Amyloid precursor protein (APP) is a synaptic single-pass transmembrane protein that is best known for its involvement in Alzheimer’s disease, a devastating neurological disorder. In Alzheimer’s disease patients, amyloid plaques containing aggregated β-amyloid peptide (Aβ) appear in specific brain regions, triggering an inflammatory response, neuronal cell death and gradual cognitive decline (reviewed in Selkoe, 1999). Aβ is derived from APP that is first cleaved at an extracellular position (β site), followed by an unusual cleavage within the APP transmembrane segment (γ site), producing ∼40–42 amino acid Aβ peptides, with the longer forms apparently causing the neurotoxicity. This processing pathway has been studied intensively with the goal of devising therapeutic interventions. In recent years, the β-site cleavage enzymes (BACE) have been identified (reviewed in Vassar and Citron, 2000), and the γ cleavage has been shown to be performed by a proteolytic complex containing Presenilin and Nicastrin (Yu et al., 2000). However, APP is also processed by an alternative pathway, in which a different, slightly more membrane-proximal extracellular position (α site) is cleaved, followed by proteolysis at the γ site, as in the Aβ pathway. The α cleavage is thought to be mediated by extracellular matrix metalloproteases of the tumour necrosis factor α-converting enzyme (TACE)/a disintegrin and metalloprotease (ADAM) family, and it generates a non-toxic peptide termed p3. Unlike the Aβ pathway, which appears to operate throughout the secretory and endosomal compartments (reviewed in Wilson et al., 1999), the p3 pathway is predominantly active at the cell surface (Parvathy et al., 1999).
In this issue of EMBO reports, Ledesma and colleagues (2000) bring attention to another protease that may influence the selection of Aβ or p3 processing pathways for APP. Starting with previous observations that Aβ is present in rafts, specialized detergent insoluble glycolipid-enriched membrane microdomains (Lee et al., 1998), that raft integrity is important for Aβ production (Simons et al., 1998) and that purified plasmin can degrade Aβ (Wnendt et al., 1997; Van Nostrand and Porter, 1999; Tucker et al., 2000), Ledesma and co-workers examined neuronal rafts for plasmin interactions with APP and Aβ. Both plasmin and its inactive precursor, plasminogen, were detected in rafts of cultured hippocampal neurons, and remarkably, active plasmin was largely restricted to the rafts themselves, suggesting a new role for rafts as sites of active proteolysis. Addition of plasmin or tissue plasminogen activator (tPA), which converts plasminogen to plasmin, increased the amount of α cleavage of APP and decreased levels of Aβ. It is not known whether plasmin may directly cleave the α site of APP or whether these effects occur as a result of the known stimulation of TACE/ADAM metalloproteases by plasmin (Kleiner and Stetler-Stevenson, 1993). Intriguingly, Ledesma et al. (2000) reported reduced plasmin levels in Alzheimer’s disease brain tissue, indicating that plasmin may be physiologically relevant as a protective factor in this disease.
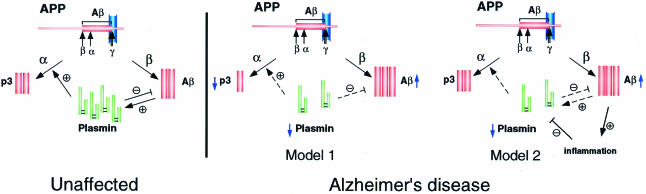
The results of Ledesma et al. (2000) are bound to re-awaken interest in the idea that upregulation of the α pathway of APP processing might reduce the toxic build-up of Aβ, either by diverting APP away from the β pathway or by directly degrading existing Aβ. Ledesma and colleagues propose that Aβ levels are regulated by a balance of different protease activities acting on the α-, β- and γ-sites of APP in conjunction with an Aβ clearance system to inhibit Aβ accumulation (Figure 1). The potential involvement of plasmin in these events was previously noted by other groups, who demonstrated that plasmin cleaves Aβ at certain sites and that exogenously added plasmin blocks Aβ neurotoxicity, supporting a physiological role for plasmin in APP/Aβ metabolism (Wnendt et al., 1997; Van Nostrand and Porter, 1999; Tucker et al., 2000). An added twist to this story is that these groups found that Aβ itself induces the plasmin system, leading to increased tPA mRNA levels, an effect caused only by aggregated, not soluble, Aβ. Thus, a feedback mechanism may exist whereby toxic Aβ stimulates the plasmin system, leading to Aβ clearance in neurons, a mechanism that breaks down due to inflammation and increased expression of tPA inhibitors in Alzheimer’s disease. The ideas that elevated Aβ accumulation may result from reduced plasmin activity (Ledesma et al., 2000) and that Aβ accumulation may initially trigger increased plasmin activity (Wnendt et al., 1997; Van Nostrand and Porter, 1999; Tucker et al., 2000) may not necessarily contradict one another. Perhaps Aβ levels are normally kept within physiological limits by regulated degradation involving a positive plasmin feedback mechanism, but high Aβ levels might overwhelm this system by causing inflammation-related tPA inhibition, suppressing plasmin activity and promoting plaque formation (Figure 1).
Fig. 1. Possible models for the role of plasmin in the development of Alzheimer’s disease. In unaffected individuals (left), plasmin influences the balance of α and β pathway activity by stimulating the α pathway (Ledesma et al., 2000) and degrading Aβ. Aβ, in turn, induces the plasmin system in a feedback mechanism (Wnendt et al., 1997; Van Nostrand and Porter, 1999; Tucker et al., 2000). In individuals with Alzheimer’s disease (right), reduced plasmin in neuronal rafts shifts the proteolytic balance towards the β pathway, leading to increased Aβ production and plaque formation (Model 1) (Ledesma et al., 2000). Alternatively, Aβ deposition may trigger inflammation, which induces inhibitors of the plasmin system, downregulating degradation and clearance of Aβ (Model 2) (Tucker et al., 2000).
Curiously, upregulation of tPA leads to enhancement of long-term potentiation and learning in mice (Madani et al., 1999), and longitudinal population studies on human subjects have uncovered a positive correlation between linguistic ability and protection from Alzheimer’s disease (Snowdon et al., 2000). These observations suggest that there may be a connection between the cognitive and memory deficits seen in Alzheimer’s disease and the activity of the plasminogen system. Taken together, these results highlight the need for further analysis of plasmin and associated factors in APP metabolism and Alzheimer’s disease. While enhanced deposition of Aβ due to aberrant γ cleavage has been well documented in relatively rare familial forms of Alzheimer’s disease caused by Presenilin gene mutations, it is conceivable that the more prevalent cases of sporadic Alzheimer’s disease may involve impaired α pathway activity or Aβ degradation, perhaps due to plasmin activity in neuronal membrane rafts.
In addition to these effects on APP metabolism, plasminogen has recently been shown to interact with disease-associated forms of the prion protein (Fischer et al., 2000), raising the possibility that the plasminogen system may influence the development or clinical severity of multiple neurodegenerative diseases.


Acknowledgments
Acknowledgements
G.P. is supported by an American Heart Association Postdoctoral Fellowship Award. M.E.F. is supported by the National Institutes of Health, the Alzheimer’s Association and a Merck Research Grant.
References
- Fischer M.B., Roeckl, C., Parizek, P., Schwarz, H.P. and Aguzzi, A. (2000) Binding of disease-associated prion protein to plasminogen. Nature, in press. [DOI] [PubMed] [Google Scholar]
- Ikezu T., Trapp, B.D., Song, K.S., Schlegel, A., Lisanti, M.P. and Okamoto, T. (1998) Caveolae, plasma membrane microdomains for α-secretase-mediated processing of the amyloid precursor protein. J. Biol. Chem., 273, 10485–10495. [DOI] [PubMed] [Google Scholar]
- Kleiner D.E. Jr and Stetler-Stevenson, W.G. (1993) Structural biochemistry and activation of matrix metalloproteases. Curr. Opin. Cell Biol., 5, 891–897. [DOI] [PubMed] [Google Scholar]
- Ledesma M.D., Da Silva, J.S., Crassaerts, K., Delacourte, A., De Strooper, B. and Dotti, C.G. (2000) Brain plasmin influences APP α-cleavage and Aβ degradation and is reduced in Alzheimer’s disease brains. EMBO rep. 1, 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-J., Liyanage, U., Bickel, P.E., Xia, W., Lansbury, P.T., Jr and Kosik, K.S. (1998) A detergent-insoluble membrane compartment contains Aβin vivo. Nature Med., 4, 730–734. [DOI] [PubMed] [Google Scholar]
- Madani R., Hulo, S., Toni, N., Madani, H., Steimer, T., Muller, D. and Vassalli, J.-D. (1999) Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J., 18, 3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathy S., Hussain, I., Karran, E.H., Turner, A.J. and Hooper, N.M. (1999) Cleavage of Alzheimer’s amyloid precursor protein by α-secretase occurs at the surface of neuronal cells. Biochemistry, 38, 9728–9734. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature, 399, A23–A31. [DOI] [PubMed] [Google Scholar]
- Simons M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C.G. and Simons, K. (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA, 95, 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon D.A., Greiner, L.H. and Markesbery, W.R. (2000) Linguistic ability in early life and the neuropathology of Alzheimer’s disease and cerebrovascular disease: findings from the nun study. Ann. N.Y. Acad. Sci., 903, 34–38. [DOI] [PubMed] [Google Scholar]
- Tucker H.M., Kihiko-Ehmann, M., Wright, S., Rydel, R.E. and Estus, S. (2000) The plasmin system is induced by and degrades amyloid-β aggregates. J. Neurosci., 20, 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand W.E. and Porter, M. (1999) Plasmin cleavage of the amyloid β-protein: alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry, 38, 11570–11576. [DOI] [PubMed] [Google Scholar]
- Vassar R. and Citron, M. (2000) Aβ-generating enzymes: recent advances in β- and γ-secretase research. Neuron, 27, 419–422. [DOI] [PubMed] [Google Scholar]
- Wilson C.A., Doms, R.W. and Lee, V.M.-Y. (1999) Intracellular APP processing and Aβ production in Alzheimer disease. J. Neuropath. Exp. Neurol., 58, 787–794. [DOI] [PubMed] [Google Scholar]
- Wnendt S., Wetzels, I. and Gunzler, W.A. (1997) Amyloid β peptides stimulate tissue-type plasminogen activator but not recombinant prourokinase. Thromb. Res., 85, 217–224. [DOI] [PubMed] [Google Scholar]
- Yu G. et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature, 407, 48–54. [DOI] [PubMed] [Google Scholar]