analysis for cluster 2 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 3. Reported are the results of the Ingenuity Pathway Analysis for cluster 3 identified over the time when comparing adverse and good outcome (HIC)
eTable 12. Cluster 4. Reported are the results of the Ingenuity Pathway Analysis for cluster 4 identified over the time when comparing adverse and good outcome (HIC)
eTable 13. Differentially expressed genes between HIE neonates versus controls over time
eTable 14. Cluster 1. Reported are the results of the Ingenuity Pathway Analysis for cluster 1 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 2. Reported are the results of the Ingenuity Pathway Analysis for cluster 2 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 3. Reported are the results of the Ingenuity Pathway Analysis for cluster 3 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 4. Reported are the results of the Ingenuity Pathway Analysis for cluster 4 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 5. Reported are the results of the Ingenuity Pathway Analysis for cluster 5 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 6. Reported are the results of the Ingenuity Pathway Analysis for cluster 6 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 7. Reported are the results of the Ingenuity Pathway Analysis for cluster 7 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 8. Reported are the results of the Ingenuity Pathway Analysis for cluster 8 identified over the time when comparing HIE neonates and healthy controls (HIC)
eTable 14. Cluster 9. Reported are the results of the Ingenuity Pathway Analysis for cluster 9 identified over the time when comparing HIE neonates and healthy controls (HIC)
Data Sharing Statement