Abstract
Transposon and marker exchange mutagenesis were used to evaluate the role of Aeromonas cytotoxic enterotoxin (Act) in the pathogenesis of diarrheal diseases and deep wound infections. The transposon mutants were generated by random insertion of Tn5-751 in the chromosomal DNA of a diarrheal isolate SSU of Aeromonas hydrophila. Some of the transposon mutants had dramatically reduced hemolytic and cytotoxic activities, and such mutants exhibited reduced virulence in mice compared to wild-type Aeromonas when injected intraperitoneally (i.p.). Southern blot data indicated that transposition in these mutants did not occur within the cytotoxic enterotoxin gene (act). The transcription of the act gene was affected drastically in the transposon mutants, as revealed by Northern blot analysis. The altered virulence of these transposon mutants was confirmed by developing isogenic mutants of the wild-type Aeromonas by using a suicide vector. In these mutants, the truncated act gene was integrated in place of a functionally active act gene. The culture filtrates from isogenic mutants were devoid of hemolytic, cytotoxic, and enterotoxic activities associated with Act. These filtrates caused no damage to mouse small intestinal epithelium, as determined by electron microscopy, whereas culture filtrates from wild-type Aeromonas caused complete destruction of the microvilli. The 50% lethal dose of these mutants in mice was 1.0 × 108 when injected i.p., compared to 3.0 × 105 for the wild-type Aeromonas. Reintegration of the native act gene in place of the truncated toxin gene in isogenic mutants resulted in complete restoration of Act’s biological activity and virulence in mice. The animals injected with a sublethal dose of wild-type Aeromonas or the revertant, but not the isogenic mutant, had circulating toxin-specific neutralizing antibodies. Taken together, these studies clearly established a role for Act in the pathogenesis of Aeromonas-mediated infections.
Aeromonas species, which have recently been placed in a new family, Aeromonadaceae, are responsible for causing a variety of human infections, including septicemia, wound infections, meningitis, pneumonia, and gastroenteritis (5). Among various virulence factors produced by Aeromonas species, enterotoxins are by far the most important in causing Aeromonas-mediated infections (1, 13, 28, 32). The cytotoxic enterotoxin gene (act) from the human diarrheal isolate SSU of Aeromonas hydrophila has been cloned, sequenced, and hyperexpressed in our laboratory (14). Four biological activities, namely, hemolytic, cytotoxic, and enterotoxic activities as well as lethality, have been shown in mice to be associated with cytotoxic enterotoxin (Act) (39). Act is a single-chain polypeptide with an estimated molecular mass of 52 kDa (40). The toxin protein is secreted as an inactive precursor (54 kDa), which is converted into the active form by proteolytic processing near the C terminus (14). Act is an aerolysin-related toxin which exhibited approximately 90% homology with an aerolysin from a fish isolate of Aeromonas bestiarum (previously designated A. hydrophila) (1, 23, 24). In contrast, comparison of Act with an aerolysin from a diarrheal isolate of Aeromonas trota revealed approximately 75% homology (1, 9, 26). Recently, an aerolysin-related toxin also was isolated from a gram-positive organism, Clostridium septicum (7).
We identified regions on Act involved in the biological functions of the toxin by deletion analysis, generation of antipeptide antibodies, and site-directed mutagenesis (16). Our data indicated that although Act had significant homology with aerolysin, there are enough differences that differential folding of these two protein molecules could occur (16, 17, 19). Further, our data suggested that there may be different loci coding for specific biological activities of Act.
Mechanism-of-action studies revealed that Act operated by creating pores, estimated to be 1.14 to 2.8 nm in diameter, in the erythrocyte membranes (17). The toxin appeared to undergo aggregation when preincubated with cholesterol, which resulted in a loss of Act’s hemolytic activity (17), indicating cholesterol to be one of the receptors for Act (17). Recently, Nelson et al. (34) reported that Thy-1, a major surface glycoprotein of T lymphocytes, is a high-affinity receptor for aerolysin from A. bestiarum.
In the present study, we have constructed transposon and isogenic mutants that were defective in the production of Act from wild-type A. hydrophila SSU to determine Act’s precise role in the overall virulence of Aeromonas. These mutants not only were devoid of Act-associated biological activities but were significantly less virulent in mice than wild-type Aeromonas, proving unequivocally the role of Act in Aeromonas-mediated infections.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A. hydrophila SSU, a diarrheal isolate, was obtained from the Centers for Disease Control and Prevention, Atlanta, Ga. The identity of this culture as A. hydrophila was confirmed by DNA-DNA hybridization and ribotyping (5). Isolate A52 of an Aeromonas species was provided by M. Kai, Tokai University, Kanagawa, Japan. A strain of Escherichia coli harboring plasmid pME9 with transposon Tn5-751 was obtained from S. P. Howard, University of Regina, Regina, Saskatchewan, Canada. The transposon Tn5-751 had two antibiotic resistance genes coding for kanamycin and trimethoprim. Rifampin- and streptomycin-resistant spontaneous mutants of A. hydrophila were prepared during these studies. Suicide vector pJQ200KS, which contained a P15A origin of replication, a sacB gene from Bacillus subtilis, and a gentamicin resistance gene, was obtained from M. K. Hynes, The University of Calgary, Calgary, Alberta, Canada (36). E. coli S17-1, with streptomycin and trimethoprim resistance and lysogenized with λpir (20, 36), was from S. J. Libby, North Carolina State University, Raleigh, N.C. Plasmid pMW1823, another suicide vector, with a chloramphenicol resistance gene from pACYC184, an origin of replication from plasmid pSC101, and the mob region from plasmid pJM703.1, was provided to us by V. L. Miller, Washington University School of Medicine, St. Louis, Mo. Plasmid pXHC95 contained a 2.8-kb BamHI DNA fragment from A. hydrophila chromosomal DNA and harbored the act gene. This plasmid had an ampicillin resistance gene and was propagated in E. coli XL1-Blue cells. Plasmid pUC4K contained a 1.2-kb kanamycin resistance gene cassette, which represented a portion of the transposon Tn903 (Pharmacia Biotech Inc., Piscataway, N.J.). The E. coli clones with recombinant plasmids, as well as Aeromonas cultures, were stored in Luria-Bertani (LB) medium containing 25% (vol/vol) glycerol at −70°C. The concentrations of antibiotics used to grow cultures were as follows: 50 μg of ampicillin per ml, 40 μg of rifampin per ml for transposon mutants and 300 μg of rifampin per ml for isogenic mutants, 25 μg of streptomycin per ml, 25 μg of trimethoprim per ml, 50 μg of kanamycin per ml, 15 μg of gentamicin per ml, and 20 μg of chloramphenicol per ml.
Transposon mutagenesis.
The transposon Tn5-751 from plasmid pME9 in E. coli was delivered to Aeromonas by conjugation as previously described (20, 38). Briefly, both E. coli(pME9) and streptomycin-resistant A. hydrophila SSU were grown under static conditions at 37°C overnight. The cultures were mixed (5 ml each) at a concentration of 8 × 106 cells/ml, centrifuged (4,000 × g for 10 min), resuspended in 200 μl of LB medium, and plated on LB plates without any antibiotic pressure. After 4 h of incubation at 37°C, the culture was removed from the plate and various dilutions (10−4 to 10−9) of the sample were plated on LB plates with streptomycin, kanamycin, and trimethoprim. The cultures were identified as Aeromonas by a positive oxidase test to differentiate them from E. coli and by an automated identification system (Vitech, St. Louis, Mo.) in the Clinical Microbiology Laboratory, The University of Texas Medical Branch, Galveston. Further, dot blot hybridization (6) was utilized to differentiate toxin-bearing Aeromonas from nontoxigenic E. coli used in the conjugation experiment. The denatured total DNA samples were applied to a nitrocellulose membrane under vacuum in a dot blot apparatus (Bio-Rad, Hercules, Calif.). The filters were dried and baked at 80°C for 2 h. The blots were prehybridized and hybridized by using Quikhyb (Stratagene, La Jolla, Calif.) at 68°C as described by the manufacturer. The probes used included a 1.4-kb DNA fragment containing the full-length act gene and a 439-bp DNA fragment representing the 5′ end of the toxin gene (14) and were labeled with [α-32P]dCTP (ICN, Irvine, Calif.) by using a random primer kit (GibcoBRL, Gaithersburg, Md.). The filters were washed at 68°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (pH 7.0) plus 0.1% sodium dodecyl sulfate (SDS) for 1 h and then in 1× SSC plus 0.1% SDS for 30 min at 68°C. The blots were exposed to the X-ray film at −70°C for 2 to 12 h.
The biological activity of Act in the culture filtrates, cell lysates, and cell debris of these transposon mutants was evaluated by hemolytic and cytotoxic assays (39). The cell lysates were prepared by resuspending the cells in phosphate-buffered saline (PBS) in the original culture volume, and the cells were sonicated (Sonifier cell disruptor 185; Branson Sonic Power Co., Danbury, Conn.). The mixture was centrifuged at 10,000 × g for 15 min at 4°C to separate cell lysate from cell debris, which then was resuspended in the original culture volume.
Construction of isogenic mutants of Aeromonas via double-crossover recombination.
Recombinant plasmid pXHC95 (14) was used to construct isogenic mutants (Fig. 1). The BstEII restriction enzyme was used to digest this plasmid and to linearize it, since there was only one BstEII site within the 2.8-kb BamHI DNA insert (Fig. 1). There were no BstEII sites in the pBluescript expression vector used to construct recombinant plasmid pXHC95. The ends of the 2.8-kb DNA fragment were made blunt by using a PCR polishing kit (Stratagene). Subsequently, a 1.2-kb kanamycin gene cartridge was isolated from plasmid pUCK4 by restriction with enzyme PstI, which bordered the kanamycin gene cassette (Fig. 1). An appropriate-sized DNA fragment was excised from a 0.8% agarose gel, extracted with phenol-chloroform, ethanol precipitated (6), and finally purified with a GeneClean II kit (Bio 101, Vista, Calif.). The ends of the kanamycin gene cassette were made blunt and ligated to the blunt-ended 2.8-kb BstEII DNA fragment by using T4 DNA ligase (Promega, Madison, Wis.).
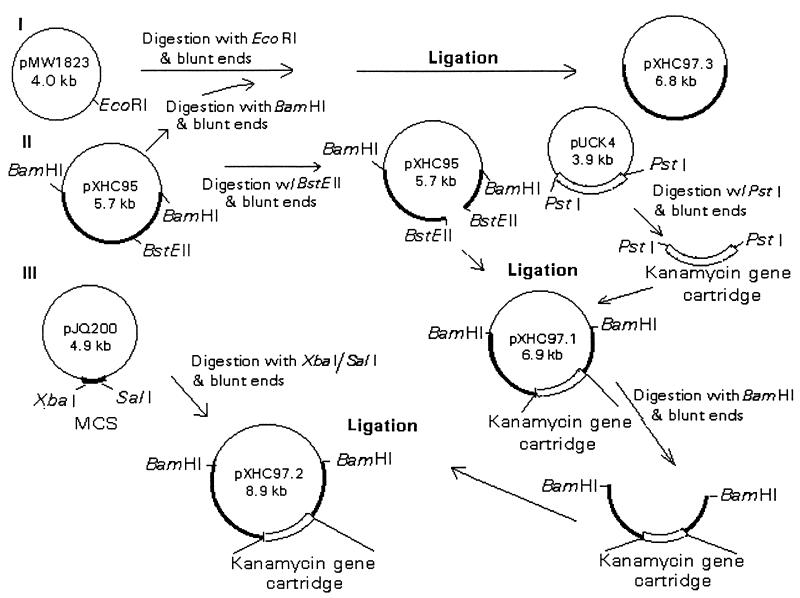
FIG. 1.
Flow diagram showing construction of various recombinant plasmids. Recombinant plasmid pXHC95 contained a 2.8-kb BamHI DNA fragment from chromosomal DNA of A. hydrophila SSU with the act gene (II). A kanamycin resistance gene cartridge from plasmid pUCK4 was introduced within the act gene to generate recombinant plasmid pXHC97.1 before ligation of the truncated act gene in the suicide vector pJQ200 to generate plasmid pXHC97.2 (III). A 2.8-kb BamHI DNA fragment containing the act gene also was subcloned in another suicide vector, pMW1823, to generate plasmid pXHC97.3 (I) for the purpose of generating a revertant of A. hydrophila with parental biological activity of Act. MCS, multiple cloning site.
The new recombinant plasmid was designated pXHC97.1 (Fig. 1) and transformed into E. coli XL1-Blue cells by electroporation (Cell-Porator Electroporation System I; GibcoBRL). The transformants were identified on LB agar plates containing ampicillin and kanamycin, and their identity was confirmed by miniplasmid isolation and digestion of the recombinant plasmid with BamHI restriction enzyme (14). Fragments of 2.9 and 4.0 kb were visualized and represented, respectively, pBluescript vector DNA and a 2.8-kb act gene-containing DNA fragment truncated with a 1.2-kb kanamycin gene cassette (total fragment size, 4.0 kb). Further, PCR was performed to demonstrate the kanamycin gene cartridge and the act-specific gene sequences within the 4.0-kb DNA fragment. The two primers used to amplify the kanamycin gene cassette sequence were 5′-CGCTGAGGTCTGCTCGTGAAGAAGGTGTT-3′ (representing bp 434 to 464) and 5′-AAAGCCACGTTGTGTCTCAAAATCTCTGATGT-3′ (representing bp 1613 to 1645) from the pUCK4 plasmid. The primer sequences which amplified the act gene were 5′-ATAGAGTCTAGACTCCATGCAAAAACTAAAAAAACTGGCTTGT-3′ (bp 884 to 911) and 5′-CATCCTGTCGACTAAGCTTTTATTGATTGGCTGCTGGCGTCACG-3′ (bp 2341 to 2365) (14). The underlined bases represented XbaI and SalI restriction sites, respectively. The deoxyoligonucleotides were synthesized by Biosynthesis, Inc., Lewisville, Tex. A Geneamp reagent kit with AmpliTaq DNA polymerase was used for PCR, as described by the manufacturer (Perkin-Elmer Cetus, Norwalk, Conn.). The sequence of the PCR product was verified by DNA sequence analysis with a Sequenase PCR sequencing kit (Amersham Life Sciences, Cleveland, Ohio).
This strategy to prepare isogenic mutants provided 1.9 and 0.9 kb of the flanking 5′ and 3′ DNA sequences, respectively, for double crossover. The biological activities (hemolytic and cytotoxic) of truncated (with the kanamycin resistance gene cassette) and native Act, with the act gene cloned in pBluescript vector under the control of a T7 promoter, were examined in E. coli (Fig. 1). The cultures were induced for 4 h at 37°C with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Subsequently, the recombinant plasmid pXHC97.1 was isolated from E. coli XL1-Blue cells by using a kit from Qiagen (Chatsworth, Calif.) and digested with the BamHI restriction enzyme. A 4.0-kb DNA fragment, which encompassed the act gene truncated with a kanamycin resistance gene cassette, was isolated, purified, and ligated to the blunt-ended suicide vector pJQ200KS, which was digested originally with the XbaI and SalI restriction enzymes. The newly constructed recombinant suicide vector was designated pXHC97.2 and transformed into E. coli S17-1 (Fig. 1). The transformants were identified on the LB agar plates supplemented with streptomycin, trimethoprim, ampicillin, kanamycin, and gentamicin, and the identity of cultures with the correct recombinant plasmid was verified by PCR with the specific primers described above.
Conjugation between A. hydrophila and E. coli.
The recombinant E. coli S17-1(pXHC97.2) (Fig. 1) was conjugated with rifampin-resistant Aeromonas, as described previously for development of transposon mutants (20, 38), and the transconjugants were selected on LB agar plates with rifampin, kanamycin, and gentamicin to select for single-crossover transconjugants or with rifampin, kanamycin, and 5% sucrose to select for double-crossover transconjugants. The integration of the recombinant suicide vector into the chromosome of Aeromonas was confirmed by Southern blot analysis.
The probes used for Southern analysis included act gene-specific sequences (as described above), the 4.9-kb plasmid pJQ200KS, and a 1.2-kb kanamycin resistance gene cassette. The filters were hybridized and washed as described for dot blot hybridization.
Northern blot analysis.
Total RNA was isolated from various transposon mutants and wild-type A. hydrophila by using the total RNA isolation kit from Qiagen. The RNA samples (6 μg) were subjected to electrophoresis on a 1.2% formaldehyde–agarose gel with 1× MOPS buffer (0.2 M MOPS [morpholinepropanesulfonic acid] [pH 7.0], 0.05 M sodium acetate, 0.01 M EDTA, pH 8.0) (6). The RNA was transferred to a nylon membrane (GibcoBRL), and after baking, the filters were prehybridized, hybridized, and washed as described for dot blot analysis. The amount of RNA in each lane was quantitated by densitometer scanning (Applied Imaging, Pittsburgh, Pa.) of 23S and 16S rRNA bands after ethidium bromide staining of the gel. All of the reagents used for Northern blot analysis were treated with diethylpyrocarbonate.
Measurement of biological activities.
The biological activity of Act was determined by cytotoxic, hemolytic, and enterotoxic assays.
(i) Cytotoxic assay.
The culture filtrates, cell lysates, and cell membranes from various Aeromonas cultures (grown for 18 h in LB medium at 37°C) were diluted twofold with PBS and added to Chinese hamster ovary (CHO) cells. After 18 to 20 h of incubation at 37°C in the presence of 5% CO2, the cytotoxic activity was recorded. The cytotoxic unit was defined as the reciprocal of the highest dilution of the toxin demonstrating 50% destruction of CHO cells (39).
(ii) Hemolytic assay.
A volume of 100 μl of PBS was added to each of the wells of a 96-well microtiter plate. Next, 100 μl of twofold-diluted toxin preparation was added, followed by 100 μl of 2% rabbit erythrocytes (39). The plate was incubated at 37°C for 1 h and observed for hemolytic activity. The hemolytic unit was defined as the reciprocal dilution of Act demonstrating 50% lysis of rabbit erythrocytes. To demonstrate that the residual cytotoxic and hemolytic activities in various transposon mutants were indeed due to Act, antibodies specific for Act were used to neutralize Act’s biological effects. The toxin preparations were mixed with antibodies and incubated at 37°C for 1 h before being placed on the erythrocytes and CHO cells. The ability of these antibodies to neutralize the biological activity of Act then was evaluated. Preimmune serum was used as a negative control in neutralization experiments.
(iii) Enterotoxic assay.
Outbred Swiss-Webster mice (25 to 30 g; Taconic Farms, Inc., Germantown, N.Y.) were anesthetized with halothane (River Edge, N.J.). An abdominal incision was made, and a single 5-cm loop was constructed, as previously described with 00 silk suture (35). A 100-μl test sample was injected into the loop. After 6 h of observation, the animals were euthanized by cervical dislocation, and the intestinal loops were removed. The amount of luminal fluid was measured and expressed as microliters per centimeter.
LD50 determination.
The wild-type A. hydrophila, its transposon and isogenic mutants, and the revertant were grown in LB medium and centrifuged, and the cells were washed twice with PBS. The bacterial cells were resuspended in PBS and injected intraperitoneally (i.p.) into Swiss-Webster mice (6 to 10 mice per group) at various doses (104 to 1010 CFU), and the mice were observed for death. The 50% lethal dose (LD50) was determined by the method of Reed and Muench (37).
Electron microscopy.
The mouse intestinal loops injected with culture filtrates from wild-type A. hydrophila and its isogenic mutants were removed after 6 h for electron microscopic studies. Small pieces (1 mm) of small intestine were fixed (17), and ultrathin sections were cut with a Sorvall MT-6000 ultramicrotome (RMC, Tucson, Ariz.) and stained with uranyl acetate and lead citrate. The sections were examined and photographed in a model 201 electron microscope (Phillips Electron Optics, Eindhoven, The Netherlands) at 60 kV.
SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Culture filtrates from A. hydrophila and its mutants were analyzed by SDS–10 to 12% polyacrylamide gel electrophoresis (30) and Western blot analysis (Bio-Rad) with toxin-specific antibodies developed in mice or rabbits. Goat anti-mouse or goat anti-rabbit immunoglobulin conjugated with alkaline phosphatase or horseradish peroxidase was used as the secondary antibody (diluted 1:3,000). The blots were developed with either an alkaline phosphatase substrate kit (Bio-Rad) or an enhanced chemiluminescence substrate (Pierce, Rockford, Ill.).
RESULTS
Transposon mutagenesis of chromosomal DNA of A. hydrophila SSU.
Approximately 4,000 transposon mutants of A. hydrophila were screened for biological activity. Culture filtrates from five mutants displayed drastically reduced biological activities, as measured by hemolytic and cytotoxic assays (Table 1). All of these mutants were confirmed to be Aeromonas. We also performed dot blot hybridization of the total DNAs from wild-type A. hydrophila and its transposon mutants by using a 439-bp act-specific gene probe. The DNAs from these cultures exhibited a positive signal, while total DNA from E. coli did not hybridize with this probe. It was crucial to differentiate Aeromonas from E. coli used in the conjugation before proceeding further, because Aeromonas and E. coli exhibited very similar biochemical profiles. We indeed obtained streptomycin-, kanamycin-, and trimethoprim-resistant colonies with no hemolytic activity, but these were identified as E. coli. The frequency at which these colonies appeared was relatively high (25 to 30%). We also confirmed that the streptomycin-resistant spontaneous mutants of Aeromonas used in the conjugation experiment exhibited biological activity similar to that of wild-type Aeromonas.
TABLE 1.
Hemolytic and cytotoxic activities of Act produced by wild-type and transposon mutants of A. hydrophila SSUa
| Culture | Hemolytic activity in:
|
Cytotoxic activity in:
|
||||
|---|---|---|---|---|---|---|
| Culture filtrates | Cell lysates | Membranes | Culture filtrates | Cell lysates | Membranes | |
| Wild-type SSU | 128 | 16 | 4 | 4,096 | 16 | 4 |
| Transposon mutants | ||||||
| 42 | 16 | 0 | 0 | 4 | 0 | 0 |
| 225 | 32 | 0 | 0 | 32 | 0 | 0 |
| 312 | 32 | 0 | 0 | 64 | 0 | 0 |
| 325 | 32 | 0 | 0 | 8 | 0 | 0 |
| 353 | 16 | 0 | 0 | 32 | 0 | 0 |
Hemolytic and cytotoxic activities were defined as the reciprocal of the highest dilution of the toxin that caused 50% lysis of erythrocytes or 50% destruction of CHO cells, respectively. All cultures were grown overnight with shaking (150 rpm) at 37°C in LB medium. The cells were harvested by centrifugation, and the culture filtrates, cell lysates, and membranes were saved for measuring the toxin activity.
Southern blot analysis was performed by using plasmid pME9 with Tn5-751 as a probe to demonstrate that transposition indeed occurred in the chromosomal DNA of A. hydrophila. Our data indicated the presence of a single copy of the transposon in the digested (SalI-BamHI) chromosomal DNAs of all five mutants of A. hydrophila tested. Digested genomic DNAs from E. coli and wild-type A. hydrophila did not react with this probe (data not shown). Similar genomic digests also were probed with the act-specific gene probe in Southern blots (Fig. 2). Interestingly, a 2.8-kb DNA fragment hybridized with this probe, irrespective of whether the chromosomal DNA was isolated from wild-type A. hydrophila or its transposon mutants (Fig. 2). These data implied that transposition might have occurred in some other region (e.g., a regulatory element) and not within the structural gene coding for the toxin.
FIG. 2.
Southern blot analysis of the genomic DNAs from wild-type and transposon mutants of A. hydrophila. The genomic DNA (15 μg) was digested with the SalI and BamHI restriction enzymes and subjected to Southern blot analysis. The probe used was a 1.4-kb XbaI/SalI DNA fragment (32P labeled), which depicts the coding region of the act gene. The blot was prehybridized, hybridized (5 × 106 cpm/ml), and washed as described in Materials and Methods. The lanes contained digested DNAs from the transposon mutants 353 (lane 1), 325 (lane 2), 312 (lane 3), 225 (lane 4), and 42 (lane 5) and from wild-type A. hydrophila SSU (lane 6) as a positive control.
The biological activity of Act in culture filtrates, cell lysates, and membranes of these transposon mutants was measured. Minimal or no toxin activity was detected in the cell lysates and membrane fractions, and reduced biological activity was observed in culture filtrates of these mutants (Table 1). These results indicated that transposition did not alter the export machinery of Aeromonas. To rule out the possibility that transposition caused delayed toxin production, both wild-type A. hydrophila SSU and its transposon mutants were grown for 96 h. Every 4 h, a culture sample was removed and total viable counts were determined. The supernatants, cell lysates, and cell membranes were examined for hemolytic and cytotoxic activities. Wild-type Aeromonas demonstrated the highest hemolytic and cytotoxic activities at 18 h. The transposon mutants similarly exhibited the highest, albeit significantly reduced, biological activity at 18 h, although the mutants had viable counts similar to those of wild-type Aeromonas. After this time point, no further increase in hemolytic and cytotoxic activities was noted for the mutant cultures. Coincident with these data was the reduced amount of Act antigen on Western blots in transposon mutants compared to wild-type Aeromonas (data not shown). By using specific polyclonal antibodies to Act, it was demonstrated that the residual biological activity in these transposon mutants was contributed by Act, since both the remaining hemolytic and cytotoxic activities of Act were abolished by Act-specific antibodies.
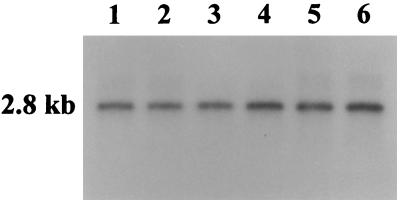
Based on the Southern blot data in Fig. 2, we performed Northern blot analysis on the total RNA isolated from wild-type A. hydrophila SSU and its transposon mutants to examine the expression of the toxin gene. A weak transcript or no transcript was detected in the transposon mutants (Fig. 3, lanes 1 to 5), whereas a transcript of approximately 1.4 kb was detected in wild-type A. hydrophila (Fig. 3, lane 6). The data in Fig. 3 and Table 1 demonstrated that the transposon mutants synthesized Act, albeit at low levels.
FIG. 3.
Northern blot analysis of the total RNAs isolated from the wild-type and transposon mutants of A. hydrophila. The total RNA was isolated by using a total RNA isolation kit (Qiagen). A 439-bp 32P-labeled DNA fragment, which represented the 5′ end of the toxin structural gene, was used as a probe in these blots. The blot was prehybridized, hybridized, and washed as described in Materials and Methods. The blots were exposed to X-ray film at −70°C for 12 h. The lanes contained RNAs from mutant cultures 353 (lane 1), 325 (lane 2), 312 (lane 3), 225 (lane 4), and 42 (lane 5) and from wild-type A. hydrophila SSU (lane 6).
Lethality studies with the Act-deficient Aeromonas strain A52 and transposon mutants of A. hydrophila SSU.
Isolate A52 of Aeromonas is naturally deficient in the act gene, as confirmed by Southern analysis and biological activity measurements. Both A. hydrophila SSU (Act positive) and Aeromonas strain A52 (Act negative) were injected i.p. into mice to demonstrate the role of Act in Aeromonas-mediated infections. The LD50 of Aeromonas strain A52 was calculated to be 3.9 × 109. However, the LD50 of wild-type A. hydrophila SSU was 2.5 × 107, indicating Act’s role in the organism’s virulence. The difference in the LD50s between these two cultures was statistically significant (P = 0.01) by the Fisher exact test. The sera from animals that survived the challenge with A. hydrophila SSU contained toxin-specific antibodies; however, the sera from animals challenged with Aeromonas strain A52 were devoid of toxin-specific antibodies as determined by Western blot analysis (data not shown).
Wild-type Aeromonas isolate SSU and all of the five transposon mutants with reduced hemolytic and cytotoxic activities (Table 1) were injected i.p. into mice to validate the data obtained with a toxin-deficient strain of Aeromonas. For these studies, we selected only one dose of bacteria (5 × 107). All mice injected with wild-type Aeromonas died within 6 to 24 h. Pure cultures of A. hydrophila could be isolated from the spleens and livers of the dead animals. However, none of the mice injected with the transposon mutants at this dose died over a 2-week observation period (data not shown).
Characterization of double-crossover mutants of Aeromonas with an altered act gene.
The strategy used to develop an isogenic mutant of Aeromonas is depicted in Fig. 1. The single-crossover transconjugants obtained after conjugation of wild-type, rifampin-resistant A. hydrophila SSU with E. coli S17-1 harboring plasmid pXHC97.2 did not grow in the presence of 5% sucrose, as it induces the sacB gene, coding for levan sucrase, which is lethal to cells when produced in large amounts. The hemolytic activity of these single-crossover mutants was similar to that of wild-type Aeromonas.
The colonies which grew in the presence of sucrose should represent genuine double-crossover mutants, since the suicide vector sequences containing sacB and gentamicin resistance genes should be lost as a result of the second crossover. Those colonies which grew in the presence of sucrose but were sensitive to gentamicin were chosen for further studies. The genuine double-crossover mutants with no hemolytic activity on 5% sheep blood agar plates were obtained at a frequency of 0.01%. These mutants were grown in LB medium for 18 h, and the culture filtrates, cell lysates, and membranes were examined for hemolytic and cytotoxic activities. Table 2 shows that the double-crossover mutants had no biological activity (e.g., hemolytic, cytotoxic, and enterotoxic activities) compared to wild-type A. hydrophila. Western blot analysis also did not demonstrate any protein band corresponding to Act in the culture filtrates of double-crossover mutants. A band corresponding to 52 kDa was detected in the culture filtrates of wild-type A. hydrophila and single-crossover mutants (data not shown).
TABLE 2.
Biological activities of Act produced in culture filtrates of A. hydrophila SSU and its isogenic mutant and revertant
| Culture | Hemolytic activitya | Cytotoxic activitya | Enterotoxic activity (μl/cm)b |
|---|---|---|---|
| Wild-type A. hydrophila with native Act | 1:512 | 1:2,048 | 118 ± 21 |
| E. coli with suicide vector pJQ200c containing a truncated act gene | 0 | 0 | NDd |
| Double-crossover mutant of A. hydrophila with a truncated act gene | 0 | 0 | 0 |
| Revertant strain of A. hydrophila in which the truncated act gene was replaced with the native act gene | 1:512 | 1:2,048 | 112 ± 12 |
The hemolytic and cytotoxic activities were defined as the reciprocal of the highest dilution of the toxin causing 50% lysis of erythrocytes or 50% destruction of CHO cells, respectively.
Enterotoxic activity was determined in mouse ligated intestinal loops. Results are means ± standard deviations.
Suicide vector pJQ200 gives a gentamicin-resistant and sucrose-sensitive phenotype to the cultures.
ND, not done.
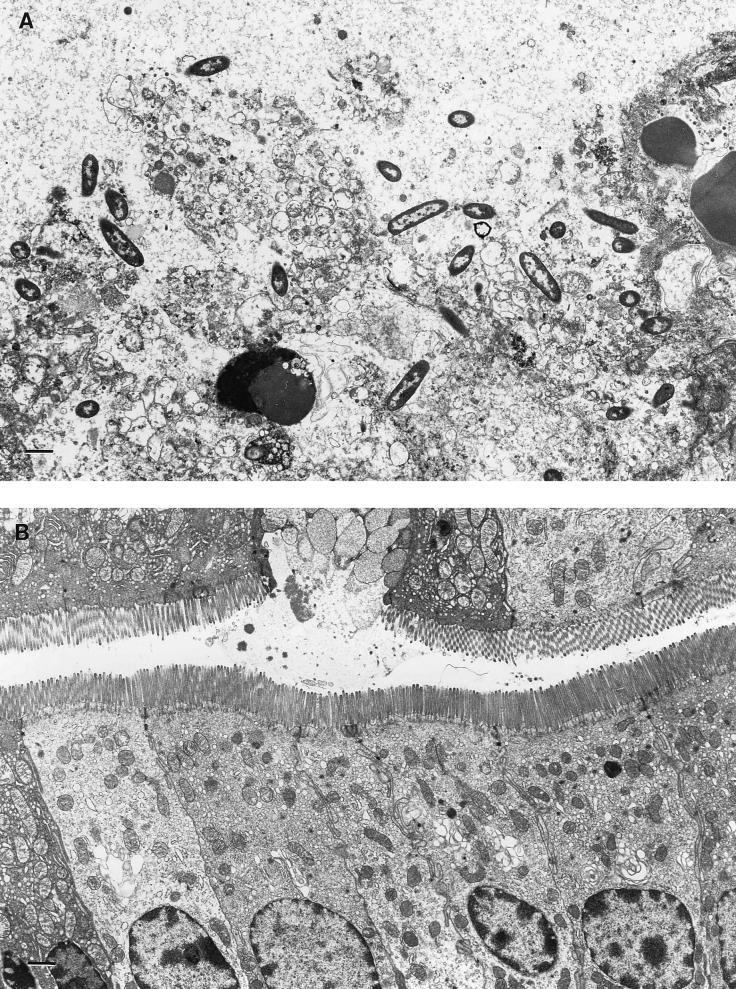
By electron microscopy, extensive tissue damage was found in the ligated small intestine injected with the culture filtrates from wild-type Aeromonas (Fig. 4A). The fluid accumulation response in a group of 10 mice was 118 ± 21 μl/cm (mean ± standard deviation) (Table 2). No tissue damage or fluid secretion was observed when the loops were challenged with culture filtrates from double-crossover mutants (Fig. 4B). The enterocytes had intact microvilli and a normal appearance.
FIG. 4.
Electron microscopy of intestinal tissues of mice injected with culture filtrates from wild-type Aeromonas and its isogenic mutant. Mouse ligated loops were placed in fixative and cut into 1-mm pieces. Ultrathin sections were stained and photographed in a Philips 201 electron microscope. (A) After administration of Act (contained in culture filtrate) from wild-type Aeromonas, enterocytes were completely destroyed. (B) Mouse loops challenged with culture filtrate from a double-crossover mutant. Normal enterocytes with intact brush borders surround the lumen. Mucus is being emptied from a goblet cell into the lumen in the upper layer of cells. Bars, 1 μm.
Southern blot analysis of the chromosomal DNAs of A. hydrophila SSU and its isogenic mutants.
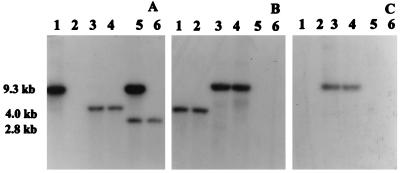
Southern blot analysis of the genomic DNAs of wild-type Aeromonas and its mutants was performed to confirm the identity of isogenic mutants. It is evident from Fig. 5A, lane 6, that a 2.8-kb chromosomal DNA fragment reacted with the act gene probe in wild-type Aeromonas. Total DNA from a single-crossover transconjugant showed bands at 9.3 and at 2.8 kb (Fig. 5A, lane 5). The band at 2.8 kb represented the native act gene of Aeromonas, whereas the band at 9.3 kb contained a truncated act gene and the suicide vector. A fragment of 4.0 kb reacted with the act gene probe in the digested DNAs of the double-crossover mutants (Fig. 5A, lanes 3 and 4), instead of a 2.8-kb fragment observed with the wild-type A. hydrophila (Fig. 5A, lane 6). The size shift was due to insertion of a kanamycin resistance gene cassette in the act gene. When similar DNA samples were probed with a kanamycin gene cassette (Fig. 5B), only the double-crossover and single-crossover mutants showed signals at 4.0 and 9.3 kb, respectively (Fig. 5B, lanes 1 to 4). Genomic DNA from wild-type Aeromonas did not react with this probe (Fig. 5B, lanes 5 and 6). When plasmid pJQ200KS was used as a gene probe, only the digested DNAs from single-crossover mutants reacted (Fig. 5C, lanes 3 and 4). As predicted, no band was detected in the digested DNAs from double-crossover mutants, indicating loss of the suicide vector sequences (Fig. 5C, lanes 1 and 2). Likewise, genomic DNA from wild-type A. hydrophila did not react with this probe (Fig. 5C, lanes 5 and 6).
FIG. 5.
Southern blot analysis of the chromosomal DNAs from A. hydrophila SSU and its isogenic mutants. Total DNAs (15 μg) from A. hydrophila and its mutants were digested with the BamHI restriction enzyme and subjected to Southern blot analysis. The probes used were a 439-bp XbaI/SalI DNA fragment, which depicts part of the coding region of the act gene (A), a 1.2-kb kanamycin resistance gene cassette (B), and a 4.9-kb pJQ200 suicide vector (C). The blots were probed and washed as described in Materials and Methods. (A) Digested DNAs from E. coli with suicide vector and truncated act gene (lane 1), double-crossover mutants of A. hydrophila (lanes 3 and 4), a single-crossover mutant of A. hydrophila (lane 5), and wild-type A. hydrophila (lane 6). (B and C) Digested DNAs from double-crossover mutants of A. hydrophila (lanes 1 and 2), single-crossover mutants of A. hydrophila (lanes 3 and 4), and wild-type A. hydrophila (lanes 5 and 6).
Reintegration of the native act gene in the double-crossover mutants with an inactive act gene.
The truncated act gene in the double-crossover mutant was replaced with the functionally active act gene by homologous recombination. To perform this experiment, the 2.8-kb BamHI DNA fragment from plasmid pXHC95 was made blunt ended and ligated to the blunt-ended suicide vector pMW1823 at the EcoRI restriction site (Fig. 1). The recombinant plasmid pXHC97.3 was transformed into E. coli S17-1. After the identity of chloramphenicol-resistant recombinant clones was confirmed, the plasmid pXHC97.3 was transferred from E. coli into an isogenic mutant of Aeromonas (rifampin resistant) which contained the truncated act gene. Colonies resistant to rifampin were inoculated on a blood agar plate and observed for a surrounding zone of hemolysis after overnight incubation at 37°C. Aeromonas revertants exhibiting hemolytic activity were obtained at a frequency of 0.05%. The hemolytic activities in the culture filtrates of wild-type and revertant strains of Aeromonas were identical, indicating that the revertant had regained the biological activity of Act (Table 2). All of the Aeromonas mutants exhibited similar growth rates in synthetic M-9 medium (data not shown).
Different doses of the wild-type A. hydrophila SSU, its isogenic mutant, and the revertant were injected i.p. into mice, which were observed for death over a 1-week period. The LD50 of wild-type Aeromonas and the revertant was 3.0 × 105, whereas the LD50 of the isogenic mutant was 1.0 × 108 (P = 0.01 by the Fisher exact test). The LD50 of wild-type Aeromonas was lower than that obtained earlier (2.5 × 107), because all of the cultures were passed through animals twice before lethality studies were performed. The cultures were injected into mouse ligated small intestine, and after 6 h, blood was drawn from the heart and spread on blood agar plates. Organisms recovered from the blood after the second passage were used in the lethality studies and were found to be more virulent than those subcultured on the synthetic medium.
The animals which survived the bacterial challenge (with wild-type Aeromonas or its mutants) were bled after 14 days, and the toxin-specific antibodies in the sera were examined by Western blot analysis. In an immunoblot in which pure Act was probed with sera from animals injected with either wild-type Aeromonas or its revertant, a band of 52 kDa was visualized, and these antibodies could effectively neutralize the tested hemolytic activity of Act. In contrast, sera from animals injected with the Aeromonas isogenic mutant, as well as the preimmune serum, did not react with Act in Western blots (data not shown).
DISCUSSION
Aeromonas species, like many other bacterial pathogens, secrete a number of extracellular proteins which play important roles in the pathogenesis of disease (3). Hemolysins have been shown to be produced by many gram-negative bacteria, and Welch and Falkow (41) were able to establish a correlation between the hemolytic titer in E. coli and lethality in rats. In contrast, Wright and Morris (42) noted that the cytolysin (with hemolytic and cytotoxic activities) produced by Vibrio vulnificus had a minimal effect on the pathogenesis of V. vulnificus infections.
Asao et al. (4) noted that the hemolysin produced by Aeromonas had multiple biological activities, including hemolytic, cytotoxic, and enterotoxic activities and lethality in mice, similar to the case for Act (14). Chakraborty et al. (9) reported that their aerolysin from A. trota reacted with antibodies to hemolysin isolated by Asao et al. (4). Hirono and Aoki (21) reported another hemolysin from Aeromonas which exhibited minimal homology with aerolysin. By marker exchange mutagenesis, Chakraborty et al. (10) showed that aerolysin-deficient mutants were less virulent in mice than wild-type Aeromonas. Molecular cloning and DNA sequence analysis of our act gene from A. hydrophila (14) revealed that it differed significantly from the aerolysin gene of A. trota and from the hemolysin purified by Asao et al. (4). The differences included the inability of one of the neutralizing Act monoclonal antibodies to react with the two other proteins in Western blot analysis and its failure to neutralize the hemolytic activity of these toxins. Site-directed mutagenesis within the act gene revealed many other differences between Act and aerolysins (16). We also have demonstrated that Act stimulated the chemotactic activity of human leukocytes and inhibited the phagocytic function of mouse phagocytes (27), clearly indicating a role for Act in Aeromonas-mediated infections. Further, in a clinical study, A. hydrophila was isolated as the sole enteropathogen from patients’ diarrheal stools and from the ready-to-eat shrimp cocktail that those patients had ingested (2), indicating a definitive epidemiological link between diarrhea and direct exposure to Aeromonas.
The LD50 of Aeromonas strain A52 (Act negative) was almost two logarithmic doses greater than the LD50 of A. hydrophila SSU when injected i.p. However, since we were unaware of the various virulence factors produced by A52 compared to SSU, we opted to generate transposon and isogenic mutants of wild-type A. hydrophila SSU. At present, no oral-challenge models are available for Aeromonas, and therefore, the current model has limitations in mimicking the true disease process in humans. Regardless, this is the first report of a study in which an act gene-deficient mutant was prepared from an authentic strain of A. hydrophila to unequivocally establish the role of Act in Aeromonas-mediated infections in mice after i.p. challenge.
The transposon mutants of A. hydrophila SSU with dramatically reduced biological activity were not lethal to mice at a dose of 5 × 107 compared to the wild type. Southern blot data suggested that transposition might not have occurred within the structural gene for Act in these mutants. Our Northern blot data demonstrated that transcription of the act gene in the transposon mutants was affected (Fig. 3). The exact location of the transposition in these mutants has not been determined and is under investigation. It is plausible that the transposition might have occurred in some regulatory element whose product was essential for the transcription of the act gene. Earlier, Chakraborty et al. (9) used transposon insertions to demonstrate that the DNA sequences flanking the aerolysin structural gene (aerA) in both the 5′ (referred to as aerC) and 3′ (referred to as aerB) regions in A. trota were important for the expression of the aerolysin gene. However, Howard et al. (24) noted that the expression of their aerolysin gene from A. bestiarum was not affected when Tn5 insertions were introduced immediately downstream of the stop codon for the aerolysin structural gene. Although regulation of the act gene is a subject of intense investigation in our laboratory, at present nothing is known about the act operon in A. hydrophila. It is therefore plausible that transposition in these mutants, although not within the structural gene, may be in the act operon.
Transposition may lead to polar mutations, and the possibility that some other virulence genes might have been affected in the transposon mutants due to a polar effect cannot be ruled out. Therefore, we generated an isogenic mutant of Aeromonas. The frequency of double-crossover events was very low (0.01%). We obtained at a high frequency colonies which acquired sucrose resistance but still were gentamicin resistant. This could have occurred as a result of various types of mutations within the sacB gene (36). Further, the DNA sequences flanking the act gene had to be increased significantly (1.9 kb at the 5′ end and 0.9 kb at the 3′ end) to obtain double-crossover mutants.
Originally, we removed 63% of the coding region of the toxin by using the BstXI restriction enzyme, which resulted in a flanking 422 bp of the DNA sequence at the 5′ end and 179 bp at the 3′ end of the act gene (14). Donnenberg and Kaper (15) similarly removed 66% of the eae gene of E. coli and had 519 and 120 bp of flanking DNA sequences in order for the double-crossover event to occur. They reported successful isolation of double-crossover mutants. However, this strategy did not provide us any genuine double-crossover mutants, although single-crossover mutants were obtained. These single-crossover transconjugants were grown without antibiotic selection to the late logarithmic phase, allowing second recombination events to accumulate. Although we obtained the desired phenotype (gentamicin sensitivity and sucrose resistance), we noticed that the suicide vector was indeed not lost and that the biological activity of the toxin remained intact. Thousands of colonies were screened on the blood agar plates for the loss of hemolytic activity, without any success. These data indicated mutations in the gentamicin resistance and sacB genes.
A dramatic difference between the LD50s of the isogenic mutant and wild-type Aeromonas when injected into animals was noted. Even with the construction of an isogenic mutant, it is possible that unlinked mutations might influence the biological effects of Act. We therefore reintroduced the native act gene in the isogenic mutant to restore the biological activity of Act. Indeed, full hemolytic, cytotoxic, and enterotoxic activities were regained by this Aeromonas revertant. Further, the revertant was as virulent in mice as wild-type A. hydrophila SSU, indicating that there was no polar effect in the isogenic mutants. Finally, we have demonstrated that Act was produced during the infection process, since antisera obtained from mice surviving infection with wild-type A. hydrophila and the revertant had Act-specific antibodies. However, the sera from animals injected with the isogenic mutant did not show an Act-specific band in Western blots.
In conclusion, we have demonstrated that elimination of the biological effects of Act by either transposon or marker exchange mutagenesis significantly affected the pathogenicity of Aeromonas in mice. These data were substantiated by using an isolate of Aeromonas that naturally did not produce Act. These observations are very provocative, since Aeromonas increasingly has been isolated from patients with peritonitis and urinary tract infections, and there have been reports in which A. hydrophila has been shown to cause multilobular lung abscesses and a fatal bacteremia with myonecrosis and gas gangrene in a hemodialysis patient treated with deferoxamine (25, 29, 31, 33). The reported isolation of Aeromonas worldwide from 5 to 7% of individuals suffering from gastroenteritis, the presence of this organism in a variety of foods, and the prevalence of Act-related molecules in most Aeromonas isolates examined have resulted in an increased awareness of their association with human infections (8, 11, 12, 18, 22). Overall, our data indicate that Act has an impact on virulence in mice, but further studies will be necessary to clearly correlate this observation with human illness.
ACKNOWLEDGMENTS
This work was supported in part by grant R01AI41611 from the National Institutes of Health. M.R.F. was supported by a McLaughlin predoctoral fellowship.
We thank S. W. Joseph, Department of Microbiology, The University of Maryland, College Park, for identifying isolate SSU as A. hydrophila and E. B. Whorton, Department of Preventive Medicine and Community Health, The University of Texas Medical Branch, for statistical analysis. Mardelle Susman is acknowledged for editorial comments.
REFERENCES
- 1.Altwegg M, Geiss H K. Aeromonas as a human pathogen. Crit Rev Microbiol. 1989;16:253–286. doi: 10.3109/10408418909105478. [DOI] [PubMed] [Google Scholar]
- 2.Altwegg M, Lucchini G M, Luthy-Hottenstein J, Rohrbach M. Aeromonas-associated gastroenteritis after consumption of contaminated shrimp. Eur J Clin Microbiol Infect Dis. 1991;10:44–45. doi: 10.1007/BF01967100. [DOI] [PubMed] [Google Scholar]
- 3.Anguita J, Rodriguez Aparicio L B, Naharro G. Purification, gene cloning, amino acid sequence analysis, and expression of an extracellular lipase from an Aeromonas hydrophila human isolate. Appl Environ Microbiol. 1993;59:2411–2417. doi: 10.1128/aem.59.8.2411-2417.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asao T, Kinoshita Y, Kozaki S, Uemura T, Sakaguchi G. Purification and some properties of Aeromonas hydrophila hemolysin. Infect Immun. 1984;46:122–127. doi: 10.1128/iai.46.1.122-127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin B, Altwegg M, Gosling P J, Joseph S, editors. The genus Aeromonas. New York, N.Y: John Wiley and Sons, Inc.; 1996. [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1989. [Google Scholar]
- 7.Ballard J, Crabtree J, Roe B A, Tweten R K. The primary structure of Clostridium septicum alpha-toxin exhibits similarity with that of Aeromonas hydrophila aerolysin. Infect Immun. 1995;63:340–344. doi: 10.1128/iai.63.1.340-344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan R L, Palumbo S A. Aeromonas hydrophila and Aeromonas sobria as potential food poisoning species: a review. J Food Safety. 1985;7:15–29. [Google Scholar]
- 9.Chakraborty T, Huhle B, Bergbauer H, Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli K-12. J Bacteriol. 1986;167:368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty T, Huhle B, Hof H, Bergbauer H, Goebel W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect Immun. 1987;55:2274–2280. doi: 10.1128/iai.55.9.2274-2280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Challapalli M, Tesss B R, Cunningham D G, Chopra A K, Houston C W. Aeromonas-associated diarrhea in children. Pediatr Infect Dis J. 1988;7:693–698. doi: 10.1097/00006454-198810000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee B D, Neogy K N. Studies on Aeromonas and Plesiomonas species isolated from cases of choleric diarrhea. Ind J Med Res. 1972;60:520–524. [PubMed] [Google Scholar]
- 13.Chopra A K, Houston C W, Kurosky A. Genetic variation in related cytolytic toxins produced by different species of Aeromonas. FEMS Microbiol Lett. 1991;78:231–238. doi: 10.1016/0378-1097(91)90163-5. [DOI] [PubMed] [Google Scholar]
- 14.Chopra A K, Houston C W, Peterson J W, Jin G F. Cloning, expression, and sequence analysis of a cytolytic enterotoxin gene from Aeromonas hydrophila. Can J Microbiol. 1993;39:513–523. doi: 10.1139/m93-073. [DOI] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson M R, Xu X-J, Houston C W, Peterson J W, Chopra A K. Amino-acid residues involved in biological functions of the cytolytic enterotoxin from Aeromonas hydrophila. Gene. 1995;156:79–83. doi: 10.1016/0378-1119(95)00043-6. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson M R, Xu X-J, Houston C W, Peterson J W, Coppenhaver D H, Popov V L, Chopra A K. Hyperproduction, purification, and mechanism of action of the cytotoxic enterotoxin produced by Aeromonas hydrophila. Infect Immun. 1997;65:4299–4308. doi: 10.1128/iai.65.10.4299-4308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gracey M, Robinson J, Burke V. Aeromonas-associated gastroenteritis. Lancet. 1982;ii:1304–1306. doi: 10.1016/s0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- 19.Green M J, Buckley J T. Site-directed mutagenesis of the hole-forming toxin aerolysin: studies on the roles of histidines in receptor binding and oligomerization of the monomer. Biochemistry. 1990;29:2177–2180. doi: 10.1021/bi00460a031. [DOI] [PubMed] [Google Scholar]
- 20.Harayama S, Tsuda M, Lino T. High frequency mobilization of the chromosome of Escherichia coli by a mutant of plasmid RP4 temperature-sensitive for maintenance. Mol Gen Genet. 1980;180:47–56. doi: 10.1007/BF00267351. [DOI] [PubMed] [Google Scholar]
- 21.Hirono I, Aoki T. Cloning and characterization of three hemolysin genes from Aeromonas salmonicida. Microb Pathog. 1993;15:269–282. doi: 10.1006/mpat.1993.1077. [DOI] [PubMed] [Google Scholar]
- 22.Hossain M A, Rahman K M, Asna S M, Rahim Z, Hussain T, Miah M R. Incidence of Aeromonas isolated from diarrhoeal children and study of some virulence factors in the isolates. Bangladesh Med Res Counc Bull. 1992;18:61–67. [PubMed] [Google Scholar]
- 23.Howard S P, Buckley J T. Molecular cloning and expression in Escherichia coli of the structural gene for the hemolytic toxin aerolysin from Aeromonas hydrophila. Mol Gen Genet. 1986;204:289–295. doi: 10.1007/BF00425512. [DOI] [PubMed] [Google Scholar]
- 24.Howard S P, Garland W J, Green M J, Buckley J T. Nucleotide sequence of the gene for the hole-forming toxin aerolysin of Aeromonas hydrophila. J Bacteriol. 1987;169:2869–2871. doi: 10.1128/jb.169.6.2869-2871.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hur T, Cheng K C, Hsieh J M. Aeromonas hydrophila lung abscess in a previously healthy man. Scand J Infect Dis. 1995;27:295. doi: 10.3109/00365549509019025. [DOI] [PubMed] [Google Scholar]
- 26.Husslein V, Huhle B, Jarchau T, Lurz R, Goebel W, Chakraborty T. Nucleotide sequence and transcriptional analysis of the AerCaerA region of Aeromonas sobria encoding aerolysin and its regulatory region. Mol Microbiol. 1988;2:507–517. doi: 10.1111/j.1365-2958.1988.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 27.Jin G-F, Houston C W. Effect of Aeromonas hydrophila enterotoxins on function of mouse phagocytes. Dig Dis Sci. 1992;37:1697–1703. doi: 10.1007/BF01299862. [DOI] [PubMed] [Google Scholar]
- 28.Keusch G T, Donta S T. Classification of enterotoxins on the basis of activity in cell culture. J Infect Dis. 1975;131:58–63. doi: 10.1093/infdis/131.1.58. [DOI] [PubMed] [Google Scholar]
- 29.Kohashi T, Sakai H, Marumo F, Sato C. Aeromonas sobria infection with severe muscle degeneration in a patient with alcoholic liver cirrhosis. Am J Gastroenterol. 1995;90Z:2234–2235. [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lin S-H, Shieh S-D, Lin, Lin Y F, De Brauwer E, Van Landuyt H W, Gordts B, Boelaert J R. Fatal Aeromonas hydrophila bacteremia in a hemodialysis patient treated with deferoxamine. Am J Kidney Dis. 1996;27:733–735. doi: 10.1016/s0272-6386(96)90112-2. [DOI] [PubMed] [Google Scholar]
- 32.Ljungh A, Kronevi T. Aeromonas hydrophila toxins-intestinal fluid accumulation and mucosal injury in animal models. Toxicon. 1982;20:397–407. doi: 10.1016/0041-0101(82)90002-2. [DOI] [PubMed] [Google Scholar]
- 33.Mani S, Sadigh M, Adriole V T. Clinical spectrum of Aeromonas hydrophila infections: report of 11 cases in a community hospital and review. Infect Dis Lin Pract. 1995;4:79–86. [Google Scholar]
- 34.Nelson K L, Raja S M, Buckley J T. The glycosylphosphatidylinositol-anchored surface glycoprotein Thy-1 is a receptor for the channel-forming toxin aerolysin. J Biol Chem. 1997;272:12170–12174. doi: 10.1074/jbc.272.18.12170. [DOI] [PubMed] [Google Scholar]
- 35.Peterson J W, Dickey W D, Saini S S, Gourley W, Klimpel G R, Chopra A K. Phospholipase A2-activating protein and idiopathic inflammatory bowel disease. Gut. 1996;39:698–704. doi: 10.1136/gut.39.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 37.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 38.Rella M, Mercenier A, Haas D. Transposon insertion mutagenesis of Pseudomonas aeruginosa with a Tn5 derivative: application to physical mapping of the arc gene cluster. Gene. 1985;33:293–303. doi: 10.1016/0378-1119(85)90237-9. [DOI] [PubMed] [Google Scholar]
- 39.Rose J M, Houston C W, Kurosky A. Bioactivity and immunological characterization of a cholera toxin-cross-reactive cytolytic enterotoxin from Aeromonas hydrophila. Infect Immun. 1989;57:1170–1176. doi: 10.1128/iai.57.4.1170-1176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose J M, Houston C W, Coppenhaver D H, Dixon J D, Kurosky A. Purification and chemical characterization of a cholera toxin-cross-reactive cytolytic enterotoxin produced by a human isolate of Aeromonas hydrophila. Infect Immun. 1989;57:1165–1169. doi: 10.1128/iai.57.4.1165-1169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch R A, Falkow S. Characterization of Escherichia coli hemolysin conferring quantitative differences in virulence. Infect Immun. 1984;43:156–161. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright A C, Morris J G. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect Immun. 1991;59:192–197. doi: 10.1128/iai.59.1.192-197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]