Abstract
The proteolytic processing of amyloid precursor protein (APP) has been linked to sphingolipid-cholesterol microdomains (rafts). However, the raft proteases that may be involved in APP cleavage have not yet been identified. In this work we present evidence that the protease plasmin is restricted to rafts of cultured hippocampal neurons. We also show that plasmin increases the processing of human APP preferentially at the α-cleavage site, and efficiently degrades secreted amyloidogenic and non-amyloidogenic APP fragments. These results suggest that brain plasmin plays a preventive role in APP amyloidogenesis. Consistently, we show that brain tissue from Alzheimer’s disease patients contains reduced levels of plasmin, implying that plasmin downregulation may cause amyloid plaque deposition accompanying sporadic Alzheimer’s disease.
INTRODUCTION
An early and invariant event in brains affected by Alzheimer’s disease (AD) is the formation of amyloid plaques. This is a consequence of the increased production or aggregation of Aβ, a 4 kDa fragment of amyloid precursor protein (APP) (reviewed in Selkoe, 1999; Sinha and Lieberburg, 1999)
APP is a single transmembrane-spanning domain protein that undergoes different proteolytic cuts during transport along the secretory pathway and at the plasma membrane. Cleavages at the so called β- or α-sites, and later at the γ-site, produce a 4 kDa (Aβ) or 3 kDa (p3) secreted peptide, respectively. Since the α-secretase cleavage prevents amyloid Aβ formation and its product is non-amyloidogenic, it is considered to be ‘non-pathological’ processing of APP. In contrast, uncontrolled β-secretase cleavage is harmful. Indeed, in patients suffering from early onset familial AD, missense mutations in the APP or presenilin gene are responsible for the production of higher levels of Aβ due to increased susceptibility to the β- or γ-secretases (reviewed in Selkoe, 1999; Sinha and Lieberburg, 1999). Although the patients with these genetic defects account for <5% of the AD population, these studies led to the hypothesis that the accumulation of Aβ in AD brains reflects increased protease activity at the β- and γ-secretase sites. While this hypothesis may be entirely correct in the case of the familial forms of AD, it is possible that non-familial AD forms (accounting for >95% of AD patients) could simply result from reduced activity of the proteases involved in α-secretase cleavage and/or in amyloid degradation once the Aβ peptide is formed.
Rafts (Simons and Ikonen, 1997) were interesting compartments in which to look for APP proteolytic activity for several reasons. First, the overexpression of caveolin, a raft structural protein, increases the α-secretase-mediated proteolysis of APP (Ikezu et al., 1998). Secondly, Aβ resides in raft microdomains (Lee et al., 1998), whose integrity is important for peptide production (Simons et al., 1998), and Aβ has been found associated with the raft marker GM1 specifically in AD brain tissue (Yanasigawa et al., 1995). The fact that GM1 is one of the receptors for the plasmin precursor, plasminogen (Miles et al., 1989), and that purified plasmin degrades synthetic Aβ in vitro (Van Nostrand and Porter, 1999; Tucker et al., 2000) led us to look for the presence of plasmin in neuronal rafts and its possible involvement in APP cleavage.
RESULTS AND DISCUSSION
Plasminogen binds to the neuron surface and is specifically activated in rafts
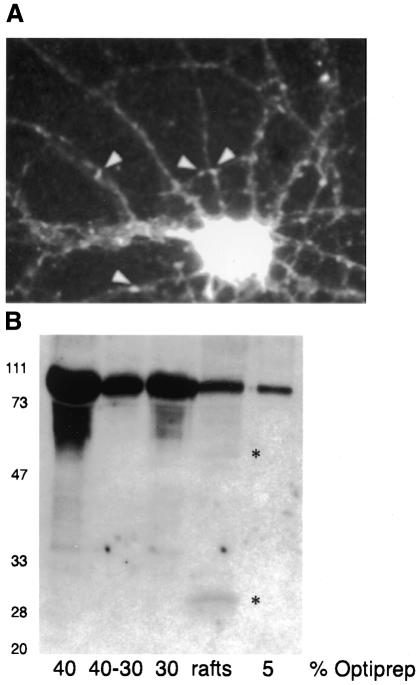
The plasminogen system and its role in fibrin degradation have been well studied in the circulatory system (Vassalli et al., 1991). In the brain, plasminogen and its proteolytic fragment plasmin are abundant in the hippocampus (Chen and Strickland, 1997), although the physiological substrate/s are not yet well defined (Chen and Strickland, 1997; Tsirka et al., 1997). The activation of plasminogen to plasmin involves a cascade of inhibitors and activators and requires binding to the cell surface (Plow et al., 1995). Therefore, we investigated whether plasminogen is present on the membranes of cultured mature hippocampal neurons. Surface staining immunofluorescence using a polyclonal antibody against plasminogen showed intense labeling of the cell body, as well as small clusters along the neurites (Figure 1A).
Fig. 1. Detection of plasminogen on the surface of and in rafts from mature hippocampal neurons, and specific activation in rafts. (A) Surface staining of mature hippocampal neurons was performed using a polyclonal antibody against plasminogen. White arrowheads indicate sites with plasminogen on the membrane. (B) Western blot analysis of all fractions of the Optiprep gradient used to obtain rafts. Plasminogen is present in all lanes (80 kDa). The 50 and 30 kDa cleavage products appear only in the raft fraction (shown by asterisks). Molecular weight markers on the left.
We then asked whether or not plasminogen is present in neuronal membrane rafts. This was addressed by detergent extraction on ice and subsequent flotation in an Optiprep gradient (see Methods). Western blot analysis revealed that not only the 80 kDa plasminogen, but also the 50 and 30 kDa fragments resulting from its activation (Figure 1B) were present in rafts. Remarkably, while plasminogen was in all of the fractions of the gradient, the 30 kDa fragment containing the active protease, plasmin, was detectable only in rafts. Hence, these first results suggest a novel role for cell rafts: proteolysis.
Plasmin and tPA increase APP α-cleavage
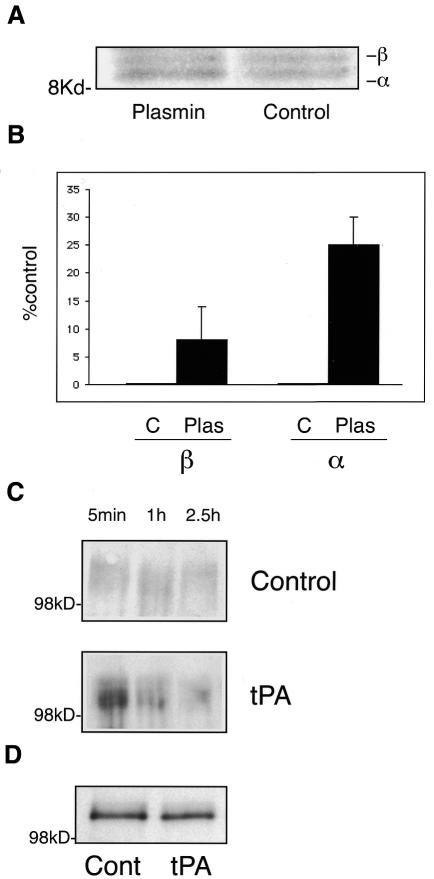
To test whether plasmin is involved in APP proteolysis, fully mature hippocampal neurons were infected with Semliki Forest virus (SFV) encoding human APP695 (Simons et al., 1998). After metabolic labeling in the presence (1 unit/ml) or absence of plasmin, APP was immunoprecipitated using an antibody against its C-terminus (De Strooper et al., 1995) (Figure 2A). Plasmin addition produced a consistent 25% increase in the amount of the α-secretase-cleaved C-terminal fragment. A slight increase (8%) in levels of β-secretase-cleaved C-terminal fragment was also observed (Figure 2B).
Fig. 2. Plasmin and tPA induce a preferential increase in APP α-cleavage in hippocampal neurons expressing human APP. (A) Autoradiography of a tricine gel loaded with [35S]methionine-labeled hippocampal neurons previously infected with SFV encoding the human APP695 isoform. The samples correspond to non-treated infected neurons (control) and infected neurons incubated with 1 unit/ml of plasmin (plasmin). The 12 kDa fragment (β) and the 10 kDa fragment (α) were immunoprecipitated using a polyclonal antibody against the C-terminal region of APP. (B) Percentage of the fragments corresponding to the β-cleavage (β) or the α-cleavage (α) with respect to the control (considered as 0). Error bars represent the standard error. While the α C-terminal fragment of APP showed a consistent 25% increase in plasmin-treated neurons, the β C-terminal fragment increased to a lower extent (only 8%) and with a higher variation. (C) Analysis of the α N-terminal secreted fragment of APP. Stage 5 neurons were infected with SFV-APP and subsequently treated with (tPA) or without (control) tPA. Equivalent aliquots from the medium were taken after 5 min, 1 h or 2.5 h incubation. The samples were analyzed by western blotting using the monoclonal antibody recognizing the APP secreted form produced by the α-cleavage. (D) Western blot of the extracted cells using the same antibody. The total amount of viral-induced APP expression is similar.
To further analyze the role of plasmin in α-secretase cleavage, we investigated the levels of N-terminal α-secreted APP (α-sAPP). Neurons were infected with human APP-SFV for 3 h and the medium replaced with either fresh medium or medium containing tissue-type plasminogen activator (tPA) (Baranes et al., 1998). Equivalent aliquots of media were taken at different times and α-sAPP analyzed by western blotting using antibody 6E10, specific for the α-secretase cleaved form of APP (Ikezu et al., 1998). tPA-treated cells showed an increased release of α-sAPP at all times analyzed compared with untreated neurons (3.4-, 2- and 1.5-fold at 5 min, 1 h and 2.5 h, respectively) (Figure 2C), while the expression of APP in the cells was similar (Figure 2D). The decline with time may reflect the inactivation of tPA in the culture media by inhibitors such as PAI-1 or neuroserpin, which are abundant in the hippocampus (not shown; see also Krueger et al., 1997; Baranes et al., 1998). This tight regulation of the plasminogen system, involving different activators and inhibitors, may also be the reason that the effects shown in Figures 2A and 3A are not more dramatic.
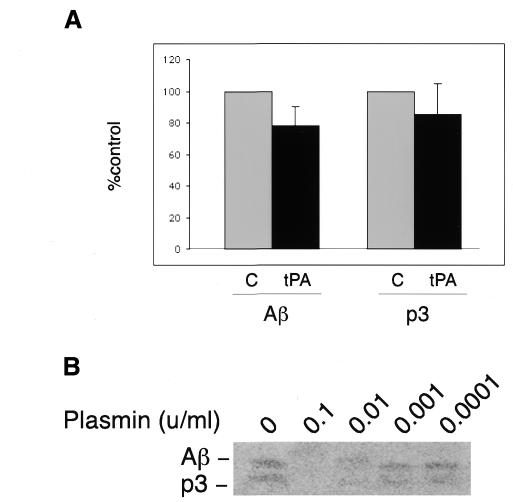
Fig. 3. Activation of plasmin reduces Aβ levels in cells overexpressing human APP. (A) The levels of Aβ and p3 secreted from HEK cells stably transfected with human APP695 in the absence (C) or presence of tPA (tPA). The mean value and standard error from three independent experiments are shown. Activation of plasmin resulted in a consistent 24% decrease in the levels of secreted Aβ with respect to the control (indicated as 100%). The results with p3 were more variable, although a 15% reduction was observed. (B) Amounts of Aβ and p3 immunoprecipitated from media of labeled HEK cells stably transfected with human APP695 and treated with different concentrations of plasmin (indicated in units/ml).
The observation that plasmin produces a preferential effect on α-cleavage is in agreement with the fact that α-cleavage of APP occurs mainly, if not exclusively, at the neuronal surface (Parvathy et al., 1999), where plasminogen is activated (Plow et al., 1995). Other pieces of evidence support a role for plasmin in α-cleavage: (i) plasmin has a natural affinity for lysine residues (Weinstein and Doolittle, 1972), and the α-cleavage site in APP is a lysine (reviewed in Selkoe, 1999); (ii) plasmin can activate metalloproteases (Kleiner and Stetler-Stevenson, 1993) and all candidate α-secretases, i.e. ADAM 10, TACE and MDC9 (Buxbaum et al., 1998; Koike et al., 1999; Lammich et al., 1999), are members of the family of disintegrin metalloproteases. Thus, plasmin could cleave APP at the α-site either directly or through the activation of other proteases.
Activation of plasmin decreases the levels of Aβ peptide
Cleavage at the α-site, in the middle of the Aβ peptide sequence, precludes the formation of the amyloidogenic APP peptide. To show that plasmin not only preferentially increases the α-processing of APP but also correlates with a decrease in secreted Aβ levels, HEK cells were metabolically labeled in the presence or absence of tPA (0.5 µg/ml). After a 4 h incubation the media were collected and immunoprecipitated using the monoclonal antibody 4G8, which recognizes Aβ and p3 peptides. Consistent with our previous finding, the addition of tPA resulted in a 24% reduction in Aβ compared with the control levels (Figure 3A). The amount of p3 was also reduced, but to a lesser extent (15%). While the reduction in the levels of secreted Aβ could be explained by the increase in α-cleavage (see Figure 2), the slight decrease in p3 could be due to the degradative effect of residual plasmin activity in the medium. To test this directly, cells overexpressing human APP were processed as above in the presence or absence of plasmin. The antibody recognizing Aβ and p3 was used to immunoprecipitate the released peptides. The addition of plasmin to the medium resulted in the degradation of both Aβ and p3, even at very low concentrations within the range of the reported physiological levels of serum plasminogen (Kwaan et al., 1992) (Figure 3B).
One likely interpretation of the above results is that plasmin enhanced α-cleavage results in higher p3 and lower Aβ levels but that, once in the medium, the still active plasmin will degrade not only Aβ but also p3.
Altogether, these results suggest that brain plasmin acts at two levels: (i) the preferential cleavage at the α-site of APP on the neuronal membrane and (ii) the degradation of released Aβ peptide.
Plasmin levels are low in brains affected by AD
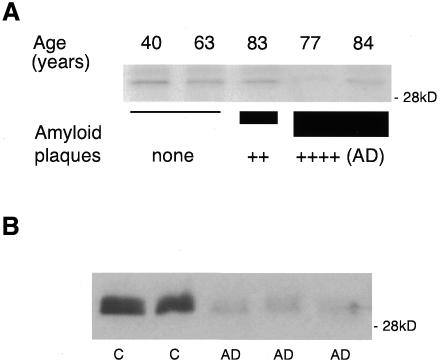
Increased release and inefficient degradation of Aβ will certainly favor amyloid plaque formation. Given the effects of plasmin activation on α-cleavage and Aβ degradation, we hypothesized that brains of patients with AD may have low levels of plasmin. This was analyzed by western blotting of human brain extracts from the neocortex using a polyclonal antibody recognizing both plasminogen and plasmin (Figure 4A). The samples analyzed represent: two control brains of different ages (40 and 63 years old) without amyloid plaques; one brain from a non-demented individual with a moderate number of plaques (aged 83); and two brains (77 and 84 years old) from diagnosed AD patients with high numbers of plaques. Quantification revealed the highest plasmin levels in the control 40 year old brain. Considering this value as 100%, the data obtained for the other samples were: 68% for the second control (63 year old); 69% for the non-demented brain with a moderate number of plaques (83 year old); 6.5 and 46% in the diagnosed AD brains from the 77 and 84 year old patients, respectively. Thus, AD and, to a lesser extent, aged cortexes have less plasmin than normal young cortexes. To investigate further the correlation between low levels of plasmin and AD, we performed the identical analysis in hippocampal tissue, one of the areas most affected by the disease. Extracts from the hippocampi of age-matched control and AD brains showed a significant reduction in the disease-affected brains (Figure 4B). The quantification of all the samples analyzed, both neocortex and hippocampus, revealed an average of 1.82 higher levels of plasmin in control than in AD-affected brains. This ratio was 1.55 in the neocortex and 1.95 in the hippocampus.
Fig. 4. Plasmin is reduced in AD brains. (A) Western blot analysis of human neocortex extracts using an antibody against plasminogen. The 30 kDa plasmin is shown for: two control brains (40 and 63 years old) without amyloid plaques (none); one brain (83 years old) corresponding to a non-demented individual with a moderate number of plaques (++); two brains (77 and 84 years old) corresponding to diagnosed AD patients containing a high number of plaques [++++ (AD)]. (B) Western blot of human hippocampus extracts from AD brains (AD) and control brains (C) using an antibody against plasminogen that recognizes the 30 kDa plasmin.
The observation that the brains of certain AD patients have low levels of plasmin is relevant in the context of amyloid plaque formation. Significantly, we found the lowest amounts in the hippocampus, the brain area most affected by the disease. Until now, no data existed regarding the levels of plasmin in human brains. However, it had been shown in the rat that mRNA levels of its activator, tPA, are low in the adult brain relative to younger brain (Thewke and Seeds, 1999). This is in agreement with our results, which show that brains not only from AD patients, but also from some aged humans have low levels of plasmin compared with younger brains. Moreover, it has been shown that the levels of α-cleaved secreted APP are significantly lower than those of the β-cleaved secreted APP, both in the cerebrospinal fluid of AD patients (Sennvik et al., 2000) and in aged rats (Anderson et al., 1999).
The above evidence, together with the raft-dependent APP cleavage (Ikezu et al., 1998; Simons et al., 1998), the presence of plasmin activity restricted to rafts (this work) and the role of plasmin in α-cleavage of human APP and in Aβ clearance (this work), encourages us to propose the following model for sporadic AD. We postulate that during early and middle life there is a balance between the different APP protease activities (α-γ and β-γ) and an active clearance system for the secreted amyloid peptide. This balance maintains physiological levels of all fragments and prevents Aβ accumulation. With increasing age, a gradual decrease in the levels or activity of the plasminogen/plasmin system occurs, resulting in the diminution of both α-cleavage and Aβ clearance. The decrease in α-cleavage increases the internalization of APP C-terminus fragments containing intact substratum for the generation of β-γ-secretase product, Aβ. The higher production of amyloid peptide together with less efficient degradation would contribute to Aβ accumulation and aggregation. Hence, the formation of amyloid plaques during senescence can be seen as a ‘passive’ consequence of a naturally occurring decrease in the levels or activity of the plasmin-mediated α-proteolytic and/or Aβ degradation systems. Environmental factors and genetic predisposition would determine who suffers drastic downregulation of plasmin activity throughout life. Regarding this last point, since long term potentiation enhances tPA production (Baranes et al., 1998), we postulate that this phenomenon may prevent the formation of plaques. This will be an interesting avenue for future research.
It has been known for some years that Aβ peptide stimulates the plasminogen system (Wnendt et al., 1997). Very recently, Tucker et al. (2000) also presented evidence that aggregated Aβ increases tPA levels, and that plasmin-mediated proteolytic activity is involved in amyloid plaque degradation. Unlike that work, our results imply that reduced brain plasmin is one of the causes of amyloid plaque formation rather than its consequence. Consistent with this view, we find low plasmin levels in AD brains. However, it is possible that once amyloid plaques are formed, they trigger the upregulation of plasminogen as a compensatory mechanism. In any case, what appears clear is that the plasminogen system is involved in APP processing, and this may create new possibilities for therapeutic approaches.
METHODS
Cell culture. Cultures of hippocampal neurons were prepared as indicated in Goslin and Banker (1991). Cells were kept in culture for 7–15 days (stage 5 neurons).
Immunofluorescence of surface membrane proteins. Neurons were incubated with the polyclonal antibody against plasminogen (Biogenesis) diluted in culture medium for 8 min at 37°C and 5% CO2. The cells were fixed with 4% paraformaldehyde and incubated with fluoresceine-conjugated anti-rabbit antibody (Amersham).
Raft purification. Stage 5 neurons were extracted for 1 h on ice in buffer A: 1% Triton X-100, 25 mM MES pH 7.00, 5 mM dithiothreitol, 2 mM EDTA and CLAP (25 µg/ml each of chymostatin, leupeptin, antipain and pepstatin A). The extracts were mixed with Optiprep (Nycomed) to reach a final concentration of 40% and overlayered in an SW40 centrifugation tube with a step gradient of 30 and 5% Optiprep in buffer A. After a 5 h centrifugation at 35 000 r.p.m., the raft fraction was obtained from the interface 30–5% Optiprep.
Western blots. Optiprep fractions or brain extracts were loaded on 12% acrylamide gels and blotted using the polyclonal antibody against plasminogen. Anti-rabbit Ig horseradish peroxidase and the ECL method (Amersham) were used for the detection of the protein. Quantification was done with the NIH program.
Expression of human APP and activation of plasminogen to plasmin in mature rat hippocampal neurons. Stage 5 neurons were infected with recombinant SFV encoding human APP695 (De Strooper, 1995) for 1.5 h before the addition of 20 mM HEPES or 1 unit/ml plasmin (Sigma). After another 1.5 h, the medium was replaced by labeling medium with 200 µCi/ml [35S]methionine, maintaining 20 mM HEPES or 1 unit/ml plasmin, respectively. After a 5 h incubation, the cells were extracted in 2% Nonidet P-40, 0.2% SDS, 5 mM EDTA, 10 mM Tris pH 7.2 and CLAP. Samples were immunoprecipitated using the polyclonal antibody B/14 against the C-terminal domain of APP (Simons et al., 1998) and loaded in Tricine gels. Radioactivity in the individual bands was determined with a PhosphorImager (Molecular Dynamics).
To analyze the α-secreted form of APP, stage 5 neurons were infected for 3 h with SFV-APP695. The medium was replaced by fresh N2 and the cells were incubated in the presence of 20 mM HEPES as a control or 0.5 µg/ml tPA (American Diagnostica). Equivalent aliquots from the media were taken after 5 min, 1 h and 2.5 h. At this time the cells were extracted in 1% Triton X-100, 0.1% SDS, 100 mM NaCl, 20 mM Tris pH 7.5 and CLAP. The cells and the aliquots from the media were precipitated in 10% trichloroacetic acid, loaded on a 6% acrylamide gel and analyzed by western blot using the monoclonal antibody 6E10 (Senetek).
Plasmin activation in HEK cells constitutively expressing APP. HEK cells stably transfected with APP695 were kindly provided by Dr Haass, Munich (Haass et al., 1992). They were labeled with [35S]methionine 100 µCi/ml for 4 h. Medium and cell extracts were processed (De Strooper et al., 1995) for the analysis of the secreted APP peptides, Aβ and p3. Plasmin or tPA were added at the indicated concentrations from the beginning of the pulse labeling. Half of the indicated amounts were added after 2 h incubation.
Human brain extracts. The samples analyzed correspond to the neocortex or to the hippocampus (10 control and 10 AD brains in total). They were homogenized in 0.2% SDS containing CLAP and centrifuged for 10 min at 1000 g. The amount of protein in the supernatants was quantified by the BCA method (Bio-Rad).
Acknowledgments
ACKNOWLEDGEMENTS
We are very grateful to B. Hellias and E. Cassin for the preparation of the rat hippocampal neurons. We also thank Drs J. Abad, R. Hodge, C. Kaether, P. Keller, F. Ruberti and E. Piddini for advice and critical reading of the manuscript. M.D.L. is a recipient of a Ramon Areces-EMBO postdoctoral fellowship.
REFERENCES
- Anderson J.J., Holtz, G., Baskin, P.P., Wang, R., Mazzarelli, L., Wagner, S.L. and Menzaghi, F. (1999) Reduced cerebrospinal fluid levels of α-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience, 93, 1409–1420. [DOI] [PubMed] [Google Scholar]
- Baranes D., Lederfin, D., Huang, Y.Y., Chen, M., Bailey, C.H. and Kandel, E.R. (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron, 21, 813–825. [DOI] [PubMed] [Google Scholar]
- Buxbaum J.D. et al. (1998) Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem., 273, 27765–27767. [DOI] [PubMed] [Google Scholar]
- Chen Z.L. and Strickland, S. (1997) Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell, 91, 917–925. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Simons, M., Multhaup, G., Van Leuven, F., Beyreuther, K. and Dotti, C.G. (1995) Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J., 14, 4932–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K. and Banker, G. (1991) Culturing Nerve Cells. MIT Press, Cambridge, MA, pp. 251–281.
- Haass C., Koo, E.H., Mellon, A., Hung, A.Y. and Selkoe, D.J. (1992) Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature, 357, 500–503. [DOI] [PubMed] [Google Scholar]
- Ikezu T., Trapp, B.D., Song, K.S., Schlegel, A., Lisanti, M.P. and Okamoto, T. (1998) Caveolae, plasma membrane microdomains for α-secretase-mediated processing of the amyloid precursor protein. J. Biol. Chem., 273, 10485–10495. [DOI] [PubMed] [Google Scholar]
- Kleiner D.E. Jr and Stetler-Stevenson, W.G. (1993) Structural biochemistry and activation of matrix metalloproteases. Curr. Opin. Cell Biol., 5, 891–897. [DOI] [PubMed] [Google Scholar]
- Koike H.et al. (1999) Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem. J., 343, 371–375. [PMC free article] [PubMed] [Google Scholar]
- Krueger S.R., Ghisu, G.P., Cinelli, P., Gschwend, T.P., Osterwalder, T., Wolfer, D.P. and Sonderegger, P. (1997) Expression of neuroserpin, an inhibitor of tissue plasminogen activator, in the developing and adult nervous system of the mouse. J. Neurosci., 17, 8984–8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan H.C. (1992) The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev., 11, 291–311. [DOI] [PubMed] [Google Scholar]
- Lammich S.et al. (1999) Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl Acad. Sci. USA, 96, 3922–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J, Liyanage, U., Bickel, P.E., Xia, W., Lansbury, P.T., Jr and Kosik, K.S. (1998) A detergent-insoluble membrane compartment contains Aβin vivo. Nature Med., 4, 730–734. [DOI] [PubMed] [Google Scholar]
- Miles L.A., Dahlberg, C.M., Levin, E.G. and Plow, E.F. (1989) Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these fibrinolytic components to the cells. Biochemistry, 28, 9337–9343. [DOI] [PubMed] [Google Scholar]
- Parvathy S., Hussain, I., Karran, E.H., Turner, A.J. and Hooper, N.M. (1999) Cleavage of Alzheimer’s amyloid precursor protein by α-secretase occurs at the surface of neuronal cells. Biochemistry, 38, 9728–9734. [DOI] [PubMed] [Google Scholar]
- Plow E.F., Herren, T., Redlitz, A., Miles, L.A. and Hoover-Plow, J.L. (1995) The cell biology of the plasminogen system. FASEB J., 9, 939–945. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature, 399, A23–A31. [DOI] [PubMed] [Google Scholar]
- Sennvik K., Fastbom, J., Blomberg, M., Wahlund, L.O., Winblad, B. and Benedikz, E. (2000) Levels of α- and β-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer’s disease patients. Neurosci. Lett., 278, 169–172. [DOI] [PubMed] [Google Scholar]
- Simons K. and Ikonen, E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C.G. and Simons, K. (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA., 95, 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. and Lieberburg, I. (1999) Cellular mechanisms of β-amyloid production and secretion. Proc. Natl Acad. Sci. USA, 96, 11049–11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewke D.P. and Seeds, N.W. (1999) The expression of mRNAs for hepatocyte growth factor/scatter factor, its receptor c-met and one of its activators tPA show a systematic relationship in the developing and adult cerebral cortex and hippocampus. Brain Res., 821, 356–367. [DOI] [PubMed] [Google Scholar]
- Tsirka S.E., Bugge, T.H., Degen, J.L. and Strickland, S. (1997) Neuronal death in the central nervous system demonstrates a non fibrin substrate for plasmin. Proc. Natl Acad. Sci. USA, 94, 9779–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker H.M. et al. (2000) The plasmin system is induced by and degrades Amyloid-β aggregates. J. Neurosci., 20, 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W.E. and Porter, M. (1999) Plasmin cleavage of the amyloid β-protein: alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry, 38, 11570–11576. [DOI] [PubMed] [Google Scholar]
- Vassalli J.D., Sappino, A.P. and Belin, D. (1991) The plasminogen activator/plasmin system. J. Clin. Invest., 88, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M.J. and Doolittle, R.F. (1972) Differential specificities of the thrombin, plasmin and trypsin with regard to synthetic and natural substrates and inhibitors. Biochim. Biophys. Acta., 258, 577–590. [DOI] [PubMed] [Google Scholar]
- Wnendt S., Wetzels, I. and Gunzler, W.A. (1997) Amyloid β peptides stimulate tissue-type plasminogen activator but not recombinant prourokinase. Thromb. Res., 85, 217–224 [DOI] [PubMed] [Google Scholar]
- Yanasigawa K., Odaka, A., Suzuki, N. and Ihara, Y. (1995) GM1 ganglioside-bound amyloid β-protein (Aβ): a possible form of preamyloid in Alzheimer’s disease. Nature Med., 1, 1062–1066. [DOI] [PubMed] [Google Scholar]