Summary
Immune-related toxicities, otherwise known as immune-related adverse events (irAEs), occur in a substantial fraction of cancer patients treated with immune checkpoint inhibitors (ICIs). Ranging from asymptomatic to life-threatening, ICI-induced irAEs can result in hospital admission, high-dose corticosteroid treatment, ICI discontinuation, and in some cases, death. A deeper understanding of the factors underpinning severe irAE development will be essential for improved irAE prediction and prevention, toward maximizing the benefits and safety profiles of ICIs. In recent work, we applied mass cytometry, single-cell RNA sequencing, single-cell V(D)J sequencing, bulk RNA sequencing, and bulk T cell receptor (TCR) sequencing to identify pretreatment determinants of severe irAE development in patients with advanced melanoma. Across 71 patients separated into three cohorts, we found that two baseline features in circulation – elevated activated CD4 effector memory T cell abundance and TCR diversity – are associated with severe irAE development, independent of the affected organ system within 3 months of ICI treatment initiation. Here we provide an extended perspective on this work, synthesize and discuss related literature, and summarize practical considerations for clinical translation. Collectively, these findings lay a foundation for data-driven and mechanistic insights into irAE development, with the potential to reduce ICI morbidity and mortality in the future.
Keywords: Immune-related adverse events, irAEs, immunotherapy, immune checkpoint inhibitors, predictive biomarkers, melanoma
1. Introduction
Over the last decade, immune checkpoint inhibitors (ICIs) have revolutionized clinical oncology, yielding impressive gains in progression-free and overall survival in patients with metastatic melanoma1–4, lung cancer5–8, and other malignancies9–11. Currently, there are 11 FDA-approved monoclonal antibody-based ICI therapies that boost anti-tumor immunity via blockade of inhibitory signaling in the tumor microenvironment12,13. Such agents include antibodies that target cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed-cell death 1 (PD-1) or its ligand (PD-L1), and more recently, lymphocyte activation gene 3 (LAG-3)14. Building upon this success, many studies are now underway to identify and translate novel immunomodulatory signaling axes and ICI treatment combinations15–19. The latter is exemplified by the recent FDA approval of dual PD-1 and LAG-3 blockade in patients with advanced melanoma20. Such efforts, along with recent advances in engineered T cells21–25, are poised to dramatically improve cancer patient outcomes in the coming decade.
Despite the efficacy of ICIs, they also carry the risk of eliciting immune-related adverse events (irAEs) – temporary or lasting inflammatory toxicities that can be debilitating or even life-threatening26. ICI-induced irAEs affect a variety of organ systems including lungs, heart, joints, thyroid, pituitary, liver, colon, nervous system, and skin30, and are clinically graded on a scale of 1 to 5, according to severity27. Symptomatic grade 2 irAEs are typically managed conservatively in the outpatient setting, while grade 3 and higher toxicities can become life-threatening, requiring inpatient management and immunosuppression with agents such as infliximab (anti-TNF-α), mycophenolate, and high-dose corticosteroids28–31. Between 10% and 60% of all ICI-treated patients develop severe irAEs (grade ≥3), with rates being highest in patients treated with combination checkpoint blockade with anti-CTLA-4 and anti-PD-1 antibodies1,2, and lower in those treated with anti-PD-1 monotherapy3. Additionally, the majority of irAEs occur early – within 14 weeks of starting ICI treatment32,33. Though irAEs may phenotypically mimic primary autoimmune disorders34, and patients with a diagnosis of autoimmunity are at increased risk for irAE development35,36, many patients with no obvious personal or family history of autoimmunity still develop severe irAEs37. Ultimately, these toxicities can cause treatment discontinuation31, hospital and intensive care unit admissions38, and occasionally deaths39, while also incurring high costs on the healthcare system at large40.
Currently, mechanisms of irAE pathophysiology are not well understood, in part because pre-clinical mouse models either underestimate irAE rates compared to human patients41 or fail to recapitulate critical features of human irAE biology42–44. Moreover, the majority of irAEs do not appear to be the result of cytotoxicity from ICIs directly interacting with normal tissue, although this mechanism appears to predominate in the case of anti-CTLA-4-mediated pituitary dysfunction45.
In addition to an incomplete mechanistic understanding of irAEs, there is also a paucity of biomarkers predictive of irAE risk. Such determinants could suggest novel avenues for targeted mechanistic studies, as they have done for markers of ICI response, including PD-L1 staining46–48, mismatch repair deficiency11, tumor mutational burden (TMB)49–51, and tumor-infiltrating lymphocyte composition52–54. Unfortunately, there is no clinical assay to determine pretreatment which patients will experience severe irAEs. Such an assay could help prevent substantial morbidity, or even mortality, and could help clinicians select alternative therapeutic measures (e.g., withholding adjuvant immunotherapy in stage III melanoma after surgical resection, or favoring anti-PD-1 monotherapy, which has a lower severe irAE rate than combination ICIs) in patients at risk for severe irAE outcomes.
In a recently published study, we showed that clonally diverse activated CD4 memory T cells are significantly elevated in pretreatment blood from patients with advanced melanoma who develop severe or life-threatening irAEs following ICI therapy55. Here we present an extended perspective on this work, contextualize it with respect to blood-based biomarkers and related literature, and discuss potential considerations for clinical translation.
2. Circulating correlates of ICI-induced irAEs
In 2011, less than 2% of US cancer patients were eligible to receive ICI therapy, but by 2018 the estimated eligibility rose to as high as 44%56. Accordingly, the number of patients suffering from debilitating ICI-induced irAEs is also projected to rise substantially over the next decade57. A clinically validated assay predictive of severe irAE development would help to identify patients at serious risk for morbidity, allowing for more precise clinical decision-making with better anticipation of these toxicities and potential preventative measures.
Blood-based predictive biomarkers of irAE development would be clinically ideal as they can be noninvasively assessed and serially monitored, similar to liquid biopsies that measure ctDNA58–64. Two general classes of circulating biomarkers include cytokines and immune cells. Among cytokine biomarker studies, Lim et al. showed that an 11-cytokine toxicity (CYTOX) score predicted irAE development in 98 melanoma patients with modest performance in their independent pretreatment and on-treatment validation cohorts (area under the curve (AUC) = 0.68 and 0.70, respectively)65. Other studies focused on cytokine fluctuations associated with specific irAEs such as thyroiditis66, colitis67,68, and dermatitis69. Among circulating immune cell biomarker studies, one group demonstrated skewed cellular proportions at baseline, namely low pre-ICI neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratios (PLR), which were associated with irAE development (OR = 2.2–2.8) across 184 lung cancer patients70. Additionally, in two studies focusing on B cells and irAEs, researchers, including members of our group, showed that a higher proportion of CD21-low B cells among total B cells at baseline71 and early on-treatment increases in CD21-low B cells72 were both associated with immune toxicity.
While these prior studies on circulating biomarkers are a vital step in the right direction, certain aspects of their experimental design, patient cohorts, and overall findings may limit their implementation in the clinic. For example, the studies showing distinct cytokine profiles associated with organ system-specific irAEs67–69,73 were all performed in small patient cohorts (n < 40) that did not include held-out validation cohorts. The lack of validation cohorts and low patient recruitment means any conclusions from these studies may not be broadly applicable. Lim and colleagues included a held-out validation cohort in their CYTOX study and assessed its ability to predict severe irAEs, defined as irAEs requiring ICI interruption and the use of high-dose steroids or other immunomodulatory drugs. Still, CYTOX performance in the validation cohort was moderate (AUC = 0.68)65. This modest performance could be driven by a lack of specificity due to inter-individual variability in baseline cytokine levels. Indeed, any potential irAE-related cytokine signal could be diluted by cytokine fluctuations caused by competing factors such as infection, inflammation, and tissue damage74. Lastly, technical limitations such as degradation during sample storage may have impacted the sensitivity of cytokine-based irAE studies75.
Earlier cellular biomarker studies also have limitations that prevent their translation into the clinic. While the study by Pavan et al. on NLRs and PLRs was completed in a large cohort of lung cancer patients (n = 184), the majority of irAEs reported were grade 1 or grade 270. Advanced knowledge of low-grade irAEs will not ultimately change the clinical management of these patients. In fact, this and other studies have shown that non-severe irAEs are prognostic of ICI clinical benefit70,76–79. Therefore, to have clinical utility, a prospective biomarker should predict severe toxicity and should lack significant association with ICI efficacy. With respect to the studies on CD21-low B cells72,80, both studies lacked substantial patient numbers and held-out validation cohorts. Therefore, similar to the organ system-specific cytokine studies, it is difficult to extrapolate the findings from these B cell studies into larger patient cohorts until additional validation studies are carried out.
3. Peripheral CD4 T cell features associated with ICI immunotoxicity
To complement and build upon previous work, we recently sought to identify cellular correlates of severe early-onset irAE development using pretreatment peripheral blood mononuclear cells (PBMCs) from patients with advanced melanoma treated with anti-PD-1 monotherapy or anti-PD-1 and anti-CTLA-4 combination therapy. Our study was based on the hypothesis that ICI-induced irAEs, similar to flare-ups in patients with primary autoimmunity, are symptomatic exacerbations that occur in the context of a baseline dysregulated immune state unleashed by ICI treatment. In support of this concept, Cebula and colleagues reported that self-reactive CD4 T cells are pervasive in the peripheral blood of healthy adult C57BL/6 mice, but are normally constrained by regulatory T cells81. We began by assembling three distinct cohorts of PBMC samples from patients with melanoma, including samples obtained from two academic medical centers. We then comprehensively profiled 20 immune cell states with mass cytometry time of flight (CyTOF) in pre-ICI PBMCs from a discovery cohort of 18 patients with advanced melanoma. Only elevated CD4 effector memory T cell (TEM) levels were significantly associated with severe irAEs following multiple hypothesis testing (P = 0.0002; Q = 0.004)55.
In addition to mass cytometry, we applied single-cell RNA sequencing (scRNA-seq) to pretreatment PBMCs from a subset of the same patients (n = 13). These data corroborated our CyTOF results showing that higher levels of CD4 TEM cells are most significantly associated with severe irAEs (P = 0.01)55. Furthermore, using canonical markers of activation (CD38+, HLA-DR+, or Ki67+) to interrogate data from both single-cell modalities, we found that activated CD4 TEM cells were more strongly associated with severe irAE development than their resting counterparts.
Normalization considerations
Notably, our results were obtained by quantifying the absolute abundance of immune subsets, including CD4 TEM cells and activated/resting subsets, relative to total PBMCs. In contrast, quantifying CD4 TEM levels from total CD3 T cells reduced the strength of the association with severe irAEs, while quantifying them among total CD4 T cells led to the loss of significance altogether (Supplementary Figure 2 in Lozano et al.55). Thus, absolute cell type abundances can yield important insights that are weakened or lost when evaluating cell type abundances relative to parental populations. These data underscore the importance of normalization considerations when analyzing cell type composition82.
TCR diversity and severe irAEs
We next wondered whether pretreatment TCR diversity might also correlate with severe ICI-induced irAEs. Since we had performed single-cell V(D)J sequencing (scV(D)J-seq) jointly with scRNA-seq, we evaluated TCR diversity of multiple CD4 and CD8 T cell subsets and correlated these with severe irAE development. TCR diversity was calculated using Shannon entropy, which captures two major components of TCR diversity: evenness (inverse of clonality) and richness (number of unique TCR clones)83,84. Because CD4 TEM cells are memory cells, they are expected to display elevated TCR clonality85,86. Indeed, CD4 TEM cells displayed elevated clonality, as assessed by 1- Pielou’s evenness83, but this population also had a high number of unique clones relative to other T cell states – enough that TCR richness eclipsed the lower evenness within the CD4 TEM population. Therefore, we observed that high TCR diversity, specifically within T cells that had CD4 TEM expression profiles, was an independent predictor of severe toxicity55. Of note, this finding extended to the aggregation of T cells pretreatment, with overall increased TCR diversity also significantly associated with severe irAEs. Still, when we subtracted diversity specific to CD4 TEM-related states from bulk TCR diversity, we observed that the majority of bulk TCR diversity elevation in patients who went on to develop severe irAEs was driven by CD4 TEM cells55. Thus, a more diverse TCR repertoire at baseline in CD4 TEM cells, broadly reflected in bulk peripheral blood, appears to be associated with the development of severe ICI-induced irAEs.
Integrative modeling for prediction of irAE risk from pretreatment blood
To validate these findings, we performed bulk RNA sequencing (RNA-seq) of pre-ICI peripheral blood from two independent cohorts with 26 and 27 melanoma patients each (total 53 patients). We then analyzed the resulting data using 1) CIBERSORTx87 deconvolution to infer cell state abundances, including activated CD4 memory T cells (CD4 TM), and 2) MiXCR88 to assemble TCRs, from which we calculated clonotypic diversity using Shannon entropy. Importantly, circulating CD4 TM cells enumerated by CIBERSORTx correlated strongly and specifically with the activated CD4 TEM subset that we identified by both CyTOF and scRNA-seq (Extended Data Figure 5 in Lozano et al.55). Moreover, patients with severe irAEs in both bulk cohorts showed significantly higher levels of activated CD4 TM cells and TCR diversity, demonstrating concordance across distinct patient cohorts and technologies. In contrast, levels of other cell subsets were not associated with severe irAE development.
We further explored whether integration of these two T cell characteristics would be more significantly associated with severe irAE development than either feature alone. Indeed, using a logistic regression framework to train a bivariable model, the resulting pretreatment model was highly predictive for severe irAE development across organ systems (AUC = 0.90, P = 0.0004), including for patients treated with combination ICI therapy (AUC = 1.0, P = 0.04) and for distinguishing different irAE grades (Kruskal–Wallis test P = 0.0006). The model remained predictive for severe irAE development across clinical and epidemiologic subgroups.
Since anti-PD-1 treatment has a markedly lower severe irAE rate (~10%) than dual anti-PD-1 and anti-CTLA-4 blockade (up to ~60%)1,89, we wondered whether the same T cell features might be predictive of irAE onset in both therapy types. To assess this, we trained the composite model using only combination therapy patients, then tested it on patients receiving anti-PD-1 alone. Strikingly, the model achieved an AUC of 0.795 in the latter group. Likewise, when training only on anti-PD-1 patients, the model achieved strong performance on combination therapy patients alone (AUC = 0.88). These data suggest that clonally diverse activated CD4 TM cells could underpin severe irAEs arising from both ICI regimens.
Independence of the composite model from ICI response
To be clinically useful, any biomarker of ICI-induced irAEs must be independent of features that predict response to ICIs. Importantly, we observed no significant association between the pretreatment composite model and durable clinical benefit from ICI treatment, whether in the held-out bulk validation cohort (AUC = 0.51, P = 0.91) or across all bulk cohort patients assessed by leave-one-out cross-validation (AUC = 0.63, P = 0.13). Still, Kagamu et al. found that in non-small cell lung cancer patients treated with anti-PD-1 monotherapy, CD62low CD4 T cells in peripheral blood were enriched pretreatment in patients who responded90. However, the CD62low CD4 T cell population identified by Kagamu et al. encompassed both TEM and TEMRA cell subsets, and classically defined CCR7−/CD45RA− CD4 TEM cells were not associated with anti-PD-1 response in this study90. Kagamu et al. also did not consider TCR diversity in their methodology, as we did with our composite model. Regardless, future work will be needed to definitively establish whether clonally diverse activated CD4 TEM cells specifically associate with severe irAE development and not durable clinical benefit.
On-treatment correlates of ICI-induced irAEs
Any T cell state that preferentially expands prior to irAE onset is likely to be linked to irAE etiology. Therefore, we applied immunoSEQ® to profile bulk TCR-ß repertoires in paired pre- and early on-treatment PBMC samples collected from 15 melanoma patients treated with combination ICIs. Strikingly, patients who developed severe irAEs experienced clonal expansion early on-treatment, with the level of TCR clonal expansion correlating with how quickly patients developed severe irAEs. Interestingly, we also found that persistent TCR clonotypes were enriched for an activated CD4 TEM transcriptomic signature55, suggesting that these cells were expanding rapidly with combination ICI administration in patients who went on to develop severe irAEs.
Severe irAEs versus frank autoimmunity
Given the phenotypic resemblance between severe irAEs and frank autoimmunity, we sought to investigate potential connections between these two entities. While none of the patients in our study had a known history of autoimmune disorder (which is considered a contraindication for checkpoint blockade91), we were able to mine publicly available peripheral blood bulk transcriptomic data from six studies covering 587 patients with either systemic lupus erythematosus or inflammatory bowel disease along with 191 healthy controls92–97. Across this large meta-dataset, which we deconvolved with CIBERSORTx, we again found that activated CD4 TM cells were significantly enriched in patients with frank autoimmunity and were enriched at higher levels than 14 other immune subsets, including CD8 T cells, Treg cells, B cells, and monocytes. These data imply that mechanisms underlying autoimmunity may share biology with those that underpin risk for severe irAE development, indicating that severe irAE risk could represent subclinical autoimmunity that becomes unmasked by immune checkpoint blockade, as has been suggested by case reports98–100.
4. Additional evidence linking CD4 TEM cells to ICI-induced irAEs
As with all correlative studies, it will be important to validate our findings prospectively, across multiple cancer types, and to delve deeper into the potential mechanisms that connect activated CD4 TM/TEM cells to ICI-induced irAEs in the affected tissues. However, in the absence of prospective validation, it is useful to synthesize data from retrospective studies published by other groups. Indeed, a number of recent studies implicate CD4 TM cell subpopulations or TCR diversity in irAE onset and/or pathology101–111. Below we highlight key results from such studies and provide a more comprehensive summary of related findings in Figure 1. We also explore potential mechanistic connections between CD4 TM/TEM cells and other factors that may underlie irAE development, including autoreactive tissue-resident memory CD8 T cells at the site of toxicity.
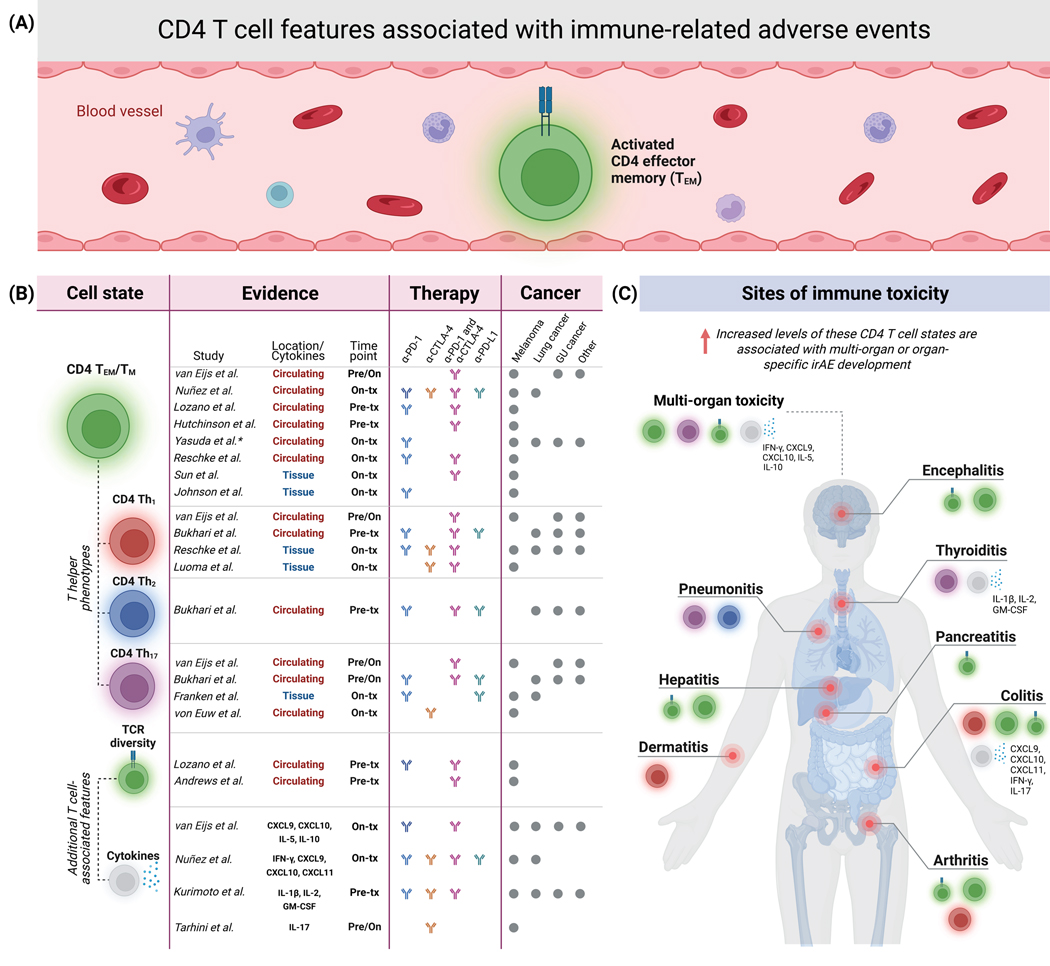
Figure 1. Studies linking CD4 T cell features to immune-related adverse events from immune checkpoint blockade.
a. Lozano et al. recently identified elevated levels of clonally diverse activated CD4 effector memory T (CD4 TEM) cells in circulation as a strong pretreatment determinant of ICI-induced severe irAEs in patients with advanced melanoma. b. Studies connecting ICI-induced irAEs in cancer patients to CD4 TEM/TM and other CD4 T cell states, T cell receptor (TCR) diversity, and circulating cytokines. Time points are relative to ICI treatment. tx, treatment. *, irAE-enriched CD4 TEM cells in Yasuda et al. 2021 also expressed CD27. c. Reported sites of organ system toxicity, related to the studies and data in panel b. CTCAE grade 3 or higher toxicities were considered for Lozano et al., while the other studies had a lower or no toxicity grade threshold.
We begin by summarizing several studies reporting a connection between CD4 TM/TEM cells and irAE incidence. For example, Hutchinson et al. recently applied clinical flow cytometry to peripheral blood derived from 44 patients with metastatic melanoma prior to combination ICIs, and found that patients who developed ICI-induced hepatitis had significantly elevated pretreatment CD4 TEM levels (P = 0.019)102 (Figure 1). The authors confirmed this finding with a validation cohort of 45 additional melanoma patients treated with combination ICIs (P = 0.018). Notably, no associations between elevated pretreatment CD4 TEM levels and other irAE types were observed. That said, the authors normalized CD4 TEM levels relative to total CD4 T cells (not total PBMCs as in Lozano et al.55) and did not specifically examine severe (grade 3+) irAEs.
In another recent study, Bukhari et al. applied scRNA-seq and cellular indexing of transcriptomes and epitopes (CITE-seq) to PBMCs from a heterogeneous cohort of 18 lung cancer patients, 4 prostate cancer patients, and 2 head and neck cancer patients treated with various ICI regimens (anti-PD-1 or anti-PD-L1 or combination ICIs). Gene set analysis revealed increased expression of cytotoxic and proinflammatory genes in CD4 T effector and memory subsets in irAE patients compared to patients with no irAEs107. The authors then characterized CD4 T cell clusters and attempted to associate them with organ-specific irAEs. A cluster composed of CD4 T helper 1/helper 2 (Th1/2) cells was significantly elevated at baseline in patients who developed ICI-induced arthritis (P = 0.015)107 (Figure 1), but did not display differences in patients who developed pneumonitis or thyroiditis. This CD4 Th1/2 population had low CCR7 and TCF7 expression, suggesting an effector memory phenotype. Further gene expression profiling of this cell population revealed significant upregulation of inflammatory genes, and gene ontology and network analysis revealed substantial overlap with genes related to autoimmune and activated CD4 T cell conditions including inflammatory arthritis. Further investigation of the data revealed that pneumonitis patients had a specific Th2 cell state enriched in peripheral blood at baseline, while thyroiditis patients had elevated baseline levels of a Th17 population107 (Figure 1). Interestingly, the Th2 and Th17 cell states associated with pneumonitis and thyroiditis, respectively, also had low CCR7 expression, again suggesting effector memory phenotypes. These data suggest that various CD4 T cell states with activated, inflammatory, effector and memory phenotypes are enriched pretreatment in irAE patients.
Similarly, Nuñez et al. recently described early on-treatment expansion of CD4 T cells expressing activation and proliferation markers (CD38 and Ki67) in a mixed cohort of lung cancer and melanoma patients who developed irAEs following treatment with anti-PD-1, anti-PD-L1, anti-CTLA-4, or combination ICIs101. As Johnson and Balko pointed out in their perspective article, it is unclear if the authors specifically assessed irAE associations with CD4 TEM cells112, despite the study mentioning this population in their FlowSOM-guided cell clustering approach101. Still, the proliferating on-treatment CD4 T cells appear to have a TEM phenotype, as they express low levels of CD27 and lack CCR7 and CD45RA expression (Figure 1). The study also reported an on-treatment increase in the Th1-associated cytokines IFN-γ, CXCL9, CXCL10, and CXCL11 in toxicity patients. Likewise, Reschke et al. 2021 used flow cytometry to serially profile PBMCs from 17 melanoma patients treated with either anti-PD-1 or combination therapy104 (Figure 1). They observed an increase in circulating activated CD4 T cells on-treatment in patients that developed irAEs with the largest spike by the second or third week after ICI treatment initiation.
Furthermore, a recently submitted pre-print by van Eijs and colleagues leveraged multicolor flow cytometry and a multiplex serum immunoassay to longitudinally profile B and T cells in a 44-patient multi-cancer cohort treated with combination therapy or PD-1 alone and found an early on-treatment increase in CD4 TEM proliferation113 (Figure 1). In support of our findings, this study found that higher pretreatment CD4 TEM proliferation was associated with a shorter time to toxicity development in patients receiving combination ICIs. Moreover, in the combination ICI group, cycling Th1 memory cells were highly proliferative and Th17 cells increased significantly in on-treatment patients that developed irAEs113 (Figure 1). These cellular findings were corroborated by increases in serum Th1-associated cytokines (CXCL9 and CXCL10), similar to Nuñez et al.101.
While there is overall consistency between the aforementioned studies implicating CD4 T cell states in irAE development, the association between activated CD4 TEM/TM cells was strongest in Lozano et al. One potential reason for this is that Lozano et al. specifically focused on identifying cellular characteristics associated with severe irAE development, defined by grade 3 or higher toxicity55. In contrast, other studies correlated with ≥ grade 2 toxicities102,107,113 or with any toxicity including grade 1101,104. A second contributing factor could be that other studies mostly normalized CD4 T cell populations relative to parent lineages (i.e., T cells, CD4 T cells, or memory CD4 T cells)101,102,104,107,113,. In contrast, we found that over-normalizing the CD4 TEM population led to a loss of association with severe irAE risk55. Many of the other studies also utilized heterogeneous patient populations with multiple cancer types, ICI treatment regimens, and diverse treatment histories. In contrast, patient cohorts in Lozano et al. were more homogeneous, including only metastatic melanoma patients treated with either anti-PD-1 or combination anti-PD-1 / anti-CTLA-4, where 90% of patients had no prior history of ICI treatment55. Nevertheless, across diverse analytical approaches, ICI treatment regimens, and patient populations, including distinct cancer types, many independent lines of evidence now implicate circulating CD4 memory T cell states, especially TEM populations, in ICI-induced irAE development (Figure 1).
Potential mechanisms connecting activated CD4 TM/TEM cells to irAEs
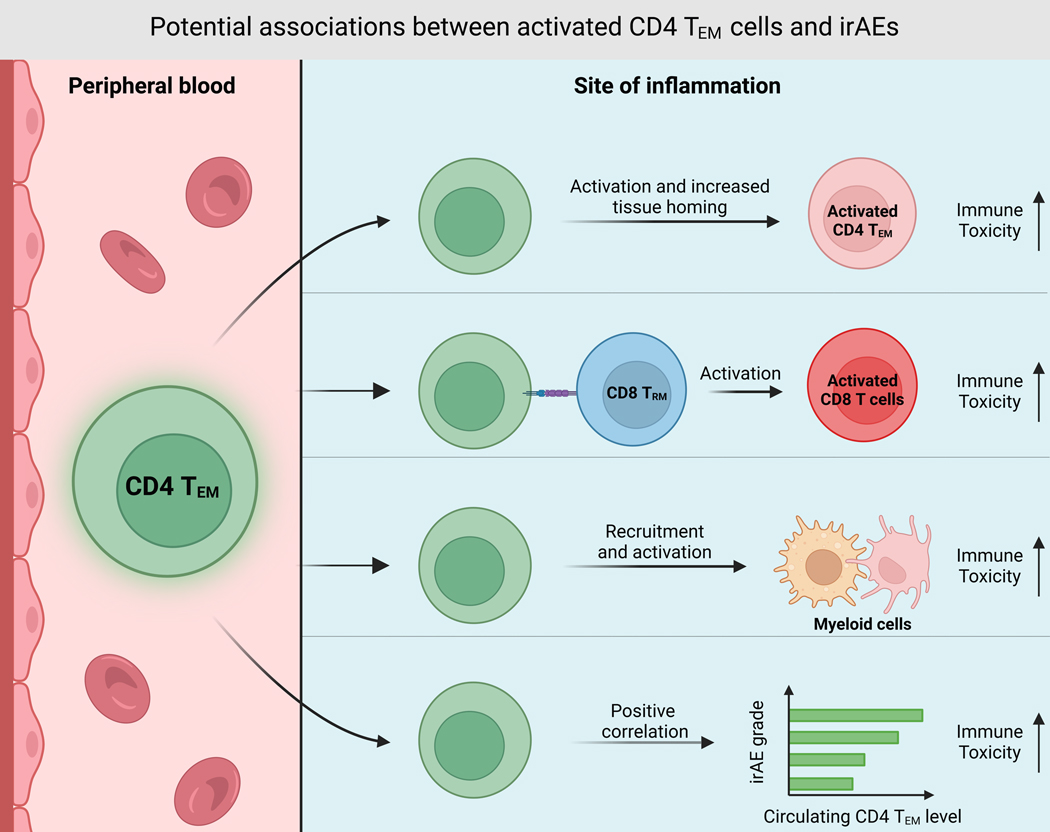
In addition to strong correlations with irAE incidence, there are multiple mechanisms by which clonally diverse activated CD4 TM/TEM cells might be causally linked to irAE development (Figure 2).
Figure 2. CD4 TEM cells as potential mediators of ICI-induced irAEs.
CD4 TEM cells may traffic to sites of future toxicity from the peripheral blood, then directly or indirectly contribute to irAE etiology via several scenarios, including 1) direct TCR-mediated cytotoxicity, 2) activation of tissue-resident CD8 memory T cells (CD8 TRM) that drive toxicity, or 3) activation of myeloid cells that exert tissue toxicity through innate proinflammatory programs or further activation of cytotoxic T cells. Alternatively, CD4 TEM cells may not be directly involved in irAE development but may instead represent a circulating biomarker with a different upstream mediator responsible for direct tissue toxicity.
One potential mechanism is via the release of proinflammatory cytokines that drive a systemic proinflammatory state. This is in line with studies that have demonstrated several circulating cytokines are associated with irAE risk65–69,101,113 (Figure 1). For instance, systemic IL-17A, produced by Th17 cells, is known to drive ICI thyroiditis in mice114 (Figures 1 and 2). Furthermore, in melanoma patients treated with anti-CTLA-4, high baseline IL-17 levels were associated with severe cases of ICI colitis68. Indeed, some steroid-resistant immune toxicities can be resolved using the anti-IL17A antibody secukinumab115. High circulating levels of other CD4 T cell-derived cytokines have also been shown to be associated with irAE development, including IFN-γ, released by Th1 cells101. Thus, CD4 TM cells with distinct T helper phenotypes could release inflammatory cytokines that drive irAE development (Figures 1 and 2).
Beyond eliciting systemic inflammation, CD4 TEM cells could underpin irAE development through a more TCR-dependent mechanism. However, this would require CD4 TEM TCRs to recognize self-tissues. Indeed, a case report on erythema nodosum (EN)-like toxicity in a metastatic melanoma patient treated with combination and anti-PD-1 ICIs revealed that the dominant TCR clones in inflammatory lesions co-localized with activated CD4 TM cells, and their TCR repertoires did not overlap with TCRs in the tumor116. This suggests that activated CD4 TM cell clones within tissue were directly driving toxicity via recognition of self-antigens. In addition, both our own data55 and Andrews et al.111 demonstrated that melanoma patients who develop severe irAEs have elevated baseline TCR diversity (Figure 1). Our analysis went further to suggest that CD4 TEM cells account for the majority of this increased TCR diversity. Given these data, we hypothesize that the expanded TCR repertoire of CD4 TM cells increases the probability that some TCR clones will recognize non-tumor targets, including self or viral antigens117,118. Data supporting the latter is evident in the study performed by Hutchinson et al., where patients who developed ICI-induced hepatitis had pretreatment cytomegalovirus (CMV) seropositivity and higher levels of CMV-reactive CD4 TEM cells compared to non-hepatitis patients102 (Figure 1). This study also showed that CD4 TEM expansion was specifically associated with ICI hepatitis102. As CMV is known to infect the liver119, Hutchinson et al. went on to show that prophylactic anti-viral treatment with valganciclovir in patients with high baseline CD4 TEM levels and CMV-seropositivity prevented ICI hepatitis from developing. Therefore, CD4 TEM cells with CMV-reactive TCRs were likely responsible for driving hepatic immune toxicity.
A similar process was described in a case study of a 70-year-old melanoma patient with no evidence of brain metastasis that died of ICI-induced encephalitis following anti-PD-1 therapy106 (Figure 1). This patient’s cerebrospinal fluid was EBV-positive throughout treatment, implying neurological EBV infection. Additionally, spatial immune profiling showed that the patient’s inflamed brain tissue was enriched with activated CD4 TM cells, and TCR sequencing identified enrichment for EBV-reactive TCR clonotypes106. Thus, both this study and the one by Hutchinson et al. suggest that CD4 TM cells specific to viral antigens expanded in response to immunotherapy and drove irAE pathology in organs commonly targeted by those viruses. In the encephalitis patient, EBV-specific TCRs comprised 10% of the TCR clones found infiltrating the site of brain toxicity106, suggesting that remaining TCR clones could have targeted tissue-specific self-antigens as observed in earlier cases of fatal ICI-induced cardiotoxicity120. Lastly, although they did not perform TCR sequencing, both Luoma et al.108 and Reschke et al.105 identified CD4 TM cells at the sites of ICI toxicity.
Once at the site of tissue destruction, activated CD4 TEM cells may directly or indirectly drive auto-inflammation. Supporting evidence comes from the identification of activated CD4 TM T cells within irAE-involved organ tissue102,105,106,108,116 (Figure 2). In the previously discussed encephalitis case study, researchers observed that activated CD4 TM cells in inflamed brain tissue expressed granzyme B (GZMB), a marker of cytotoxicity106. Additionally, a single-cell RNA-seq study of eight ICI colitis patients showed that GZMA, which encodes granzyme A, was a top differentially expressed gene in CD4 T cells from colitis tissue108. This study also found that T cell clusters expressing high GZMA in irAE-inflamed colitis tissue had low CCR7 expression, suggesting memory differentiation108. Both studies illustrate how activated CD4 TM cells might use granzymes to directly cause ICI-associated tissue damage. Furthermore, relevant to the ICI encephalitis and colitis cases, direct CD4 T cell-mediated cytotoxicity is implicated in phenotypically similar autoimmune conditions including multiple sclerosis121,122 and ulcerative colitis123.
CD4 TM/TEM cells might also drive inflammation indirectly following tissue invasion, by providing help to cytotoxic CD8 T cells (Figure 2). Indeed, among 15 melanoma patients treated with either anti-PD-1 or combination ICIs, Reschke et al. detected a spike in activated CD8 T cells alongside the increase in CD4 TEM cells at the time of irAE onset104. Moreover, Nuñez et al. characterized a time-dependent relationship between the proportion of CD38+ Ki67+ CD8 T cells and severe irAE onset101. This study also demonstrated a spike in IFN-γ in addition to the chemokines CXCL9, CXCL10, and CXCL11 in patients that developed irAEs. IFN-γ, secreted by Th1 cells, is a known inducer of CXCL9, CXCL10, and CXCL11, which are CD8 T cell chemoattractants124. Thus, if CD4 TEM cells adopt a Th1 phenotype and secrete IFN-γ, they could increase cytotoxic CD8 T cell traffic to the site of inflammation. Indeed, the same publications on ICI-induced encephalitis106 and colitis108 that observed enrichment of activated CD4 memory T cells also observed enrichment of cytotoxic CD8 T cells within irAE-involved tissues106,108. Furthermore, in the colitis study by Luoma et al., there was a high proportion of shared TCRs between cytotoxic CD8 T cells and tissue-resident CD8 memory T cells (CD8 TRM), suggesting that CD8 TRM cells could have differentiated into cytotoxic CD8 T cells to exert toxicity125. Thus, activated CD4 TEM cells could have provided proinflammatory cytokines to these dormant CD8 TRM cells, differentiating them into autoinflammatory effectors126.
Another potential mechanism by which activated CD4 TEM cells might exert ICI-induced toxicity is via interaction with myeloid cells within the affected tissue127 (Figure 2). Studies investigating cytokines associated with irAE development provide evidence of myeloid involvement. Notably, GM-CSF and G-CSF, two of the 11 cytokines comprising the CYTOX score65, are known myeloid growth factors128,129. In contrast to the role cytotoxic CD8 T cells likely play in ICI immune toxicity, the interaction between activated CD4 TM cells and monocytes/macrophages may involve reciprocal activation. Luoma et al. transcriptionally demonstrated that patients with ICI colitis have activated myeloid cells that express high levels of TNF-α, IL-1β, and other pro-inflammatory cytokines108. As mentioned before, Nuñez et al. characterized that IFN-γ, secreted by Th1 cells, and CXCL9–11101 were associated with irAEs. One potential source of these cytokines could be M1-polarized monocytes/macrophages124,130. Although the Nuñez study was unable to confirm that monocytes were the source of these increased cytokines, they did observe an early on-treatment increase in monocytes among melanoma patients responding to ICIs that had toxicity relative to ICI responders with no toxicity101. Moreover, in a study by Gudd et al. on ICI-related hepatitis, cytotoxic GZMB+ CD8 T cells were found to co-localize near activated CD163+ macrophages in inflamed liver tissue131. Thus, Th1-polarized autoreactive CD4 memory T cells could use IFN-γ to stimulate monocytes and macrophages to secrete proinflammatory cytokines, such as TNF-α, alongside T cell chemokines to recruit cytotoxic CD8 T cells and more activated CD4 T cells that continue to activate monocytes in a feed-forward loop. As noted above, steroid-resistant irAEs can be successfully treated with infliximab, a therapeutic monoclonal antibody against TNF-α, suggesting that breaking this feed-forward loop can be utilized therapeutically132,133.
Lastly, expansion of the CD4 TEM compartment could simply be caused by an upstream driver of immune toxicity, and thus CD4 TEM cells might represent a passenger biomarker associated with irAE development, without playing a direct role in exerting toxicity in the affected tissue (Figure 2). Future studies using systematically collected tissue and blood before and after irAE development, both in humans and in mice, will be required to determine which of these possibilities is correct.
5. Considerations for clinical translation
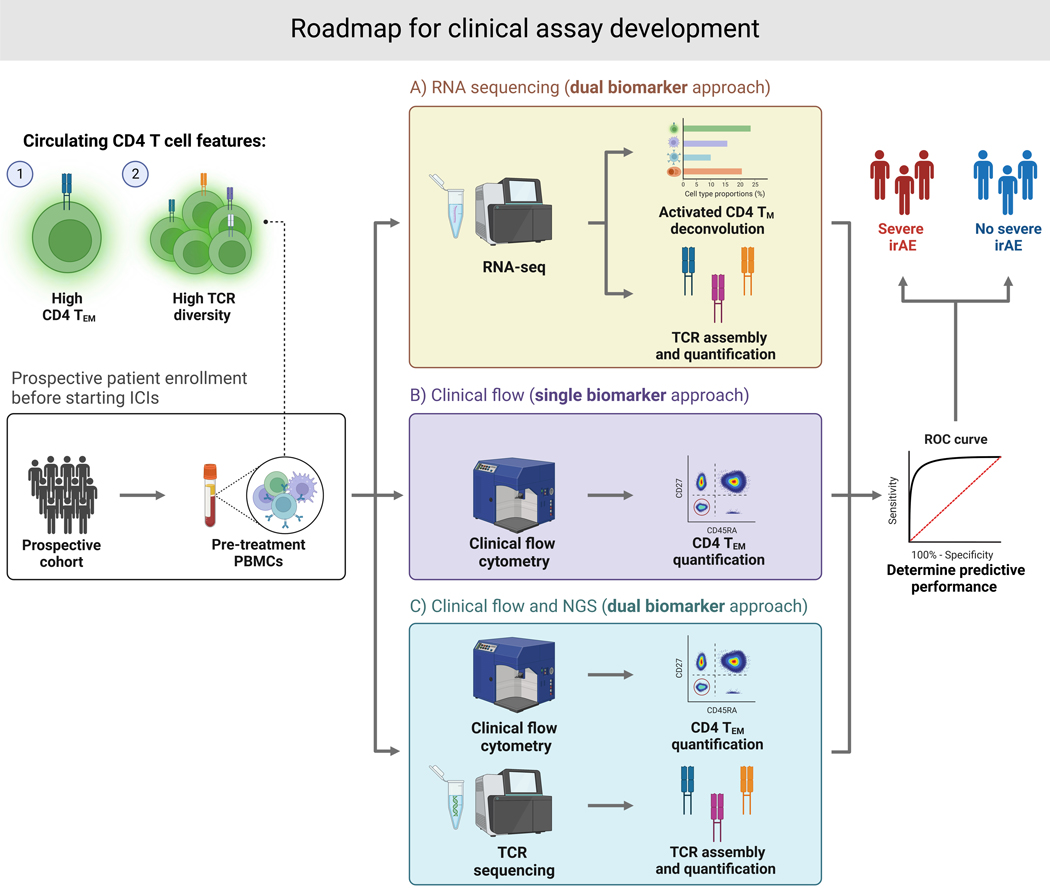
As detailed above, CD4 TM/TEM cells and TCR diversity are promising pretreatment correlates of severe irAE development (Figure 1), with important mechanistic implications (Figure 2). Based on these data, we propose the following roadmap for evaluating blood-based assays to predict severe irAEs in the clinic (Figure 3).
Figure 3. Potential roadmap for clinical assay development.
Following prospective enrollment and peripheral blood collection pretreatment, ICI-induced irAE risk may be predicted using (A) a dual biomarker approach with bulk RNA sequencing to quantify both activated CD4 TEM abundance and TCR diversity, (B) a single biomarker approach using clinical flow cytometry to quantify CD4 TEM abundance, or (C) a dual biomarker approach that pairs clinical flow cytometry with TCR sequencing.
An ideal clinical test, in our view, would be a bulk RNA sequencing assay. Indeed, cell type composition and TCR diversity can be simultaneously assessed from peripheral blood using bulk RNA-seq, with comparable performance across distinct batches (Lozano et al.)55. This would facilitate an off-the-shelf workflow for calculating the composite model reported in our study. Crucially, bulk RNA-seq is a mature, economical technology that can faithfully and reproducibly capture gene expression from even partially degraded material (down to ~10–50 ng of input), while also being amenable to targeted sequencing panels that can increase sensitivity while reducing costs even further. Additionally, the FDA has already granted Breakthrough Device designation for several cancer-focused RNA-seq panels, including MI Transcriptome™ CDx (Caris Life Sciences), TruSight™ Oncology Comprehensive (Illumina), and CancerDetect™ (Viome), paving the way for the eventual approval of RNA-seq-based companion diagnostics.
Flow cytometry is an alternative to bulk RNA-seq that already has considerable FDA precedent for use in clinical settings134, making it a potentially attractive assay for activated CD4 TEM quantification (Figure 3). Moreover, pretreatment activated CD4 TEM quantification by mass cytometry strongly correlated with severe irAE development in a discovery cohort55. Separately, the composite model could be rederived from activated CD4 TEM cell levels enumerated by flow cytometry and TCR diversity measured by immunoSEQ®, an assay that is commercially available for peripheral blood TCR clonotypic analysis (Figure 3). Future studies will be needed to test these possibilities and prospectively validate the leading approach.
Lastly, it will also be critical in the future to combine circulating biomarkers of severe irAE development with biomarkers of durable ICI benefit. This will yield a comprehensive clinical framework to jointly predict both the risk of severe toxicity and the probability of benefit. It might be possible to develop such a comprehensive blood-based assay by profiling only CD4 and CD8 T cell states. Indeed, it has been shown that CD8 T cell levels are lower pretreatment in melanoma and lung cancer patients that respond to PD(L)1 checkpoint blockade135,136. This type of comprehensive blood-based assay could have a tremendous clinical impact across diverse cancer patient populations.
6. Concluding remarks
In summary, activated CD4 TEM cells and TCR diversity represent important candidate biomarkers for predicting severe ICI-induced irAEs, with implications that point to the mechanistic core of irAE development. The literature we have synthesized here overall support this assertion, however, it will be important to perform prospective validation and assess translational potential. Overall, we hypothesize that clonally diverse CD4 TEM cells are at the center of a latent autoinflammatory potential that exists at baseline and is unmasked by ICI treatment. If prospectively validated, this autoinflammatory state has the potential to be detected using practical technologies such as bulk RNA sequencing or flow cytometry in order to predict who is most at risk of suffering from severe ICI-induced toxicity.
Despite growing evidence connecting CD4 TM/TEM cells to various manifestations of ICI-induced irAEs, many questions remain. For example, it is unclear how pervasive this association is across diverse ICI regimens, cancer types, and irAE types, especially those with late-onset (occurring after 3 months) or that rarely occur (e.g., myocarditis).
If clinicians had a practical assay that could reliably predict susceptibility to severe irAEs, it could be leveraged to more closely monitor at-risk patients, and potentially personalize early administration of targeted immunosuppressive therapeutics such as infliximab28–31. Additionally, the administration of immune checkpoint blockade itself could be further personalized, for example by considering active surveillance rather than adjuvant immune checkpoint blockade in stage II-III melanoma patients following surgery who are found to be at high risk for severe irAE development, or similarly for consideration of neoadjuvant checkpoint blockade prior to definitive resection. A deeper mechanistic understanding of irAE development could also yield further insights into pathophysiology and point toward the development of entirely new classes of irAE-specific preventative and therapeutic measures.
Acknowledgments
This work was in part supported by grants from the National Cancer Institute (no. K08CA237727 to D.Y.C., no. R21CA218950 to R.H. and no. R00CA187192 to A.M.N.), National Heart, Lung, and Blood Institute (no. T35HL007649 to A.N.), Melanoma Research Alliance Team Science Award (D.Y.C., M.S., R.H., A.A.C., and A.M.N., Yale Cancer Center Meyers Award (M.S. and R.H.), V Foundation for Cancer Research V Scholar Award (A.A.C.), Washington University Alvin J. Siteman Cancer Research Fund (A.A.C.), Virginia and D.K. Ludwig Fund for Cancer Research (A.M.N.), Stinehart-Reed Foundation (A.M.N.), Stanford Bio-X Interdisciplinary Initiatives Seed Grants Program (IIP) (A.M.N.), and the Donald E. and Delia B. Baxter Foundation (A.M.N.). All figures were created with BioRender.com.
M.S. has consulted for Idera Pharmaceuticals, Regeneron Pharmaceuticals, Apexigen, Alligator Bioscience, Verastem Oncology, Agenus, Rubius Therapeutics, Bristol Myers Squibb, Genentech-Roche, Boston Pharmaceuticals, Servier Laboratories, Adaptimmune Therapeutics, Immunocore, Dragonfly Therapeutics, Pierre Fabre Pharmaceuticals, Molecular Partners, Boehringer Ingelheim, Innate Pharma, Nektar Therapeutics, Pieris Pharmaceuticals, Numab Therapeutics, Abbvie, Zelluna Immunotherapy, Seattle Genetics/Seagen, Genocea Biosciences, GI Innovation, Chugai-Roche, BioNTech, Eli Lilly, Modulate Therapeutics, Array Biopharma, AstraZeneca and Genmab. M.S. has stock options in EvolveImmune, NextCure, Repertoire Immune Medicines, Adaptive Biotechnologies, Actym Therapeutics, and Amphivena Therapeutics and has stock ownership in GlaxoSmithKline and Johnson & Johnson. A.A.C. has patent filings related to cancer and immunological biomarkers and has served as an advisor/consultant to Illumina, Roche, AstraZeneca, Daiichi Sankyo, Invitae, Tempus, Geneoscopy, AlphaSights, DeciBio, and Guidepoint. A.A.C. has received honoraria from Agilent, Roche, and Dava Oncology. A.A.C. has stock options in Geneoscopy, research support from Roche, Illumina, and Tempus, and ownership interests in LiquidCell Dx and Droplet Biosciences. A.M.N. has patent filings related to expression deconvolution, digital cytometry, and cancer biomarkers, has served as an advisor/consultant to Roche, Merck, and CiberMed, and has ownership interests in CiberMed and LiquidCell Dx.
Footnotes
Competing interests
The other authors declare no conflicts of interest.
References
- 1.Hodi FS et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 19, 1480–1492 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Larkin J. et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373, 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O. et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 30, 582–588 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schachter J. et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390, 1853–1862 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 383, 1328–1339 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Carbone DP et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. The New England journal of medicine vol. 376 2415–2426 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 373, 123–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 373, 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan E. et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci. Transl. Med. 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EK & Konstantinopoulos PA Combined PARP and Immune Checkpoint Inhibition in Ovarian Cancer. Trends Cancer Res. 5, 524–528 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Le DT et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372, 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster RM The immune checkpoint inhibitors: where are we now? Nat. Rev. Drug Discov. 13, 883–884 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Ziogas DC, Theocharopoulos C, Koutouratsas T, Haanen J. & Gogas H. Mechanisms of resistance to immune checkpoint inhibitors in melanoma: What we have to overcome? Cancer Treat. Rev. 113, 102499 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Qian W, Sun X. & Jiang S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J. Hematol. Oncol. 15, 143 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soliman H. et al. A phase-1/2 study of adenovirus-p53 transduced dendritic cell vaccine in combination with indoximod in metastatic solid tumors and invasive breast cancer. Oncotarget 9, 10110–10117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X. et al. Tumor antigen-specific CD8+ T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancer. Cell. Immunol. 313, 43–51 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Azpilikueta A. et al. Successful Immunotherapy against a Transplantable Mouse Squamous Lung Carcinoma with Anti-PD-1 and Anti-CD137 Monoclonal Antibodies. J. Thorac. Oncol. 11, 524–536 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Yang R. et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 12, 832 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan P. et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 385, 1196–1206 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Tawbi HA et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 386, 24–34 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chodon T. et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20, 2457–2465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y-C et al. Treatment of Patients With Metastatic Cancer Using a Major Histocompatibility Complex Class II-Restricted T-Cell Receptor Targeting the Cancer Germline Antigen MAGE-A3. J. Clin. Oncol. 35, 3322–3329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins PF et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019–1027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foy SP et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature 615, 687–696 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doran SL et al. T-Cell Receptor Gene Therapy for Human Papillomavirus-Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 37, 2759–2768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postow MA, Sidlow R. & Hellmann MD Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 27.NCI. Common Terminology Criteria for Adverse Events (CTCAE) v6.0. [Google Scholar]
- 28.Puzanov I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for ImmunoTherapy of Cancer vol. 5 Preprint at 10.1186/s40425-017-0300-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson JA et al. Management of Immunotherapy-Related Toxicities, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 17, 255–289 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Brahmer JR, Lacchetti C. & Thompson JA Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. Journal of Oncology Practice vol. 14 247–249 Preprint at 10.1200/jop.18.00005 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Kumar V. et al. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 8, 49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Tang S-Q et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients. Cancer Res. Treat. 53, 339–354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siakavellas SI & Bamias G. Checkpoint inhibitor colitis: a new model of inflammatory bowel disease? Curr. Opin. Gastroenterol. 34, 377 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Plaçais L. et al. Risk of irAEs in patients with autoimmune diseases treated by immune checkpoint inhibitors for stage III or IV melanoma: results from a matched case–control study. Annals of the Rheumatic Diseases vol. 81 1445–1452 Preprint at 10.1136/ard-2022-222186 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Cortellini A. et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. The Oncologist vol. 24 e327–e337 Preprint at 10.1634/theoncologist.2018-0618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang DY et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 4, 1721–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balaji A. et al. Immune-Related Adverse Events Requiring Hospitalization: Spectrum of Toxicity, Treatment, and Outcomes. J. Oncol. Pract. 15, e825–e834 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang DY et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 4, 1721–1728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu JN et al. Cost of inpatient admissions for immune-related adverse effects from immune checkpoint inhibitor therapy: A single center experience. J. Clin. Orthod. 36, 3060–3060 (2018). [Google Scholar]
- 41.Morad G, Helmink BA, Sharma P. & Wargo JA Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shultz LD et al. Humanized mouse models of immunological diseases and precision medicine. Mamm. Genome 30, 123–142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulendran B. & Davis MM The science and medicine of human immunology. Science 369, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mestas J. & Hughes CCW Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Caturegli P. et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 186, 3225–3235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolled-Filhart M. et al. Development of a Companion Diagnostic for Pembrolizumab in Non-Small Cell Lung Cancer Using Immunohistochemistry for Programmed Death Ligand-1. Arch. Pathol. Lab. Med. 140, 1243–1249 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Mok TSK et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393, 1819–1830 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Balar AV et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Hellmann MD et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 33, 853–861.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizvi NA et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hellmann MD et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 378, 2093–2104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loi S. et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann. Oncol. 28, v608 (2017). [Google Scholar]
- 53.Pagès F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Gooden MJM, de Bock GH, Leffers N, Daemen T. & Nijman HW The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Cancer 105, 93–103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lozano AX et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 28, 353–362 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haslam A. & Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open 2, e192535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalinich M. et al. Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA. J Immunother Cancer 9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moding EJ, Nabet BY, Alizadeh AA & Diehn M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 11, 2968–2986 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pantel K. & Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Wan JCM et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Chaudhuri AA et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 7, 1394–1403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellini B. & Chaudhuri AA Circulating Tumor DNA Minimal Residual Disease Detection of Non-Small-Cell Lung Cancer Treated With Curative Intent. J. Clin. Oncol. 40, 567–575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chin R-I et al. Detection of Solid Tumor Molecular Residual Disease (MRD) Using Circulating Tumor DNA (ctDNA). Mol. Diagn. Ther. 23, 311–331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellmann MD et al. Circulating Tumor DNA Analysis to Assess Risk of Progression after Long-term Response to PD-(L)1 Blockade in NSCLC. Clin. Cancer Res. 26, 2849–2858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim SY et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin. Cancer Res. 25, 1557–1563 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Kurimoto C. et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 111, 1468–1477 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshino K, Nakayama T, Ito A, Sato E. & Kitano S. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: potential role of Th1-dominant immune response in immune-related adverse events: two case reports. BMC Cancer 19, 1019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarhini AA et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 3, 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka R. et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J. Dermatol. Sci. 86, 71–73 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Pavan A. et al. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 24, 1128–1136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimura K. et al. CD21lo B Cells Could Be a Potential Predictor of Immune-Related Adverse Events in Renal Cell Carcinoma. J Pers Med 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurimoto C. et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 111, 1468–1477 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu D, Dinh TL, Bausk BP & Walt DR Long-Term Measurements of Human Inflammatory Cytokines Reveal Complex Baseline Variations between Individuals. Am. J. Pathol. 187, 2620–2626 (2017). [DOI] [PubMed] [Google Scholar]
- 75.de Jager W, Bourcier K, Rijkers GT, Prakken BJ & Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 10, 52 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X. et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 18, 87 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xing P. et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer 7, 341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maher VE et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 37, 2730–2737 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Eggermont AMM et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 6, 519–527 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishimura K. et al. CD21lo B Cells Could Be a Potential Predictor of Immune-Related Adverse Events in Renal Cell Carcinoma. J Pers Med 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cebula A. et al. Dormant pathogenic CD4+ T cells are prevalent in the peripheral repertoire of healthy mice. Nat. Commun. 10, 4882 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newman AM, Gentles AJ, Liu CL, Diehn M. & Alizadeh AA Data normalization considerations for digital tumor dissection. Genome biology vol. 18 128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiffelle J. et al. T-cell repertoire analysis and metrics of diversity and clonality. Curr. Opin. Biotechnol. 65, 284–295 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Rényi A. On the Foundations of Information Theory. Revue de l’Institut International de Statistique / Review of the International Statistical Institute 33, 1–14 (1965). [Google Scholar]
- 85.Qi Q. et al. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. U. S. A. 111, 13139–13144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian Y. et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 8, 1473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newman AM et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 37, 773–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolotin DA et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Haanen JBAG et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv119–iv142 (2017). [DOI] [PubMed] [Google Scholar]
- 90.Kagamu H. et al. CD4+ T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol Res 8, 334–344 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Pitsios C. et al. Contraindications to immunotherapy: a global approach. Clin. Transl. Allergy 9, 45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burczynski ME et al. Molecular classification of Crohn’s disease and ulcerative colitis patients using transcriptional profiles in peripheral blood mononuclear cells. J. Mol. Diagn. 8, 51–61 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carpintero MF et al. Diagnosis and risk stratification in patients with anti-RNP autoimmunity. Lupus 24, 1057–1066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung T. et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 350, 455–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy WP et al. Association of the interferon signature metric with serological disease manifestations but not global activity scores in multiple cohorts of patients with SLE. Lupus Sci Med 2, e000080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palmer NP et al. Concordance between gene expression in peripheral whole blood and colonic tissue in children with inflammatory bowel disease. PLoS One 14, e0222952 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters LA et al. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat. Genet. 49, 1437–1449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bieber AK, Yin L. & Lo Sicco K. Pruritus and Tense Bullae After Discontinuation of Pembrolizumab in a Patient With Renal Cell Carcinoma. JAMA 324, 1453–1454 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Lopez AT & Geskin L. A Case of Nivolumab-Induced Bullous Pemphigoid: Review of Dermatologic Toxicity Associated with Programmed Cell Death Protein-1/Programmed Death Ligand-1 Inhibitors and Recommendations for Diagnosis and Management. Oncologist 23, 1119–1126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singer S, Nelson CA, Lian CG, Dewan AK & LeBoeuf NR Nonbullous pemphigoid secondary to PD-1 inhibition. JAAD Case Rep 5, 898–903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nuñez NG et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Med (N Y) 4, 113–129.e7 (2023). [DOI] [PubMed] [Google Scholar]
- 102.Hutchinson JA et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 12, 1439 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yasuda Y. et al. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci. Transl. Med. 13, (2021). [DOI] [PubMed] [Google Scholar]
- 104.Reschke R. et al. Distinct Immune Signatures Indicative of Treatment Response and Immune-Related Adverse Events in Melanoma Patients under Immune Checkpoint Inhibitor Therapy. Int. J. Mol. Sci. 22, 8017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reschke R. et al. Checkpoint Blockade-Induced Dermatitis and Colitis Are Dominated by Tissue-Resident Memory T Cells and Th1/Tc1 Cytokines. Cancer Immunol Res 10, 1167–1174 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson DB et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 25, 1243–1250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bukhari S. et al. Single-cell RNA sequencing reveals distinct T cell populations in immune-related adverse events of checkpoint inhibitors. Cell Rep Med 4, 100868 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luoma AM et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655–671.e22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franken A. et al. Single-cell transcriptomics identifies pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in immune checkpoint inhibitor-related pneumonitis. J Immunother Cancer 10, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.von Euw E. et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J. Transl. Med. 7, 35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andrews MC et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson DB & Balko JM T cell dynamism and immune-related adverse events. Cancer Cell (2023) doi: 10.1016/j.ccell.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 113.van Eijs MJM et al. Toxicity-specific peripheral blood T and B cell dynamics in anti-PD-1 and combined immune checkpoint inhibition. medRxiv (2023) doi: 10.1101/2023.01.20.23284818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lechner MG et al. Inhibition of IL-17A Protects against Thyroid Immune-Related Adverse Events while Preserving Checkpoint Inhibitor Antitumor Efficacy. J. Immunol. 209, 696–709 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Monsour EP, Pothen J. & Balaraman R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus 11, e5824 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun X. et al. 812 Erythema nodosum-like toxicity in an immunotherapy treated patient is accompanied by oligoclonal memory activated CD4 T cells. J Immunother Cancer 9, (2021). [Google Scholar]
- 117.Johnson DB & Balko JM Primed for toxicity: CD4+ T cells and immune checkpoint inhibitors. Med 3, 155–156 (2022). [DOI] [PubMed] [Google Scholar]
- 118.Johnson DB, Nebhan CA, Moslehi JJ & Balko JM Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinzger C. et al. Hepatocytes are permissive for human cytomegalovirus infection in human liver cell culture and In vivo. J. Infect. Dis. 180, 976–986 (1999). [DOI] [PubMed] [Google Scholar]
- 120.Johnson DB et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 375, 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raveney BJE et al. Involvement of cytotoxic Eomes-expressing CD4+ T cells in secondary progressive multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peeters LM et al. Cytotoxic CD4+ T Cells Drive Multiple Sclerosis Progression. Front. Immunol. 8, 1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu Y. et al. CD4+CD29+T cells are blamed for the persistent inflammatory response in ulcerative colitis. Int. J. Clin. Exp. Pathol. 8, 2627–2637 (2015). [PMC free article] [PubMed] [Google Scholar]
- 124.Tokunaga R. et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat. Rev. 63, 40–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dougan M, Luoma AM, Dougan SK & Wucherpfennig KW Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 184, 1575–1588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bello E. & Dougan M. Elevated circulating memory T cells precede immunotherapy toxicities in melanoma. Trends in cancer research vol. 8 347–349 (2022). [DOI] [PubMed] [Google Scholar]
- 127.McDaniel MM et al. Effector memory CD4+ T cells induce damaging innate inflammation and autoimmune pathology by engaging CD40 and TNFR on myeloid cells. Sci Immunol 7, eabk0182 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wicks IP & Roberts AW Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol. 12, 37–48 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Bendall LJ & Bradstock KF G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 25, 355–367 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Marcovecchio PM, Thomas G. & Salek-Ardakani S. CXCL9-expressing tumor-associated macrophages: new players in the fight against cancer. J Immunother Cancer 9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gudd CLC et al. Activation and transcriptional profile of monocytes and CD8+ T cells are altered in checkpoint inhibitor-related hepatitis. J. Hepatol. 75, 177–189 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Paparoupa M, Stupperich S, Goerg-Reifenberg L, Wittig A. & Schuppert F. Successful Treatment of an Immune-Mediated Colitis Induced by Checkpoint Inhibitor Therapy in a Patient with Advanced Melanoma. Case Rep. Gastroenterol. 14, 554–560 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harris JP, Postow MA & Faleck DM Efficacy of Infliximab Dose Escalation in Patients with Refractory Immunotherapy-Related Colitis: A Case Series. Oncologist 27, e350–e352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pedreira CE, Costa ES, Lecrevisse Q, van Dongen JJM & Orfao A. Overview of clinical flow cytometry data analysis: recent advances and future challenges. Trends Biotechnol. 31, 415–425 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Krieg C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nature medicine vol. 24 144–153 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Nabet BY et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell 183, 363–376.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]