Abstract
Rab5 and phosphatidylinositol 3-kinase (PI3K) have been proposed to co-regulate receptor endocytosis by controlling early endosome fusion. However, in this report we demonstrate that inhibition of epidermal growth factor (EGF)-stimulated PI3K activity by expression of the kinase-deficient PI3K p110 subunit (p110Δkin) does not block the lysosomal targeting and degradation of the EGF receptor (EGFR). Moreover, inhibition of total PI3K activity by wortmannin or LY294002 significantly enlarges EGFR-containing endosomes and dissociates the early-endosomal autoantigen EEA1 from membrane fractions. However, this does not block the lysosomal targeting and degradation of EGFR. In contrast, transfection of cells with mutant Rab5 S34N or microinjection of anti-Rabaptin5 antibodies inhibits EGFR endocytosis. Our results, therefore, demonstrate that PI3K is not universally required for the regulation of receptor intracellular trafficking. The present work suggests that the intracellular trafficking of EGFR is controlled by a novel endosome fusion pathway that is regulated by Rab5 in the absence of PI3K, rather than by the previously defined endosome fusion pathway that is co-regulated by Rab5 and PI3K.
INTRODUCTION
Phosphatidylinositol 3-kinases (PI3Ks) are grouped into three classes (Vanhaesebroeck et al., 1997). Class I PI3Ks include p85/p110, which are activated by receptor tyrosine kinases, including EGFR and the platelet-derived growth factor receptor (PDGFR) (Hu et al., 1992; Rodriguez-Viciana et al., 1994). Class II PI3Ks are larger in size and contain a C-terminal C2 domain. Class III PI3Ks include yeast Vps34P and their homologues in other species. Both class I and class III PI3Ks are inhibited by low nanomolar concentrations of wortmannin (Vanhaesebroeck et al., 1997), whereas the inhibition of class II PI3Ks requires high nanomolar to micromolar concentrations of wortmannin (Domin et al., 1997). All three classes of PI3Ks have been implicated in the regulation of vesicular trafficking. In particular, mutant PDGFR lacking its high-affinity binding sites for PI3Ks fails to reach lysosomes and instead becomes distributed to the cell periphery in response to PDGF stimulation (Joly et al., 1994). This suggests that PDGF-stimulated PI3K activity is essential for PDGFR targeting to lysosomes and degradation there. However, microinjection of inhibitory anti-hVP34 antibodies, rather than anti-p110α antibodies, blocks the transit of internalized PDGFR to perinuclear compartments (Siddhanta et al., 1998).
Rab5 regulates receptor endocytosis by controlling early endosome fusion (Stenmark et al., 1994). Several Rab5 effectors, including Rabaptin5 (Stenmark et al., 1995), Rabaptin5β (Gournier et al., 1998) and EEA1 (Christoforidis et al., 1999), have been identified. Recent studies have shown that Rab5 and PI3K co-regulate endosome fusion by controlling the association of EEA1with endosomes (Simonsen et al., 1998). EEA1 interacts directly with phosphatidylinositol(3)-phosphate (PI-3-P) through its FYVE finger domain (Burd and Emr, 1998; Gaullier et al., 1998; Patki et al., 1998) and the localization of EEA1 on early endosomes depends on an intact FYVE domain (Stenmark et al., 1996; Gaullier et al., 1998) and PI3K activity (Patki et al., 1997). In addition to PI-3-P, EEA1 associates with GTP-bound Rab5 through independent domains, and the interactions between EEA1and both PI-3-P and GTP-Rab5 are necessary for the stable association of EEA1 with early endosomes in vivo (Simonsen et al., 1998). Based on these results, an endosome fusion model has been proposed. In this model, early endosome fusion is mediated by EEA1 that is co-regulated by Rab5 and PI3K (Simonsen et al., 1998).
We have studied the role of PI3Ks and Rab5 in the intracellular trafficking of EGFR. We found that inhibition of EGF-stimulated PI3K activity by the kinase-deficient catalytic p110α subunit (p110Δkin) did not block the lysosomal targeting and degradation of EGFR, neither did inhibition of total PI3K activity by wortmannin or LY294002. However, transfection of cells with a Rab5 mutant, Rab5 S34N, or microinjection of cells with anti-Rabaptin5 antibodies inhibited EGFR endocytosis. Our results demonstrate that the intracellular trafficking of EGFR is regulated by a novel endosome fusion pathway that is controlled by Rab5 in the absence of PI3K, rather than co-regulated by Rab5 and PI3K.
RESULTS
Inhibition of EGF-stimulated p85/p110 PI3K activity and its effects on EGFR endocytosis
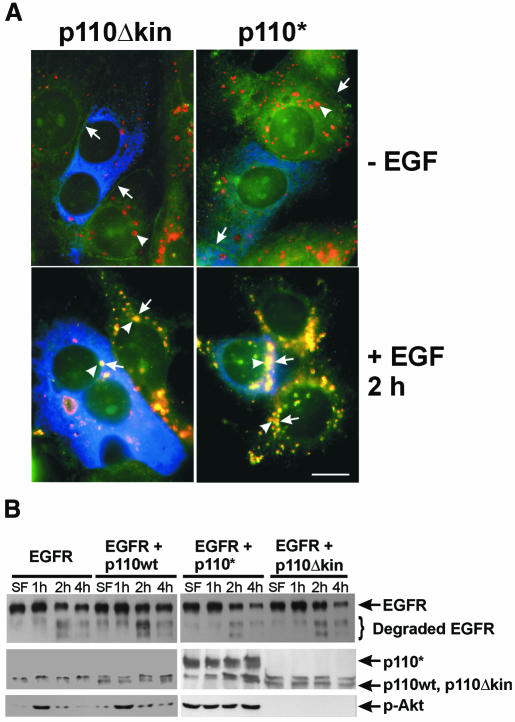
To block EGF-stimulated PI3K activation, we transfected SKBR-3 cells with the kinase-deficient catalytic p110α subunit, p110Δkin, and then examined the effects on EGF-induced targeting of EGFR to lysosomes. The lysosomal localization of EGFR was determined by co-localization of EGFR and Lamp, a lysosomal marker. As shown in Figure 1A, endocytosis of EGFR followed the same pattern in both transfected and non-transfected cells. EGFR was localized to the plasma membrane without EGF stimulation and co-localized with Lamp1 in lysosomes following EGF stimulation for 2 h. Interestingly, increasing PI3K activity by transfection of cells with the constitutively activated p110α subunit p110* also had no effect on EGF-induced targeting of EGFR to lysosomes (Figure 1A).
Fig. 1. EGFR endocytosis after inhibition of EGF-stimulated PI3K activity. (A) SKBR-3 cells were transfected with myc-tagged mutant p110Δkin or p110* for 48 h and then stimulated with EGF for 2 h or not stimulated. Indirect immunofluorescence was performed as described in Methods. Photographs are triple exposures with EGFR localization in the green channel (arrows), Lamp1 localization in the red channel (arrowheads) and transfected p110 in the blue channel. Size bar = 15 µm. (B) 293T cells were transfected with human EGFR, wild-type PI3K p110, mutant p110Δkin and/or p110* for 48 h and then stimulated with EGF for the indicated time. Cell lysates were subjected to immunoblot analysis with anti-EGFR, anti-myc or anti-phospho-Akt (p-Akt) antibodies.
EGF-induced EGFR degradation was examined in 293T cells transfected with EGFR or co-transfected with EGFR and wild-type p110, mutant p110Δkin or p110* (Figure 1B). Following EGF stimulation for 2–4 h, intact EGFR (represented by the top 170 kD band) decreased significantly, while degraded EGFR (represented by the lower bands) increased significantly to the same degree in cells transfected with or without the p110 mutants. To determine whether transfection of the p110 mutants modulates PI3K activity, we assayed the activation of Akt in transfected cells with anti-phospho-Akt antibodes. We found that transfection of p110Δkin significantly reduced Akt phoshorylation, whereas transfection of p110* significantly enhanced Akt phosphorylation (Figure 1B). Together, these results suggest that EGF-stimulated PI3K activity is not required for the lysosomal targeting and degradation of EGFR.
Inhibition of total PI3K activity and its effects on EGFR endocytosis
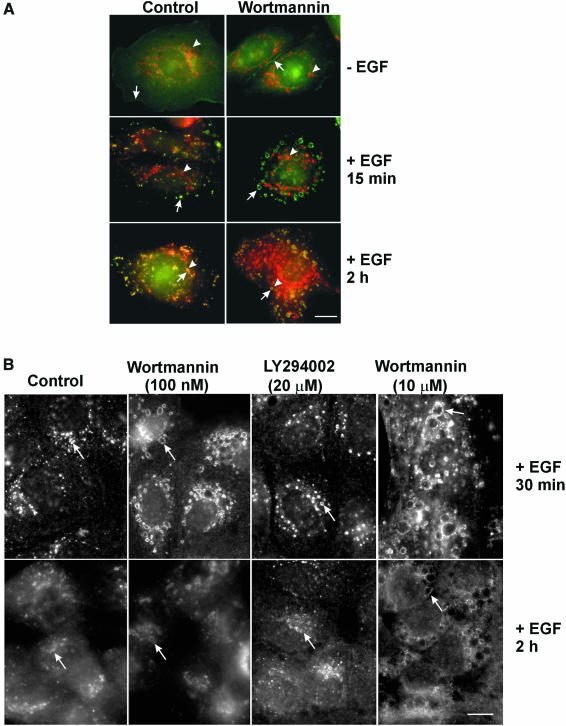
Next, we investigated whether basal PI3K activity is required for the lysosomal targeting and degradation of EGFR. We treated SKBR-3 cells with wortmannin and examined the co-localization of EGFR and Lamp1 in response to EGF (Figure 2A). In both the control and wortmannin-treated cells, without EGF stimulation, EGFR was localized to the plasma membrane, while Lamp1 was localized to lysosomes. Following EGF stimulation for 15 min, EGFR was internalized into endosomes, but there was no significant co-localization of EGFR and Lamp1. After incubation with EGF for 2 h, EGFR was endocytosed to lysosomes and co-localized with Lamp1. Wortmannin treatment dramatically enlarged EGFR-containing endosomes, but did not significantly enlarge the Lamp1-positive lysosomes.
Fig. 2. Effects of wortmannin on the intracellular trafficking of EGFR in SKBR-3 and MDCK cells. (A) SKBR-3 cells. Left panel, control. Right panel, cells treated with wortmannin (100 nM). Cells were either kept in serum-deprived media or stimulated with EGF for 15 min or 2 h. Photographs are double exposures with EGFR localization in the green channel (arrows), Lamp1 localization in the red channel (arrowheads). (B) MDCK cells. Left most panels: control. Second panels: cells treated with wortmannin (100 nM). Third panels: cells treated with LY294002 (20 µM). Right most panels: cells treated with a high concentration of wortmannin (10 µM). Cells were stimulated with EGF for 30 min or 2 h. EGFR localization (arrows) was revealed by indirect immunofluorescence. Size bar = 20 µm.
Wortmannin or LY294002 treatment also did not block EGFR targeting to lysosomes in MDCK cells (Figure 2B) and BT20 cells (data not shown). Both wortmannin and LY294002 enlarged EGFR-containing endosomes, but the effect of LY294002 was not as dramatic as that of wortmannin. Similar effects of LY294002 were observed in BT20 and SKBR-3 cells (data not shown).
In the above experiments, the wortmannin concentration was 100 nM. At this low concentration, wortmannin effectively inhibits class I and class III, but not class II, PI3K activity (Domin et al., 1997; Vanhaesebroeck et al., 1997). To completely inhibit class II PI3K activity, we treated MDCK cells with 10 µM wortmannin and examined the effects on the lysosomal targeting and degradation of EGFR (Figure 2B). While 10 µM wortmannin had much stronger effects on cell morphology than 100 nM wortmannin, it did not block the lysosomal targeting and degradation of EGFR. We also treated BT20 and SKBR-3 cells with 10 µM wortmannin and obtained similar results (data not shown).
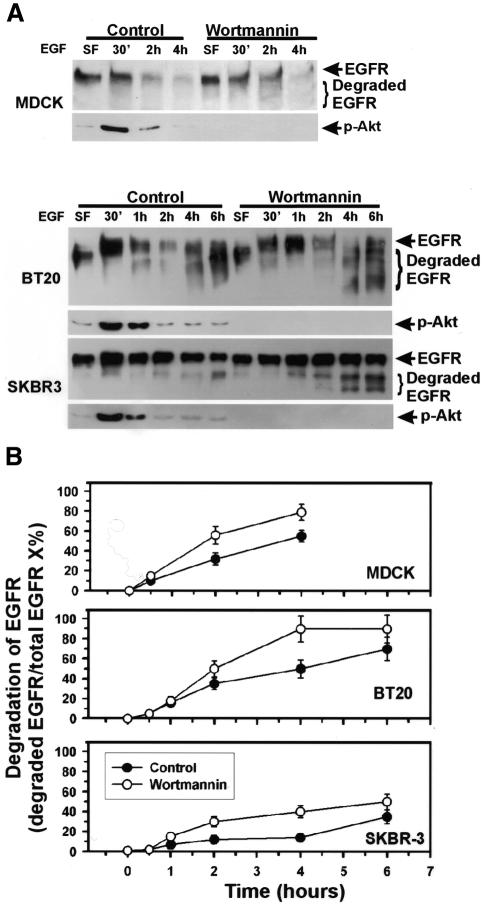
We then examined the effects of wortmannin on EGF-induced EGFR degradation in MDCK, BT20 and SKBR-3 cells by immunoblotting (Figure 3). Wortmannin did not inhibit, but rather moderately enhanced, EGF-induced EGFR degradation. Immunoblotting of the same membrane with anti-phospho-Akt antibodies confirmed that wortmannin treatment abolished Akt phosphorylation. Similar results were obtained when the concentration of wortmannin was increased to 10 µM (data not shown) and when wortmannin was substituted by 20 µM LY294002 (data not shown). Together, these results suggest that even basal PI3K activity is not required for the EGF-induced lysosomal targeting and degradation of EGFR.
Fig. 3. Effects of wortmannin on the lysosomal degradation of EGFR in MDCK, SKBR-3 and BT20 cells. (A) Cells were treated with wortmannin and then stimulated with EGF for the indicated times. Cell lysates were subjected to immunoblot analysis with anti-EGFR or anti-phospho-Akt (p-Akt) antibodies. (B) Quantification of the results from (A). Quantification was performed with a digital imaging system from Alpha Innotech Corporation (San Leandro, CA).
Requirement for Rab5 and Rabaptin5 activity in EGFR endocytosis
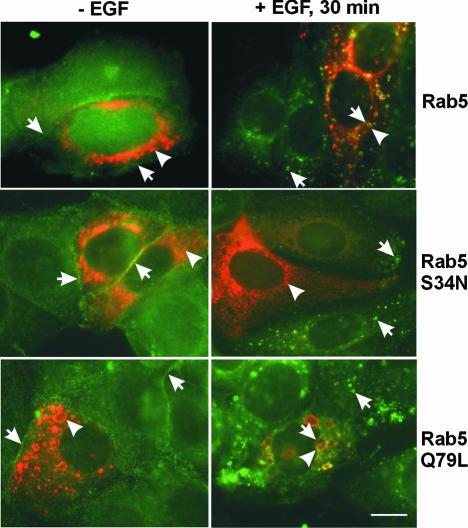
Two Rab5 mutants, Rab5 S34N and Q79L, were used to modulate Rab5 activity in vivo. Rab5 S34N has a preferential affinity for GDP, while Rab5 Q79L is defective in GTP hydrolysis (Stenmark et al., 1994). SKBR-3 cells were transfected with wild-type Rab5, mutant Rab5 S34N or mutant Q79L, and EGF-stimulated EGFR endocytosis was examined 48 h post-transfection (Figure 4). Consistent with previous reports, transfection with wild-type Rab5 resulted in normal endosomes, while transfection with Rab5 S34N resulted in fragmented endosomes and transfection with Rab5 Q79L resulted in enlarged endosomes. Without EGF stimulation, EGFR was localized to the plasma membrane of the transfected and non-transfected cells. Following EGF stimulation, EGFR was endocytosed into Rab5-positive endosomes in cells transfected with wild-type Rab5 or Rab5 Q79L. However, in Rab5 S34N-transfected cells, EGFR was localized to the plasma membrane and to some very small Rab5-negative peripheral vesicles. This suggests that Rab5 S34N blocks intracellular trafficking of EGFR to endosomes and lysosomes.
Fig. 4. Regulation of the intracellular trafficking of EGFR by Rab5. SKBR-3 cells were transfected with wild-type Rab5, mutant Rab5 S34N or Q79L for 48 h and then stimulated with EGF for 30 min (right panels) or kept in serum-deprived media (left panels). Photographs are double exposures with EGFR localization in the green channel (arrows) and Rab5 localization in the red channel (arrowheads). Size bar = 20 µm.
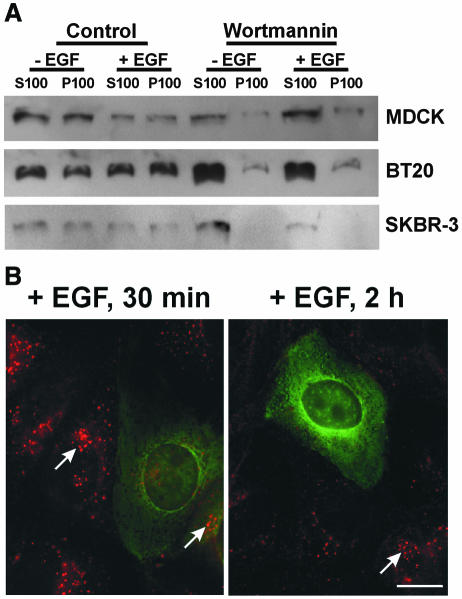
We then examined whether the Rab5 effectors, EEA1 and Rabaptin5, were required for EGFR endocytosis. Wortmannin has been reported to significantly inhibit the membrane association of EEA1 and thus to disrupt endosome fusion (Patki et al., 1997). We investigated whether wortmannin affects the membrane association of EEA1 in our cell systems. Treatment with 100 nM wortmannin resulted in a significant dissociation of EEA1 from the membrane fractions of MDCK, BT20 and SKBR-3 cells, whereas a small portion of EEA1 remained associated with membrane fractions in MDCK and BT20 cells (Figure 5A). When the concentration of wortmannin was increased to 10 µM, the membrane association of EEA1 was further reduced, but not completely abolished, in MDCK and BT20 cells (data not shown). Thus, inhibition of PI3K by wortmannin significantly reduced the association of EEA1 with endosomes, but did not block the lysosomal targeting and degradation of EGFR.
Fig. 5. Effects of Rab5 effectors on the intracellular trafficking of EGFR. (A) Dissociation of EEA1 from membrane fractions by wortmannin treatment of MDCK, SKBR-3 and BT20 cells. Cells were subjected to subcellular fractionation into a soluble fraction (S100) and a particulate fraction (P100) after treatment with wortmannin (100 nM) and EGF (30 min), as indicated. Subcellular fractions were subjected to immunoblot analysis with anti-EEA1 antibodies. (B) Effects of microinjection with anti-Rabaptin5 antibodies on EGFR endocytosis in MDCK cells. Cells were stimulated with EGF for 15 min or 2 h after injection. Photographs are double exposures, with EGFR localization in the red channel (arrows) and microinjected antibodies in the green channel. Size bar = 20 µm.
To investigate whether Rabaptin5 is required for the intracellular trafficking of EGFR, we microinjected MDCK cells with a Rabaptin5 monoclonal antibody and then stimulated with EGF. Following EGF stimulation for 30 min and 2 h, EGFR staining in perinuclear endosomes was much weaker in the injected cells than that in the non-injected cells (Figure 5B), which suggests that inhibition of Rabaptin5 blocks the intracellular trafficking of EGFR.
DISCUSSION
In contrast to what has been reported regarding PDGFR (Joly et al., 1994), our results indicate that the intracellular trafficking of EGFR does not require EGF-stimulated PI3K activity. Among the three classes of PI3Ks, only class I p85/p110 PI3Ks are activated by EGFR and PDGFR (Hu et al., 1992; Rodriguez-Viciana et al., 1994). To completely inhibit EGF-stimulated PI3K activity, SKBR-3 and 293T cells were transfected with the kinase-deficient p110α subunit p110Δkin (Hu and Schlessinger, 1994). We showed that transfection of cells with p110Δkin had no effect on the EGF-stimulated lysosomal targeting and degradation of EGFR (Figure 1). It has been reported that microinjection of inhibitory anti-p110α antibodies did not block the transit of internalized PDGFR to perinuclear compartments (Siddhanta et al., 1998).
In addition, we showed that inhibition of PI3K activity by 100 nM or 20 µM LY294002 does not block the EGF-induced lysosomal targeting and degradation of EGFR (Figures 2 and 3), but rather moderately enhances the lysosomal degradation of EGFR (Figure 3). However, 100 nM wortmannin blocks both the transfer of the PDGFR from peripheral compartments to juxtanuclear vesicles and its subsequent degradation (Shpetner et al., 1996). Microinjection of inhibitory anti-hVP34 antibodies blocks the transit of internalized PDGFR to perinuclear compartments (Siddhanta et al., 1998). Given that 100 nM wortmannin inhibits both p85/p110 PI3K and hVP34 activity (Schu et al., 1993; Vanhaesebroeck et al., 1997), our results suggest that the targeting of EGFR and PDGFR to lysosomes are regulated differently. Moreover, a high concentration of wormannin (10 µM) that blocks class II PI3K activity was still unable to inhibit the lysosomal targeting and degradation of EGFR (Figure 2 and data not shown). Together, these results indicate that PI3K activity is not required for the lysosomal targeting and degradation of EGFR.
However, we found that the inhibition of Rab5 activity with Rab5 S34N blocked the intracellular trafficking of EGFR (Figure 4). Thus, the early endosome fusion during EGFR endocytosis is controlled by Rab5. Our results do not suggest that Rab5 specifically interacts with EGFR to regulate the intracellular trafficking of EGFR, but rather indicate that EGFR, like many other receptors, requires functional Rab5 for its intracellular trafficking.
It is widely accepted that the interactions of EEA1 with both PI-3-P and GTP-Rab5 are necessary for the stable association of EEA1 with early endosomes in vivo (Simonsen et al., 1998). Wortmannin has been reported to significantly inhibit the membrane association of EEA1, thereby disrupting endosome fusion (Patki et al., 1997). Our finding that PI3K activity is not required for the intracellular trafficking of EGFR to lysosomes raises an interesting question: does inhibition of PI3K activity abolish the membrane association of EEA1, thereby blocking the function of EEA1? We examined the effects of wortmannin on the membrane association of EEA1 in MDCK, BT20 and SKBR-3 cells. We found that in SKBR3 cells, the membrane association of EEA1 became undetectable when total PI3K activity was inhibited by wortmannin or LY294002. However, treatment of BT20 and MDCK cells with wortmannin or LY294002 significantly reduced, but did not completely abolished, the membrane association of EEA1 (Figure 5A). The fact that inhibition of PI3K activity by wortmannin significantly blocked the membrane association of EEA1, but did not block the intracellular trafficking of EGFR, suggests that EEA1 may not be important for the intracellular trafficking of EGFR. On the other hand, the persistent minimum membrane association of EEA1 in wortmannin- or LY294002-treated BT20 and MDCK cells may suggest that this membrane association of EEA1 is required for the intracellular trafficking of EGFR. The increased levels of Rab5-GTP have been reported to restore membrane fusion even when PI3K activity is inhibited (Jones et al., 1998; Simonsen et al., 1998). Thus, it is possible that the minimum membrane association of EEA1 is controlled by Rab5 in the absence of PI3K activity.
In either case, our results strongly suggest that the intracellular trafficking of EGFR may be regulated by a novel endosome fusion pathway that is regulated by Rab5 in the absence of PI3K, rather than by the previously defined endosome fusion pathway that is co-regulated by Rab5 and PI3K. Our results do not exclude the possible involvement of PI3Ks in the regulation of the intracellular trafficking of EGFR in vivo, but rather suggest that the function of PI3Ks is dispensable.
Given that EEA1 may or may not be involved in the intracellular trafficking of EGFR, we examined the involvement of another Rab5 effector, Rabaptin5, in the intracellular trafficking of EGFR. Rabaptin5 activity is essential for Rab5-mediated endosome fusion (Stenmark et al., 1995). Indeed, our results demonstrate that Rabaptin5 activity is required for the intracellular trafficking of EGFR (Figure 5B). However, whether Rabaptin5 functions together with EEA1 or functions independently in regulating endosome fusion needs to be investigated further.
METHODS
Antibodies and chemicals. Sheep anti-EGFR antibodies were purchased from Upstate Technology (Lake Placid, NY). Rabbit anti-EGFR and rabbit anti-myc antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antibodies to Rab5, EEA1 and Rabaptin5 were from BD PharMingen Research (San José, CA). Rabbit anti-phospho-Akt antibodies were from New England Biolabs. Mouse anti-Lamp1 antibodies were from Research Diagnostics Inc. (Flanders, NJ). Unless otherwise specified, all chemicals were from Sigma.
Wortmannin or LY294002 treatment. Cells were treated with wortmannin (100 nM or 10 µM) or LY294002 (20 µM) for 15 min at 37°C and then treated with EGF (100 ng/ml) for the indicated time intervals in the continuous presence of wortmannin or LY294002. For treatments longer than 2 h, medium was replaced with fresh medium containing the same concentrations of wortmannin or LY294002. Cells were fixed and assayed for EGFR endocytosis.
Transient transfection. SKBR-3 and 293T cells were transiently transfected by calcium phosphate precipitation with the vectors encoding myc-tagged wild-type p110, mutant p110Δkin, mutant p110*, wild-type Rab5, mutant Rab5 Q79L, Rab5 S34N, or EGFR. Forty-eight hours after transfection, SKBR-3 cells were used for indirect immunofluorescence while 293T cells were used for immunoblot analysis.
Microinjection and Indirect immunofluorescence. Microinjection and indirect immunofluorescence were carried out as described previously (Wang and Moran, 1996). For the SKBR-3 cells transfected with the vector encoding myc-tagged wild-type or mutant p110, cells were incubated first with sheep anti-EGFR, rabbit anti-myc and mouse anti-Lamp1 antibodies, and then with a mixture of FITC-labelled donkey anti-sheep, rhodamine-labelled donkey anti-mouse and AMCA-labelled donkey anti-rabbit IgG. For SKBR-3 cells transfected with the wild-type or mutant Rab5, the primary antibodies were sheep anti-EGFR and mouse anti-Rab5 antibodies. The secondary antibodies were FITC-labelled donkey anti-sheep IgG and rhodamine-labelled donkey anti-mouse IgG. For SKBR-3 cells treated with wortmannin or LY294002, sheep anti-EGFR and mouse anti-Lamp1 antibodies were used as the primary antibodies. For MDCK cells treated with wortmannin or LY294002, the primary antibody was sheep anti-EGFR antibody, and the secondary antibody was rhodamine-labelled donkey anti-sheep IgG. Colour photographs were taken with a digital camera by superimposing the monochrome graphs of two or three channels.
Immunoblotting. Immunoblotting was performed as described previously (Wang and Moran, 1996). For EGFR degradation assay, following treatment of wortmannin/LY294002 and EGF, cells were lysed with RIPA buffer in the presence of 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM AEBSF, 1 µM pepstatin A and 10 µg of aprotinin/ml. To determine the membrane association of EEA1, following treatment of wortmannin/LY294002 and EGF, cells were fractionated into a soluble fraction (S100) and a particulate fraction (P100) as described previously (Fam et al., 1997). Aliquots containing 20 µg of protein from each cell lysate were used for electrophoresis. EGFR, phospho-Akt and the myc-tagged p110 were probed with polyclonal rabbit ant-EGFR, anti-phospho-Akt and anti-myc antibodies, respectively, followed by HRP-conjugated goat anti-rabbit IgG. EEA1 was probed with an EEA1 monoclonal antibody, followed by HRP-conjugated goat-anti-mouse IgG. Protein bands were detected by enhanced chemiluminescence development (Pierce Chemical) and quantitated with a FluorChem digital imaging system (Alpha Innotech Corporation).
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. Zerial for Rab5 reagents, L.T. Williams and A. Klippel for PI3K p110 reagents, and R. Rachubinski and D. Brindley for comments on the manuscript. This work was supported in part by a grant from the Canadian Institutes of Health Research (CIHR). Z.W. is a CIHR Scholar and an Alberta Heritage Foundation for Medical Research Scholar.
REFERENCES
- Burd C.G. and Emr, S.D. (1998) Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell, 2, 157–162. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., McBride, H.M., Burgoyne, R.D., and Zerial, M. (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Domin J., Pages, F., Volinia, S., Rittenhouse, S.E., Zvelebil, M.J., Stein, R.C., and Waterfield, M.D. (1997) Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J., 326, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam N.P., Fan, W.T., Wang, Z., Zhang, L.J., Chen, H., and Moran, M.F. (1997) Cloning and characterization of Ras-GRF2, a novel guanine nucleotide exchange factor for Ras. Mol. Cell Biol., 17, 1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier J.M., Simonsen, A., D’Arrigo, A., Bremnes, B., Stenmark, H., and Aasland, R. (1998) FYVE fingers bind PtdIns(3)P. Nature, 394, 432–433. [DOI] [PubMed] [Google Scholar]
- Gournier H., Stenmark, H., Rybin, V., Lippe, R., and Zerial, M. (1998) Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO J., 17, 1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. and Schlessinger, J. (1994) Direct association of p110β phosphatidylinositol 3-kinase with p85 is mediated by an N-terminal fragment of p110β. Mol. Cell Biol., 14, 2577–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Margolis, B., Skolnik, E.Y., Lammers, R., Ullrich, A., and Schlessinger, J. (1992) Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell Biol., 12, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M., Kazlauskas, A., Fay, F.S., and Corvera, S. (1994) Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science, 263, 684–687. [DOI] [PubMed] [Google Scholar]
- Jones A.T., Mills, I.G., Scheidig, A.J., Alexandrov, K., and Clague, M.J. (1998) Inhibition of endosome fusion by wortmannin persists in the presence of activated Rab5. Mol. Biol. Cell, 9, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V., Virbasius, J., Lane, W.S., Toh, B.H., Shpetner, H.S., and Corvera, S. (1997) Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA, 94, 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V., Lawe, D.C., Corvera, S., Virbasius, J.V., and Chawla, A. (1998) A functional PtdIns(3)P-binding motif. Nature, 394, 433–434. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Warne, P.H., Dhand, R., Vanhaesebroeck, B., Gout, I., Fry, M.J., Waterfield, M.D., and Downward, J. (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature, 370, 527–532. [DOI] [PubMed] [Google Scholar]
- Schu P.V., Takegawa, K., Fry, M.J., Stack, J.H., Waterfield, M.D., and Emr, S.D. (1993) Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science, 260, 88–91. [DOI] [PubMed] [Google Scholar]
- Shpetner H., Joly, M., Hartley, D., and Corvera, S. (1996) Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol., 132, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhanta U., McIlroy, J., Shah, A., Zhang, Y., and Backer, J.M. (1998) Distinct roles for the p110α and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol., 143, 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Lippe, R., Christoforidis, S., Gaullier, J.M., Brech, A., Callaghan, J., Toh, B.H., Murphy, C., Zerial, M., and Stenmark, H. (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton, R.G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J., 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Vitale, G., Ullrich, O., and Zerial, M. (1995) Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell, 83, 423–432. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Aasland, R., Toh, B.H., and D’Arrigo, A. (1996) Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem., 271, 24048–24054. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers, S.J., Panayotou, G., and Waterfield, M.D. (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci. Sci., 22, 267–272. [DOI] [PubMed] [Google Scholar]
- Wang Z. and Moran, M.F. (1996) Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science, 272, 1935–1939. [DOI] [PubMed] [Google Scholar]