Abstract
Background
Only a few comprehensive studies have been carried out on parasites in amphibians and reptiles in Ukraine. This has resulted in identifying over 100 helminth species across these vertebrate groups. However, most of the studies were performed in the 20th century and the taxonomy of many parasites and their hosts has changed ever since, in addition to the discovery of new species and registrations of species that had not been previously known for Ukraine. In recent decades, there have been very few publications on helminths from amphibian or reptile hosts in this region. Notably, just one of these recent studies is a faunistic study, providing a list of helminths found in two species of green frogs – Pelophylaxridibundus (Pallas, 1771) and Pelophylaxesculentus (Linnaeus, 1758). Therefore, it is clear that publishing datasets of modern records of helminths in these vertebrate groups, based on modern taxonomy, is an essential step in further studies of their parasitic diversity. Additionally, such study is important in terms of global climate change, the growing number of possibilities of invasion of alien species (both hosts and parasites) that might potentially become a threat to native biota and growing anthropogenic pressure on local populations of hosts that affect the parasites as well. In future, this study is planned to be used for the creation of a checklist of helminths of the herpetofauna of Ukraine. The present dataset is an inventory of various species of helminths parasitising common species of the herpetofauna in central, northern, western and southern Ukraine recorded during field studies in the 2021-2023 period.
New information
The dataset is the first one to represent the up-to-date and unified data on helminths of reptiles and amphibians of Ukraine. Previously, records of this group of organisms with reference to their hosts were presented as several separate records within the country. Currently, this is the largest dataset presenting geocoded records of non-human-related helminths in the fauna of Ukraine. It reports helminth species from 15 hosts (205 individuals), including eight amphibians and seven reptilian species found in various Ukrainian regions. A total of 47 helminth species have been documented in the research and during 2021-2023 period on the territory of northern (Kyiv and Zhytomyr), western (Lviv, Zakarpattia Ivano-Frankivsk), central (Vinnytsia, Dnipropetrovsk, Cherkasy, Zaporizhzhia and Poltava) and southern (Odesa) regions of Ukraine. The identified helminth species belong to the following phyla: Acanthocephala (Centrorhynchidae (2), Echinorhynchidae (2)); Nematoda (Acuariidae, Anisakidae, Cosmocercidae (3), Dioctophymatidae, Gnathostomatidae (1), Kathlanidae (1), Molineidae (7), Onchocercidae (1), Pharyngodonidae (1), Rhabdiasidae (6), Strongyloididae); Platyhelminthes (Diplodiscidae (1), Diplostomidae (2), Encyclometridae (1), Haematoloechidae (1), Leptophallidae (2), Macroderidae (1), Mesocestoididae, Opisthorchiidae (2), Plagiorchiidae (3), Pleurogenidae (2), Polystomatidae (3), Proteocephalidae (1), Strigeidae (1) and Telorchiidae (3)). Only some helminths in the dataset were not identified to species level. Material is stored in the collection of the department of Parasitology of the I. I. Schmalhausen Institute of Zoology NAS of Ukraine.
Keywords: endoparasites, biodiversity, herpetofauna, common species, helminths, parasitic worms, geocoded occurrence
Introduction
According to the known literature (Lindenmayer et al. 2011, Koprivnikar et al. 2011, Kopecký et al. 2013, Vasyliuk et al. 2015, García‐Díaz et al. 2016, Demkowska-Kutrzepa et al. 2018, Nekrasova et al. 2019, Marushchak et al. 2021), amphibians are currently the most threatened class of vertebrate animals and nearly a third of amphibian species are already extinct or are on the edge of extinction due to critical decline of their populations. Amphibians play an important role in food chains, serving as predators and prey for many other animals. This characteristic is related to the infection of amphibians with various parasites of larval and adult stages. This makes studying amphibian parasites a valuable tool for ecological studies, as the intensity and prevalence of infection, i.e. the abundance and occurrence of parasites can reflect the state of the whole ecosystem. Reptiles often serve as apex predators (e.g. monitors) or tertiary consumers (e.g. snakes) and rarely occupy lower positions in the food chains (Lindeman 1942, Bannikov et al. 1977). Nevertheless, reptiles often share habitats with amphibians and frequently harbour closely-related parasite species, making them important for parasitological studies. Moreover, both amphibians and reptiles are known to have limited capacity for long-distance migrations (Bannikov et al. 1977, Kuzmin 2012). Thus, it makes their parasitofauna suitable for representing the current state of separate local parts of the ecosystem and interactions between them, too (Okulewicz et al. 2014, Kuzmin et al. 2020, Čeirāns et al. 2021, Čeirāns et al. 2023). Particular interest in recent decade was drawn to studies of parasitofauna of those exotic (therefore potentially invasive) amphibians and reptiles kept in terrariums that might potentially become sources of invasive species of helminths to native herpetofauna of Ukraine (Kostko and Barkar 2019, Stets 2019).
Several significant studies dealing with terrestrial cold-blooded vertebrate parasites were performed on Ukraine's territory in the 20th century. Mazurmovich (1951) and Maguza (1973) investigated helminths of amphibians in the northern part of Ukraine. The study of Mazurmovich (1951) focused on the fauna of frogs' helminths, their life cycles and their impact on their hosts. A subsequent study by Maguza (1973) mainly focused on the helminths fauna and morphology of species. Unfortunately, at the time, the taxonomy of helminths, as well as some frogs (e.g. P.esculentus was not recognised) was incomplete and many of the species identifications (especially trematodes and nematodes) in both studies were incorrect. Later on, Ryzhikov et al. (1980) summarised all available data on amphibian helminths from broad territories of the former USSR. In their work, the Ukrainian species of amphibians were hosts to a total of 92 species of helminths (23 in urodelans and 90 in anurans), which included 41 species of trematodes, 37 nematodes, eight acanthocephalans, three cestodes and two monogeneans. During the same period, only scattered findings of helminths from reptiles in Ukraine were published (Ivanitskiy 1927, Vlasenko 1930, Modrzejewska 1938, Ivanitskiy 1940). Nonetheless, a monumental study by Sharpilo (1976) comprehensively compiled all the data available at that time on reptiles from the former USSR and included information on about 100 species of helminths parasitising snakes, lizards and turtles in Ukraine. These species included around 35 trematodes, around 50 nematodes, six cestodes, five acanthocephalans and one monogenean. Many of these species were found in larval stages (thus frequently identified to the genus or higher level), frequently sharing amphibian and reptile host species. Both latter studies included the identification keys and descriptions of species of trematodes, nematodes, cestodes, monogeneans and acanthocephalans. At the beginning of the 21st century, no all-encompassing studies of helminths on the territory of Ukraine took place. All research was focused mainly on separate morphological, ecological and biological characteristics of individual species or groups of helminths (Kuzmin 1993, Kuzmin 2000, Kuzmin et al. 2020, Kuzmin et al. 2023) or helminthofauna of particular host species from different locations (e.g. helminths of Ranidae frogs from north-western part of the Polissia Region of Ukraine (Kuzmin et al. 2012) or description of Hexametraquadricornis (Wedl, 1861) from the Crimean Peninsula (Kuzmin and Kukushkin 2012). Even if the territory of Ukraine had been studied in general, it appeared to be only a part included in a much larger geographical area, like Palearctica in the summarising study of helminths of sand lizards (Sharpilo et al. 2001).
In total, over 100 different helminth species were recorded from amphibians and reptiles in Ukraine, nearly a third of which were registered in the larval stage (using herpetofauna as intermediate or paratenic hosts) (Sharpilo 1976, Ryzhikov et al. 1980). However, the systematics of these parasites have undergone major changes (for example, nematodes were referred to as Class in both studies) and some species were described from studied hosts in Europe (and Ukraine as well): (e.g. Oswaldocruzialisnykiensis Svitin, 2017, described from a forest massif near Kyiv, Ukraine by Svitin (2017)); Rhabdiasesculentarum Cipriani, Mattiucci, Paoletti, Santoro & Nascetti, 2012, described in Europe by Cipriani et al. (2012)); synonymised (e.g. Oswaldocruziagoezei Skrjabin & Schultz, 1952 (synonym of Oswaldocruziafiliformis Goeze, 1782), Hexadontophorus spp., Paraentomelas spp.) or reinstated (e.g. Oswaldocruziaskrjabini Travassos, 1917) since the mentioned studies were published (Baker 1980, Baker 1987, Cipriani et al. 2012, Svitin 2015, Svitin 2017). Moreover, several host species were re-identified within their known natural range (e.g. Anguiscolchica (Nordmann, 1840) (Jablonski et al. 2021) and Hylaorientalis Bedriaga, 1890 (Manilo et al. 2014) have not been known in Ukraine at that time), as well as parasite species being synonymised or renamed. The only recent study dealing with helminths of Ukrainian amphibians with up-to-date systematic included 19 species of trematodes, seven species of nematodes and one acanthocephalan collected from frogs P.esculentus and P.ridibundus (Kuzmin et al. 2020). Therefore, the new checklist, based on currently-recognised parasite species from different Ukrainian amphibians (currently 22 known species: Anura - 15, Caudata - 7) and reptiles (currently 24 known species: Testudines - 1, Squamata - 23) (Bannikov et al. 1977, Kuzmin 2012, Manilo et al. 2014, Jablonski et al. 2021), will be helpful for further ecological studies in the area.
General description
Purpose
The primary objective of this study is to investigate helminth diversity in the predominant species of amphibians and reptiles in Ukraine, except for those listed in the Red Data Book of Ukraine. Accordingly, we reduced our sampling efforts when the likelihood of discovering additional helminth species within specific hosts became low. Additionally, we did not sample many host species that do not typically have specific helminths. For instance, after studying 23 specimens of Ranatemporaria Linnaeus, 1758, we only dissected three specimens of Ranaarvalis (Nilsson, 1842) because both frog species usually share the same helminth species (Ryzhikov et al. 1980).
Of all studied host species, only the common newt did not have any helminths detected (however, it should be noted that only one specimen was examined). While the common newt is not listed as an endangered species, we could not locate an area with a dense population of this species. According to literature data (Ryzhikov et al. 1980), the common newt has no specific helminth parasites. Due to that, we chose to limit the dissection of this host to avoid putting additional pressure on its populations.
Additional information
In the study, we collected a total of 47 species of helminths. That includes nearly all common parasite species, some of which have not been documented previously for the territory of Ukraine. For instance, two specimens of the acanthocephalan A.ranae were obtained from the intestine of the European pond turtle. Both were found attached, suggesting that the turtle most likely became infected by ingesting their intermediate host, Asellusaquaticus (Linnaeus, 1758) (Malacostraca, Asellidae). Additionally, for the first time, we recorded larvae of two distinct Mesocestoides species in the body cavity of common vipers. Notably, only one of them, namely Mesocestoideslineatus Goeze, 1782, had been previously reported in Ukrainian reptiles (Sharpilo 1976).
In our study, we tried to identify all parasites at the species level. However, we frequently encountered metacercariae and cystacanths in amphibians and early-stage nematode larvae in both amphibians and reptiles. These helminth specimens could only be identified to the family, class or phylum level. Amongst these records (n = 48) identified to a higher-than-specific level, there are 13 records identified to the level of genus (Oswaldocruzia - 5, Eustrongylides - 1, Strongyloides - 1, Neyraplectana - 1, Contracaecum - 2, Tylodelphys - 1, Mesocestoides - 2), 22 records - to the level of family (Strigeidae - 9, Cosmocercidae - 9, Acuariidae - 2, Plagiorchiidae - 1, Centrorhynchidae - 1), five records - to the level of superfamily (Metastrongyloidea - 5), two records - to the level of order (Spirurida - 2), one record - to the level of class (Trematoda - 1) and five records - to the level of phylum (Nematoda - 5). We believe that molecular methods can assist in their identification in the future, assuming data on adult parasites are deposited in GenBank.
The dataset presented provides current information on parasite species found in regions of Ukraine that are readily accessible. In addition to a list of parasites, the data encompasses their geographical location, site of infection and infection intensity (Svitin et al. 2023). While exploring other regions could potentially reveal more parasite species, we believe this dataset offers a valuable background for further research, including analyses of parasite communities amongst the examined hosts and comparative studies of other hosts and in other regions.
Project description
Title
Creating the genetic database of helminths from common species of amphibians and reptiles from the territory of Ukraine
Personnel
Yaroslav Syrota (Point of contact)
Study area description
The project covers different territories of Ukraine and includes the invasive study of communities of helminths presented in the target studied species of herpetofauna.
The aims of the projects include:
collecting material from the maximum possible number of species of amphibians and reptiles from different regions of Ukraine;
identifying the collected parasites using light microscopy methods;
obtaining the genetic sequences of the most widely used nuclear (18S) and mitochondrial (cox1) markers.
Funding
The project is mainly funded by the Grant for Young Scientists (0123U100296) of the National Academy of Sciences of Ukraine and is performed in the I. I. Schmalhausen Institute of Zoology NAS of Ukraine. The publishing of the study is supported by the EU 508 NextGenerationEU through the Recovery and Resilience Plan for Slovakia under projects No. 09I03-03-V01-00046 and No. 09I03-03-V01-00016. Additionally, the research was partly funded by the project Emys-R https://emysr.cnrs.fr through the 2020-2021 Biodiversa & Water JPI joint call for research proposals, under the BiodivRestore ERA-Net COFUND programme and with the funding organisations Agence Nationale de la Recherche (ANR, France), Bundesministerium für Bildung und Forschung (BMBF, Germany), State Education Development Agency (VIAA, Latvia) and National Science Center (NSC, Poland).
Sampling methods
Study extent
The authors of this project collected 440 records of helminths, of which 48 records were identified only to higher taxonomic categories of parasites from common species of amphibians and reptiles from various localities within Ukraine. All species were studied by light microscopy methods and the geocoded dataset was created. The presented dataset expands the knowledge on the recorded helminths' species of amphibians and reptiles, as there had been less than 50 records of these representatives of the world's fauna in Ukraine known before the dataset was published.
Sampling description
During the reporting period, several field trips were performed to various regions of Ukraine in order to collect material. The most favourable period for collecting amphibians is spring when they gather in large groups during spawning. However, due to security reasons as a result of the current Russian aggression, most of the hosts for this project were collected in late April and early May and until the beginning of November. This period is not critical since the level of helminth infection of the hosts increases due to active feeding, movement and completion of the spawning period (parasites that have entered the bodies of the hosts in the early larval stages had enough time to develop to adult stages) compared to the period immediately after wintering and at the time of spawning, when the hosts hardly eat, while the individuals weakened by hyperinfection of parasites died during wintering. During the warm periods of 2021, 2022 and 2023, a total of 205 amphibians and reptiles (Marushchak et al. 2023) from Zakarpattia, Lviv, Ivano-Frankivsk, Poltava, Zhytomyr, Vinnytsia, Odesa, Cherkasy and Kyiv Regions and the City of Kyiv (Fig. 1) were captured and examined. These hosts included anuran amphibians: 23 grass frogs R.temporaria, three moor frogs R.arvalis, 12 common toads Bufobufo (Linnaeus, 1758), 22 green toads Bufotesviridis (Laurenti, 1768), 12 common spadefoots Pelobatesfuscus (Laurenti, 1768), six marsh frogs P.ridibundus, five eastern tree frogs H.orientalis, seven European fire-bellied toads Bombinabombina (Linnaeus, 1761) and one common newt Lissotritonvulgaris (Linnaeus, 1758); and reptiles: 23 sand lizards Lacertaagilis Linnaeus, 1758, 21 viviparous lizards Zootocavivipara (Lichtenstein, 1823), six eastern slowworms A.colchica, 23 grass snakes Natrixnatrix (Linnaeus, 1758), seven dice snakes Natrixtessellata (Laurenti, 1768), 27 European pond turtles Emysorbicularis (Linnaeus, 1758) and seven common vipers Viperaberus (Linnaeus, 1758). Most amphibians and reptiles were caught by hand or with a net (using a modified hydrobiological net or strong fishing net) and several terrapins were captured in traps. The turtle trap used was the modified crab trap with wider openings and an attached plastic container for the bait. The most effective bait was freshly euthanised fish caught in the same water, though spoiled fish and the visceral organs of other turtles were successfully used.
Figure 1.
Map of records of helminths of herpetofauna on the territory of Ukraine.
For visualising the records, the points of herpetofauna registrations were collected (with the indication of latitude 00.00000 N and longitude 00.00000 E) using the field off-line orientation programme MAPS.ME (version 12.0.1-Google) and Google Earth Pro (version 7.3.3). Visualisation of records and creation of maps was carried out in the QGIS program (v.2.181, QGIS Development Team, 2016. QGIS Geographic Information System. Open Source Geospatial Foundation. URL http://qgis.org). Species were identified using methodological materials (Bannikov et al. 1977, Nekrasova et al. 2005, Kuzmin 2012).
The dataset visualisation was conducted using the R programming language (R core Team 2023). Initially, the dataset containing information about various hosts was merged with the parasites' dataset. Following this, parasitological metrics, such as total prevalence, richness, mean intensity and host sample size, were computed for each host species utilising versatile functions from the dplyr package. After that, the obtained data were visualised using the ggplot2 package.
Autopsies were performed in field laboratories during expedition trips. Otherwise live animals were transported to the laboratory of the I. I. Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine. Plastic containers of suitable sizes with holes for ventilation lined with moist plant substrate, paper towels or soft cloth were used to transport the hosts. Amphibians and reptiles were euthanised by injecting 10% lidocaine in the brain or bloodstream (only terrapins) and examined for helminths. In amphibians, the spinal cord was cut and the canal was flushed with saline using a thin Pasteur pipette to detect trematode larvae.This could not be done for reptile parasites due to a thin spinal canal that is too narrow and long. All organs were removed, washed in saline and checked for parasites. The body cavity, body and limb muscles and subcutaneous space were also checked for parasites in selected individuals. Found parasites were carefully removed, placed in small Petri dishes with saline and then fixed accordingly to the taxonomic group. Nematodes and smaller trematodes were fixed with hot 70% ethanol; monogeneans, large trematodes and cestodes were euthanised with hot water and then fixed with 70% ethanol; acanthocephalans were left in distilled water for up to 24 hours for the proboscis to extend and then fixed with 70% ethanol. For larval stages found in cysts, some specimens were removed using forceps and needles and fixed with 70% ethanol, other specimens were fixed in cysts or (in case of hyperinfection with a single species) counted and only a sample was taken. All parasites were transferred to vials with 70% ethanol and subsequently stored in the fridge. Several monogeneans were fixed in 10% formalin and stored at room temperature.
For the microscopic studies, nematodes and smaller trematodes were placed in distilled water for about 20-60 min, cleared in lactophenol for 30-120 min and studied as temporary mounts. Larger trematodes were stained with Mayer's haematoxylin in the following stages: soaking the material preserved in 70% ethanol in water (5 min); immersion in dye (5–10 min depending on the size of the individuals); immersion in a solution of hydrochloric acid (5 min); ammonia solution (5 min); passing through alcohols of increasing concentration (70%, 80%, 90%, 96%, 100% – 5(7) min for each stage); cleared in clove oil (10–15 min) and inclusion in Canadian balm on a permanent slide.
Cestodes were stained with carmine according to the following scheme: immersion in dye (5–10 min); acidified alcohol (5 min); passing through alcohols of increasing concentration (80%, 90%, 96%, 100% - 5(7) min for each stage); clearing in clove oil (10–15 min) and mounted in Canada balm on a permanent slide.
A study on the morphology of helminths was performed under an AmScope T690B light microscope with a digital camera; photomicrographs were obtained on a ZEISS Axio Imager M1 System microscope at the Center for Collective Use of Scientific Instruments "Animalia" (https://www.izan.kiev.ua/cen-coll.htm) at the Institute of Zoology. The image plate was composed using Photoshop (CS5 v.12.1.0) software. The original descriptions and latest redescriptions of species were used for the identification, as well as the identification keys in the monographs by Ryzhikov et al. (1980) and Sharpilo and Iskova (1989) and in separate articles (Svitin and Kuzmin 2012, Kuzmin and Kukushkin 2012, Svitin 2017). Some individuals of the larval stages of nematodes and trematodes were not identified at the species level due to the lack of diagnostic features.
Quality control
The authors of the dataset are fully responsible for the quality of data provided: accuracy of identification, counting etc.
Step description
Conducting field trips in search of the hosts;
Collecting the living hosts by hand, traps or net and field identification of their species;
Autopsies of the hosts, dissections and study of helminths;
Extracting and identification of helminths according to the standard methods;
Georeferencing;
Organising the dataset according to the Darwin Core standards.
Geographic coverage
Description
The dataset represents the records of helminths made on the territory of Ukraine, namely those regions that could be reached by the field expeditions under the conditions of war: Zaporizhzhia, Zhytomyr, Lviv, Vinnytsia, Cherkasy, Zakarpattia, Ivano-Frankivsk, Poltava, Odesa, Dnipropetrovsk and Kyiv administrative regions.
Coordinates
44.402 and 52.376 Latitude; 22.17 and 40.188 Longitude.
Taxonomic coverage
Description
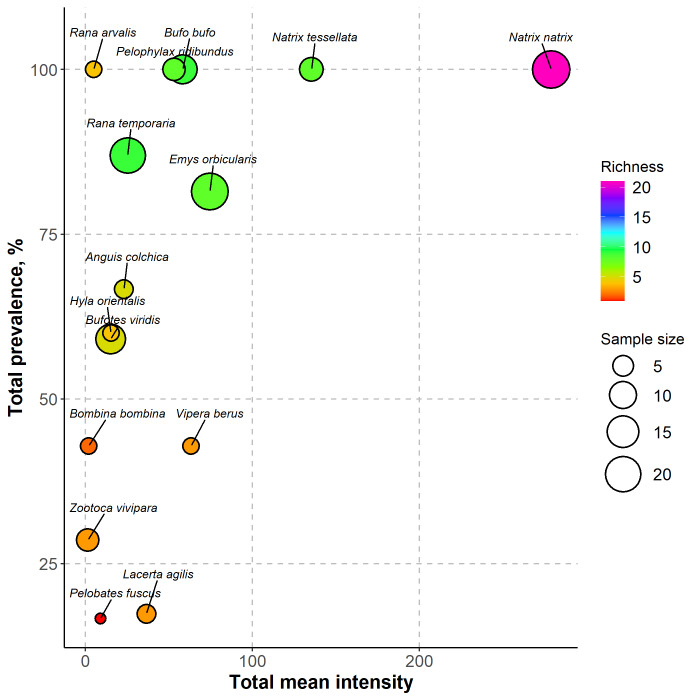
The dataset consists of the records of helminths from the three most represented phyla: Nematoda, Acanthocephala and Platyhelminthes. It represents the findings of 47 different helminth species from 15 different hosts (8 from class Amphibia and 7 from class Reptilia (Fig. 2)) from different areas of Ukraine. Fourteen remaining species of amphibians and 17 species of reptiles were not included in the study because of their conservation status or the presence of their populations within the occupied territories where the authors had no access. Some helminths were identified only to the higher taxonomic levels: seven genera, five families, one superfamily, two orders, one class and one phylum (Fig. 3, Table 1).
Figure 2.
Visualising the helminth community of common herpetofauna species in Ukraine. Each point represents a helminth component community of a host species. The x-axis shows the prevalence of infection, which is the proportion of hosts infected with at least one helminth species. The y-axis shows the mean intensity of infection, which is the average number of helminth individuals per infected host. The colour of the points indicates the richness of the helminth community, which is the number of helminth species found in each host. The size of the points shows the host sample size.
Figure 3.
Representatives of the major taxa (Monogenea, Acanthocephala, Trematoda, Nematoda and Cestoda) found in the present study: A Polystomoidesocellatum; B Pseudoacanthocephalusbufonis; C Telorchisstossichi; D Entomelasentomelas; E Ophiotaeniaeuropaea.
Table 1.
Helminth species identified to species level (ordered alphabetically within each phylum) recorded in the present study, their hosts and sites of infection: msc - mesocercariae of trematodes, mtc - metacercariae of trematodes, L3 - third-stage juveniles of nematodes.
| Parasite | Site of infection | Host species | Prevalence, % | Mean intensity |
| Acanthocephala: Palaeacanthocephala | ||||
| Acanthocephalusranae (Schrank, 1788) | intestine | B.bombina | 42.9 | 1.7 |
| E.orbicularis | 3.7 | 2 | ||
| R.temporaria | 47.8 | 4.2 | ||
| R.arvalis | 33.3 | 1 | ||
| P.ridibundus | 33.3 | 7.5 | ||
| Centrorhynchusspinosus (Kaiser, 1893) (cystacanth) | body cavity | H.orientalis | 20 | 3 |
| Pseudoacanthocephalusbufonis (Shipley, 1903) | intestine | B.bufo | 16.7 | 6 |
| Sphaerirostrispicae (Rudolphi, 1819) (cystacanth) | body cavity & muscles | Z.vivipara | 4.8 | 1 |
| Nematoda: Chromadorea | ||||
| Cosmocercacommutata (Diesing, 1851) | rectum | B.bufo | 16.7 | 33 |
| intestine & rectum | B.viridis | 18.2 | 20.8 | |
| Cosmocercaornata (Dujardin, 1845) | intestine & rectum | R.temporaria | 69.6 | 8.1 |
| intestine | P.ridibundus | 16.7 | 1 | |
| Entomelasdujardini (Maupas, 1916) | lungs | A.colchica | 66.7 | 6.5 |
| Entomelasentomelas (Dujardin, 1845) | pharynx | A.colchica | 50 | 8 |
| Falcaustraarmenica Massino, 1924 | intestine | E.orbicularis | 22.2 | 8.8 |
| Icosiellaneglecta (Diesing, 1851) | limb muscles | P.ridibundus | 33.3 | 7.5 |
| Oswaldocruziabialata (Molin, 1860) | intestine | R.temporaria | 73.9 | 9.9 |
| R.arvalis | 33.3 | 3 | ||
| Oswaldocruziaduboisi Ben Slimane, Durette-Desset & Chabaud, 1993 | intestine &stomach | N.natrix | 8.7 | 8 |
| Oswaldocruziafiliformis (Goeze, 1782) | intestine | B.bufo | 100 | 25.2 |
| Oswaldocruzialacertica Svitin, 2017 | intestine | L.agilis | 8.7 | 10.5 |
| Oswaldocruzialisnykiensis Svitin, 2017 | intestine | A.colchica | 33.3 | 18.5 |
| Oswaldocruziaskrjabini Travassos, 1937 | intestine & stomach | Z.vivipara | 23.8 | 1.3 |
| Oswaldocruziaukrainae Iwanitzky, 1928 | intestine &rectum | B.viridis | 40.9 | 5.8 |
| Oxysomatiumbrevicaudatum (Zeder, 1800) | lungs (L3) | R.temporaria | 8.7 | 3 |
| intestine | R.temporaria | 8.7 | 17.5 | |
| A.colchica | 16.7 | 4 | ||
| stomach | N.natrix | 4.3 | 48 | |
| Parapharyngodonszczerbaki Radchenko & Sharpilo, 1975 | intestine | L.agilis | 4.3 | 123 |
| Rhabdiasbufonis (Schrank, 1788) | lungs | R.temporaria | 100 | 2.7 |
| R.arvalis | 30.4 | 9.3 | ||
| Rhabdiasrubrovenosa (Schneider, 1866) | lungs | B.viridis | 22.7 | 8.2 |
| Rhabdiassphaerocephala Goodey, 1924 | lungs | B.bufo | 91.7 | 7.2 |
| Serpentirhabdiasfuscovenosa (Railliet, 1899) | lung | N.natrix | 91.3 | 14.9 |
| N.tessellata | 100 | 40.9 | ||
| V.berus | 14.3 | 1 | ||
| Spiroxyscontortus (Rudolphi, 1819) | stomach | E.orbicularis | 85.2 | 22.2 |
| intestine | N.natrix | 8.7 | 1 | |
| Platyhelminthes: Trematoda | ||||
| Alariaalata (Goeze, 1782) (msc) | body cavity | N.natrix | 39.1 | 136.8 |
| Astiotremaemydis Ejsmont, 1930 | intestine | E.orbicularis | 18.5 | 9.4 |
| Astiotremamonticellii Stossich, 1904 | intestine | N.natrix | 43.5 | 146.5 |
| N.tessellata | 28.6 | 5 | ||
| Diplodiscussubclavatus (Pallas, 1760) | intestine | H.orientalis | 40 | 1 |
| intestine & rectum | P.ridibundus | 100 | 12 | |
| Encyclometracolubrimurorum (Rudolphi, 1819) | stomach | N.natrix | 52.2 | 30.2 |
| body cavity (mtc) | H.orientalis | 40 | 6 | |
| Haematoloechusvariegatus (Rudolphi, 1819) | lungs | P.ridibundus | 14.3 | 1 |
| B.bombina | 16.7 | 1 | ||
| Leptophallusnigrovenosus (Bellingham, 1844) | oesophagus & intestine | N.natrix | 13 | 17.3 |
| Macroderalongicollis (Abildgaard, 1788) | lung | N.natrix | 52.2 | 6 |
| N.tessellata | 28.6 | 1.5 | ||
| Metaleptophallusgracillimus (Luhe, 1909) | oesophagus & intestine | N.natrix | 43.5 | 14.9 |
| Opisthioglypheranae (Frolich, 1791) | intestine | P.ridibundus | 83.3 | 27.4 |
| R.temporaria | 4.3 | 1 | ||
| B.bufo | 8.3 | 1 | ||
| N.natrix | 4.3 | 3 | ||
| Paralepodermacloacicola (Luhe, 1909) | intestine | N.natrix | 52.2 | 26.7 |
| N.tessellata | 42.9 | 29 | ||
| Plagiorchiselegans (Rudolphi, 1802) | intestine | L.agilis | 4.3 | 2 |
| Pleurogenesclaviger (Rudolphi, 1819) | intestine | R.temporaria | 4.3 | 1 |
| B.bufo | 8.3 | 17 | ||
| Prosotocusconfusus (Looss, 1894) | intestine | P.ridibundus | 16.7 | 10 |
| Skrjabinoecessimilis (Looss, 1899) | lungs | R.arvalis | 33.3 | 3 |
| Strigeasphaerula (Rudolphi, 1803) (mtc) | body cavity | N.natrix | 52.2 | 125.9 |
| N.tessellata | 28.6 | 42.5 | ||
| Telorchisassula (Dujardin, 1845) | stomach | N.natrix | 69.6 | 40.6 |
| intestine | N.tessellata | 100 | 62.9 | |
| Telorchisstossichi Goldberger, 1911 | intestine | E.orbicularis | 33.3 | 118 |
| Tylodelphysexcavata (Rudolphi, 1803) (mtc) | spine | P.ridibundus | 33.3 | 34 |
| Platyhelminthes: Cestoda | ||||
| Ophiotaeniaeuropaea Odening, 1963 | intestine | N.natrix | 82.6 | 6.8 |
| N.tessellata | 71.4 | 5.8 | ||
| Platyhelminthes: Monogenea | ||||
| Polystomaintegerrimum (Frolich, 1791) | urinary bladder | R.temporaria | 39.1 | 1.8 |
| Polystomaviridis Euzet, Combes & Batchvarov, 1974 | urinary bladder | B.viridis | 4.5 | 3 |
| Polystomoidesocellatum (Rudolphi, 1819) | pharynx | E.orbicularis | 25.9 | 1.4 |
Taxa included
| Rank | Scientific Name | |
|---|---|---|
| kingdom | Animalia | |
| phylum | Acanthocephala | |
| class | Palaeacanthocephala | |
| order | Echinorhynchida | |
| family | Echinorhynchidae | |
| order | Polymorphida | |
| family | Centrorhynchidae | |
| phylum | Nematoda | |
| class | Chromadorea | |
| order | Dioctophymatida | |
| family | Dioctophymatidae | |
| order | Rhabditida | |
| family | Acuariidae | |
| family | Anisakidae | |
| family | Cosmocercidae | |
| family | Gnathostomatidae | |
| family | Kathlanidae | |
| family | Molineidae | |
| family | Onchocercidae | |
| family | Pharyngodonidae | |
| family | Rhabdiasidae | |
| superfamily | Metastrongyloidea | |
| family | Strongyloididae | |
| order | Spirurida | |
| phylum | Platyhelminthes | |
| class | Cestoda | |
| order | Cyclophyllidea | |
| family | Mesocestoididae | |
| order | Onchoproteocephalidea | |
| family | Proteocephalidae | |
| class | Monogenea | |
| order | Polystomatidea | |
| family | Polystomatidae | |
| class | Trematoda | |
| order | Diplostomida | |
| family | Diplostomidae | |
| family | Strigeidae | |
| order | Plagiorchiida | |
| family | Diplodiscidae | |
| family | Encyclometridae | |
| family | Haematoloechidae | |
| family | Leptophallidae | |
| family | Macroderidae | |
| family | Opisthorchiidae | |
| family | Plagiorchiidae | |
| family | Pleurogenidae | |
| family | Telorchiidae |
Temporal coverage
Formation period: 2021/2023.
Notes
The parasites' hosts were collected during the vegetation period, when the hosts began to be active, to the period of seasonal decreasing of the activity: from March to October.
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
Data resources
Data package title
Particular records of helminths from common species of herpetofauna of Ukraine
Resource link
https://doi.org/10.15468/v45tya
Alternative identifiers
https://www.gbif.org/uk/dataset/ec73e150-38ce-46ff-9268-70bc0dd86a60
Number of data sets
1
Data set 1.
Data set name
Particular records of helminths from common species of herpetofauna of Ukraine
Data format
Darwin Core
Data format version
1.8
Description
The dataset consists of 440 records of helminths belonging to 47 species; 48 records were identified only to higher taxonomic categories. A total of 11305 parasites individuals were recorded from 205 hosts belonging to eight amphibian and seven reptile species. Such stages as mature individuals, larvae and cysts were taken into account after examining such parts of the hosts' bodies as muscles, stomach, liver, lungs, body cavity, intestine, rectum, spine, pharynx, oesophagus and bladder (Svitin et al. 2023).
The dataset presented provides current information on parasite species found in regions of Ukraine that are readily accessible. In addition to a list of parasites, the data encompasses their geographical location, site of infection and infection intensity. While exploring other regions could potentially reveal more parasite species, we believe this dataset offers a valuable background for further research, including analyses of parasite communities amongst the examined hosts and comparative studies with other hosts and regions.
Data set 1.
| Column label | Column description |
|---|---|
| occurrenceID | http://rs.tdwg.org/dwc/terms/occurrenceID; a unique identifier of a particular occurrence within this dataset. |
| scientificName | http://rs.tdwg.org/dwc/terms/scientificName; the full scientific name, with authorship and date information, if known, of the identified species or other taxonomic level. |
| basisOfRecord | http://rs.tdwg.org/dwc/terms/basisOfRecord; a specific nature of the way in which the data were recorded. |
| eventDate | http://rs.tdwg.org/dwc/terms/eventDate; the date-time or interval during which the observation was made. |
| verbatimeventDate | http://rs.tdwg.org/dwc/terms/verbatimEventDate; an original version of the recorded date-time or interval during which the observation was made. |
| taxonRank | http://rs.tdwg.org/dwc/terms/taxonRank; the taxonomic rank of the record made. |
| decimalLatitude | http://rs.tdwg.org/dwc/terms/decimalLatitude; the geographic latitude (in decimal degrees, using the spatial reference system given in dwc:geodeticDatum) of the geographic centre of a location, where the observed individual (its host actually) was spotted. |
| decimalLongitude | http://rs.tdwg.org/dwc/terms/decimalLongitude; the geographic longitude (in decimal degrees, using the spatial reference system given in dwc:geodeticDatum) of the geographic centre of a location, where the observed individual (its host actually) was spotted. |
| coordinatesUncertaintyInMetres | http://rs.tdwg.org/dwc/terms/coordinateUncertaintyInMeters; the horizontal distance (in metres) from the given latitude and longitude describing the smallest circle containing the whole of the location. |
| geodeticDatum | http://rs.tdwg.org/dwc/terms/geodeticDatum; the ellipsoid, geodetic datum or spatial reference system (SRS), upon which the geographic coordinates given in decimalLatitude and decimalLongitude are based. |
| language | http://purl.org/dc/terms/language; a language of the resource. |
| taxonRemarks | http://rs.tdwg.org/dwc/terms/taxonRemarks; comments or notes about the taxon or name. |
| organismRemarks | http://rs.tdwg.org/dwc/terms/organismRemarks; comments or notes about the registered organism(s). |
| occurrenceRemarks | http://rs.tdwg.org/dwc/terms/occurrenceRemarks; comments or notes about the occurrence of the organism. In this dataset - localisation of the helminths in different parts of the host's body. |
| organismQUantity | http://rs.tdwg.org/dwc/terms/organismQuantity; a number or enumeration value for the quantity of the registered organisms. |
| organismQuantityType | http://rs.tdwg.org/dwc/iri/organismQuantityType; the type of quantification system used for the quantity of organisms. |
| georeferenceProtocol | http://rs.tdwg.org/dwc/terms/georeferenceProtocol; a description or reference to the methods used to determine the spatial footprint, coordinates and uncertainties. |
| recordedBy | http://rs.tdwg.org/dwc/iri/recordedBy; a person, group or organisation responsible for recording the original registration of the species. |
| identifiedBy | http://rs.tdwg.org/dwc/terms/identifiedBy; a list (concatenated and separated) of names of people, groups or organisations who assigned the taxon to the registered organism. |
| georeferencedBy | http://rs.tdwg.org/dwc/iri/georeferencedBy; a person, group or organisation who determined the georeference (spatial representation) for the location where the host of the parasite(s) was caught. |
| materialSampleID | http://rs.tdwg.org/dwc/terms/materialSampleID; an identifier for the sampled hosts, where the parasites were found. |
| associatedTaxa | http://rs.tdwg.org/dwc/terms/associatedTaxa; a list (concatenated and separated) of identifiers or names of taxon records and the associations of this occurrence to each of them. |
| countryCode | http://rs.tdwg.org/dwc/terms/countryCode; the standard code for the country in which the location of record occurs. |
| country | http://rs.tdwg.org/dwc/terms/country; the name of the country or major administrative unit in which the location of record occurs. |
| stateProvince | http://rs.tdwg.org/dwc/terms/stateProvince; the name of the next smaller administrative region than country. |
| locality | http://rs.tdwg.org/dwc/terms/locality; the specific description of the place. |
| kingdom | http://rs.tdwg.org/dwc/terms/kingdom; the full scientific name of the kingdom in which the taxon is classified. |
| phylum | http://rs.tdwg.org/dwc/terms/phylum; the full scientific name of the phylum or division in which the taxon is classified. |
| class | http://rs.tdwg.org/dwc/terms/class; the full scientific name of the class in which the taxon is classified. |
| order | http://rs.tdwg.org/dwc/terms/order; the full scientific name of the order in which the taxon is classified. |
| family | http://rs.tdwg.org/dwc/terms/family; the full scientific name of the family in which the taxon is classified. |
| genus | http://rs.tdwg.org/dwc/terms/genus; the full scientific name of the genus in which the taxon is classified. |
| specificEpithet | http://rs.tdwg.org/dwc/terms/specificEpithet; the name of the first or species epithet of the scientificName. |
| verbatimTaxonRank | http://rs.tdwg.org/dwc/terms/verbatimTaxonRank; the taxonomic rank of the most specific name (here - superfamily, as this option was absent in the mapping tool) used in the dataset. |
| type | http://purl.org/dc/elements/1.1/type; the nature or genre of the resource. |
Acknowledgements
The authors wish to express their sincere thanks to Dr. Oksana Greben for her help with staining and identification of cestodes and to Dr. Volodymyr Gorobchyshyn, Dr. Oksana Nekrasova, Ms. Valeria Dupak for their invaluable help during the field studies. The authors are grateful to the NGO "Ukrainian Nature Conservation Group" (UNCG; https://uncg.org.ua/) for providing a place to publish data on their profile as a publisher on the GBIF platform. Additionally, the authors are extremely grateful to the Armed Forces of Ukraine for protection and the opportunity to conduct scientific research and publish scientific works, supporting science in Ukraine even during the time of the full-scale Russian aggression.
Funding Statement
The work was supported by the project "Creating of genetic base of helminths from common species of amphibians and reptiles from the territory of Ukraine" № 29/02-2022(4) funded by National Academy of Sciences of Ukraine and was done in the I. I. Schmalhausen institute of zoology NAS of Ukraine. Also, the study was partially supported by the VEGA grant 2/0099/22 and the EU 508 NextGenerationEU through the Recovery and Resilience Plan for Slovakia under projects No. 09I03-03-V01-00046 and No. 09I03-03-V01-00016. Additionally the research was partly funded by the project <tn type="lower" obkms_id="42430402-BA0B-4283-9B00-63838C557CF3"><tn-part type="genus" full-name="Emys">Emys</tn-part></tn>-R https://emysr.cnrs.fr through the 2020-2021 Biodiversa & Water JPI joint call for research proposals, under the BiodivRestore ERA-Net COFUND programme, and with the funding organizations Agence Nationale de la Recherche (ANR, France), Bundesministerium für Bildung und Forschung (BMBF, Germany), State Education Development Agency (VIAA, Latvia), and National Science Center (NSC, Poland).
References
- Baker M. R. Revision of Entomelas Travassos, 1930 (Nematoda: Rhabdiasidae) with a review of genera in the family. Systematic Parasitology. 1980;1(2):83–90. doi: 10.1007/bf00009853. [DOI] [Google Scholar]
- Baker M. R. Synopsis of the Nematoda parasitic in amphibians and reptiles. Memorial University of Newfoundland. St. John's; Newfoundland: 1987. 325. [Google Scholar]
- Bannikov A. G., Darevsky I. S., Ishchenko V. G., Rustamov A. K., Shcherbak N. N. Определитель земпноводных и пресмыкающихся фауны СССР. Prosveshchenie; Moscow: 1977. 415. Russian. [Google Scholar]
- Čeirāns A., Gravele E., Gavarane I., Pupins M., Mezaraupe L., Rubenina I., Kvach Y., Skute A., Oskyrko O., Nekrasova O., Marushchak O., Kirjushina M. Helminth communities in amphibians from Latvia, with an emphasis on their connection to host ecology. Journal of Helminthology. 2021;95 doi: 10.1017/s0022149x2100047x. [DOI] [PubMed] [Google Scholar]
- Čeirāns Andris, Pupins Mihails, Kirjusina Muza, Gravele Evita, Mezaraupe Ligita, Nekrasova Oksana, Tytar Volodymyr, Marushchak Oleksii, Garkajs Alberts, Petrov Iurii, Skute Arturs, Georges Jean-Yves, Theissinger Kathrin. Top-down and bottom-up effects and relationships with local environmental factors in the water frog–helminth systems in Latvia. Scientific Reports. 2023;13(1) doi: 10.1038/s41598-023-35780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani Paolo, Mattiucci Simonetta, Paoletti Michela, Santoro Mario, Nascetti Giuseppe. Rhabdiasesculentarum n. sp. (Nematoda: Rhabdiasidae) from green frogs of the Ranaesculenta species complex in Italy: molecular evidence, morphological description and genetic differentiation from its congeners in frogs and toads. Systematic Parasitology. 2012;82(2):131–146. doi: 10.1007/s11230-012-9355-x. [DOI] [PubMed] [Google Scholar]
- Demkowska-Kutrzepa Marta, Studzińska Maria, Roczeń-Karczmarz Monika, Tomczuk Krzysztof, Abbas Zahrai, Różański Paweł. A review of the helminths co-introduced with Trachemysscriptaelegans – a threat to European native turtle health. Amphibia-Reptilia. 2018;39(2):177–189. doi: 10.1163/15685381-17000159. [DOI] [Google Scholar]
- García‐Díaz Pablo, Ross Joshua V., Woolnough Andrew P., Cassey Phillip. The lliegal wildlife trade is a likely source of alien species. Conservation Letters. 2016;10(6):690–698. doi: 10.1111/conl.12301. [DOI] [Google Scholar]
- Ivanitskiy S. V. On trematode fauna of vertebrates of Ukraine. Veterinarne dilo. 1927;8:23–34. Russian. [Google Scholar]
- Ivanitskiy S. V. Materials on helminthofauna of vertebrate animals of Ukraine (fauna of cestodes, nematodes, acanthocephalans) Sbornik Trudov Kharkovskogo Veterinatnogo Instituta. 1940;19(1):129–154. Russian. [Google Scholar]
- Jablonski Daniel, Sillero Neftalí, Oskyrko Oleksandra, Bellati Adriana, Čeirāns Andris, Cheylan Marc, Cogălniceanu Dan, Crnobrnja-Isailović Jelka, Crochet Pierre-André, Crottini Angelica, Doronin Igor, Džukić Georg, Geniez Philippe, Ilgaz Çetin, Iosif Ruben, Jandzik David, Jelić Dušan, Litvinchuk Spartak, Ljubisavljević Katarina, Lymberakis Petros, Mikulíček Peter, Mizsei Edvárd, Moravec Jiří, Najbar Bartłomiej, Pabijan Maciej, Pupins Mihails, Sourrouille Patricia, Strachinis Ilias, Szabolcs Márton, Thanou Evanthia, Tzoras Elias, Vergilov Vladislav, Vörös Judit, Gvoždík Václav. The distribution and biogeography of slow worms (Anguis, Squamata) across the Western Palearctic, with an emphasis on secondary contact zones. Amphibia-Reptilia. 2021;42(4):519–530. doi: 10.1163/15685381-bja10069. [DOI] [Google Scholar]
- Kopecký O., Kalous L., Patoka J. Establishment risk from pet-trade freshwater turtles in the European Union. Knowledge and Management of Aquatic Ecosystems. 2013;410 doi: 10.1051/kmae/2013057. [DOI] [Google Scholar]
- Koprivnikar Janet, Gibson Chris H., Redfern Julia C. Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proceedings of the Royal Society B: Biological Sciences. 2011;279(1733):1544–1550. doi: 10.1098/rspb.2011.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostko P. P., Barkar V. A. Monitoring reptiles diseases. https://abbsl.osau.edu.ua/index.php/visnuk/article/download/12/6 Agrarian Bulletin of the Black Sea Region. Veterinary sciences. 2019;91:35–40. Ukrainian. [Google Scholar]
- Kuzmin S. Земноводные бывшего СССР. 2nd Revised Edition. Vol. 1. КМК Scientific Press Ltd.; Moscow: 2012. 370. Russian. [Google Scholar]
- Kuzmin Y., Khymyn M., Svitin R. Parasitic nematodes and acanthocephalans of frogs (Ranaarvalis, Pelophylax kl. esculentus) from the territory of National Park «Prypiat-Stokhid». Scientific Bulletin of the National Park "Prypiat-Stokhid". 2012:17–23. Ukrainian.
- Kuzmin Yuriy, Dmytrieva Ivanna, Marushchak Oleksiy, Morozov-Leonov Svyatoslav, Oskyrko Oleksandra, Nekrasova Oksana. Helminth species and infracommunities in frogs Pelophylaxridibundus and P.esculentus (Amphibia: Ranidae) in Northern Ukraine. Acta Parasitologica. 2020;65(2):341–353. doi: 10.2478/s11686-019-00164-3. [DOI] [PubMed] [Google Scholar]
- Kuzmin Yu., Dmytriieva I., Svitin R. Icosiellaneglecta (Nematoda, Onchocercidae) in Ukraine: occurrence, hosts, morphological and molecular characterisation. Zoodiversity. 2023;57(1):75–92. doi: 10.15407/zoo2023.01.075. [DOI] [Google Scholar]
- Kuzmin Y. I. The development of Rhabdias fuscovenosa (Nematoda: Rhabdiasidae) under the experimental conditions. In: , editor. Proceedings of XI Conference of the Ukrainian Society of Parasitologists; XI Conference of the Ukrainian Society of Parasitologists; Kyiv. Kyiv: 1993. 2. Russian. [Google Scholar]
- Kuzmin Y. I. Investigations on the parasitic nematodes of Ranatemporaria during the hybernation period. Acta Parasitologica. 2000;45(3):175–175. [Google Scholar]
- Kuzmin Y. I., Kukushkin O. V. Hexametraquadricornis (Nematoda, Ascaridida) from Leopard Snake (Reptilia, Squamata) in Crimea (Ukraine) https://www.researchgate.net/publication/323073661_Hexametra_quadricornis_Nematoda_Ascaridida_from_Leopard_Snake_Reptilia_Squamata_in_Crimea_Ukraine Vestnik Zoologii. 2012;46(6):550–550. [Google Scholar]
- Lindeman R. L. The trophic-dynamic aspect of ecology. http://links.jstor.org/sici?sici=0012-9658%28194210%2923%3A4%3C399%3ATTAOE%3E2.0.CO%3B2-P. Ecology. 1942;23(4):399–417. doi: 10.2307/1930126. [DOI] [Google Scholar]
- Lindenmayer D. B., Wood J. T., McBurney L., MacGregor C., Youngentob K., Banks S. C. How to make a common species rare: a case against conservation complacency. Biological Conservation. 2011;144(5):1663–1672. doi: 10.1016/j.biocon.2011.02.022. [DOI] [Google Scholar]
- Maguza V. S. Гельминты амфибий Полесья Украины. I. I. Schmalhausen Institute of Zoology NAS of Ukraine; Kyiv: 1973. 27. Russian. [Google Scholar]
- Manilo V. V., Smirnov N. A., Manuilova O. N. Comparative analysis of morphological and karyological features of European tree frog, Hylaarborea, and the eastern tree frogs, Hylaorientalis (Anura, Hylidae) from Ukraine. https://www.researchgate.net/publication/284442834_Comparative_analysis_of_morphological_and_karyological_features_of_European_tree_frog_Hyla_arborea_and_the_eastern_tree_frogs_Hyla_orientalis_Anura_Hylidae_from_Ukraine Proceedings of Ukrainian Herpetological Scoity. 2014;(5):55–72. Russian.
- Marushchak OY, Nekrasova OD, Tytar VM, Smirnov NA, Korshunov OV, Pupins M, Mykytynets G, Skute A, Henle K, Kaiser H. A GIS approach to the study of colour anomalies in amphibians of Ukraine reveals the deleterious effect of human impacts. https://www.biotaxa.org/hn/article/view/62048 Herpetology Notes. 2021;14:1239–1251. [Google Scholar]
- Marushchak O., Svitin R., Syrota Y., Kuzmin Y., Dmytrieva I., Nechai A., Lisitsyna O. Ukrainian Nature Conservation Group; 2023. [2023-09-13T00:00:00+03:00]. Records of common Ukrainian herpetofauna species for which the infestation with helminths was studied in 2021-2023. 1.0. [Google Scholar]
- Mazurmovich BN. Паразитические черви амфибий и их взаимоотношения с хозяевами и внешней средой. Izdatelstvo Kievskogo Gosudarstvennogo Universiteta im. T. G. Shevchenko; Kyiv: 1951. 100. Russian. [Google Scholar]
- Modrzejewska H. On the parasitic worms of Emysorbicularis from Polish Polesie. Zoologica Poloniae. 1938;3:125–139. German. [Google Scholar]
- Nekrasova O., Mezhzherin S., Morozov-Leonov S. In: Herpetologia Petropolitana. Ananjeva N., Tsinenko O., editors. Vol. 1. Russian Journal of Herpetology; St. Petersburg – Moscow: 2005. Diagnostic traits in the morphology of green frogs (Rana esculenta complex) in the Middle Dnepr basin.3. Russian. [Google Scholar]
- Nekrasova O, Smirnov N, Marushchak O, Korshunov O, Kotserzhynska I. Change in the protection status and points of finds of the crested newt Trituruscristatus (Amphibia, Salamandridae) in Ukraine. https://uncg.org.ua/tvarynnyj-svit/ Materials for the 4th edition of the Red Book ofUkraine. Fauna, 3. Conservation Biology in Ukraine, Kyiv, 24.09.2019. I. I.Schmalhause institute of zoology NAS of Ukraine. 2019;3:10. Ukrainian] [Google Scholar]
- Okulewicz A., Hildebrand J., Łysowski R., Buńkowska K., Perec-Matysiak A. Helminth communities of green and brown frogs from Poland (Lower Silesia Region) Journal of Herpetology. 2014;48(1):34–37. doi: 10.1670/12-108. [DOI] [Google Scholar]
- Team R core. R Foundation for Statistical Computing; 2023. R: A language and environment for statistical computing. [Google Scholar]
- Ryzhikov K. M., Sharpilo V. P., Shevchenko N. N. Гельминты амфибий фауны СССР. Nauka; Moscow: 1980. 275. Russian. [Google Scholar]
- Sharpilo V. P. Паразитические черви пресмыкающихся фауны СССР. Naukova Dumka; Kyiv: 1976. 287. Russian. [Google Scholar]
- Sharpilo V. P., Iskova N. I. Плагиорхиаты (Plagiorchiata). Фауна Украины. Т. 34. Трематоды 3. Naukova Dumka; Kyiv: 1989. 280. Russian. [Google Scholar]
- Sharpilo V. P., Biserkov V. V., Kostadinova A., Behnke J. M., Kuzmin Y. I. Helminths of the sand lizard, Lacertaagilis (Reptilia, Lacertidae), in the Palaearctic: faunal diversity and spatial patterns of variation in the composition and structure of component communities. Parasitology. 2001;123(4):389–400. doi: 10.1017/s0031182001008587. [DOI] [PubMed] [Google Scholar]
- Stets O. Disinfection of terrariums after dehelminthization of reptiles. Ukrainian journal of veterinary sciences. 2019;10(4):80–85. doi: 10.31548/ujvs2019.04.010. Ukrainian. [DOI] [Google Scholar]
- Svitin R., Kuzmin Y. Oswaldocruziaduboisi (Nematoda, Molineidae): Morphology, hosts and distribution in Ukraine. Vestnik Zoologii. 2012;46(3):195–20. doi: 10.2478/v10058-012-0017-x. [DOI] [Google Scholar]
- Svitin Roman. Two new species of Oswaldocruzia (Nematoda, Molineidae) parasitising lizards in Ukraine. Zootaxa. 2017;4263(2) doi: 10.11646/zootaxa.4263.2.9. [DOI] [PubMed] [Google Scholar]
- Svitin R, Syrota Y, Kuzmin Y, Marushchak O, Dmytrieva I, Nechai A., Lisitsyna O. Ukrainian Nature Conservation Group (NGO); 2023. [2023-09-13T00:00:00+03:00]. Particular records of helminths from common species of herpetofauna of Ukraine. 1.8. [Google Scholar]
- Svitin R. S. New data on the morphology and distribution of Oswaldocruziaskrjabini (Nematoda, Molineidae) Vestnik Zoologii. 2015;49(5):447–452. doi: 10.1515/vzoo-2015-0052. [DOI] [Google Scholar]
- Vasyliuk O. V., Nekrasova O. D., Shyriaieva D. V., Kolomytsev G. O. A review of major impact factors of hostilities influencing biodiversity in the eastern Ukraine (modeled on selected animal species) Vestnik Zoologii. 2015;49(2):145–158. doi: 10.1515/vzoo-2015-0016. [DOI] [Google Scholar]
- Vlasenko P. V. To the fauna of trematodes of amphibians and reptiles from the outskirts of Kharkiv. Trudy Kharkivskogo Tovarystva Doslidnykiv Pryrody. 1930;1:53–53. Ukrainian. [Google Scholar]