Abstract
High levels of nitric oxide (NO) are produced by inducible nitric oxide synthase (iNOS) in response to activating signals from Th1-associated cytokines and play an important role in cytotoxicity and cytostasis against many pathogenic microorganisms. In addition to its direct effector function, NO serves as a potent immunoregulatory factor. NO produced by gamma interferon-activated macrophages immobilizes and kills Schistosoma mansoni larvae, and several studies have indicated a role for this pathway in protective immunity against this parasite. The potential regulatory influence of NO in immunity to S. mansoni is less well understood. In this study, we have used iNOS-deficient mice to determine the role of NO in mice vaccinated with irradiated cercariae of S. mansoni. We show by enzyme-linked immunosorbent assay and reverse transcriptase PCR analysis that vaccinated iNOS-deficient mice develop exacerbated type 1 cytokine responses in the lungs, the site where resistance to infection is primarily manifested. In addition, parasite-specific immunoglobulin G2a (IgG2a) and IgG2b antibody responses were significantly increased in vaccinated iNOS-deficient animals and total IgE antibody levels in serum were decreased relative to those in wild-type controls. Surprisingly, since resistance in this vaccine model is largely Th1 dependent and since Th1-related cellular and humoral immune responses were found to be exacerbated in vaccinated iNOS-deficient mice, vaccine-elicited protective immunity against challenge infection was found to be reduced. These findings demonstrate that iNOS plays a paradoxical role in immunity to S. mansoni, both in the effector mechanism of resistance and in the down regulation of the type 1 cytokine response, which is ultimately required for NO production.
Production of nitric oxide (NO) is induced in multiple cell types by the action of cytokines, including gamma interferon (IFN-γ), interleukin-1β (IL-1β), and tumor necrosis factor alpha (TNF-α), and bacterial products, such as lipopolysaccharide (LPS) and staphylococcal enterotoxin B (22). In response to these activating signals, NO production from l-arginine is catalyzed by the enzyme inducible NO synthase (iNOS or NOS2) (22). Numerous studies have documented the potent antimicrobial activity of NO against intracellular and extracellular pathogens, including parasitic protozoa, viruses, fungi, and bacteria (18, 20, 22). In addition to its direct cytotoxic or cytostatic activity, NO is an important immunoregulatory factor, displaying potent immunosuppressive activity (21, 26) and also influencing Th-cell differentiation (25, 36).
Studies from our laboratory and others have suggested that NO plays a role in protective immunity to the helminth parasite Schistosoma mansoni. In a murine vaccination model, a single exposure to radiation-attenuated parasites enables mice to eliminate 60 to 80% of the worms that ordinarily develop from a challenge infection (27). Resistance in this model is dependent on CD4+ T cells (38) and is associated with induction of Th1-type cytokine patterns (31). In particular, in vivo depletion of IFN-γ causes a significant reduction in vaccine-induced resistance (34). IFN-γ-activated macrophages and endothelial cells kill larval schistosomes in vitro via an arginine-dependent mechanism involving NO production (15, 30). Previous studies in this model indicate that the majority of challenge parasites are eliminated as they traverse the lungs of vaccinated mice (9, 40). Peak IFN-γ and iNOS mRNA expression occurs in the lungs of vaccinated and challenged mice at the time when challenge parasites are believed to be eliminated, and iNOS can be identified in the pulmonary inflammatory foci around the migrating larvae (43), as would be predicted if this mechanism plays a role in resistance in vivo. Further support of this hypothesis is gained from the observation that in genetic crosses between mouse strains that are high or low responders to the irradiated cercariae vaccine, the ability to develop resistance to challenge infection segregated with the ability to develop activated larvicidal macrophages (14). Moreover, treatment of vaccinated animals with an inhibitor of NO production, aminoguanidine, markedly decreased the level of resistance to challenge infection (43). Together, these findings are consistent with a role for NO in the effector mechanism of the protective immune response induced by vaccination with attenuated cercariae. Nevertheless, direct NO-mediated killing of challenge parasites within the lungs in vivo remains controversial (7), and other mechanisms of vaccine-induced parasite attrition have been postulated, including antibody-dependent mechanisms (24, 46), physical entrapment within inflammatory foci (7), and deflection into the alveolar spaces (9, 19).
In the present study, we sought to further define the role of NO in vaccine-induced resistance to S. mansoni through examination of immune responses in mice that are genetically iNOS deficient. Particular attention was paid to the pattern of cytokine expression within the lung itself, the proposed site of elimination of challenge parasites. The findings reported here demonstrate that in addition to a possible effector function, NO plays an important role in the regulation of the type 1/type 2 cytokine balance after vaccination with irradiated cercariae.
MATERIALS AND METHODS
Laboratory hosts, parasites, and parasite antigen preparation.
iNOS-deficient mice were originally constructed by gene targeting in embryonic stem cells as described previously (23) and were generously provided by John D. MacMicking and Carl Nathan (Cornell University Medical College) and John S. Mudgett (Merck Research Laboratories). The mice were generated from a mixed background of 129/SvEv × C57BL/6, and female mice at the F2 generation were used between 6 and 8 weeks of age. Age-matched wild-type WT (129/SvEv × C57BL/6) mice at the F2 generation were used as controls. The knockout (KO) mice were bred and maintained in a National Institutes of Health American Association for the Accreditation of Laboratory Animal Care-approved animal facility. Cercariae of a Puerto Rican strain of S. mansoni (NMRI) were obtained from infected Biomphalaria glabrata snails (Biomedical Research Institute, Rockville, Md.). Soluble worm antigen preparation (SWAP) was derived from homogenized adult parasites as previously described (15).
Infections and immunizations.
S. mansoni cercariae were attenuated with 40 kilorads of γ-irradiation from a 137Cs source. Mice were vaccinated by immersion of the tail for 40 min in water containing 500 irradiated cercariae. Exposed mice and age- and sex-matched controls were used 4 to 5 weeks after vaccination, a time when they display high levels of immunity (27). The mice were challenged percutaneously with 120 cercariae for all immunity studies and with 500 cercariae for all histological and cytokine measurements (46). The animals were perfused 6 weeks later to determine the degree of protective immunity. The level of resistance for vaccinated mice was calculated from the mean worm burdens of control mice by using the formula percent resistance, R = 100 × (worm recovery from controls − worm recovery from vaccinees)/worm recovery from controls.
Measurement of schistosome-specific antibody responses.
The mice were bled by orbital puncture on day 28 after vaccination. In some experiments, serum was also collected on days 10, 14, and 18 after challenge infection. Immulon 4 (Dynatech Laboratories Inc., Chantilly, Va.) microtiter plates were coated overnight at 37°C with SWAP (1 μg in 50 μl/well). Dilutions of SWAP were made in 0.2 M sodium carbonate–bicarbonate buffer (pH 9.4). The plates were blocked with 200 μl of 5% nonfat dried milk–phosphate-buffered saline (PBS)–0.05% Tween 20 per well for 90 min at 37°C. The blocking solution was aspirated, and the wells were rinsed six times with PBS–0.05% Tween 20. Individual mouse sera were diluted fourfold starting at a 1/20 dilution in 1% bovine serum albumin (BSA)–PBS and 50 μl was added to appropriate wells. Normal mouse serum served as a negative control. The plates were kept at 4°C overnight and then washed six times with PBS–0.05% Tween 20. Isotype-specific horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG), IgA, IgM, IgG1, IgG2a, or IgG2b (Zymed Laboratories) antibodies (50 μl) was added at a 1/1,000 to 1/2,000 dilution in 1% BSA–PBS, and the mixture was incubated at 37°C for 60 min. The wells were washed six times with PBS–0.05% Tween 20, and 100 μl of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)-H2O2 one-step substrate (Kirkegaard and Perry Laboratories Inc., Gaithersburg, Md.) was added per well. After development at room temperature, the absorbance was read at 405 nm. The total IgE level in serum was quantitated by enzyme-linked immunosorbent assay, (ELISA) using a protocol provided by Pharmingen. The plates were coated with anti-mouse IgE capture monoclonal antibody (MAb) from clone R35-72 in 0.1 M NaHCO3 (pH 8.2) overnight at 4°C. The secondary MAb was a biotinylated anti-mouse IgE from clone R35-92. The streptavidin-peroxidase was diluted 1:1,000 in PBS–1% BSA. A purified mouse trinitrophenol-specific IgE MAb from Pharmingen was used as a standard.
Histopathologic testing.
The left lung was inflated with Bouin-Hollande fixative and processed routinely. The size and cell composition (percentage of eosinophils) of the inflammatory foci was determined in histological sections stained with Wright’s Giemsa stain. The diameters of all lesions (3 to 12 lesions) per lung were measured with an ocular micrometer, and the volume of each focus was calculated by assuming a spherical shape.
RT-PCR detection of cytokine mRNAs.
One lobe of the right lung was homogenized in 1 ml of RNA STAT-60 by using a tissue polytron (Omni Int., Waterbury, Conn.), and total RNA was isolated as recommended by the manufacturer. The RNA was resuspended in diethylpyrocarbonate-treated water and quantitated spectrophotometrically. A reverse transcriptase PCR (RT-PCR) procedure was performed as described previously (42) to determine the relative quantities of IL-4, IL-5, TNF-α, IL-1β, IFN-γ, and hypoxanthine phosphoribosytransferase (HPRT) mRNA. The primers and probes for all genes have been published (42, 44). The PCR conditions were strictly defined for each cytokine primer pair such that a linear relationship between input RNA and the final PCR product was obtained. Positive and negative controls were included in each assay to confirm that only cDNA PCR products were detected and that none of the reagents was contaminated with cDNA or previous PCR products. The number of PCR cycles for each gene was as described previously (44). The amplified DNA was analyzed by electrophoresis, Southern blotting, and hybridization with cytokine-specific probes. The chemiluminescent signals were quantified with a 600 ZS scanner (Microtek International, Torrance, Calif.). The amount of PCR product was determined by comparison of signal density to that of standard curves generated from simultaneously amplified stepwise dilutions of cDNA obtained from samples with a large amount of specific cytokine mRNA. The fold increase was calculated as the reciprocal of the equivalent dilution of control (unchallenged mouse lung) cDNA. Amplification of HPRT served as an internal control for the amount of RNA and cDNA from each sample.
Lymphocyte culture.
Cells from the spleens and lung-associated lymph nodes (LALN) (parathoracic) were cultured in 24-well tissue culture plates at 3 × 106 cells/ml in RPMI 1640 (Biofluids, Rockville, Md.) containing 10% heat-inactivated fetal calf serum (Sterile Systems, Inc. Logan, Utah), 2 mM glutamine, 100 U of penicillin, 100 μg of streptomycin/ml, 10 mM HEPES, and 5 × 10−5 M 2-mercaptoethanol in the presence of SWAP (50 μg/ml) or concanavalin A (ConA) (5 μg/ml). Spleens were processed individually, while LALN were pooled from four to five animals in each experiment. Supernatant fluids were collected at 72 h for lymphokine assays. IFN-γ and IL-5 were measured by specific two-site ELISA as described previously (45). IL-4 levels were determined by proliferation of CT.4S cells. Cytokine levels were calculated from standard curves constructed by using recombinant-murine cytokines.
Macrophage larvacidal and TNF-α assays.
Peritoneal cells were collected from immunized mice injected 18 to 20 h previously with 250 μg of SWAP in 0.5 ml of buffered saline. Total cell numbers were determined by hemacytometer counts. The cultures were composed of >80% macrophages, with the remainder being primarily monocytes and a small percentage of neutrophils (1, 15). Three hour schistosomula were prepared as previously described (15). The cells were incubated with schistosomula at a macrophage-to-target ratio of 104: in Dulbecco’s modified Eagle’s medium containing 4.5 mg of glucose/ml (Advanced Biotechnology, Silver Spring, Md.), 10% fetal calf serum, and antibiotics. After 40 h at 37°C, larval viability was determined microscopically by the criteria of motility and internal granularity (15). The background mortality of larvae cultured in the absence of cells averaged 10%. Nitric oxide production was assessed by the Griess reaction with 100 μl of supernatant collected after 40 h of culture. In some cultures, the cells were stimulated with 100 U of IFN-γ per ml in the presence or absence of the iNOS inhibitor N-monomethyl-l-arginine (l-NMMA) (15) to block the production of NO. For TNF-α measurements, thioglycolate-elicited macrophages were used. Mice were injected with 1.5 ml of sterile thioglycolate, and peritoneal exudate cells (PECs) were obtained 4 days later. The cells were placed in 24-well plates and stimulated for 0, 3, 6, and 24 h with 100 U of IFN-γ per ml, 250 ng of LPS per ml, or combination of the two activators. TNF-α in the culture supernatants was assayed by using a murine TNF-α ELISA kit (Genzyme Corp., Cambridge, Mass.).
Statistics.
Statistical significance was determined by Student’s two-tailed t test or by analysis of variance, and significance was set at P < 0.05. All experiments were repeated at least once with similar results.
RESULTS
Peritoneal macrophages from vaccinated iNOS-deficient mice fail to kill schistosomula in vitro.
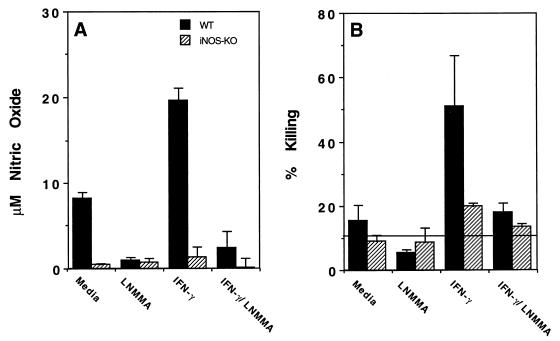
NO produced by activated macrophages has been shown to kill S. mansoni schistosomula in vitro (15). In additional proof of the effector role of NO in this system, we vaccinated both WT and iNOS-deficient (iNOS-KO) mice and examined the ability of their macrophages to kill schistosomula in vitro. Macrophage populations were elicited by specific antigen challenge with SWAP. Antigen-elicited cells from WT mice produced measurable nitric oxide (Fig. 1A) and showed slight larvicidal activity (Fig. 1B) as isolated, consistent with a low level of in vivo immune system activation. In vitro IFN-γ treatment further stimulated WT cells to increased NO production and much higher levels of parasite killing. Both parameters, NO production and killing, were returned to near baseline levels by inclusion of l-NMMA, a competitive inhibitor of NO production, in the culture medium. In contrast, antigen-elicited cells obtained from iNOS-KO mice were almost completely deficient in NO production and showed little or no killing of parasites, even after ex vivo activation with IFN-γ. Together, these data reaffirm that macrophage-derived NO is the major mediator of in vitro killing of schistosomula and demonstrate that antigen-elicited macrophages from iNOS-deficient animals have no apparent other mechanism for killing the parasites in vitro.
FIG. 1.
Peritoneal macrophages from vaccinated iNOS-deficient mice fail to kill schistosomula in vitro. Larvicidal activity and NO production in SWAP-elicited macrophages isolated from vaccinated WT and iNOS-KO mice was evaluated. Mice were vaccinated with 500 cercariae irradiated with 40 kilorads and were injected intraperitoneally with 250 μg of SWAP 5 weeks later. The 18-h antigen-elicited cells were assayed for their ability to kill 3-h schistosomula and to secrete NO. Separate cultures also contained IFN-γ (100 U/ml) or a combination of IFN-γ and the inhibitor of NO synthase (l-NMMA). The data reported are the means and standard deviations of triplicate determinations and are representative of three experiments performed. The background spontaneous death of schistosomula in cultures without peritoneal cells added is indicated by the horizontal line in panel B.
Vaccine-induced protection is reduced but not eliminated in iNOS-deficient mice.
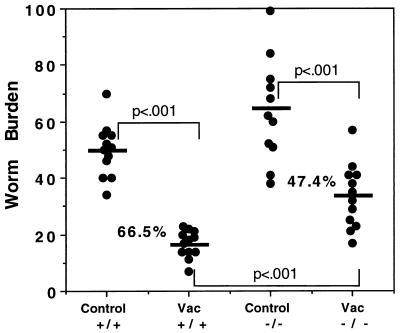
To examine the role of NO in vaccine-induced resistance to S. mansoni infection in vivo, we vaccinated WT and iNOS-KO mice by a single exposure to radiation-attenuated cercariae and challenged them 4 weeks later with unattenuated parasites to assess protective immunity. As shown in Fig. 2, WT mice displayed a 60 to 70% reduction in worm burden at 6 weeks postinfection, consistent with published reports (27). iNOS-KO mice displayed a reduced overall level of protection, with a great deal of overlap between control and vaccinated groups.
FIG. 2.
Vaccine-induced protection is reduced but not eliminated in iNOS-deficient mice. Groups of C57BL/6 × 129SvEv F2 (WT, +/+) and iNOS-KO (−/−) mice were vaccinated (Vac) with 500 cercariae irradiated with 40 kilorads and were challenged percutaneously 5 weeks later (12 animals/group) with 120 nonattenuated cercariae. Nonvaccinated and challenged mice were included as controls. Worm recovery was assessed 6 weeks postchallenge and is illustrated as individual worm burdens. The average worm burden is indicated by the horizontal line in each group. Statistical comparisons were made by using Student’s t test. An almost identical level of protection was observed in a second experiment.
Type 1-associated cytokine expression is increased in iNOS-deficient mice.
To further elucidate the role of NO in vivo, we vaccinated WT and iNOS-KO mice, challenged the animals with unattenuated parasites 4 weeks later, and examined their immune response in detail over the next 3 weeks. In initial studies, we sacrificed vaccinated animals at 0, 10, 14, and 18 days postchallenge and examined the evolving cytokine response in the LALN and spleen.
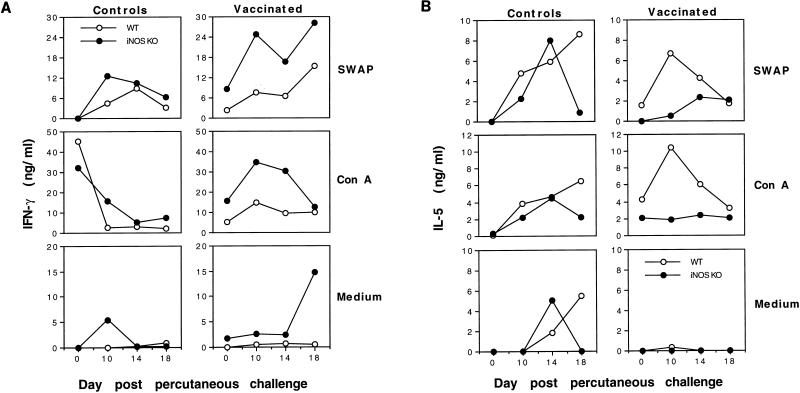
In these experiments, isolated cells were cultured in medium alone or restimulated in vitro with SWAP or ConA, and the 72-h supernatants were examined for IFN-γ and IL-5 as markers of type 1 and type 2 cytokine responses, respectively. As shown in Fig. 3A, LALN cells from vaccinated WT and iNOS-KO mice both displayed significant IFN-γ production after challenge, in response to either SWAP or ConA. Nevertheless, cells obtained from iNOS-KO mice consistently displayed substantially elevated production of IFN-γ. By day 18 postchallenge, even non-restimulated cells from vaccinated iNOS-KO mice showed a marked and highly significant IFN-γ response, in contrast to those from WT animals. A similar trend was observed with cells from nonvaccinated and challenged mice, either as isolated or after restimulation with SWAP, although the differences between WT and iNOS-KO mice were less obvious at some time points.
FIG. 3.
Type 1-associated cytokine expression is increased in iNOS-deficient mice. C57BL/6 × 129SvEv F2 (WT) and iNOS-KO vaccinated and control nonvaccinated mice were challenged with 500 unattenuated cercariae, as described in Materials and Methods. The animals were sacrificed on days 0, 10, 14, or 18 postchallenge. LALN were isolated, placed in culture at 3 × 106 LALN cells/ml, and restimulated with medium alone, SWAP (50 μg/ml), or ConA (5 μg/ml). Culture supernatants were isolated at 72 h and assayed for production of IFN-γ (A) or IL-5 (B). The reactivities of lymph node cells pooled from four mice per time point are illustrated.
As a reflection of a type 2 response, IL-5 levels were evaluated in the same culture supernatants. As seen in Fig. 3B, restimulated cells from vaccinated WT mice displayed a highly significant IL-5 response at nearly every time point examined postchallenge. These data thus concur with published reports showing a mixed type 1/type 2 response in vaccinated and challenged WT mice (43). In contrast, vaccinated iNOS-KO mice exhibited a reduction in both SWAP- and ConA-induced IL-5 production, with the most striking difference being observed on day 10 postchallenge, when IL-5 production peaked in WT animals. Although both nonvaccinated control groups displayed similar increases in IL-5 production at early time points (Fig. 3B), there was a marked decrease in IL-5 expression by day 18 postchallenge in iNOS-KO mice, which was not observed in WT animals. Together, these data suggest that NO plays an important role regulating the type 1/type 2 cytokine balance in both naive and vaccinated mice. In the absence of NO, the mice developed a more highly polarized type 1 response. Similar alterations in IFN-γ and IL-5 expression were also observed in spleen cell cultures (data not shown).
Type 1-associated humoral response is more prominent in vaccinated iNOS-deficient mice.
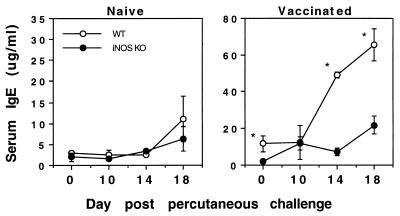
Although protective immunity in mice vaccinated a single time with attenuated parasites is thought to be dependent in large part on a cell-mediated immune response (31, 34, 38), recent studies have suggested that parasite-specific antibodies do participate (18a), particularly in mice displaying a Th1-skewed immune response (46). Therefore, we analyzed the antibody response in vaccinated WT- and iNOS-deficient mice to determine whether there was any alteration in the antibody isotype profile. As shown in Fig. 4, total IgE levels in serum were significantly reduced in iNOS-KO mice at most time points postchallenge compared with the levels in WT animals. It should also be noted that IL-4 levels were down-modulated in the iNOS-deficient mice (data not shown), which probably explains the marked reduction in the level of IgE antibodies in these animals.
FIG. 4.
IgE levels in serum are decreased in vaccinated iNOS-deficient mice. C57BL/6 × 129SvEv F2 (WT) and iNOS-KO vaccinated and control nonvaccinated mice were challenged with 500 unattenuated cercariae. On days 0, 10, 14, and 18 postchallenge, four animals per group were bled and IgE levels in serum were determined by ELISA. The total IgE level in serum was recorded as the mean and standard error. Statistical comparisons were made by using Student’s t test, and the asterisk indicates that the groups are significantly different at that time point (P < 0.05).
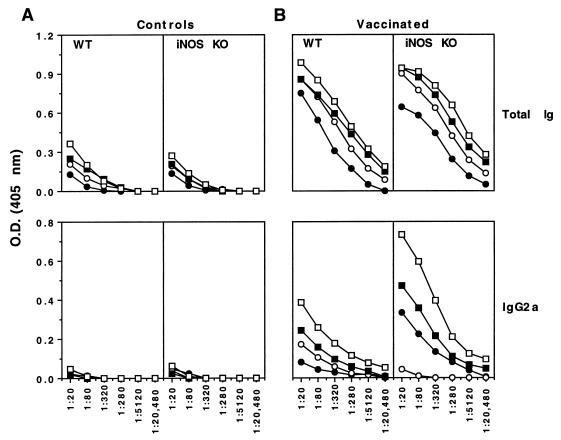
As shown in Fig. 5B, total parasite-specific Ig titers were increased approximately two- to four-fold in iNOS-KO mice compared with WT animals at nearly all time points postchallenge while the high titers of IgG1 antibodies were almost identical in both groups of mice (data not shown). Most strikingly, IgG2a antibody titers were increased more than 16-fold in the iNOS-deficient animals. A similar but only two- to fourfold increase in IgG2b titers was also detected (data not shown). There was little or no antibody response in any of the nonvaccinated animals except for total IgG levels in serum, which were modestly but similarly elevated in both WT and iNOS-KO mice by day 18 postchallenge (Fig. 5A). These findings thus correlate with the altered type 1/type 2 cytokine balance observed in the draining lymph nodes and spleens of the same animals (Fig. 3).
FIG. 5.
The Th1-associated humoral response is more prominent in vaccinated iNOS-deficient mice. C57BL/6 × 129SvEv F2 and iNOS-KO vaccinated and control nonvaccinated mice were challenged with 500 unattenuated cercariae. On days 0 (solid circles), 10 (open circles), 14 (solid squares), and 18 (open squares) postchallenge, four animals per group were bled and SWAP-specific antibody isotypes were determined by ELISA. The serum from individual mice was diluted as illustrated in the figure, and the average optical density (O.D.) for each group is presented.
Inflammatory foci around schistosomula in the lungs of iNOS-deficient mice are diminished in size.
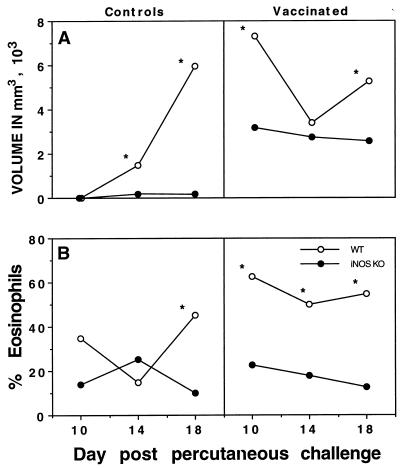
We examined the size and cellular phenotype of the inflammatory foci which form around the parasites as they migrate through the lungs, the predominant site of attrition of challenge infection in vaccinated mice. iNOS is expressed almost exclusively in these pulmonary inflammatory foci (43), and it has been suggested that the phenotype of these pulmonary lesions may significantly influence the successful onward migration and maturation of the parasites (7). In previous studies, we found that use of exogenous IL-12 to modulate the type 1/type 2 cytokine balance resulted in a reduction in the size of the inflammatory foci that form around challenge parasites as they migrate through the lungs (45). Similar to published results obtained with vaccinated and IL-12-treated animals, vaccinated iNOS-KO mice developed smaller inflammatory foci (Fig. 6A) and displayed >60% reduction in the number of tissue eosinophils within the lesions (Fig. 6B). Even more striking differences in the formation of foci were detected in the nonvaccinated control challenge groups. The changes in size as well as in eosinophil content correlate well with the altered IL-5 response observed in the iNOS-deficient animals (Fig. 3B).
FIG. 6.
The size of inflammatory foci induced by schistosomula in the lungs of iNOS-deficient mice are diminished and eosinophil accumulation in the lung is also reduced. C57BL/6 × 129SvEv F2 (WT) and iNOS-KO vaccinated and control nonvaccinated mice were challenged with 500 unattenuated cercariae. On days 10, 14, and 18 postchallenge, the left lungs were placed in fixative and processed routinely to measure the size of inflammatory foci (A) and the degree of tissue eosinophilia (B). The diameters of all lesions (3 to 12 lesions) per lung per mouse (four mice per group) were measured with an ocular micrometer, and the volume of each focus was calculated by assuming a spherical shape. The averages for each group at each time point are shown. Statistical comparisons were made by using Student’s t test, and the asterisk indicates that the groups are significantly different at that time point (P < 0.05).
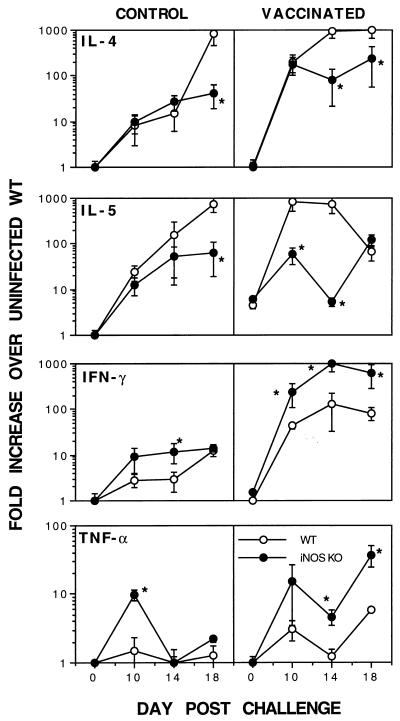
Type 1 cytokine mRNA production is enhanced and type 2 cytokine mRNA levels are reduced in the lungs of iNOS-deficient mice.
Although the lymph node and antibody data suggested that there was a strong skewing toward a more dominant type 1 immune response in iNOS-deficient animals, it was of interest to determine the immune response in the lungs at the time of parasite elimination. We therefore isolated total RNA from the lungs of vaccinated mice at 0, 10, 14, and 18 days postchallenge and performed quantitative RT-PCR to examine the changes in expression of several type 1 and type 2 cytokine mRNAs. In agreement with the ELISA results, vaccinated WT mice displayed a marked increase in the levels of both Th1- and Th2-associated cytokines at all time points postchallenge (Fig. 7). In contrast, vaccinated iNOS-KO mice showed significantly lower IL-4 and IL-5 mRNA levels at most time points postchallenge but developed almost a 1-log-unit increase in IFN-γ mRNA expression. Again, similar findings were observed in the nonvaccinated control groups, although the changes were less marked. Together, these data confirm a strong skewing toward a more pronounced type 1 immune response in iNOS-deficient mice. Interestingly, the TNF-α mRNA level was also significantly increased at two of three time points examined.
FIG. 7.
Type 1 cytokine mRNA production is enhanced and type 2 cytokine mRNA levels are reduced in the lungs of iNOS-deficient mice. C57BL/6 × 129SvEv F2 (WT) and iNOS-KO vaccinated and control nonvaccinated mice were challenged with 500 unattenuated cercariae, as described in Materials and Methods. Four animals per group were sacrificed on days 0, 10, 14, and 18 postchallenge, and total lung RNA isolated for RT-PCR analysis. Changes in cytokine-specific mRNAs were calculated from standard curves and are reported as fold increases in mRNA expression over the average background expression observed in untreated (unexposed WT control) mouse lungs ± standard error. Fold changes in mRNA levels which were significantly (P < 0.05) different between groups at the same time point are indicated with an asterisk.
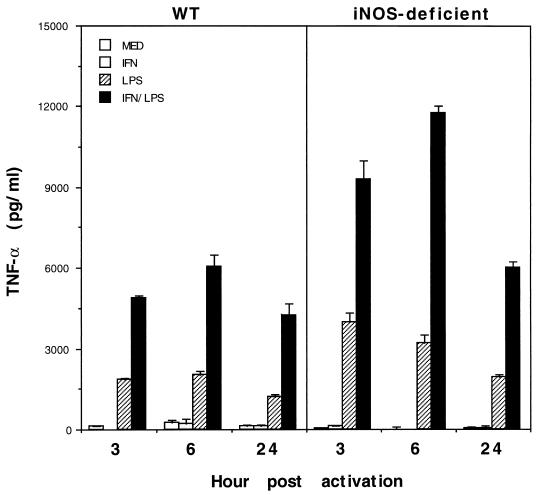
Thioglycolate-elicited PECs from iNOS-deficient mice produce elevated quantities of TNF-α in response to IFN-γ and LPS.
To begin to address the mechanism of the enhanced TNF-α and type 1 immune response in iNOS-deficient animals, we compared the pattern of TNF-α expression in thioglycolate-elicited macrophages from WT and iNOS-deficient mice. For these experiments, animals were injected intraperitoneally with 1.5 ml of sterile thioglycolate and PECs were obtained by lavage 4 days later. The PECs were stimulated in vitro with IFN-γ, LPS, or a combination of IFN-γ and LPS for 3, 6, or 24 h, and the culture supernatants were analyzed by ELISA for TNF-α. IFN-γ stimulation alone caused a slight or no increase in TNF-α expression in WT or iNOS-deficient PECs (Fig. 8). LPS triggered a significant TNF-α response in both WT and iNOS-deficient cells, although the levels in iNOS-deficient cultures were on average twofold higher. A similar significant increase in TNF-α expression was also detected in IFN-γ- and LPS-stimulated cultures from iNOS-deficient animals.
FIG. 8.
Thioglycolate-elicited peritoneal macrophages from vaccinated iNOS-deficient mice produce increased levels of TNF-α in response to IFN-γ and LPS. The production of TNF-α by PECs was evaluated in WT and iNOS-deficient mice. Mice were injected with 1.5 ml of sterile thioglycolate, and PECs were harvested 4 days later and placed in 24-well plates at 5 × 106 cells/ml. Individual wells were stimulated with IFN-γ (100 U/ml), LPS (250 ng/ml), or both. Culture supernatants were collected at 3, 6, and 24 h postactivation and examined for TNF-α by ELISA. The data reported are the means ± standard deviations for four mice per group.
DISCUSSION
Identification of the immune mechanisms by which attenuated infection protects mice against subsequent virulent infection should be beneficial in the design of a vaccine to prevent human schistosomiasis, a parasitic disease that currently afflicts an estimated 200 million people (5). Previous observations of a correlation between macrophage activity and resistance to S. mansoni infection in the radiation-attenuated vaccine model (17), along with the larvicidal function of NO produced by IFN-γ-activated macrophages and endothelial cells (1, 18) and the reduction in resistance resulting from in vivo treatment with an inhibitor of NO production (43), led us to postulate that the effector mechanism of vaccine-induced resistance involves Th1-associated immune responses leading to NO production. Mice genetically deficient in the iNOS enzyme provided an opportunity for definitive assessment of the function of NO in vaccine-induced immunity. As with other “gene knockout” models, however, since the enzyme is absent during the entire period when immunity is evolving, the role of NO in immune system effector function could not be separated from any potential role in the development of immunity. In the course of these experiments, we identified a paradoxical function for NO. It plays an effector role in resistance but may also promote parasite survival by down-regulating the vaccine-induced type 1 response that is required for its production.
The results presented here show that iNOS deficiency eliminated macrophage killing of larval parasites in vitro (Fig. 1) and diminished the level of vaccine-induced resistance by approximately 25 to 30% (Fig. 2). Similar findings were also recently reported by another group studying vaccine-induced immunity to schistosomiasis in iNOS-deficient mice (8). These findings confirm a role for NO in resistance but raise the possibility that other mechanisms are also equally if not more important. Indeed, it has been shown that erythrocytes can almost completely abolish the schistosomulacidal activity of NO in in vitro larvicidal assays (8). Nevertheless, ultrastructural studies have shown that macrophages are in extremely close proximity to schistosomula, both intravascularly and intra-alveolarly, in the lungs (7), and there is abundant iNOS expression within inflammatory foci surrounding the parasite in vivo (43); therefore, it seems unlikely that the erythrocyte quenching effect plays a substantial role in vivo. Also, the question remains whether the unknown NO-independent mechanisms play a role in vaccine-induced resistance to S. mansoni in WT animals. The 25 to 30% abrogation of resistance seen in iNOS-KO mice is consistent with the inhibition of resistance previously observed in aminoguanidine-treated animals (43) and could be interpreted to affirm the general importance of other mechanisms. However, in those experiments it was not confirmed that aminoguanidine treatment totally abrogated NO production in vivo. Moreover, it has not been shown that the vaccine-induced immune responses which evolve in the complete absence of NO are also present in WT animals. In this regard, the experiments presented here reveal a role for NO in the regulation of immune system reactivity, particularly of type-1 responses, in this model. Thus, this study significantly extends the findings reported by Coulson et al., since the effects of iNOS deficiency on the evolving immune response were not reported in their study (8). While the ability of NO to suppress T- and B-cell proliferation has been known for some time (2, 11), the potential to influence Th-cell differentiation is not as well understood and may vary with different pathogens. Various studies suggested a preferential suppressive effect of NO on either Th1 cells (20, 36, 39) or Th2 cells (28) or, alternatively, no differential effect (4). Increased spleen cell proliferation and/or release of IFN-γ in the absence of NO in vivo has been reported in mouse models of leishmaniasis (21, 39), toxic shock syndrome (12, 23), and bacterial septic arthritis (25). In these last three studies, exaggerated production of TNF-α was also observed. The results presented here (Fig. 3) clearly support a role for NO in modulation of type 1-associated cytokine responses in vivo. The mechanism whereby NO exerts this influence is unknown. NO has been reported to increase the production of IL-4, which promotes Th2 responses (6). However, measurement of cytokine mRNA expression in the lungs of vaccinated or control iNOS-KO mice showed decreased IL-4 levels only 2 to 3 weeks after challenge infection, whereas an enhanced IFN-γ mRNA response was observed within 10 days in vaccinated animals (Fig. 7). These observations do not seem consistent with a pivotal role for IL-4. We have observed increased production of TNF-α in IFN-γ- and LPS-activated thioglycolate-elicited macrophages from iNOS-deficient mice (Fig. 8). Thus, increased expression of TNF-α alone by antigen-presenting cells could explain the increased type 1 response, given the known role of TNF-α as an important cofactor for Th1-cell development (32). The reported effects of NO on major histocompatibility complex class II expression (33), apoptosis (3), endothelial-cell activation (10), and adhesion molecule expression (10) could also play a role in the alteration of immune system reactivity observed in iNOS-KO mice.
The nature of the residual but significant protective effector mechanisms in vaccinated iNOS-deficient mice likewise remains to be defined. It is generally agreed that resistance in mice vaccinated by a single exposure to irradiated cercariae is Th1 associated and that IFN-γ plays a key role (34). While we have postulated that the role of IFN-γ in this model is primarily that of effector cell activation (17), others have proposed that the main function of this cytokine is to up-regulate adhesion molecules on cells within the inflammatory foci surrounding parasites in the lungs, thereby impeding larval migration (35). Our histopathologic analyses of parasites in the lungs of vaccinated iNOS-KO mice revealed no obvious differences in either their number or their location with respect to WT animals. Pulmonary inflammatory foci were significantly smaller in iNOS-KO mice but were compact; no evidence was found for the type of diffuse leukocytic infiltration reported in IFN-γR–KO mice, which has been proposed as the basis for ineffective parasite entrapment in those animals (41). Therefore, the results presented here for iNOS-deficient mice, in which vaccine-induced resistance was significantly decreased despite a substantial increase in IFN-γ levels, argue against a direct effect of IFN-γ in the immune effector mechanism operating in this system. Pulmonary foci were composed predominantly of mononuclear cells in both WT and iNOS-KO mice, with the most obvious difference being a dearth of eosinophils in the latter (Fig. 6). Previous studies with IL-5-depleted (31) or transgenic (13) mice argue against a role for eosinophils in protective immunity, however, since IL-5-depleted animals were fully protected in the absence of an eosinophil response.
One possible contributor to the resistance to challenge infection observed in iNOS-KO mice might be TNF-α (29). Whereas an increase in TNF-α mRNA production was observed in vaccinated and challenged WT mice (Fig. 7), levels of TNF-α mRNA in the lungs of vaccinated iNOS-KO mice were approximately 10-fold higher. This increased production of TNF-α, produced chiefly by monocytes/macrophages, probably reflects heightened IFN-γ activation in the absence of feedback regulation by iNO. There may also be more direct effects of iNO on macrophage-derived TNF-α given the finding that TNF-α expression was increased in IFN-γ and LPS-activated iNOS-derived PECs (Fig. 8). In addition to its role as an inflammatory mediator, TNF-α is a potent effector molecule for the killing of certain tumor cell targets (37) and has been shown to have larvicidal activity at high concentrations (16).
Enhanced IgG2a levels observed in iNOS-KO mice (Fig. 5) might also play a role in the residual vaccine-induced resistance developed by these animals. We have previously shown that mice which have been both treated with IL-12 and multiply immunized with irradiated cercariae are almost entirely protected against challenge infection (46). Serum from these animals contained elevated levels of parasite-specific IgG2a, IgG2b, and IgG1 compared with sera from mice vaccinated in the absence of IL-12 and was better able to passively transfer resistance to naive recipients (46). More recently, experiments with B cell-deficient (μMT-KO) mice have reaffirmed a role for humoral response in the single-dose radiation-attenuated vaccine model (18a).
The fact that iNOS-deficient mice displayed an immune response to the irradiated-cercariae vaccine which, in comparison to that of WT animals, was greatly skewed toward type 1 reactivity reveals the difficulties inherent in interpreting results obtained from gene knockout mice. Our original objective was to assess the role of NO in the effector mechanism of S. mansoni resistance induced by an attenuated vaccine. In this regard, the results presented here suggest that NO plays only a modest (25 to 30%) role in resistance. However, because of the immunoregulatory role of NO, this modest decrease in protection is manifested in the presence of substantial increases in virtually every other mediator of resistance proposed in this model, including IFN-γ, TNF-α, and antibody. Therefore, it remains unclear whether the results obtained with iNOS-deficient animals accurately reflect the magnitude of the contribution of NO to resistance in WT mice. It seems paradoxical that NO, one of the most potent effector molecules against schistosome parasites yet discovered, might at the same time promote parasite survival by down-regulating not only its own production but also the induction of related protective type 1 responses. However, limitation of excessive NO production and the resulting tissue damage is no doubt of general benefit to the host. These results provide insight into the complicated nature of the protective immune response elicited by the attenuated vaccine. They further suggest that a defined vaccine based purely on induction of an NO-mediated effector mechanism is unlikely to provide maximal protection, due to the self-limiting nature of this response.
ACKNOWLEDGMENTS
We thank Sara Hieny for excellent technical assistance. We also acknowledge Alan Sher, Karl Hoffmann, Matthias Hesse, and Monica Chiaramonte for their helpful advice and comments.
REFERENCES
- 1.Ahmed S F, Oswald I P, Caspar P, Hieny S, Keefer L, Sher A, James S L. Developmental differences determine larval susceptibility to nitric oxide-mediated killing in a murine model of vaccination against Schistosoma mansoni. Infect Immun. 1997;65:219–226. doi: 10.1128/iai.65.1.219-226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albina J E, Abate J A, Henry W L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 3.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 4.Bauer H, Jung T, Tsikas D, Stichtenoth D O, Frolich J C, Neumann C. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology. 1997;90:205–211. doi: 10.1046/j.1365-2567.1997.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist N R. Schistosomiasis vaccine development: approaches and prospects. Mem Inst Oswaldo Cruz Rio J. 1995;90:221–227. doi: 10.1590/s0074-02761995000200017. [DOI] [PubMed] [Google Scholar]
- 6.Chang R H, Feng M H, Liu W H, Lai M Z. Nitric oxide increased interleukin-4 expression in T lymphocytes. Immunology. 1997;90:364–369. doi: 10.1111/j.1365-2567.1997.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulson P S. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 8.Coulson P S, Smythies L E, Betts C, Mabbott N A, Sternberg J M, Wei X-G, Liew F Y, Wilson R A. Nitric oxide produced in the lungs of mice immunized with the radiation-attenuated schistosome vaccine is not the major agent causing challenge parasite elimination. Immunology. 1998;93:55–63. doi: 10.1046/j.1365-2567.1998.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean D A, Mangold B L. Evidence that both normal and immune elimination of Schistosoma mansoni take place at the lung stage of migration prior to parasite death. Am J Trop Med Hyg. 1992;47:238–248. doi: 10.4269/ajtmh.1992.47.238. [DOI] [PubMed] [Google Scholar]
- 10.DeCaterina R, Libby P, Peng H B, Thannickal V J, Rajavashisth T B, Gimbrone M A, Jr, Shin W S, Liao J K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falzarano G, Krenger W, Snyder K M, Delmonte J, Jr, Karandikar M, Ferrara J L. Suppression of B-cell proliferation to lipopolysaccharide is mediated through induction of the nitric oxide pathway by tumor necrosis factor-alpha in mice with acute graft-versus-host disease. Blood. 1996;87:2853–2860. [PubMed] [Google Scholar]
- 12.Florquin S, Amraoui Z, Dubois C, Decuyper J, Goldman M. The protective role of endogenously synthesized nitric oxide in staphylococcal enterotoxin B-induced shock in mice. J Exp Med. 1994;180:1153–1158. doi: 10.1084/jem.180.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman G L, Jr, Tominaga A, Takatsu K, Secor W E, Colley D G. Elevated innate peripheral blood eosinophilia fails to augment irradiated cercarial vaccine-induced resistance to Schistosoma mansoni in IL-5 transgenic mice. J Parasitol. 1995;81:1010–1041. [PubMed] [Google Scholar]
- 14.James S L, Skamene E, Meltzer M S. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J Immunol. 1983;131:948–953. [PubMed] [Google Scholar]
- 15.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 16.James S L, Glaven J, Goldenberg S, Meltzer M S, Pearce E. Tumour necrosis factor (TNF) as a mediator of macrophage helminthotoxic activity. Parasite Immunol. 1990;12:1–9. doi: 10.1111/j.1365-3024.1990.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 17.James S L, Boros D L. Immune effector role of macrophages in experimental schistosomiasis mansoni. Immunol Ser. 1994;60:461–473. [PubMed] [Google Scholar]
- 18.James S L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Jankovic, D. Personal communication.
- 19.Kassim O O, Dean D A, Mangold B L, Von Lichtenberg F. Combined microautoradiographic and histopathologic analysis of the fate of challenge Schistosoma mansoni schistosomula in mice immunized with irradiated cercariae. Am J Trop Med Hyg. 1992;47:231–237. doi: 10.4269/ajtmh.1992.47.231. [DOI] [PubMed] [Google Scholar]
- 20.Liew F Y. Nitric oxide in infectious and autoimmune diseases. Ciba Found Symp. 1995;195:234–44. [PubMed] [Google Scholar]
- 21.Liew F Y. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995;7:396–399. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 22.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 23.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 24.Mangold B L, Dean D A. Passive transfer with serum and IgG antibodies of irradiated cercaria-induced resistance against Schistosoma mansoni in mice. J Immunol. 1986;136:2644–2648. [PubMed] [Google Scholar]
- 25.McInnes I B, Leung B, Wei X-Q, Gemmell C C, Liew F Y. Septic arthritis following Staphylococcus aureus infection in mice lacking inducible nitric oxide synthase. J Immunol. 1998;160:308–317. [PubMed] [Google Scholar]
- 26.Mills C D. Molecular basis of “suppressor” macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991;146:2719–2723. [PubMed] [Google Scholar]
- 27.Minard P, Dean D A, Jacobson R H, Vannier W E, Murrell K D. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978;27:76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- 28.Nukaya I, Takagi K, Kawabe T, Suketa Y. Suppression of cytokine production in T helper type 2 cells by nitric oxide in comparison with T helper type 1 cells. Microbiol Immunol. 1995;39:709–714. doi: 10.1111/j.1348-0421.1995.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 29.Oswald I P, Wynn T A, Sher A, James S L. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald I P, Eltoum I, Wynn T A, Schwartz B, Caspar P, Paulin D, Sher A, James S L. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sher A, Coffman R L, Hieny S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 32.Shibuya K, Robinson D, Zonin F, Hartley S B, Macatonia S E, Somoza C, Hunter C A, Murphy K M, O’Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- 33.Sicher S C, Vazquez M A, Lu C Y. Inhibition of macrophage Ia expression by nitric oxide. J Immunol. 1994;153:1293–1300. [PubMed] [Google Scholar]
- 34.Smythies L E, Coulson P S, Wilson R A. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149:3654–3658. [PubMed] [Google Scholar]
- 35.Smythies L E, Coulson P S, Wilson R A. Immunity to Schistosoma mansoni in mice vaccinated with irradiated cercariae: cytokine interactions in the pulmonary protective response. Ann Trop Med Parasitol. 1993;87:653–657. doi: 10.1080/00034983.1993.11812825. [DOI] [PubMed] [Google Scholar]
- 36.Taylor-Robinson A W. Counter-regulation of T helper 1 cell proliferation by nitric oxide and interleukin-2. Biochem Biophys Res Commun. 1997;233:14–19. doi: 10.1006/bbrc.1997.6386. [DOI] [PubMed] [Google Scholar]
- 37.Urban J L, Shepard H M, Rothstein J L, Sugarman B J, Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci USA. 1986;83:5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vignali D A, Crocker P, Bickle Q D, Cobbold S, Waldmann H, Taylor M G. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology. 1989;67:466–472. [PMC free article] [PubMed] [Google Scholar]
- 39.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 40.Wilson R A, Coulson P S, Dixon B. Migration of the schistosomula of Schistosoma mansoni in mice vaccinated with radiation-attenuated cercariae, and normal mice: an attempt to identify the timing and site of parasite death. Parasitology. 1986;92:101–116. doi: 10.1017/s0031182000063484. [DOI] [PubMed] [Google Scholar]
- 41.Wilson R A, Coulson P S, Betts C, Dowling M A, Smythies L E. Impaired immunity and altered pulmonary responses in mice with a disrupted interferon-gamma receptor gene exposed to the irradiated Schistosoma mansoni vaccine. Immunology. 1996;87:275–282. doi: 10.1046/j.1365-2567.1996.465550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 43.Wynn T A, Oswald I P, Eltoum I A, Caspar P, Lowenstein C J, Lewis F A, James S L, Sher A. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J Immunol. 1994;153:5200–5209. [PubMed] [Google Scholar]
- 44.Wynn T A, Eltoum I, Oswald I P, Cheever A W, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wynn T A, Jankovic D, Hieny S, Cheever A W, Sher A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995;154:4701–4709. [PubMed] [Google Scholar]
- 46.Wynn T A, Reynolds A, James S, Cheever A W, Caspar P, Hieny S, Jankovic D, Strand M, Sher A. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J Immunol. 1996;157:4068–4078. [PubMed] [Google Scholar]