Abstract
Background
The phase III RATIONALE-302 study evaluated tislelizumab, an anti-programmed cell death protein 1 antibody, as second-line (2L) treatment for advanced/metastatic esophageal squamous cell carcinoma (ESCC). This prespecified exploratory analysis investigated outcomes in patients from Europe and North America (Europe/North America subgroup).
Patients and methods
Patients with tumor progression during/after first-line systemic treatment were randomized 1 : 1 to open-label tislelizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or irinotecan).
Results
The Europe/North America subgroup comprised 108 patients (tislelizumab: n = 55; chemotherapy: n = 53). Overall survival (OS) was prolonged with tislelizumab versus chemotherapy (median: 11.2 versus 6.3 months), with a hazard ratio (HR) of 0.55 [95% confidence interval (CI) 0.35-0.87]; HR was similar irrespective of programmed death-ligand 1 score [≥10%: 0.47 (95% CI 0.18-1.21); <10%: 0.55 (95% CI 0.30-1.01)]. Median progression-free survival was 2.3 versus 2.7 months with tislelizumab versus chemotherapy [HR: 0.97 (95% CI 0.64-1.47)]. Overall response rate was greater with tislelizumab (20.0%) versus chemotherapy (11.3%), with more durable response (median duration of response: 5.1 versus 2.1 months). Tislelizumab had a favorable safety profile versus chemotherapy, with fewer patients experiencing ≥grade 3 treatment-related adverse events (13.0% versus 51.0%). Those on tislelizumab experienced less deterioration in health-related quality of life, physical functioning, and/or disease- and treatment-related symptoms (i.e. fatigue, pain, and eating problems) as compared to those on chemotherapy, per the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30) and QLQ-OES18 scores.
Conclusions
As a 2L therapy for advanced/metastatic ESCC, tislelizumab improved OS and had a favorable safety profile as compared to chemotherapy in European/North American ESCC patients in the randomized phase III RATIONALE-302 study.
Key words: tislelizumab, esophageal squamous cell carcinoma, anti-programmed cell death protein 1 antibody
Highlights
-
•
2L tislelizumab for advanced/metastatic ESCC prolonged OS versus chemotherapy in a Europe/North America subgroup.
-
•
Tislelizumab had a more favorable safety profile versus chemotherapy in the Europe/North America patient subgroup.
-
•
Tislelizumab’s efficacy and safety in the Europe/North America subgroup were consistent with the overall trial population.
-
•
Results support the potential for tislelizumab to become a 2L option for advanced ESCC in Western countries.
Introduction
Esophageal squamous cell carcinoma (ESCC) represents the most common histological type of esophageal cancer globally, accounting for 85% of cases.1 Although survival rates have improved over recent decades, esophageal cancers, including ESCC, continue to have a poor prognosis.1 Prognosis is particularly poor for patients with metastatic ESCC, for whom the 5-year survival rate has been reported to be 6.9%.2
While the incidence of ESCC is highest in East Asia, ESCC also affects a substantial number of patients in Europe and North America.1,3 In the United States, data from the National Cancer Institute’s Surveillance, Epidemiology and End Results database and the Centers for Disease Control and Prevention's National Program of Cancer Registries indicate that there were over 64 000 cases of ESCC recorded between 2001 and 2015.4 More recent estimates from the International Agency for Research on Cancer GLOBOCAN database suggest that in 2020 there were over 6000 ESCC cases in North America and over 31 000 ESCC cases across European regions.1
Internationally, disease characteristics and the treatment approach recommended in clinical practice guidelines are broadly consistent for patients with unresectable locally advanced or metastatic ESCC.5, 6, 7, 8, 9, 10, 11 However, regional nuances between Western and Asian countries have been reported in the etiology of ESCC, patient characteristics, and treatment selection.3,8,9,12, 13, 14, 15, 16 For example, while the etiology of ESCC is multifactorial and yet to be fully elucidated, in Western countries smoking and excessive alcohol consumption are believed to be the main risk factors of ESCC, while hot beverage consumption and lack of fresh fruit and vegetable intake are thought to be key risk factors in Asian countries.13,14,17 In addition, ESCC has been reported to be diagnosed at an earlier age and stage in Asian countries than in Western countries.12,16 Furthermore, differences in treatment patterns have also been noted—for example, more prevalent use of curative surgery in Asian versus Western patients, and more prevalent use of immunotherapy and targeted therapies as second-line (2L) treatments in Western versus Asian countries.16 Therefore, it is important to investigate the efficacy of new interventions both globally and in specific regions, including in Europe and North America.
Tislelizumab is a humanized IgG4 monoclonal antibody with high affinity and specificity for programmed cell death protein 1 (PD-1).18 Tislelizumab is under investigation in a variety of tumor types, including in patients with ESCC at various lines of therapy and disease stages.19, 20, 21 In the global phase III RATIONALE-302 study in patients with advanced or metastatic ESCC who had disease progression during or after first-line systemic treatment, tislelizumab was shown to significantly prolong overall survival (OS) with a tolerable safety profile compared with investigator’s choice of chemotherapy (paclitaxel, docetaxel, or irinotecan) in the overall study population.21 The analysis reported here presents the efficacy and safety of tislelizumab in patients enrolled in this study from European countries and North America, which represented ∼20% of the study population.21
Patients and methods
Study design
RATIONALE-302 (ClinicalTrials.gov identifier: NCT03430843) was a randomized, controlled, two-arm, open-label, phase III study that recruited patients from 11 countries/regions (Belgium, mainland China, France, Germany, Italy, Japan, Republic of Korea, Spain, Taiwan, the UK, and the United States) and compared the efficacy and safety of tislelizumab versus chemotherapy as 2L treatment in patients with advanced or metastatic ESCC, as reported previously.21 The study was conducted in conformance with the International Conference on Harmonisation Good Clinical Practice Guideline, the principles of the Declaration of Helsinki, and applicable local laws and regulations. The protocol was approved by the relevant Institutional Review Board/Independent Ethics Committee for participating study sites. All patients provided written informed consent before participation in the study. A full copy of the protocol is provided in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.102202.
The present report focuses on results for patients recruited in European countries (i.e. Belgium, France, Germany, Italy, Spain, and the UK) and the United States (referred to as the Europe/North America subgroup hereafter).
Participants
Adult patients (≥18 years of age) with histologically confirmed advanced or metastatic ESCC whose disease progressed during or after first-line systemic treatment were eligible. Patients who had tumor progression during or within 6 months after definitive chemoradiotherapy, neoadjuvant, or adjuvant therapy were also eligible. The study excluded patients who had received ≥2 lines of systemic therapy for advanced or metastatic disease, or previously received treatment with a PD-1 or programmed death-ligand 1 (PD-L1) inhibitor. Full inclusion and exclusion criteria are described in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Interventions
Investigator’s choice of chemotherapy was determined for all patients before randomization. Patients were subsequently randomized 1 : 1 to receive treatment with tislelizumab or investigator’s choice of chemotherapy. Randomization was stratified by region [Asia (excluding Japan) versus Japan versus Europe/North America], Eastern Cooperative Oncology Group performance status (ECOG PS; 0 versus 1), and investigator-chosen chemotherapy (paclitaxel versus docetaxel versus irinotecan).21
Patients in the tislelizumab arm received tislelizumab 200 mg intravenously (IV) once every 3 weeks (Q3W). In the chemotherapy arm, patients received one of the following: paclitaxel 135-175 mg/m2 IV Q3W (or 80-100 mg/m2 IV once weekly, according to local and/or country-specific standard-of-care guidelines); docetaxel 75 mg/m2 IV Q3W; or irinotecan 125 mg/m2 IV on days 1 and 8, Q3W.
Study treatments were administered until disease progression, intolerable toxicity, or withdrawal for other reasons. Cross-over between chemotherapy regimens or between chemotherapy and tislelizumab treatment arms was not allowed during the study treatment period. There was no blinding of study treatments.
Endpoints and assessments
The primary endpoint was OS in the intent-to-treat (ITT) population, which included all randomized patients (analyzed according to their treatment arm), and the key secondary endpoint was OS in the PD-L1-positive population [PD-L1 score ≥10% using the VENTANA PD-L1 (SP263) Assay].21 The PD-L1 score was defined as the total percentage of the tumor area (tumor and any desmoplastic stroma) covered by tumor cells with PD-L1 membrane staining at any intensity and tumor-associated immune cells with PD-L1 staining at any intensity, as visually estimated.
Other secondary efficacy endpoints included progression-free survival (PFS) and tumor response outcomes, both assessed by investigators, per Response Evaluation Criteria in Solid Tumors version 1.1, and patient-reported health-related quality of life (HRQoL). HRQoL assessments included global health status (GHS)/QoL, physical functioning, and fatigue scales of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30), and the index score (indicating overall ESCC symptoms) and symptoms scores for dysphagia, reflux, eating, and pain scales of the esophageal cancer-specific module (QLQ-OES18).22,23 Increases in scores for GHS/QoL and physical functioning and decrease in symptom scale scores indicate improvement.
Safety was assessed as a secondary endpoint through monitoring of the incidence and severity of treatment-emergent adverse events (TEAEs) according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. Safety analyses were carried out using the safety population, which included all randomized patients who received at least one dose of the study drug, analyzed by actual treatment received.
Further detail on the study endpoints and their assessment is provided in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Statistical analyses
Sample size calculations and statistical considerations for the primary analyses in the overall study population have been reported previously.21 Analysis of results by subgroups defined by geographic region, including Europe/North America, was prespecified as part of exploratory subgroup analyses for: OS within the ITT and PD-L1-positive populations; PFS, overall response rate (ORR), and duration of response (DoR) within the ITT population; and safety within the safety population.
No formal hypothesis testing was carried out for the Europe/North America subgroup analysis and all statistical analyses reported herein are descriptive. Median OS was estimated by the Kaplan–Meier method with 95% confidence intervals (CIs) estimated using the method of Brookmeyer and Crowley. Hazard ratios (HRs) for OS analyses were based on an unstratified Cox regression model, including only treatment as a covariate, accompanied by two-sided 95% CIs. In addition, a post hoc OS sensitivity analysis was carried out that adjusted for imbalances in baseline ECOG PS and PD-L1 expression between treatment groups, with either baseline ECOG PS (0 or 1) or PD-L1 expression (PD-L1 score ≥10%, <10%, or unknown) included as an additional covariate within the Cox regression model.
PFS and DoR were analyzed using similar methodology to OS. ORR was calculated, accompanied by two-sided 95% CIs calculated using the Clopper–Pearson method. In addition, the common odds ratio for ORR was calculated using an unstratified Cochran–Mantel–Haenszel test, accompanied by two-sided 95% CIs. Evaluation of least squares (LS) mean change from baseline to week 12 in the EORTC QLQ-C-30 and QLQ-OES18 patient-reported HRQoL assessments was based on a mixed-effect model for repeated measurements, with the patient-reported outcomes endpoint score as the response variable. Safety outcomes were analyzed using descriptive statistics.
Results
Patients and treatment
In the overall population, 512 patients were enrolled between January 2018 and March 2020 and randomized to tislelizumab or chemotherapy (ITT population).21 Of these patients, 108 (21.1%) were recruited from European and North American countries,21 which constituted the Europe/North America subgroup (tislelizumab: n = 55; chemotherapy: n = 53) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.102202). Among these patients, 54 (98.2%) in the tislelizumab group and 49 (92.5%) in the chemotherapy group received at least one dose of study treatment (the safety population).
In the Europe/North America subgroup, the median age was 65.0 years, most patients had metastatic disease (92.6%), and 28.7% had PD-L1 score ≥10% (Table 1). All patients except one in the tislelizumab group had received prior platinum-based chemotherapy. Baseline characteristics in the Europe/North America subgroup were generally balanced between the two treatment arms, with the exception that a smaller proportion of patients in the tislelizumab arm (58.2%) had an ECOG PS of 1 compared with the chemotherapy arm (66.0%). In addition, more patients in the tislelizumab arm (40.0%) in the Europe/North America subgroup had PD-L1 score ≥10% compared with the chemotherapy arm (17.0%). Baseline characteristics of the overall population are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Table 1.
Patient demographics and baseline characteristics in the Europe/North America subgroup
| Tislelizumab (n = 55) | Chemotherapy (n = 53) | |
|---|---|---|
| Age | ||
| Median, years (range) | 65.0 (41-86) | 65.0 (35-80) |
| <65 years, n (%) | 25 (45.5) | 24 (45.3) |
| ≥65 years, n (%) | 30 (54.5) | 29 (54.7) |
| Sex, n (%) | ||
| Male | 37 (67.3) | 36 (67.9) |
| Female | 18 (32.7) | 17 (32.1) |
| Race, n (%) | ||
| Asian | 0 (0.0) | 4 (7.5) |
| White/Caucasian | 53 (96.4) | 44 (83.0) |
| Black/African American | 0 (0.0) | 2 (3.8) |
| Othera | 2 (3.6) | 3 (5.7) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 2 (3.6) | 2 (3.8) |
| Not Hispanic or Latino | 51 (92.7) | 49 (92.5) |
| Unknown/not reported | 2 (3.6) | 2 (3.8) |
| ECOG performance status, n (%) | ||
| 0 | 23 (41.8) | 18 (34.0) |
| 1 | 32 (58.2) | 35 (66.0) |
| PD-L1 scoreb, n (%) | ||
| ≥10% | 22 (40.0) | 9 (17.0) |
| <10% | 25 (45.5) | 35 (66.0) |
| Unknown | 8 (14.5) | 9 (17.0) |
| Smoking status, n (%) | ||
| Never | 13 (23.6) | 15 (28.3) |
| Former | 27 (49.1) | 23 (43.4) |
| Current | 15 (27.3) | 15 (28.3) |
| Previous anticancer interventions/therapies, n (%) | ||
| Surgery | 9 (16.4) | 10 (18.9) |
| Radiotherapy | 34 (61.8) | 34 (64.2) |
| Platinum-based chemotherapy | 54 (98.2) | 53 (100.0) |
| Disease stage at study entry, n (%) | ||
| Locally advanced | 2 (3.6) | 6 (11.3) |
| Metastatic | 53 (96.4) | 47 (88.7) |
Data presented for the Europe/North America subgroup of the intent-to-treat population, which included all randomized patients, analyzed according to their randomized treatment arm.
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1.
Including categories of ‘not reported’, ‘unknown’, and ‘other’.
Assessed using the VENTANA SP263 assay [PD-L1 positive is defined as PD-L1 score ≥10%, PD-L1 negative is defined as PD-L1 score <10%, unknown refers to patients without sample collection, or with non-evaluable samples, or with scored unqualified sample (patients with scored unqualified sample were identified and reclassified as unknown after database lock)].
At the time of the data analysis (cut-off: 1 December 2020), 48 patients (87.3%) in the tislelizumab arm and 49 (92.5%) in the chemotherapy arm had discontinued treatment, with disease progression being the most common cause in both arms (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.102202). Median duration of study follow-up was 9.26 months in the tislelizumab arm (range: 0.6-21.4 months) and 5.82 months (range: 0.2-21.6 months) in the chemotherapy arm. Among patients who had received at least one dose of study treatment, the median duration of exposure was 2.8 months (range: 0.6-14.3 months) for tislelizumab and 2.0 months (range: 0.2-10.1 months) for chemotherapy.
In the Europe/North America subgroup, post-study treatment anticancer therapies were received after study treatment discontinuation by 49.1% of patients in the tislelizumab arm and by 45.3% of patients in the chemotherapy arm (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102202). This included immunotherapy in one patient (1.8%) in the tislelizumab arm and in seven patients (13.2%) in the chemotherapy arm.
Efficacy: OS
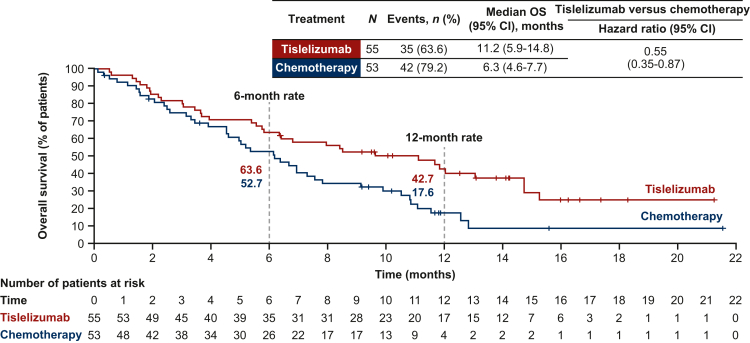
In the Europe/North America subgroup, 35 deaths (63.6% of patients) occurred in the tislelizumab arm and 42 (79.2%) in the chemotherapy arm. OS was prolonged in the tislelizumab arm [median: 11.2 months (95% CI 5.9-14.8 months)] versus the chemotherapy arm [median: 6.3 months (95% CI 4.6-7.7 months)] (Figure 1). The OS HR for tislelizumab versus chemotherapy was 0.55 (95% CI 0.35-0.87).21 The post hoc sensitivity analyses adjusted for baseline ECOG PS and PD-L1 expression status confirmed that the baseline imbalance between treatment arms in these parameters had little impact on the estimate of the treatment effect on OS [HR for analysis adjusted for baseline PD-L1 expression: 0.54 (95% CI 0.33-0.87); HR for analysis adjusted for baseline ECOG PS: 0.51 (95% CI 0.32-0.82)].
Figure 1.
Overall survival in the Europe/North America subgroup. CI, confidence interval; OS, overall survival. Data presented for the Europe/North America subgroup of the intent-to-treat population, which included all randomized patients, analyzed according to their treatment arm. Hazard ratio was based on unstratified Cox regression model including treatment as a covariate. Medians were estimated by the Kaplan–Meier method with 95% CIs estimated using the Brookmeyer and Crowley method. OS rates (cumulative probability of OS) were estimated by the Kaplan–Meier method. Graph presents Kaplan–Meier survival plots.
When analyzed by subgroups defined by baseline PD-L1 expression, the OS HR for tislelizumab versus chemotherapy was 0.47 (95% CI 0.18-1.21) in patients with PD-L1 score ≥10%, and 0.55 (95% CI 0.30-1.01) in patients with PD-L1 score <10% (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102202). When analyzed by subgroups defined by investigator-chosen chemotherapy, the HRs for tislelizumab (n = 55) versus paclitaxel (n = 28), docetaxel (n = 5), and irinotecan (n = 20) were 0.60 (95% CI 0.35-1.03), 0.63 (95% CI 0.22-1.80), and 0.48 (95% CI 0.27-0.88), respectively (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102202).
OS results in the overall population are summarized in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Efficacy: PFS
In the Europe/North America subgroup, the median PFS was 2.3 months (95% CI 1.5-2.8 months) in the tislelizumab arm and 2.7 months (95% CI 1.4-3.9 months) in the chemotherapy arm [HR 0.97 (95% CI 0.64-1.47)] (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.102202). PFS results in the overall population are summarized in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Efficacy: tumor responses
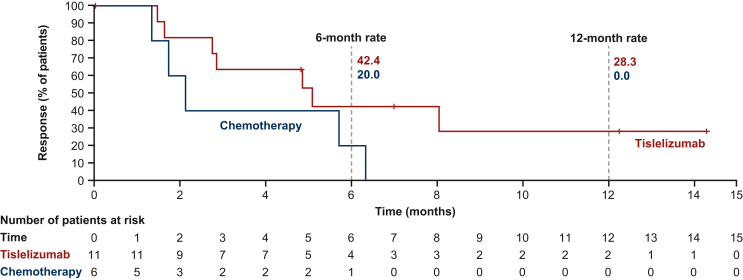
In the Europe/North America subgroup, the ORR was 20.0% (95% CI 10.4% to 33.0%) with tislelizumab and 11.3% (95% CI 4.3% to 23.0%) with chemotherapy (Table 2). Responses in the tislelizumab arm included two patients (3.6%) with complete responses and nine (16.4%) with partial responses. There were no complete responses and six partial responses (11.3%) in the chemotherapy arm. Median DoR was 5.1 months (95% CI 1.6 months-not evaluable) with tislelizumab and 2.1 months (95% CI 1.3-6.3 months) with chemotherapy (Figure 2). Tumor response outcomes in the overall population and the Europe/North America subgroup are presented in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Table 2.
Summary of tumor response outcomes in the Europe/North America subgroup
| Tislelizumab (n = 55) | Chemotherapy (n = 53) | |
|---|---|---|
| ORR, n (%) | 11 (20.0) | 6 (11.3) |
| 95% CI | 10.4-33.0 | 4.3-23.0 |
| Odds ratio for ORR (95% CI) | 1.96 (0.67-5.75) | |
| Best overall response, n (%) | ||
| Complete response | 2 (3.6) | 0 (0.0) |
| Partial response | 9 (16.4) | 6 (11.3) |
| Stable disease | 17 (30.9) | 20 (37.7) |
| Progressive disease | 23 (41.8) | 16 (30.2) |
| Not evaluablea | 0 (0.0) | 2 (3.8) |
| Not assessableb | 4 (7.3) | 9 (17.0) |
| Median duration of response, months (95% CI) | 5.1 (1.6-NE) | 2.1 (1.3-6.3) |
| Patients with ongoing response, n/N (%) | 4/11 (36.4) | 0/6 (0.0) |
Data presented for the Europe/North America subgroup of the intent-to-treat population, which included all randomized patients, analyzed according to their randomized treatment arm. ORR and duration of response were based on unconfirmed tumor responses. ORR and odds ratios between arms were calculated using the unstratified Cochran–Mantel–Haenszel method. Two-sided 95% CIs were calculated for ORR using the Clopper–Pearson method. Median duration of response was estimated by the Kaplan–Meier method with 95% CIs estimated using the Brookmeyer and Crowley method.
CI, confidence interval; NE, not estimable; ORR, overall response rate; RECIST, Response Evaluation Criteria in Solid Tumors.
Not evaluable based on RECIST v1.1.
Not assessable owing to no post-baseline tumor assessment by the data cut-off, including patients who discontinued for any reason, or died without having any post-baseline tumor assessment.
Figure 2.
Duration of response in the Europe/North America subgroup. CI, confidence interval; DoR, duration of response. Data presented for the responders within the Europe/North America subgroup of the intent-to-treat population, which included all randomized patients, analyzed according to their treatment arm. Medians were estimated by the Kaplan–Meier method with 95% CIs estimated using the Brookmeyer and Crowley method. DoR rates (cumulative probability of DoR) were estimated by the Kaplan–Meier method. Graph presents Kaplan–Meier plots.
Health-related quality of life
In the Europe/North America subgroup, patients in the tislelizumab-treated arm reported less deterioration in GHS/QoL, physical function, and disease- and treatment-related symptoms as compared to those on chemotherapy (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.102202). More specifically, patients from the Europe/North America subgroup in the tislelizumab arm reported less deterioration, based on change from baseline to week 12, on the EORTC QLQ-C30 fatigue item [LS mean difference −11.6 (95% CI −25.8 to 2.6)], and EORTC QLQ-OES18 pain item [−7.5 (−16.9 to 2.0)], index score [−5.3 (−13.3 to 2.7)], and eating item [−3.3 (−18.6 to 12.0)], as compared to those on chemotherapy. Results for the overall study population (i.e. QLQ-C30 GHS/QoL, physical functioning, and fatigue, and QLQ-OES18 pain, eating, and other disease- and treatment-related symptom items/domains) are also presented in Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.102202.
Safety and tolerability
In the Europe/North America subgroup, most patients in both the tislelizumab and chemotherapy treatment arms experienced at least one TEAE (96.3% and 95.9%, respectively) (Table 3). The incidence of treatment-related TEAEs was lower in the tislelizumab arm (70.4%) compared with the chemotherapy arm (87.8%). The most commonly reported treatment-related TEAEs in the tislelizumab arm were asthenia (16.7%), fatigue (16.7%), diarrhea (13.0%), decreased appetite (9.3%), arthralgia (9.3%), and hypothyroidism (9.3%) (Table 3). The most commonly reported treatment-related TEAEs in the chemotherapy arm were diarrhea (32.7%), anemia (26.5%), decreased appetite (24.5%), nausea (24.5%), and neutropenia (24.5%). Fewer patients experienced ≥grade 3 treatment-related TEAEs or serious treatment-related TEAEs in the tislelizumab arm (13.0% and 7.4%, respectively) compared with the chemotherapy arm (51.0% and 14.3%, respectively).
Table 3.
Summary of treatment-emergent adverse event incidence in the Europe/North America subgroup
| Tislelizumab (N = 54) n (%) | Chemotherapy (N = 49) n (%) | |
|---|---|---|
| Any TEAE | 52 (96.3) | 47 (95.9) |
| Treatment-related | 38 (70.4) | 43 (87.8) |
| Any ≥grade 3 TEAE | 30 (55.6) | 35 (71.4) |
| Treatment-related | 7 (13.0) | 25 (51.0) |
| Any serious TEAE | 21 (38.9) | 23 (46.9) |
| Treatment-related | 4 (7.4) | 7 (14.3) |
| Any TEAE leading to treatment discontinuation | 8 (14.8) | 15 (30.6) |
| Treatment-related | 2 (3.7) | 2 (4.1) |
| Any TEAE leading to dose modificationa | 11 (20.4) | 26 (53.1) |
| Treatment-related | 5 (9.3) | 23 (46.9) |
| Any TEAE leading to deathb | 3 (5.6) | 5 (10.2) |
| Treatment-related | 2 (3.7) | 3 (6.1) |
| Most common treatment-related TEAEs occurring in ≥10% of patients by preferred termc | ||
| Asthenia | 9 (16.7) | 8 (16.3) |
| Fatigue | 9 (16.7) | 11 (22.4) |
| Diarrhea | 7 (13.0) | 16 (32.7) |
| Decreased appetite | 5 (9.3) | 12 (24.5) |
| Nausea | 3 (5.6) | 12 (24.5) |
| Anemia | 2 (3.7) | 13 (26.5) |
| Constipation | 2 (3.7) | 9 (18.4) |
| Peripheral sensory neuropathy | 2 (3.7) | 8 (16.3) |
| Neutropenia | 0 (0.0) | 12 (24.5) |
| Alopecia | 0 (0.0) | 11 (22.4) |
| Neutrophil count decreased | 0 (0.0) | 5 (10.2) |
| Stomatitis | 0 (0.0) | 6 (12.2) |
Data presented for the Europe/North America subgroup of the safety population, which included all randomized patients who received at least one dose of the study drug, analyzed by actual treatment received. Adverse event grades were evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03, and coded using the Medical Dictionary for Regulatory Activities version 23.0.
TEAE, treatment-emergent adverse event.
Dose modification include dose held, dose interruption and dose reduction for chemotherapy, and dose held and dose interruption for tislelizumab.
Death events because of disease progression were excluded.
Reported in ≥10% of patients in either the tislelizumab or chemotherapy arm ordered by incidence in the tislelizumab arm.
The incidence of treatment-related TEAEs leading to treatment discontinuation was similar in the tislelizumab and chemotherapy arms (3.7% and 4.1%, respectively) (Table 3). More patients required dose modification due to treatment-related TEAEs in the chemotherapy arm (46.9%), than with tislelizumab (9.3%).
Treatment-related TEAEs leading to death were reported in two patients in the tislelizumab arm (due to pulmonary arterial hypertension and hemoptysis, in one patient for each) and in three patients in the chemotherapy arm (due to general physical health deterioration, septic shock, and multiple organ dysfunction syndrome, in one patient for each) (Table 3).
The incidence of TEAEs and treatment-related TEAEs was similar between the Europe/North America subgroup and the overall population (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.102202).
Discussion
The RATIONALE-302 study enrolled patients with advanced or metastatic ESCC with disease progression during or after first-line systemic treatment (typically platinum-based chemotherapy), who were randomized to receive tislelizumab or investigator’s choice of chemotherapy.21 The present analysis of the RATIONALE-302 study indicates that tislelizumab prolonged OS versus chemotherapy in patients from Europe and North America, consistent with the results previously reported in the overall study population.21
The RATIONALE-302 trial enrolled 79% of patients from Asia and 21% from Europe and North America.21 This regional representation is consistent with the global geographic distribution of ESCC cases.17 Baseline characteristics in the Europe/North America subgroup were generally similar to the overall population of the study (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102202), except for a few regional variations. Of note, compared with the overall population, the Europe/North America subgroup had higher proportions of patients aged ≥65 years, female patients, and patients with a baseline ECOG PS of 0. These small regional variations were consistent with known clinicopathological nuances between the Western and Asian ESCC populations.12
In the Europe/North America subgroup, an improvement in OS was observed with tislelizumab versus chemotherapy [median OS: 11.2 months (95% CI 5.9-14.8 months) versus 6.3 months (95% CI 4.6-7.7 months); HR: 0.55 (95% CI 0.35-0.87)]. The OS benefit with tislelizumab versus chemotherapy was accompanied by higher ORR (20.0% versus 11.3%) and prolonged DoR (median: 5.1 versus 2.1 months). These results are consistent with those observed in the overall population of the RATIONALE-302 trial (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102202), indicating that the global treatment benefits of tislelizumab also apply to the Europe/North America subgroup.21
The improvement in OS with tislelizumab versus chemotherapy in the Europe/North America subgroup was observed regardless of PD-L1 expression. In patients with PD-L1 score ≥10% the OS HR was 0.47, and in those with PD-L1 score <10% the OS HR was 0.55, consistent with the trend in the overall population.21 These results are also in line with prior studies demonstrating OS benefits regardless of PD-L1 expression status for nivolumab and camrelizumab versus chemotherapy as 2L treatment in advanced/metastatic ESCC.24,25 In addition, in the Europe/North America subgroup, the OS HRs for tislelizumab versus chemotherapy in each chemotherapy option subgroup (HRs of 0.60, 0.63, and 0.48 in the paclitaxel, docetaxel, and irinotecan subgroups, respectively) indicated that the OS benefit with tislelizumab was observed regardless of chemotherapy selection, consistent with results in the overall population.
In a meta-analysis that investigated the efficacy of anti-PD-1 or anti-PD-L1 antibodies in various tumor types, it was observed that even though such therapies showed efficacy benefit over comparators in both Asian and non-Asian populations, the benefit observed in Asian patients was greater than in non-Asian patients.26 However, the meta-analysis only included one study in patients with esophageal cancers (KEYNOTE-181), which enrolled both ESCC and esophageal adenocarcinoma.7,26 Other studies investigating treatment with anti-PD-1 antibodies in various lines of therapy in esophageal cancers support a consistent benefit of anti-PD-1 therapy in Asian and non-Asian patients, in line with our findings.27, 28, 29 These studies include the RATIONALE-306 study of tislelizumab in combination with chemotherapy as first-line treatment for advanced or metastatic ESCC, which showed a consistent OS benefit of tislelizumab plus chemotherapy over chemotherapy alone in Asian and non-Asian regional subgroups.29
Overall, the safety and tolerability of tislelizumab in the Europe/North America subgroup was more favorable than chemotherapy, with a notably lower incidence of ≥grade 3 treatment-related TEAEs (in 13.0% versus 51.0%, respectively). This is consistent with the safety and tolerability observed in the overall population,21 and with that previously reported for other PD-1 inhibitors in ESCC.7,24
The RATIONALE-302 study and the present subgroup analysis have some limitations. Treatments administered were open-label and given the exploratory nature of the subgroup analyses, there was no formal statistical testing of treatment effects in the Europe/North America subgroup. It should also be noted that although anti-PD-1/PD-L1 agents plus chemotherapy were not recommended as first-line therapeutic regimens for advanced ESCC during the design and conduct of the present study, these combination regimens have since become first-line treatment options.30 Patients with prior exposure to PD-1/PD-L1 therapies were excluded from this study and, therefore, the results are not applicable to patients who have received these combination regimens in prior first-line treatment. In addition, although region was a stratification factor for the study, due to the limited sample size, there were imbalances between treatment arms in baseline ECOG PS and PD-L1 score in the Europe/North America subgroup. However, sensitivity analyses adjusting for these imbalances showed an OS benefit for tislelizumab versus chemotherapy that was consistent with the unadjusted analyses.
Conclusions
In conclusion, among patients from Europe and North America with advanced or metastatic ESCC whose disease progressed during or after first-line systemic therapy, tislelizumab prolonged OS versus chemotherapy, and was associated with a more favorable safety profile, consistent with the results seen in the overall study population. These results support the potential for tislelizumab to become a 2L treatment option for patients with advanced or metastatic ESCC in Western countries.
Acknowledgements
The authors would like to thank the patients for their participation in the study, and the global investigators and site personnel for their contributions to the conduct of the study. The authors would also like to thank Hongqian Wu for her continued support in the development of this publication. A list of investigators is provided in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.102202. Medical writing support, under the direction of the authors, was provided by Arezou Seyed Hossein, MPharm, Helena Crisford, PhD, and Victoria Dagwell, MSc, of Ashfield MedComms, an Inizio company, and was funded by BeiGene, Ltd.
Funding
This work was supported by BeiGene, Ltd. In collaboration with the academic authors, the sponsor participated in the study design, in the collection, analysis and interpretation of data, and in the writing of the report. The authors led the writing of the report, with professional medical writing assistance funded by the sponsor, and the authors were responsible for the decision to submit the article for publication.
Disclosure
JA declares honoraria: Eli Lilly and Company, Bristol Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech Biopharma, Zymeworks, Astellas Pharma, Amgen, OncoTherics, Daiichi Sankyo, Novartis, Servier, Gilead Sciences, BeiGene, Fresenius Kabi, Boehringer Ingelheim, GRAIL; consulting or advisory role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol Myers Squibb; research funding: Novartis, Bristol Myers Squibb, Taiho Pharmaceutical, Roche/Genentech, Amgen, Eli Lilly and Company/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, ProLynx, Zymeworks, Daiichi Sankyo, Astellas Pharma; and patents, royalties, other intellectual property: Genentech, Roche, Bristol Myers Squibb, Taiho, MedImmune, Merck, Amgen, and Eli Lilly and Company. FEH declares honoraria: Servier, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, and Pierre Fabre. DC declares research grants payable to The Royal Marsden NHS Foundation Trust: MedImmune/AstraZeneca, Clovis, Eli Lilly and Company, 4SC, Bayer, Celgene, and Roche; on Scientific Advisory Board for OVIBIO. MA declares consulting/advisory roles: Bristol Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, and Servier; and honorarium for speaking: Amgen, Bristol Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, and Servier. PT-P declares consulting/advisory roles: Astellas, AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Eli Lilly and Company, Daiichi, Novartis, Roche; and study support: Merck Serono. GVS declares relationship(s) and consulting fees: AstraZeneca, Eli Lilly and Company, Merck Sharp & Dohme, Pfizer, Roche, Johnson & Johnson, and Takeda; consulting or advisory role: Eli Lilly and Company, BeiGene, and AstraZeneca; institutional grants: Eli Lilly and Company, Merck Sharp & Dohme, and Tesaro; and travel and accommodation: Bayer. MVdE declares consulting or advisory roles: Bayer, Eli Lilly and Company, Roche, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, AstraZeneca, Daiichi Sankyo, Pierre Fabre, and Taiho Pharmaceutical; and research funding: Amgen, Roche, and Merck KGaA. S-BK declares stock and other ownership interests: Neogene TC Corp and Genopeak; honoraria: DAEHWA Pharmaceutical, and ISU Abxis; consulting or advisory role: Eli Lilly and Company, AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis, BeiGene, and Daiichi Sankyo/AstraZeneca; research funding: Novartis, Dongkook Pharma, and Genzyme. KK declares honoraria: Eli Lilly and Company, Bristol Myers Squibb, and Ono Pharmaceutical; consulting or advisory roles: Ono Pharmaceutical, BeiGene, Merck Sharp & Dohme, Oncolys BioPharma, and Bayer; speakers' bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, and Merck Sharp & Dohme; research funding: Ono Pharmaceutical, Shionogi, Merck Sharp & Dohme Oncology, BeiGene, Chugai Pharma, Bayer, AstraZeneca, and Taiho Pharmaceutical. LS declares consulting or advisory roles: Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbour BioMed, and Merck; speakers' bureau: Hutchison Whampoa, and Merck Sharp & Dohme; research funding: Nanjing Yaojieankang Biotechnology, Baiji Shenzhou (Beijing) Biotechnology Co Ltd., Beijing Xiantong Biomedical Technology Co Ltd., QiLu Pharmaceutical, and Zaiding Pharmaceutical. LL declares employment: BeiGene, and Johnson & Johnson/Janssen; stock and other ownership interests: BeiGene; travel, accommodations, expenses: BeiGene, and Johnson & Johnson/Janssen. ND declares employment: BeiGene. JS declares employment: BeiGene. GB declares employment: BeiGene. EVC declares consulting or advisory role: Bayer, Eli Lilly and Company, Roche, Servier, Bristol Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GSK, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte, and Astellas Pharma; research funding: Amgen, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Roche, Celgene, Ipsen, Merck, Merck KGaA, Servier, and Bristol Myers Squibb.
Data sharing
On request, and subject to certain criteria, conditions, and exceptions, BeiGene, Ltd., will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (i) for indications that have been approved or (ii) in programs that have been terminated. BeiGene will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data requests may be submitted to DataDisclosure@beigene.com.
Supplementary data
References
- 1.Morgan E., Soerjomataram I., Rumgay H., et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e2. doi: 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute SEER Program Cancer stat facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html Available at.
- 3.Abnet C.C., Arnold M., Wei W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel N., Benipal B. Incidence of esophageal cancer in the United States from 2001-2015: a United States Cancer Statistics analysis of 50 states. Cureus. 2018;10 doi: 10.7759/cureus.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of The People’s Republic of China Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version) Chin J Cancer Res. 2019;31(2):223–258. doi: 10.21147/j.issn.1000-9604.2019.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obermannová R., Alsina M., Cervantes A., et al. ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992–1004. doi: 10.1016/j.annonc.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Kojima T., Shah M.A., Muro K., et al. KEYNOTE-181 Investigators. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi K., Kanamori K., Ninomiya Y., et al. Progress in multimodal treatment for advanced esophageal squamous cell carcinoma: results of multi-institutional trials conducted in Japan. Cancers (Basel) 2020;13(1):51. doi: 10.3390/cancers13010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muro K., Lordick F., Tsushima T., et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):34–43. doi: 10.1093/annonc/mdy498. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa Y., Uno T., Oyama T., et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 2. Esophagus. 2019;16(1):25–43. doi: 10.1007/s10388-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa Y., Uno T., Oyama T., et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16(1):1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Jiang Y., Wu C., et al. Comparison of clinicopathologic features and survival between eastern and western population with esophageal squamous cell carcinoma. J Thorac Dis. 2015;7(10):1780–1786. doi: 10.3978/j.issn.2072-1439.2015.10.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Xu J., Zheng Y., et al. Esophageal cancer: epidemiology, risk factors and screening. Chin J Cancer Res. 2021;33(5):535–547. doi: 10.21147/j.issn.1000-9604.2021.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang H., Fan J.H., Qiao Y.L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14(1):33–41. doi: 10.20892/j.issn.2095-3941.2016.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Y., Qin S., Luo S., et al. Pembrolizumab versus chemotherapy for patients with esophageal squamous cell carcinoma enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open. 2022;7(1) doi: 10.1016/j.esmoop.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe D.H., Gricar J., DeCongelio M., Mackie D.S. A global perspective in second-line treatment patterns for patients with advanced esophageal squamous cell carcinoma. Thorac Cancer. 2022;13(9):1240–1257. doi: 10.1111/1759-7714.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold M., Ferlay J., van Berge Henegouwen M.I., Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T., Song X., Xu L., et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J., Bai Y., Xu N., et al. Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2020;26(17):4542–4550. doi: 10.1158/1078-0432.CCR-19-3561. [DOI] [PubMed] [Google Scholar]
- 20.Yu R., Wang W., Li T., et al. RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. 2021;17(31):4081–4089. doi: 10.2217/fon-2021-0632. [DOI] [PubMed] [Google Scholar]
- 21.Shen L., Kato K., Kim S.B., et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol. 2022;40:3065–3076. doi: 10.1200/JCO.21.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fayers P., Bottomley A. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(suppl 4):S125–S133. doi: 10.1016/s0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 23.Blazeby J.M., Conroy T., Hammerlid E., et al. European Organisation for Research and Treatement of Cancer Gastrointestinal and Quality of Life Groups. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39(10):1384–1394. doi: 10.1016/s0959-8049(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 24.Kato K., Cho B.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 26.Peng L., Qin B.D., Xiao K., et al. A meta-analysis comparing responses of Asian versus non-Asian cancer patients to PD-1 and PD-L1 inhibitor-based therapy. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1781333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly R.J., Ajani J.A., Kuzdzal J., et al. CheckMate 577 Investigators. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 28.Doki Y., Ajani J.A., Kato K., et al. CheckMate 648 Trial Investigators. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 29.Yoon H., Kato K., Raymond E., et al. LBA-1 RATIONALE-306: randomized, global, placebo-controlled, double-blind phase 3 study of tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma (ESCC) Ann Oncol. 2022;33(suppl 4):S375. doi: 10.1016/S1470-2045(23)00108-0. [DOI] [PubMed] [Google Scholar]
- 30.Ma X., Ding Y., Qian J., et al. Nomogram based on monocyte-to-lymphocyte ratio to predict survival of unresectable esophageal squamous cell carcinoma who receive first-line PD-1/PD-L1 inhibitors combined with chemotherapy. Curr Oncol. 2022;29(11):8937–8954. doi: 10.3390/curroncol29110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.