Abstract
Culture filtrate from Mycobacterium tuberculosis contains molecules which promote high levels of protective immunity in animal models of subunit vaccination against tuberculosis. We have used two-dimensional electrophoresis for analysis and purification of six novel M. tuberculosis culture filtrate proteins (CFPs): CFP17, CFP20, CFP21, CFP22, CFP25, and CFP28. The proteins were tested for recognition by M. tuberculosis-reactive memory cells from different strains of inbred mice and for their capacity to induce a skin test response in M. tuberculosis-infected guinea pigs. CFP17, CFP20, CFP21 and CFP25 induced both a high gamma interferon release and a strong delayed-type hypersensitivity response, and CFP21 was broadly recognized by different strains of inbred mice. N-terminal sequences were obtained for the six proteins, and the corresponding genes were identified in the Sanger M. tuberculosis genome database. In parallel we established a two-dimensional electrophoresis reference map of short-term culture filtrate components and mapped novel proteins as well as already-known CFP.
For a number of years, efforts to develop a subunit vaccine against tuberculosis (TB) have focused on proteins released from growing mycobacteria into the extracellular medium (3, 31). These released proteins are generally believed to be responsible for the high efficacy of live vaccine, Mycobacterium bovis BCG, and recognition of these molecules may lead to early immunological detection of the infected macrophages and control of the disease. Subunit vaccines based on mixtures of culture filtrate proteins (CFPs) from Mycobacterium tuberculosis have, in a number of studies, resulted in protective immunity in animal models of TB (1, 25, 32, 39), and the molecules are recognized strongly during M. tuberculosis infection in various animal models (22, 31), as well as in early stages of pulmonary TB in humans (11).
Culture filtrate is therefore an attractive source of candidate antigens for a new vaccine and diagnostic reagents. Short-term culture filtrate (ST-CF) from M. tuberculosis is composed of numerous components, and so far only a minority of these have been isolated and characterized. In total, approximately 15 proteins have been purified from culture filtrate; most of them were initially identified by use of murine monoclonal antibodies (MAbs) (13, 15, 19, 30). In general, these proteins have been isolated among the abundant culture filtrate components which are accessible for conventional purification (24, 30, 42). Studies of T-cell recognition and direct analysis of the potential of these molecules in experimental vaccines have so far pointed to only a few culture filtrate antigens, notably Ag85 and ESAT-6, as candidate antigens for a novel TB vaccine (2, 24). Attempts to screen human cellular responses to separated CFPs, on the other hand, have demonstrated that there are still numerous uncharacterized antigens of various molecular masses to be identified (11).
In this study, we have focused on purifying new immunologically active proteins from ST-CF by preparative two-dimensional electrophoresis (2-DE). Eleven proteins were purified from ST-CF, and six of these (CFP17, CFP20, CFP21, CFP22, CFP25, and CFP28) were previously uncharacterized proteins. An analytical 2-DE reference system for CFPs was established, in which previously characterized culture filtrate antigens as well as the newly purified proteins were mapped. The genes encoding the novel proteins were identified, and the biological activities of the proteins were evaluated in animal models of TB.
MATERIALS AND METHODS
Bacteria and preparation of ST-CF.
ST-CF was produced as described previously (3). Briefly, M. tuberculosis H37Rv (8 × 106 CFU/ml) was grown in modified Sauton medium on an orbital shaker for 7 days. The culture supernatants were sterile filtered and concentrated on a YM3 membrane (Amicon, Danvers, Mass.).
Purification of native proteins from ST-CF.
ST-CF was precipitated with ammonium sulfate at 80% saturation. The precipitated proteins were removed by centrifugation and after being washed were resuspended in buffer containing 8 M urea, 0.5% (wt/vol) CHAPS {3-[(3-cholamidopropyl)-dimethyl ammonio]-1-propanesulfonate}, and 5% (vol/vol) glycerol. Protein (250 mg) was separated on a Rotofor Isoelectric Cell (Bio-Rad, Richmond, Calif.) in a pH gradient with 3% Biolyt 3/5 and 1% Biolyt 4/6 (Bio-Rad). Fractions 9 to 15 were pooled and refractionated on the Rotofor in the same buffer. The fractions obtained were analyzed by silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis phosphate-buffered saline (SDS-PAGE), and fractions with similar band patterns were pooled, buffer exchanged to (PBS), and concentrated to 1 to 3 ml on a Centriprep concentrator (Amicon) with a 3-kDa-cutoff membrane. An equal volume of sample buffer (63 mM Tris-HCl [pH 6.8], 10% glycerol, 2% SDS) was added, and the protein solution was boiled for 5 min before further separation on a Prep-Cell column (Bio-Rad) in a matrix of 16% polyacrylamide at 200 V overnight. Fractions containing pure proteins were collected. Samples used for testing of in vivo or in vitro biological activity were washed three times with PBS on a Centricon concentrator (Amicon). The fractions were stabilized with 0.5% fetal calf serum (Gibco Life Technology, Inchinnan, Scotland), and SDS was removed by passing the sample twice through an Extracti-Gel D column (Pierce, Rockford, Ill.).
Cloning, expression, and purification of rCFP22 and rCFP25.
All primers used for cloning and sequencing were synthesized with an ABI-391 DNA synthesizer (Applied Biosystems).
By using the cfp22 and cfp25 gene sequences found in the Sanger database, the following PCR primers were synthesized: cfp22 forward, ACAGATCTGTAATGGCAGACTGTGAT; cfp22 reverse, TTTTCCATGGTCAGGAGATGGTGATCGA; cfp25 forward, ACAGATCTGCGCATGCGGATCCGTGT; and cfp25 reverse, TTTTCCATGGTCATCCGGCGTGATCGAG. Both forward primers create BglII sites, and both reverse primers create NcoI sites DNA fragments were obtained by PCR amplification of M. tuberculosis H37Rv chromosomal DNA with these primers and were purified on agarose gels and cloned into the pT7Blue T vector (Novagen, Abingdon, United Kingdom). Plasmid DNA was subcloned into the expression vector pMCT6 (18) in frame with eight histidines at the N termini of the expressed proteins, and the resulting clones were sequenced.
Expression and metal affinity purification of recombinant CFP22 (rCFP22) and rCFP25 on a TALON column (Clontech Laboratories, Palo Alto, Calif.) were done essentially as described by the manufacturers.
The recombinant protein preparations were pooled and dialyzed against 3 M urea in 10 mM Tris-HCl, pH 8.5. The dialyzed protein was further purified by fast protein liquid chromatography (Pharmacia, Uppsala, Sweden) with a 1-ml Mono-Q column and eluted with a linear 0 to 1 M gradient of NaCl. Fractions were analyzed by SDS-PAGE and dialyzed against 25 mM HEPES buffer, pH 8.5.
The lipopolysaccharide (LPS) contents in the rCFP22 and rCFP25 preparations were determined by the Limulus amoebocyte lysate clot test (7).
SDS-PAGE, Western blot analysis, and 2-DE.
Analytical SDS-PAGE was done with 10 to 20% gradient gels (16 by 16 by 0.075 cm) as described by Laemmli (26) under reducing conditions unless otherwise indicated. For calibration, low-molecular-weight standard mixtures (Bio-Rad) were run in parallel with the samples. The gels were either silver stained (10) or transferred to nitrocellulose (Schleicher and Schuell, Dassel, Germany) as previously described (44). For immunoblot analysis, the nitrocellulose membranes were incubated with mouse MAbs followed by alkaline phosphatase-labeled rabbit antimouse antibodies (D314; DAKO, Glostrup, Denmark). A panel of MAbs defining known CFPs was used: Hyb 76-8 (ESAT-6), Hyb 76-1 (GroES), K12 (MPT63), HBT2 (CFP20), L24.b3 (MPT64), HYT6 (19-kDa lipoprotein), HYT27 (Ag85 complex), HBT12 (PstS), HBT10 (Ald), I10 (MPT32), and HAT3 (DnaK). The antibodies K12 and I10 were kindly provided by M. Gennaro and G. Marchal, respectively.
2-DE in polyacrylamide gels was carried out as described by Hochstrasser et al. (23), except that in the first dimension, Nonidet P-40 was replaced by Tween 80. The first-dimension isoelectric focusing tube gels (14 by 0.15 cm) contained Biolyt 4/6 and Biolyt 5/7 (2:3) (Bio-Rad). After the first-dimension electrophoresis, samples were separated on 10 to 20% gradient gels. The pI scale was calibrated by measuring the pH of 0.5-cm pieces of focusing gel soaked in 1 ml of degassed Milli Q water.
Identification of the positions of individual proteins in 2-DE analysis of ST-CF was achieved by two methods: (i) comparative computer analysis (Phoretix International, Newcastle, United Kingdom) of the 2-DE spot pattern of ST-CF with and without addition of the purified protein and (ii) immunoblotting with MAbs defining known CFPs as described above.
N-terminal sequencing.
For N-terminal sequencing, the protein fractions were washed with Milli Q water on a Centricon concentrator (Amicon) with a cutoff at 3 kDa, and 10 to 50 pmol was applied to a polyvinylidene difluoride membrane in a ProSpin concentrator (Applied Biosystems). The membrane was washed three times with 20% methanol and subjected to N-terminal sequence analysis by automated Edman degradation with a Procise 494 sequencer (Applied Biosystems) as described by the manufacturer. The SWISSPROT database was searched with FASTA algorithms (33).
Animals and experimental infections.
Female C57BL/10 mice and congenic B10.BR mice (haplotype H-2k), and B10.HTG mice (haplotype H-2g) were purchased from Harlan Olac Ltd. (Bicester, United Kingdom).
Memory-immune mice were generated as previously described (12). Briefly, mice received a primary infection with 5 × 104 CFU of M. tuberculosis H37Rv via the lateral tail vein, after which they were treated with isoniazid (Merck, Rahway, N.J.) and rifabutin (Farmatalia Carlo Erba, Milan, Italy) in their drinking water for 2 months to clear the infection. The mice were rested for a period of 4 to 6 months before challenge with 106 CFU of bacteria intravenously, and the animals were sacrificed on day 4 postinfection.
Female outbred Ssc:AL strain guinea pigs were bred at Statens Serum Institut (Copenhagen, Denmark) and were infected via an ear vein with M. tuberculosis H37Rv in 0.2 ml of PBS containing 5 × 104 CFU.
Lymphocyte cultures.
Spleen lymphocytes were isolated from memory-immune mice during the recall of protective immunity as previously described (12). Briefly, cells were pooled from three mice and cultured in microtiter wells (2 × 105 cells/200 μl) in RPMI 1640 medium supplemented with β-mercaptoethanol, penicillin-streptomycin, glutamine, and fetal calf serum. Recombinant mouse interleukin-2 (2.5 U/ml; Genzyme, Cambridge, Mass.) was added to all cultures. ST-CF, purified native proteins, and recombinantly produced proteins were all buffer exchanged into PBS and tested in various concentrations (0.5 to 8 μg/ml) in cultures (results not shown). On the basis of these results, we chose to use the purified proteins at 2 μg/ml and ST-CF at 5 μg/ml throughout the study. Supernatants were harvested after 48 h of incubation, and gamma interferon (IFN-γ) levels were quantified by enzyme-linked immunosorbent assay as described previously (12). Experimental values are given as means of duplicate or triplicate cultures ± standard errors. Toxicity tests were performed for all protein preparations as follows. Twofold dilutions of the antigens (8 to 0.5 μg/ml) were tested for toxicity in coculture with a suboptimal concentration of concanavalin A (0.32 μg/ml). The proliferative responses were compared to those of cell cultures stimulated with concanavalin A alone, and no suppression of the response was observed at any of the antigen concentrations used.
Skin testing.
Four weeks after infection of the guinea pigs, skin testing was performed with proteins diluted to 1 μg/ml in 0.1 ml of PBS and injected intradermally in the shaved flanks. Tuberculin purified protein derivative (PPD) RT23 (10 tuberculin units; Statens Serum Institut) was used as a positive control. Reaction diameters were measured at 24 h after injection, and reaction diameters of less than 3 mm were considered negative.
RESULTS
Purification of M. tuberculosis CFPs by preparative 2-DE.
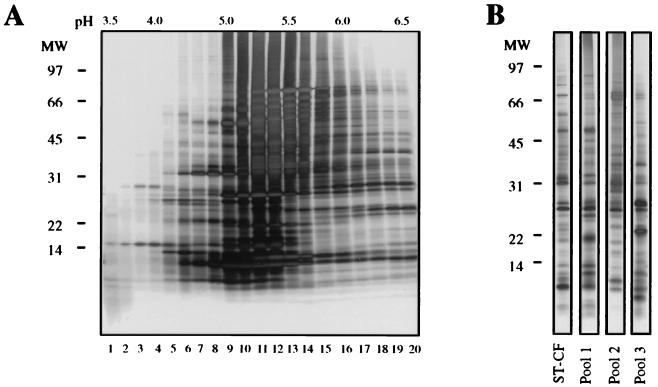
Single CFPs were purified by using a strategy based on preparative 2-DE with isoelectric focusing as the first step followed by separation according to size in SDS-PAGE. Pilot experiments demonstrated that CFPs focused within a narrow pI range (pI 4 to 7), with a large number of molecules with pIs of around 5.5. Isoelectric focusing of ST-CF was done with a Rotofor Cell, and the pH range of 3.5 to 6.5 was chosen. The proteins were separated into 20 fractions; the majority of the protein bands were in fractions within the pH range of 5 to 5.8, whereas the peripheral fractions had a lower protein content but also a markedly different band composition (Fig. 1A). Fractions with similar band patterns were combined into three pools as follows. Fractions 6 to 8 were sampled as pool 1, and fractions 16 to 20 were sampled as pool 3. The remaining fractions, 9 to 15, were pooled and refractionated on the Rotofor Cell. The resulting fractions in the pH range of 5.0 to 5.7 were collected and samples as pool 2. By this method three pools with markedly different band composition were obtained and used for further fractionation (Fig. 1B).
FIG. 1.
Fractionation of CFPs from M. tuberculosis by preparative isoelectric focusing. (A) ST-CF was fractionated on a Rotofor Cell, and each fraction was analyzed by SDS-PAGE and silver staining. The fraction number is indicated below each lane, and the pHs of selected fractions are indicated at the top. (B) The fractions were pooled into three major pools. All fractions were analyzed by silver staining after SDS-PAGE, and the protein profiles were compared to that of ST-CF. Sizes of molecular mass (MW) markers (in kilodaltons) are indicated at the left.
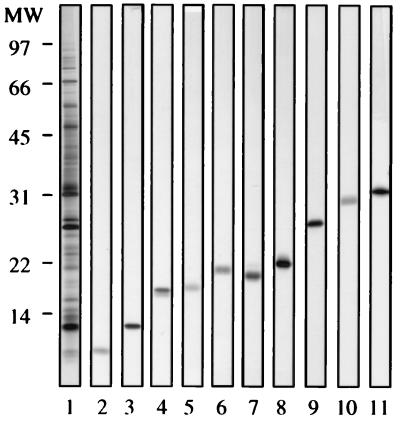
The proteins in each pool were separated according to size by preparative SDS-PAGE on a Prep-Cell column. A polyacrylamide concentration of 16% was chosen, as it gives optimal resolution of molecules below 35 kDa, a region previously demonstrated to contain highly stimulatory molecules in animal models as well as in human donors (2, 5, 11, 36). The fractions obtained were analyzed by SDS-PAGE, and 10 fractions chosen for further investigation, as they contained only one protein band (Fig. 2). These 10 single purified CFPs, with molecular masses of 8 to 30 kDa, were tested by Western blot analysis with a panel of MAbs defining previously characterized CFPs (results not shown). Five of the proteins were identified as already-known proteins: ESAT-6, GroES, MPT63, MPT64, and MPT59 (Fig. 2, lanes 2, 3, 4, 9, and 11, respectively). The remaining five proteins appeared to be novel proteins and were designated CFP17 (Fig. 2, lane 5), CFP20 (lane 6), CFP21 (lane 7), CFP22 (lane 8), and CFP28 (lane 10). GroES, CFP17, and CFP20 were obtained from pool 1; MPT63, CFP21, MPT64, and CFP28 were obtained from pool 2; and ESAT-6, CFP22, and MPT59 were obtained from pool 3.
FIG. 2.
Purified CFPs obtained by preparative size separation. Lane 1, protein profile of ST-CF; lanes 2 to 11, migrations of the individual purified proteins. The proteins were separated by nonreducing SDS-PAGE, which was followed by silver staining. Sizes of molecular mass (MW) markers (in kilodaltons) are indicated at the left.
CFP20 was recognized by MAbs HBT2 and HBT11. An antigen recognized by these MAbs has previously been isolated by affinity chromatography, but the protein has not been characterized (27, 48). None of the other novel proteins were recognized by any of the MAbs available.
2-DE of CFPs.
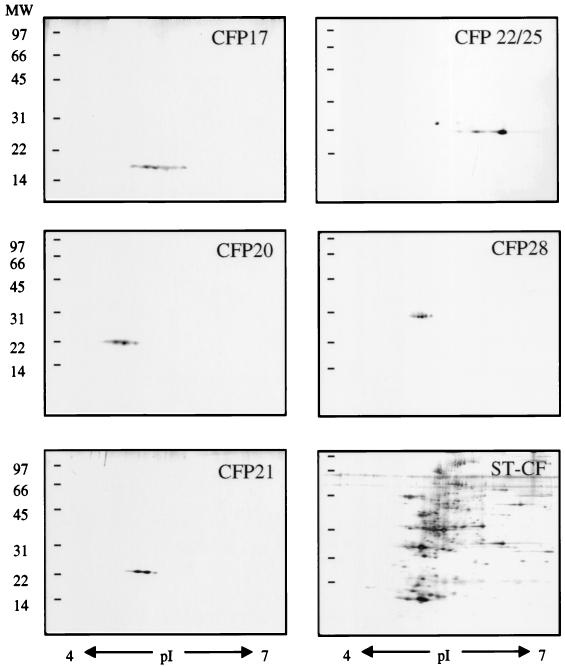
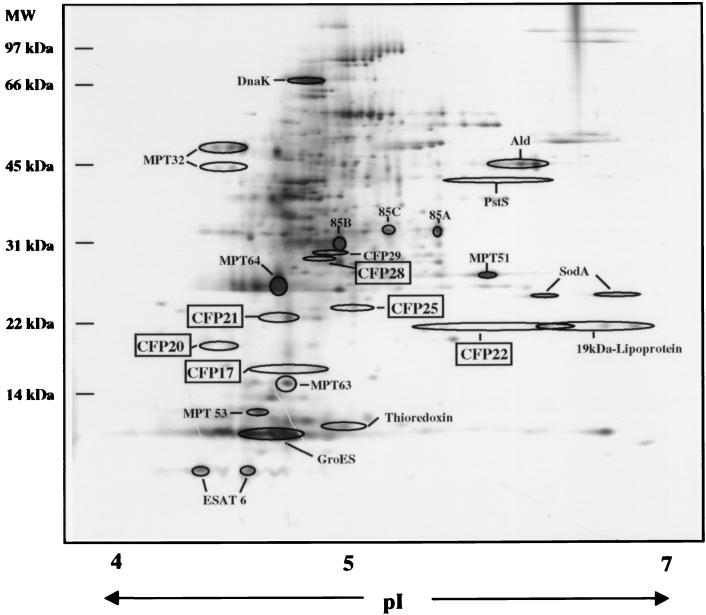
Mycobacterial proteins are being identified at an increasing rate, and analytical tools are needed to perform a systematic evaluation of newly purified proteins and to distinguish them from the already-characterized proteins. We have utilized the analytical power of 2-DE to establish a 2-DE map of ST-CF components in which the positions of previously characterized CFPs as well as those of the panel of novel CFPs were mapped. This was achieved by visual inspection as well as computer-assisted evaluation of parallel 2-DE gels with purified antigens, ST-CF, or purified antigens added to ST-CF. The positions of the previously characterized proteins were also confirmed by Western blotting with specific MAbs. The analysis confirmed that CFP17, CFP20, CFP21, CFP22, and CFP28 all mapped as previously uncharacterized proteins with molecular masses of 17 to 28 kDa (Fig. 3). Of the five proteins, CFP17, CFP20, CFP21, and CFP28 all focused at the expected molecular mass as a cluster of spots within a narrow pI range, indicating the presence of only one protein in the preparation. All of the proteins focused as more than one spot, which may be due to microheterogeneity caused by posttranslational modification, e.g., deamidation or oxidation of side chains, as previously observed for both mycobacterial and nonmycobacterial proteins (9, 17, 43). Interestingly, 2-DE analysis of the CFP22 preparation revealed that a protein of slightly higher molecular mass was copurified and seen as a spot at 25 kDa and at a slightly lower pI (Fig. 3). The protein of 25 kDa was seen only when a reducing agent was introduced in the SDS-PAGE analysis, which explained the copurification of the two molecules during the nonreducing separation on the Prep-Cell column. The preparation was accordingly designated CFP22/25. All of this information was integrated into a 2-DE reference map of ST-CF components (Fig. 4).
FIG. 3.
2-DE analysis of the novel CFPs. The CFP17, CFP20, CFP21, CFP22/25, and CFP28 preparations were separated by 2-DE, which was followed by silver staining. The migrations of the purified CFPs are compared to the spot pattern of the complex mixture ST-CF. The CFP22 preparation is seen to contain another molecule of 25 kDa. This preparation was accordingly designated CFP22/25. MW, molecular masses in kilodaltons.
FIG. 4.
2-DE pattern of M. tuberculosis CFPs. ST-CF was analyzed by 2-DE in the pH range of 4 to 7. The proteins were separated according to isoelectric point in the first dimension and then by size in the second dimension. The gel was silver stained. The positions of proteins mapped are indicated; known proteins are designated by the most commonly used name, and the novel CFPs identified in this study are marked by boxes. MW, molecular mass.
N-terminal sequence analysis and identification of the genes encoding the novel proteins.
The five protein preparations were transferred to polyvinylidene difluoride membranes, which were subjected to N-terminal sequencing. CFP17, CFP20, CFP21, and CFP28 all gave one main sequence, which is highly indicative of a pure protein preparation. Fifteen amino acids were determined for each of the proteins (Table 1). For the CFP22/25 preparation, N-terminal sequencing of the two individual bands separated by reducing SDS-PAGE confirmed the existence of two protein species with different N-terminal sequences.
TABLE 1.
N-terminal sequences and genomic identification of the purified proteins
| Protein | N-terminal amino acid sequencea | Corresponding cosmid in the Sanger database | Theoretical mol wt/pIb | Homology to other proteinsc |
|---|---|---|---|---|
| CFP17 | SELDAPAQAGTEXAV | MTCY1A11.16c | 13,833/4.4 | None |
| CFP20 | AQITLRGNAINTVGE | MTCY9F9.32c | 16,897/4.2 | 52% identity in 166-aa overlap to E. coli scavengease p20 (EC 93212), 52% identity in 166-aa overlap to E. coli thiol peroxidase (EC 33213), 51% identity in 166-aa overlap to Haemophilus influenzae ToxR regulon (HI32759) |
| CFP21 | DPXSDIAVVFARGTH | MTCY39.35c | 18,657/4.6 | 33% identity in 193-aa overlap to fungal cutinase precursor (P41744) |
| CFP22 | TNSPLATATATLHTN | MTCY10H4.08c | 18,517/6.8 | 90% identity in 185-aa overlap to M. leprae peptidyl-propyl cis-trans isomerase (E235739) |
| CFP25 | AXPDAEVVFARGRFE | MTCY339.08c | 19,665/4.9 | 43% identity in 217-aa overlap with CFP21, 32% identity in 190-aa overlap with cutinase (P41744) |
| CFP28 | XXQKSLELIXXTAXE | NFd | NAe | NA |
N-terminal sequences of the proteins found in culture filtrate. X, the amino acid could not be determined.
Calculated from the deduced sequence of the mature protein.
Only homologies of >30% identity are shown, except CFP20, for which only homologies of >50% identity are shown. aa, amino acid.
NF, not found.
NA, not applicable.
Each of the six N-terminal sequences obtained was used for a homology search of the Sanger M. tuberculosis database with the Blast program. For CFP17, CFP20, CFP21, CFP22, and CFP25 the N-terminal amino acid sequence was found to be identical to the deduced amino acid sequence for an open reading frame identified in the Sanger database, whereas no similarity for CFP28 was found in the database (Table 1). The five open reading frames identified in the Sanger database were examined and found to code for mature proteins ranging from 132 to 187 amino acids (Fig. 5). The first amino acid identified in the mature CFP17, CFP20, CFP21, CFP22, and CFP25 were residues 31, 2, 33, 8, and 33 in the deduced sequences, respectively. The stretch of deduced amino acids upstream of the N-terminal sequences of the mature CFP17, CFP21, and CFP25 suggested the presence of a putative leader sequence cleaved after secretion. However, only the sequences of CFP21 and CFP25 had the typical characteristics of a signal peptide (approximately 20 to 40 amino acids with a stretch of largely hydrophobic residues) (46).
FIG. 5.
Deduced amino acid sequences of the CFPs. Full-length sequences are shown for the proteins CFP17, CFP20, CFP21, CFP22, and CFP25. The amino acids determined by N-terminal sequencing in this study are in boldface.
The theoretical molecular weight and pI were calculated from the sequences, and in each case the theoretical molecular weight and pI were somewhat less than those observed (Table 1). This slight difference may arise from the presence of urea in the first dimension of the 2-DE, as previously described (30).
The identified sequences were used for homology searches in the EMBL database with the TFASTA algorithm (33). None of the identified proteins were identical to previously described proteins from M. tuberculosis, whereas homology to proteins from other bacteria was found for four of the proteins (Table 1).
CFP22 showed 90% identity in a 182-amino-acid overlap to a peptidyl-prolyl isomerase from Mycobacterium leprae and is most likely the M. tuberculosis homolog of this protein. CFP20 exhibited identity to a number of outer cell wall proteins and enzymes from other bacteria. CFP21 and CFP25 are homologous proteins (43% in a 217-amino-acid overlap), and both are homologous to a cutinase from fungi (29). In addition, the analysis of the open reading frame for CFP21 revealed that this protein was encoded within the translated region RD2, which is not present in some strains of M. bovis BCG (28).
Cloning and expression of rCFP22 and rCFP25.
CFP22 and CFP25 were purified together, and the biological activities of the individual antigens therefore could not be evaluated. As CFP25 is present in only trace amounts in ST-CF, purification and evaluation of this single protein from culture filtrate was considered impractical. Therefore, the genes encoding CFP22 and CFP25 were cloned, and the proteins were expressed as recombinant proteins. cfp22- and cfp25-containing DNA fragments were amplified from M. tuberculosis H37Rv chromosomal DNA by PCR with cfp22- and cfp25-specific primers and cloned into the Escherichia coli expression vector pMCT6 in frame with eight N-terminal histidine codons.
Recombinant, histidine-tagged rCFP22 and rCFP25 were expressed in E. coli XL1Blue cells and purified by metal affinity chromatography followed by anion-exchange (Mono-Q) chromatography, concentration, and dialysis against a suitable buffer. The resulting clones were sequenced and found to be 100% in agreement with the cfp22 and cfp25 sequences obtained from the Sanger database.
Before the proteins were used in immunological tests, the preparations were analyzed for contamination with LPS. In both cases, LPS was present in amounts that are not suspected to interfere with either T-cell or skin test experiments (<1.0 ng of LPS/mg of rCFP22 and <25 ng of LPS/mg of rCFP25).
Immunological activities of the CFPs.
The immunological activities of the six CFPs were investigated in mice and guinea pigs infected with M. tuberculosis. Mice of three congenic strains on the B10 background representing the H-2b, H-2k, and H-2g-haplotypes were rendered memory immune by primary M. tuberculosis infection followed by chemotherapy, as previously described (2). Recognition of the purified proteins by memory effector cell lymphocytes isolated at day 4 of rechallenge was investigated. All molecules were recognized in the C57BL/10 strain, and CFP17 and CFP21 were the most potent inducers of IFN-γ release, giving rise to 40 to 60% of the response to total ST-CF (Tables 2 and 3). In the B10.BR mice, CFP20 and CFP21 induced the highest IFN-γ release, although at a level somewhat lower than that in the C57BL/10 mice (Table 2). For B10.HTG mice the amount of IFN-γ released in response to CFP17, CFP20, and CFP22/25 was negligible; however, CFP21 also induced a marked IFN-γ release in this strain, almost at the level of ST-CF (Table 2). No IFN-γ release was detected when the antigens were tested in spleen cell cultures isolated from naive mice (results not shown).
TABLE 2.
Recognition of purified CFPs by memory effector cells from B10 congenic strains of mice
| Antigenb | IFN-γ release (ng/ml)a in:
|
||
|---|---|---|---|
| C57BL/10 mice (H-2b) | B10.BR mice (H-2k) | B10.HTG mice (H-2g) | |
| Controlc | <0.1 | 0.3 | <0.1 |
| ST-CF | 11.68 ± 0.3 | 11.05 ± 0.02 | 4.46 ± 0.02 |
| CFP17 | 5.33 ± 0.2 | 0.78 ± 0.03 | 0.48 ± 0.02 |
| CFP20 | 3.20 ± 0.1 | 1.73 ± 0.09 | 0.15 ± 0.02 |
| CFP21 | 7.34 ± 0.1 | 2.98 ± 0.04 | 3.57 ± 0.4 |
| CFP22/25 | 3.38 ± 0.3 | 0.25 ± 0.09 | 0.14 ± 0.01 |
| CFP28 | 1.11 ± 0.1 | 0.49 ± 0.08 | 0.60 ± 0.07 |
IFN-γ release was measured in spleen cell cultures isolated from three memory-immune mice 4 days after rechallenge with 106 CFU of M. tuberculosis H37Rv. Results are means ± standard errors.
Single proteins were tested at 2 μg/ml, and ST-CF was tested at 5 μg/ml.
Stimulation without antigen.
TABLE 3.
Immunological activities of the purified CFPs in M. tuberculosis-infected mice and guinea pigs
| Protein | IFN-γ release (ng/ml)a | DTH reaction diam (mm)b |
|---|---|---|
| Controlc | <0.10 ± 0.0 | <3 ± 0.3 |
| PPD/ST-CFd | 20.96 ± 1.1 | 11.7 ± 0.5 |
| CFP17 | 9.25 ± 0.1 | 12.9 ± 1.0 |
| CFP20 | 2.39 ± 1.8 | 12.3 ± 0.8 |
| CFP21 | 10.73 ± 0.04 | 10.4 ± 0.5 |
| CFP22/25 | 5.34 ± 0.3 | 4.2 ± 0.2 |
| rCFP22 | <0.10 ± 0.0 | <3 ± 0.2 |
| rCFP25 | 9.87 ± 0.1 | 10.3 ± 0.6 |
| CFP28 | 2.82 ± 0.2 | 5.8 ± 0.8 |
IFN-γ release from memory effector cells was measured in spleen cell cultures isolated from three memory-immune C57BL/10 mice 4 days after rechallenge with 106 CFU of M. tuberculosis. The proteins were used at 2 μg/ml. Results are means ± standard errors.
Skin testing was done 4 weeks after infection with 5 × 104 CFU of M. tuberculosis. Values shown are means ± standard errors; for eight readings. The proteins were tested at 1 μg/ml.
Stimulation without antigen.
PPD (10 tuberculin units) was used in the skin test experiment, while ST-CF (5 μg/ml) was used in the lymphocyte cultures.
The response to the rCFP22 and rCFP25 was compared to that to the native antigen preparations isolated from culture filtrate. The recognition of these antigens in the mouse model and the ability to induce a DTH response in guinea pigs infected with M. tuberculosis were evaluated (Table 3).
In these experiments rCFP25 was demonstrated to be responsible for the activity of the mixed preparation, while rCFP22 induced neither IFN-γ release nor a significant DTH reaction. The ranking of the antigens’ immunological activities in the C57BL/10 strain was in agreement with the results of the other experiment (Table 2), confirming the high reactivity of CFP17 and CFP21, both of which induced IFN-γ release in this model at levels above those for the well-known T-cell antigen MPT59 (6.5 ± 0.1 ng/ml). In the guinea pig model CFP17 and CFP21 also demonstrated a high activity, but this model in addition identified CFP20 and CFP25 as potent preparations which gave rise to DTH reactions at levels comparable to those for PPD (Table 3). CFP28 induced a weak skin test reaction, and no responses to rCFP22 were found. These experiments were repeated three times with the same overall result, leading to the same relative ranking of the immunological activities of the antigens.
Taken together, these data support the overall conclusion that CFP17, CFP20, CFP21, and CFP25 are antigens strongly recognized in animals infected with M. tuberculosis. CFP21, in particular, is broadly recognized in animals of different major histocompatibility complex class II compositions.
DISCUSSION
The aim of the present study was to identify proteins from M. tuberculosis with potential for use as a TB vaccine or diagnostic reagents. ST-CF was used as the source of antigens, since this preparation has been the basis of several successful studies of experimental subunit vaccines (reviewed in reference 14) and contains antigens recognized in the early stage of M. tuberculosis infection in animals as well as in humans (11, 36).
ST-CF is a complex mixture composed of a large number of components present in different concentrations, ranging from proteins barely detectable in silver-stained gels to components present in abundant quantities. Using classical chromatographic methods, Nagai et al. (30) were the first to isolate and characterize a number of the major CFPs. More recently, conventional purification has resulted in the identification of novel culture filtrate antigens (40, 42, 44), but such studies have also demonstrated that in many cases antigens that are already known are obtained (21, 24). These abundant CFPs were originally identified as the 33 major proteins in ST-CF (3), but as demonstrated in this study as well as in another very recent report (43), sensitive 2-DE allows the detection of at least 150 different protein species. 2-DE separation followed by direct excision of spots and N-terminal sequence analysis is obviously an attractive and rapid method, but again this method has the disadvantage that only proteins present in high quantities will be obtained.
In this regard, ESAT-6 and the recently identified T-cell antigen CFP29 are both present only in very small amounts in culture filtrate but are still very potent T-cell antigens (12, 36, 40, 44). It is therefore clear that there is no direct correlation between the relative representation of an antigen in culture filtrate and its immunological relevance, as has been suggested elsewhere (21, 24), and this may reflect differences in the protein expression in vivo and in vitro. We therefore decided to employ a preparative 2-DE method in which a preseparation of proteins by isoelectric focusing enables the subsequent purification of novel proteins, including molecules present in only small quantities in culture filtrate.
Boesen et al. (11) showed that patients with minimal TB are characterized by a strong cellular reactivity to a range of ST-CF proteins, with a recognition of molecules ranging from 5 to 35 kDa. This predominant recognition of low-mass CFPs prevails in different species and has been reported for mice, guinea pigs, and cattle infected with M. tuberculosis (12, 22, 36). We therefore optimized our size separation to enable maximal separation of this region. Six proteins not previously described were obtained, and four of these proteins, CFP17, CFP20, CFP21, and CFP25, were immunologically very active and induced either a high IFN-γ release from murine memory effector cells or a pronounced DTH reaction. One of the proteins purified, CFP20, was recognized by the MAbs HBT2 and HBT11. This antigen has previously been purified by affinity chromatography and was found to induce a strong proliferative response in humans and mice (4, 6, 27, 48), but until now no biochemical or sequence data on this antigen have been available.
Interestingly, the present study led to the identification of CFP21, which is encoded in the RD2 region of the genome, a region reported to be absent from several strains of BCG (28). CFP21 elicited a strong skin test reaction and was broadly recognized in genetically different strains of inbred mice. The value of this protein as a diagnostic reagent either alone or in combination with other antigens also absent in BCG, such as MPT64 and ESAT-6, will be the subject of future studies.
The establishment of 2-DE reference maps of mycobacterial proteins from different subcellular locations will greatly complement the biochemical and genetic characterization of M. tuberculosis proteins. In the coming years the complete sequence of the M. tuberculosis genome (35) and the use of 2-DE to characterize the proteome, i.e., the total set of expressed proteins, will change TB research dramatically and allow a direct analysis of genes expressed under different conditions and of importance for host-parasite interactions. A recent report describes the resolution of more than 600 spots in 2-DE analysis of a whole-cell extract of M. tuberculosis (45), and another very recent study has addressed this important subject by mapping a number of CFPs defined by the World Health Organization standard panel of MAbs (43). The results obtained in that study are generally in good agreement with the 2-DE map presented in this study. In our early culture filtrate, however, we cannot detect the KatG molecule found in abundant quantities in the culture filtrate used by Sonnenberg and Belisle (43). This discrepancy could be explained by the different culture periods used in the two studies (7 versus 14 days), as we can detect this protein in culture filtrates harvested at late time points (data not shown). The reproducibility of 2-DE maps established in different laboratories emphasizes the potential of this method for future purification and characterization of mycobacterial proteins in complex mixtures.
In the present study, the proteins CFP22 and CFP25 were copurified as one band at 22 kDa by preparative SDS-PAGE performed under nonreducing conditions. Separation of the recombinant CFP25 under reducing and nonreducing conditions confirmed that the relative migration of the nonreduced molecule is faster, reflecting a smaller total hydrophodynamic volume. Analysis of the deduced sequence for CFP25 revealed the presence of four cysteines, and we propose, therefore, that there are one or two internal disulfide bonds in this molecule.
ST-CF consists of proteins released from the bacterium into the medium before significant autolysis of the bacterium has taken place (3). Most proteins destined for translocation across the cytoplasmic membrane, in both gram-negative and gram-positive bacteria, are synthesized as preproteins containing an NH2-terminal signal sequence which is cleaved from the mature protein by specific peptidases (34, 46). However, several proteins without a signal peptide have been found in ST-CF, e.g., superoxide dismutase, ESAT-6, and CFP29 (40, 44, 49). In this study only two of the six molecules identified, CFP21 and CFP25, contain the typical consensus sequence for a signal peptide (46).
Export of proteins lacking classical signal peptides has been described for several bacterial species (for reviews, see references 37, 38, and 41), but not much is known about the actual translocation of these proteins across the plasma membrane. In E. coli, one signal peptide-independent pathway, the ABC protein-mediated export mechanism, involves three proteins located in the membrane. Both proteins secreted by this pathway, as well as the Yop proteins secreted from yersiniae, contain particular sequences involved in secretion but lacking the classical features of a signal peptide (8, 47). Whether similar mechanisms exists in mycobacteria is yet to be established, but a recent report indicated that the signal for secretion in some cases may be encoded internally in mycobacterial proteins (20). It is interesting that both CFP17 and CFP22 are preceded by peptides apparently cleaved from the mature protein and that none of them have the characteristics of typical signal peptides. These peptides could play a role in protein secretion or, alternatively, may be cleaved off as a result of nonspecific degradation.
Another possible explanation for the seven amino acids preceding the CFP22 sequence found in ST-CF could be that the start codon of this open reading frame is GTG (coding for Val in position 7) and not the predicted ATG (coding for Met in position 1). If this is the case, then the predicted first amino acid of the mature protein will be Thr (in position 8), in agreement with the N-terminal sequence of the protein present in ST-CF. Analysis of the DNA sequence upstream from the two possible start codons did not clarify this, as no consensus Shine-Dalgarno sequence could be identified (data not shown).
There is no doubt that although ST-CF is produced from very early cultures, some autolysis will take place, leading to release of proteins directly from the cytoplasm. CFP22 is 90% identical to an M. leprae peptidyl-prolyl isomerase which functions as an intracellular housekeeping enzyme during protein synthesis (for a review, see reference 16), and if this protein serves the same function in M. tuberculosis, the small amount of CFP22 in ST-CF must be a result of autolysis. In this regard, it is noteworthy that CFP22 is not recognized in any of the experimentally infected animal models used. This finding, together with the strong recognition of the rest of the proteins isolated, supports our present understanding that extracellular antigens are the main targets recognized in the first phase of M. tuberculosis infection, leading to early control of disease.
ACKNOWLEDGMENTS
This investigation received financial support from The European Community (project no. TS3*CT94-0313 and BNH-4-CT97-2167) and the Center for Advanced Food Research.
We are grateful to Charlotte Adamzcky Bak, Annette Hansen, Birgitte Smedegaard, and Bente Isbye for excellent technical assistance.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen P, Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen P, Ljungqvist L, Haslov K, Bentzon M W, Heron I. Proliferative response to seven affinity purified mycobacterial antigens in eight strains of inbred mice. Int J Lepr Mycobact Dis. 1991;59:58–67. [PubMed] [Google Scholar]
- 7.Baek L. New, sensitive rocket immunoelectrophoretic assay for measurement of the reaction between endotoxin and Limulus amoebocyte lysate. J Clin Microbiol. 1983;17:1013–1020. doi: 10.1128/jcm.17.6.1013-1020.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 9.Bini L, Sanchez-Campillo M, Santucci A, Magi B, Marzocchi B, Comanducci M, Christiansen G, Birkelund S, Cevenini R, Vretou E, Ratti G, Pallini V. Mapping of Chlamydia trachomatis proteins by immobiline-polyacrylamide two-dimensional electrophoresis: spot identification by N-terminal sequencing and immunoblotting. Electrophoresis. 1996;17:185–190. doi: 10.1002/elps.1150170130. [DOI] [PubMed] [Google Scholar]
- 10.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 11.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt L, Oettinger T, Holm A, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 13.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhay M J, Andersen P. Immunological requirements for a subunit vaccine against tuberculosis. Immunol Cell Biol. 1997;74:595–603. doi: 10.1038/icb.1997.94. [DOI] [PubMed] [Google Scholar]
- 15.Engers H D, Bennedsen J, Buchanan T M, Chaparas S D, Kadival G, Closs O, David J R, van-Embden J, Godal T, Mustafa A S, Ivanyi J, Young D B, Kaufmann S H E, Khoenko A G, Kolk A H J, Kubin M, Louis J A, Minden P, Shinnick T M, Trnka L. Results of a World Health Organization-sponsored workshop to characterize antigens recognized by Mycobacterium-specific monoclonal antibodies. Infect Immun. 1986;51:718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker J, Fischer G. Immunophilins: structure-function relationship and possible role in microbial pathogenicity. Mol Microbiol. 1993;10:445–456. doi: 10.1111/j.1365-2958.1993.tb00917.x. [DOI] [PubMed] [Google Scholar]
- 17.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harth G, Clemens D L, Horwitz M A. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc Natl Acad Sci USA. 1994;91:9342–9346. doi: 10.1073/pnas.91.20.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harth G, Horwitz M A. Expression and efficient export of enzymatically active Mycobacterium tuberculosis glutamine synthetase in Mycobacterium smegmatis and evidence that the information for export is contained within the protein. J Biol Chem. 1997;272:22728–22735. doi: 10.1074/jbc.272.36.22728. [DOI] [PubMed] [Google Scholar]
- 21.Harth G, Lee B Y, Horwitz M A. High-level heterologous expression and secretion in rapidly growing nonpathogenic mycobacteria of four major Mycobacterium tuberculosis extracellular proteins considered to be leading vaccine candidates and drug targets. Infect Immun. 1997;65:2321–2328. doi: 10.1128/iai.65.6.2321-2328.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslov K, Andersen A, Nagai S, Gottschau A, Sorensen T, Andersen P. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochstrasser D F, Harrington M G, Hochstrasser A C, Miller M J, Merril C R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988;173:424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Ljungqvist L, Andersen A B, Andersen P, Haslov K, Worsaae A, Bennedsen J, Heron I. Affinity purification, biological characterization and serological evaluation of defined antigens from Mycobacterium tuberculosis. Trop Med Parasitol. 1990;41:333–335. [PubMed] [Google Scholar]
- 28.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez C, De-Geus P, Lauwereys M, Matthyssens G, Cambillau C. Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature. 1992;356:615–618. doi: 10.1038/356615a0. [DOI] [PubMed] [Google Scholar]
- 30.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 32.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlman D, Halvorson H O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983;167:391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- 35.Philipp W J, Poulet S, Eiglmeier K, Pascopella L, Balasubramanian V, Heym B, Bergh S, Bloom B R, Jacobs W R, Cole S T. An integrated map of the genome of the tubercle bacillus, Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc Natl Acad Sci USA. 1996;93:3132–3137. doi: 10.1073/pnas.93.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pugsley A P, Schwartz M. Export and secretion of proteins by bacteria. FEMS Microbiol Rev. 1985;32:3–38. [Google Scholar]
- 39.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenkrands I, Rasmussen P B, Carnio M, Jacobsen S, Theisen M, Andersen P. Identification and characterization of a 29-kilodalton protein from Mycobacterium tuberculosis culture filtrate recognized by mouse memory effector cells. Infect Immun. 1998;66:2728–2735. doi: 10.1128/iai.66.6.2728-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonen M, Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993;57:109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha R K, Verma I, Khuller G K. Immunobiological properties of a 30 kDa secretory protein of Mycobacterium tuberculosis H37Ra. Vaccine. 1997;15:689–699. doi: 10.1016/s0264-410x(96)00230-7. [DOI] [PubMed] [Google Scholar]
- 43.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen A L, Nagai S, Houen G, Andersen P, Andersen A B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urquhart B L, Atsalos T E, Roach D, Basseal D J, Bjellqvist B, Britton W L, Humphrey-Smith I. Proteomic contigs of Mycobacterium tuberculosis and Mycobacterium bovis (BCG) using novel immobilised pH gradients. Electrophoresis. 1997;18:1384–1392. doi: 10.1002/elps.1150180813. [DOI] [PubMed] [Google Scholar]
- 46.von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 47.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 48.Worsaae A, Ljungqvist L, Heron I. Monoclonal antibodies produced in BALB.B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J Clin Microbiol. 1988;26:2608–2614. doi: 10.1128/jcm.26.12.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young D B, Kaufmann S H, Hermans P W, Thole J E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]