Abstract
Mammalian artificial chromosomes (MACs) provide a new tool for the improvement of our knowledge of chromosome structure and function. Moreover, they constitute an alternative and potentially powerful tool for gene delivery both in cultured cells and for the production of transgenic animals. In the present work we describe the molecular structure of MC1, a human minichromosome derived from chromosome 1. By means of restriction and hybridization analysis, satellite-PCR, in situ hybridization on highly extended chromatin fibres, and indirect immunofluorescence, we have established that: (i) MC1 has a size of 5.5 Mb; (ii) it consists of 1.1 Mb alphoid, 3.5 Mb Sat2 DNA, and telomeric and subtelomeric sequences at both ends; (iii) it contains an unusual region of interspersed Sat2 and alphoid DNAs at the junction of the alphoid and the Sat2 blocks; and (iv) the two alphoid blocks and the Sat2-alphoid region bind centromeric proteins suggesting that they participate in the formation of a functional kinetochore.
INTRODUCTION
Mammalian artificial chromosomes (MACs) represent one of the most powerful research tools for understanding chromosome structure and function. Furthermore, they provide an excellent material for developing chromosome-based vectors to be used in basic biological studies and for gene delivery to animal cells, and also for generating transgenic animals. Attempts to isolate the functional elements, namely the centromere, the telomere and the origins of replication, from higher eukaryotic chromosomes have been the object of considerable attention for many years, ever since yeast artificial chromosome (YAC) technology made its appearance. By far the best understood element, at least at the level of sequence requirement for function, is the telomere (Farr et al., 1991). Much attention is now focused on centromeres, and less on the origins of replication, although information concerning these elements is also very limited. Furthermore, MACs can find useful applications in the study of the territorial organization of chromosomes in interphase nuclei.
By means of various approaches it has been shown that the centromere is formed over alphoid DNA although a few cases of non-alphoid centromeres have been described (Lee et al., 1997; du Sart et al., 1997). This finding leads to the postulation of the existence of epigenetic mechanisms which could play a central role in determining centromere function independently from the primary DNA sequences (Karpen and Allshire, 1997).
The analysis of mammalian chromosomes from a structural and functional point of view is hindered by the huge amount of DNA which underlies chromosome function. Minichromosomes, which contain only a subset of the chromosomal DNA, are an excellent material for this purpose. Examples of such analyses have been provided recently by studies that characterize minichromosomes of diverse origin (Mills et al., 1999; Shen et al., 1999; Yang et al., 2000). Current minichromosomes belong to two classes: (i) molecules assembled de novo using alphoid and telomeric DNAs (Harrington et al., 1997; Ebersole et al., 2000); (ii) molecules derived by cutting back natural chromosomes using telomere-directed fragmentation (Mills et al., 1999) or exposure to irradiation (Mascarello et al., 1980). Since minichromosomes of class (i) seem to be quite different structurally from the input elements, they are not related in a simple manner to the natural chromosomes. Thus molecules of class (ii) would appear to be more suitable for the study of chromosome function, for this reason our attention was focused on the minichromosome MC1, which was obtained by the breakage of human chromosome 1.
Previously we have described MC1 as being very stable in the hamster cell background, since no minichromosome loss was detected even after growth without selection for 200 generations (Guiducci et al., 1999). In the study reported here, we provide the physical map of MC1 along with information regarding the sequences underlying centromere and telomere function.
RESULTS AND DISCUSSION
Structure of MC1
MC1 was derived from human chromosome 1 by γ-irradiation of a monosomatic CHO hybrid cell line. It was shown to be linear and to contain alphoid DNA from human chromosome 1, some of the pericentromeric Sat2 DNA, the SDHC gene (1q21 in human chromosome 1) and an estimated 1–2 Mb of unique sequences (Carine et al., 1989; Au et al., 1999). The size of MC1 has not been precisely determined; it was originally thought to be 5–10 Mb (Carine et al., 1986; 1989); a recent paper suggests a size of 2–3 Mb (Au et al., 1999), whereas our previous data indicate a size of 5.5 Mb (Guiducci et al., 1999).
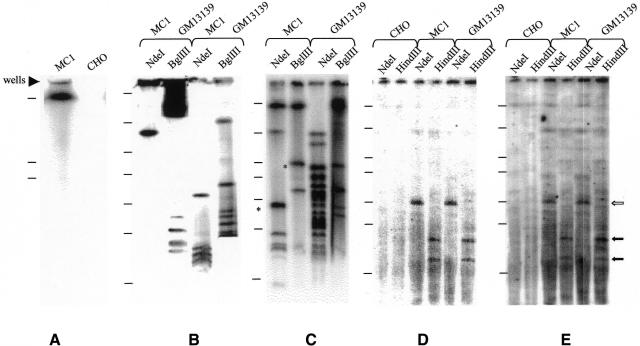
We have undertaken restriction and hybridization analysis, using two rare cutting enzymes NdeI, and BglII, to assemble the map of MC1 and to accurately determine its size. Intact DNA, prepared from the CHO hybrid cells containing MC1, was digested and hybridized with three probes: alphoid DNA (Figure 1B), obtained by PCR using primers specific for human chromosome 1 (Aleksandrov et al., 1988; see Methods); pericentromeric Sat2 DNA (Figure 1C) mapped to the 1q12 region (Cooke and Hindley, 1979) and subtelomeric DNA (Figure 1D), free of telomeric repeats and specific for all human chromosomal ends (Ascenzioni et al., 1990). In order to compare MC1 with a natural chromosome 1 the same analysis was performed on the monosomatic CHO/human chromosome 1 hybrid (GM13139). Although the two molecules are of different origins (the HT1080 fibrosarcoma cell line and the MRC5 fibroblast line, respectively) it is reasonable to compare them since no cell type specificity has been described for the structural chromosome elements, at least at the level of the DNA sequences that underlie centromere and telomere function.
Fig. 1. Long range mapping of MC1 and human chromosome 1. (A) undigested MC1 and CHO plugs hybridized to the Sat2 probe. (B–E) MC1 and GM13139 plugs digested with the indicated enzymes hybridized with: (B) chromosome 1 alphoid probe, (C) Sat2 DNA, (D) subtelomeric probe and (E) telomeric probe. The asterisk indicates double bands which were resolved under different run conditions. S. pombe chromosome markers in (A) were: 5.7, 4.6 and 3.5 Mb. Markers in (B–E) were: 2.2 Mb, 1.12 Mb, 750 kb, 610 kb, 450 kb, 225 kb and 48 kb. At the right side of the figure the filled and open arrows indicate HindIII and NdeI fragments, respectively.
BglII cuts MC1 into nine fragments ranging in size from 120 kb to 2 Mb, which add up to 4.98 Mb (Table I). Two of these fragments (530 and 170 kb) hybridized with both Sat2 and alphoid probes, whereas only one fragment hybridized to the subtelomeric probe. The remaining fragments showed specific hybridization with Sat2 or alphoid DNA. NdeI gave the same number of fragments, which add up to 5.08 Mb (Table I). The 1.1 Mb fragment hybridized strongly to the alphoid probe and weakly to Sat2 DNA. Seven of the remaining fragments hybridized with Sat2 DNA. It is usually assumed that satellite DNAs are organized as single arrays (Haaf and Ward, 1994), but in the case of MC1 the presence of two BglII fragments hybridizing to both the Sat2 and alphoid probes opened the possibility that the alphoid and Sat2 arrays are interspersed in the region defined by the two fragments. The subtelomeric probe revealed one 450 kb fragment, which is the same size as the one obtained with BglII digestion (data not shown). The MC1 subtelomeric fragments revealed by BglII and NdeI digestions have also been found in human chromosome 1 (Figure 1D and data not shown).
Table I. Summary of data on MC1 structure. Structural and cytological analysis of MC1 containing CHO cells. The data reported were obtained by Southern hybridization with alphoid, Sat2 and subtelomeric probes; FISH on extended chromatin fibres with pAL1, pZ5.1 and Sat2 probes; indirect immunofuorescence with CREST and anti-CENP-F antibodies, combined with FISH with alphoid and Sat2 probes.
| BglII fragments | NdeI fragments | Total length | Relative orientation of satellite blocks | Localization of CENPs | |
|---|---|---|---|---|---|
| |
|
BglII |
NdeI |
|
|
| pZ5.1 + pAL1 | CREST | ||||
| 2 Mbb, 720 kbb | 2 Mbb, 1.1 Mba,b | 4.98 Mb | 5.08 Mb | adjacent blocks | localized on |
| 700 kbb | 450 kbc | pAL1 + Sat2 | alphoid and alphoid/Sat2 | ||
| 530 kba,b | 440 kbb | partially overlapped | regions | ||
| 450 kbc | 420 kbb, 220 kbb | blocks | CENP-F | ||
| 170 kba,b | 175 kbb, 160 kbb | pZ5.1 + Sat2 | localized on | ||
| 150 kba, 140 kba | 120 kbb | separated | alphoid region | ||
| 120 kba | blocks | ||||
aAlphoid fragments.
bSat2 fragments.
cSubtelomeric fragments.
By comparing the size of the NdeI and BglII fragments in MC1 and in human chromosome 1, it was possible to establish that the alphoid arrays in MC1 had undergone pronounced structural changes, whereas the Sat2 DNA region was only partially modified. Moreover, the total amount of alphoid and Sat2 DNAs was reduced in MC1 by 60 and 30%, respectively.
The total length of MC1, as determined from the BglII and NdeI restriction fragments and taking into consideration only one 450 kb terminal fragment, is 500 kb smaller than the size estimated by PFGE, which makes use of Schizosaccharomyces pombe chromosomes as molecular markers (Figure 1A). It could be hypothesized that this divergence is caused by the presence in the 450 kb band of two terminal fragments, one for each MC1 end, or that unknown sequences are present in MC1. To answer this question HindIII digested MC1 samples were hybridized to the subtelomeric probe. As shown in Figure 1D, two fragments of 150 and 80 kb were revealed by the subtelomeric probe. That these fragments represent the ends of MC1 was demonstrated by showing that the two fragments hybridized also to the telomeric probe (Figure 1E). It is therefore reasonable to surmise that MC1 contains two 450 kb terminal fragments which consist in part of subtelomeric and telomeric sequences, and in part of as yet uncharacterized sequences, presumably containing the SDHC gene. The subtelomeric and telomeric regions of MC1 have been conserved intact from chromosome 1, since digestion of this chromosome with BglII, NdeI and HindIII produces fragments identical in size and sequence composition to those obtained with MC1. Thus, taken together, the restriction data are consistent with a size for MC1 of 5.5 Mb.
The restriction and hybridization data suggest that MC1 consists predominantly of Sat2 and alphoid DNA. That this is indeed the case was further demonstrated by reverse painting of GM13139 chromosomes with the MC1 probe. This experiment was carried out under high stringency conditions (see Methods) in order to avoid hybridization of MC1 telomeres to intrachromosomal telomere-like sequences of hamster chromosomes (Bertoni et al., 1996), which would have masked possible signals due to hamster sequences present in MC1. Only one hybridization signal was detected in the central region of one chromosome (Figure 2), which was identified as human chromosome 1 by means of a second fluorescence in situ hybridization (FISH) with a coatasome 1 probe (data not shown). Moreover, since no hybridization signals were detected in the hamster chromosomes, it is possible to surmise that MC1 is almost free of CHO DNA.
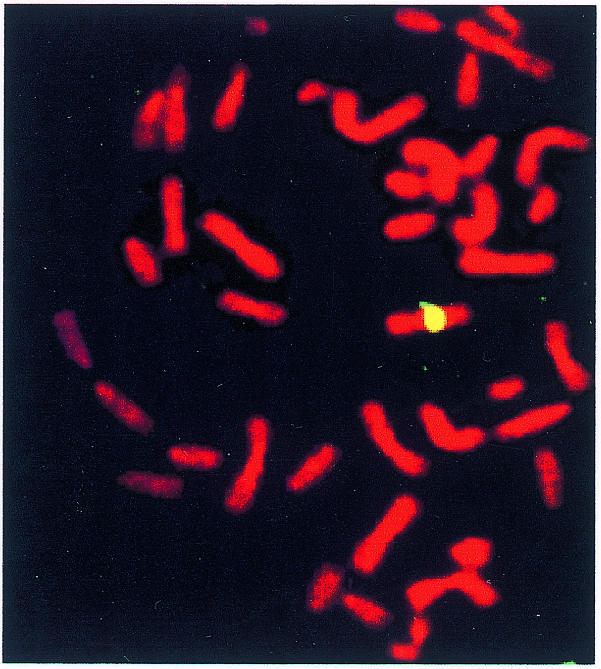
Fig. 2. Reverse painting of GM13139 chromosomes with the biotin-labelled MC1 probe revealed with avidin-FITC. The chromosomes were counterstained with propidium iodide.
Satellite PCR mapping
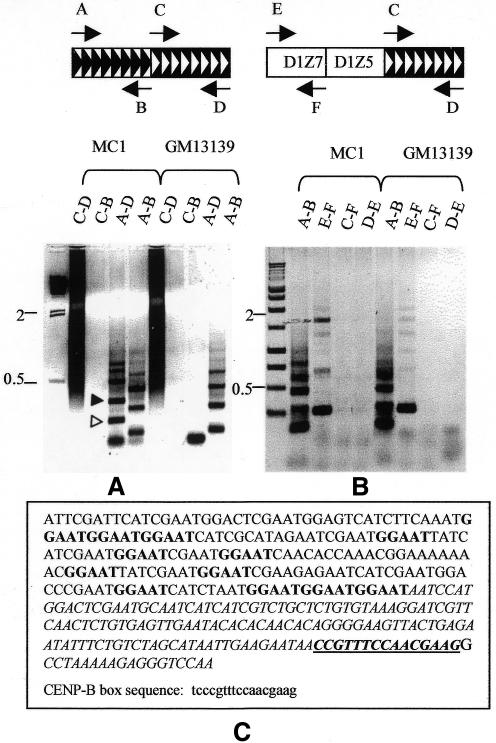
In order to study the structural organization of MC1 satellite DNA regions we have designed PCR by combining primers specific for the alphoid and Sat2 repeats (satellite-PCR). This analysis is based on the following rationale: where alphoid and Sat2 blocks are adjacent, satellite-PCR is expected to amplify only one fragment; conversely if a region of interspersed alphoid and Sat2 repeats exists, satellite-PCR should be expected to skip some priming sites, thereby amplifying multiple fragments. We have performed this analysis with MC1 and with human chromosome 1 as a control. Furthermore, the proper choice of the primers was confirmed by means of alphoid and Sat2 PCR. The former, because of the higher order repeat organization of the alphoid units (Lee et al., 1997), gave rise to a typical amplification pattern. A similar pattern was obtained with both DNAs (Figure 3A and B, lanes A-B), demonstrating that the alphoid DNA of MC1 maintained the same higher order repeat structure of chromosome 1. A continuous smear of amplified DNA was obtained with the Sat2 primers C-D (Figure 3A, lanes C-D) confirming the heterogeneous composition of the Sat2 block. No amplification was obtained with C-B primers either in MC1 or chromosome 1, whereas the A-D primers yielded multiple bands in MC1 and only one band in chromosome 1 (Figure 3A, lanes A-D). The specificity of the PCR products was demonstrated by sequencing two MC1 fragments obtained with A-D primers (Figure 3A). The resulting sequences (Figure 3C; EMBL accession No. AJ302782) share features of both Sat2 and alphoid DNAs. In fact it was possible to show that each fragment contains a region related to Sat2 DNA flanked by alphoid-related sequences which contain the CENP-B box. The two fragments differ from each other by the length of the Sat2 DNA. This result strongly suggests the presence of an interspersed alphoid/Sat2 region in which the satellite repeats follow the orientation reported in Figure 3. The A and B primers were designed on the basis of the chromosome 1 alphoid sequence (see Methods), but more recently it has been shown that the chromosome 1 centromere contains two alphoid subsets: one, D1Z5, specific for human chromosome 1 and most probably similar to the GenBank accession No. M38468 sequence, and the other, D1Z7, coexisting on chromosomes 1, 5 and 19. These loci are organized as distinct domains running as follows: 1p–D1Z5–D1Z7–D1Z5–1q (Finelli et al., 1996). To map D1Z7 with respect to the Sat2 DNA region, we carried out the PCR reported in Figure 3B. As expected, PCR E-F yielded an amplification pattern that is very similar in MC1 and chromosome 1 (lanes E-F), but is different from the one obtained in the A-B reaction; furthermore, no amplification was obtained with the combined D1Z7–Sat2 primers (lanes C-F and D-E), suggesting that D1Z7 is distant from the Sat2 region.
Fig. 3. Satellite-PCR mapping and sequencing. Gel electrophoresis of MC1 and GM13139 PCR obtained with the primers indicated at the top of each panel. Amplified region in (A) and (B): (A) alphoid/Sat2; (B) D1Z7-Sat2. (C) Sequence of the fragment marked with a filled triangle in (A); the GGAAT repeat, characteristic of Sat2 DNA is shown in bold; the region homologous to the alphoid DNA is italicized; the CENP-B box is underlined, and the consensus sequence is reported below in lower case. The first slot in (A) and (B) contains λ-HindIII size markers and a 1 kb ladder, respectively. The size markers indicated are in kb. Open and filled triangles in (A) indicate the two sequenced fragments. Symbols: black chevrons, alphoid block; white chevrons, Sat2 block; both oriented as in GenBank.
Mapping MC1 by FISH on extended chromatin fibres
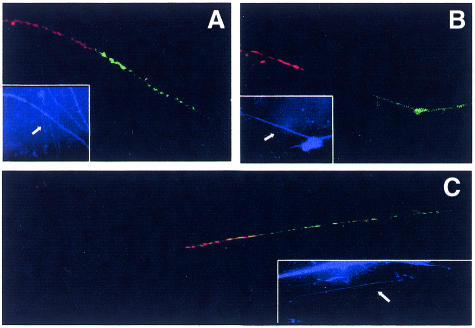
Minichromosomes, due to their small size, appear as dots in metaphase chromosome preparations, which makes conventional FISH unsuitable for ordering the various probes on these molecules. To overcome this problem we have utilized FISH on extended chromatin fibres. The two alphoid subsets of human chromosome 1 are recognized by the pAL1 and pZ5.1 probes, which identify the D1Z5 and D1Z7 loci, respectively (Finelli et al., 1996). Dual colour FISH on extended chromatin fibres, carried out with the pAL1 and pZ5.1 probes, revealed the presence of both alphoid subsets arranged as two distinct domains, one for each subset (Figure 4A). We have also investigated the arrangement of the Sat2 DNA with respect to the alphoid domains. The data described in the previous sections suggest that Sat2 and alphoid DNA sequences are interspersed along a region of ∼700 kb (530 + 170) BglII fragments. To confirm this result we have carried out two-colour FISH on extended chromatin fibres with Sat2–green/pZ5.1–red and Sat2–green/pAL1–red probes (Figure 4). It can be seen that Sat2 and D1Z7 are distinct domains arranged as separated blocks (Figure 3B) whereas the Sat2 and D1Z5 domains partially overlap along the fibre (Figure 4C). Thus, MC1 consists of alphoid and satellite blocks running as follows: D1Z7–D1Z5–D1Z5/Sat2–Sat2. This structure indicates that one of the two break points which generated MC1 occurred in the D1Z7 alphoid subset; the second break point was suggested to be located at the 1q side of the SDHC gene.
Fig. 4. Dual colour fibre-FISH of MC1. Extended chromatin fibres were simultaneously hybridized with: (A) pAL1–green/pZ5.1–red; (B) pZ5.1–red/Sat2–green; (C) pAL1–red/Sat2–green. The insert shows DAPI counterstained fibres, the white arrow points to the hybridized fibre. Digoxigenin-labelled pAL1 and Sat2 probes were revealed by anti-digoxigenin-FITC, biotin-labelled pZ5.1 and pAL1 were revealed by avidin-Cy3.
Analysis of MC1 centromeric proteins
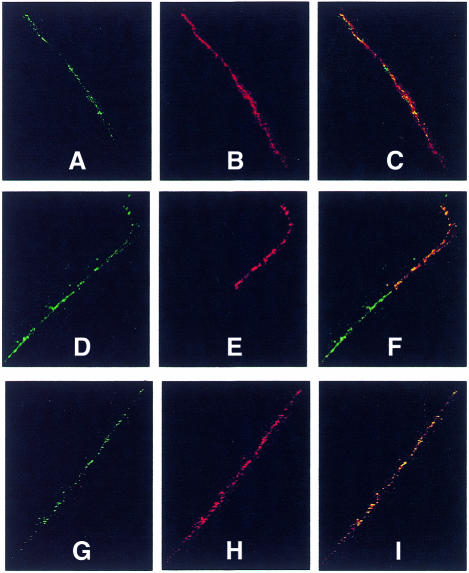
The functional status of a centromere can be addressed by looking at the presence of centromere proteins (CENPs) that are components of the centromere/kinetocore complex on the metaphase chromosome. CENPs proteins can be revealed by utilizing CREST autoimmune sera (Earnshaw and Rothfield, 1985). We have carried out CREST immunofluorescence followed by FISH with both alphoid and Sat2 DNA on extended chromatin fibres. Figure 5 shows that CREST colocalizes with alphoid (Figure 5A–C) and partially with Sat2 DNAs (Figure 5D–F).
Fig. 5. Immunofluorescence/FISH analysis of MC1. Centromeric proteins were detected on extended chromatin fibres with (A–F) CREST and (G–I) anti-CENP-F, and subsequently the fibres were hybridized in situ to alphoid (A-C) and Sat2 (D-F) probes. CREST immunofluorescence (red) partially overlaps the Sat2 block (green), whereas it completely overlaps the alphoid region (green). CENP-F immunofluorescence (red) extends over the entire alphoid fibre (green). Merged images are shown in (C), (F) and (I). CREST and CENP-F were revealed with Cy3 and Texas-red, respectively; biotin-labelled Sat2 and alphoid probes with avidin-FITC.
It has been shown that CREST binds both functional and non-functional centromeres. In order to obtain information about MC1 centromere activity we have thus used immunosera against CENP-F. This protein gradually accumulates during the cell cycle and reaches a peak in the G2 and M phases, after which it is rapidly degraded upon completion of mitosis. It has been postulated that CENP-F plays a central role in establishing and maintaining the connection with the spindle microtubules, and in fact its presence correlates only with active centromeres in dicentric chromosomes (Faulkner et al., 1998). CENP-F was localized by indirect immunofluorescence with antibodies specific for the human CENP-F protein followed by in situ hybridization with an alphoid probe. As shown in Figure 5G–I, CENP-F and alphoid signals are perfectly merged, demonstrating that the entire alphoid containing region takes part in centromere function.
The data reported here demonstrate that MC1 is a 5.5 Mb molecule composed of telomeres with adjacent subtelomeric sequences derived in intact form from chromosome 1, and a centromere which is assembled over two blocks of alphoid repeats plus an interspersed region of alphoid/Sat2 DNA. The centromeric and pericentromeric regions of the minichromosome are reduced in size by almost a factor of two, and present a region of interspersion of alphoid and Sat2 sequences at the junction between the two sequence blocks. In spite of these structural dissimilarities, centromere function is conserved, as attested by mitotic stability. The region of interspersion has been shown to participate in the function of the centromere. A small fraction of the minichromosome remains to be fully characterized; this region presumably contains the SDHC gene that had been previously assigned to MC1.
METHODS
DNA preparation, digestion and analysis
Intact DNA, prepared by means of the agarose plug procedure (Guiducci et al., 1999) was digested overnight at 37°C with 40 U for 100 µl plug, using the recommended buffer (Boehringer Mannheim). Digested samples were electrophoresed in a CHEF Mapper apparatus (Bio-Rad) under conditions which separate Saccharomyces cerevisiae chromosomes. Undigested MC1 and CHO plugs were electrophoresed by the prolonged PFGE run described by Guiducci, (Guiducci et al., 1999). Staining and hybridization conditions have been described previously (Ascenzioni et al., 1990).
Preparation of extended chromatin fibres
Chromatin fibres were prepared according to Haaf and Ward (1994). For immunolocalization of CENPs the following procedure was utilized: 106 cells were cytospun (120 g for 4 min) onto glass slides, and after air drying the slides were incubated for 5 min in lysis buffer (0.5% SDS, 50 mm EDTA, 200 mM Tris–HCl pH 7.4). Subsequently the slides were tilted so as to allow the buffer to run down the surface. The resulting fibres were fixed with 80% ethanol for 30 min at room temperature.
FISH/immunofluorescence
Immunofluorescence was carried out as follows: after two washes with PBST (PBS, 0.05% Tween 20), the slides were incubated with 20% FBS for 30 min. Primary antibody was added and the slides were incubated at 37°C for 2 h in a humidified chamber. The slides were washed once with PBST, incubated with the secondary antibody at 37°C for 30 min and washed once before further processing. In situ hybridization was performed after fixing the slides with 3:1 methanol:acetic acid (Guiducci et al., 1999). High-stringency hybridization was carried out with the following post-hybridization washes: three times 50% formamide in 2× SSC at 42°C, 5 min each; three times 2× SSC at 42°C. Images were captured using a Zeiss Axioscope and a digital Kodak MDS 120 camera. When necessary the images were merged using Adobe Photoshop 4.0.
Probes, primers and immunological reagents
Probes for DNA hybridization: Sat2 DNA purified from EcoRI-digested pUC1.77 (Cooke and Hindley, 1979); the subtelomeric fragment obtained from pL8 (Ascenzioni et al., 1990); 50 bp of human telomeric DNA purified from pHuTel (kindly provided by Dr E. Gilson); chromosome 1 alphoid DNA, amplified from GM13139 DNA with primers A and B. Probes for FISH: pAL1 and pZ5.1 DNAs, gel purified from the corresponding plasmids after cutting with HindIII and EcoRI–SalI, respectively; Sat2 and alphoid chromosome 1 DNAs as described above; MC1 DNA, purified from preparative PFGE gels using the running conditions described previously (Guiducci et al., 1999); coatasome for human chromosome 1 (Oncor).
Primers: A (TGGAGATTTGGACCTCTTTGAGGCC) and B (GAATGTTCAGCTCTGTGAGTTGAAC) from the chromosome 1 alphoid DNA (GenBank: M38468); C (CCATTCGATGATGATTCCATTCG) and D (CATCGAATGGACTCGAATGGAGTC) from ppd17 (GenBank accession No. J00296); E (TGCACGTGGATAAGTTGTCC) and F (CACCACGCAGTTTGTGGGAA) from the alpha-satellite D1Z7 (GenBank accession No. M26920).
The following antisera were used: CREST (Società Italiana Chimici) used at 1/100 dilution in PBS; polyclonal anti-CENP-F (Novus Biologicals) used at 1/750 dilution in PBS; anti-human-IgG conjugated with Cy3 (Amersham); anti-rabbit-IgG conjugated with Texas-red (Novous Biologicals).
Cloning and sequencing of PCR
The PCR products, after gel purification, were cloned in pGEM-T (Promega) following the supplier’s specifications. Sequencing was done by the fmol DNA cycle sequencing system (Promega) with pUC/M13 forward and reverse primers.
Acknowledgments
ACKNOWLEDGEMENTS
Thanks are due to: M. Rocchi and E. Gilson for providing the chromosome 1 alphoid subsets and the pHuTel plasmid, respectively, and to Dr Maria Carmela Savarese for sequencing. This work was supported by a grant (97.01096.PF49) from the CNR and a grant from the MURST (Modalità alternative di transgenesi animale: ricerche scientifiche sulla costruzione di mini- e micro-cromosomi artificiali). The financial support of Telethon-Italy (Grant No. A164) is also gratefully acknowledged.
REFERENCES
- Aleksandrov I.A., Akopian, T.A., Vinnik, E.A., Mitkevich, S.P. and Kiselev, L.L. (1988) Primary structure of chromosome-specific human alpha-satellite DNA. Dokl. Akad. Nauk SSSR, 300, 1235–1239. [PubMed] [Google Scholar]
- Ascenzioni F., Della Valle, G., Guerrini, A.M., Pisani, G., Biondi, O. and Donini, P. (1990) A human DNA telomeric fragment containing yeast ARS and mitotic stabilizing sequences. Res. Microbiol., 141, 1117–1129. [DOI] [PubMed] [Google Scholar]
- Au H.C., Mascarello, J.T. and Scheffler, I.E. (1999) Targeted integration of a dominant neoR marker into a 2- to 3-Mb Human minichromosome and transfer between cells. Cytogenet. Cell Genet., 86, 194–203. [DOI] [PubMed] [Google Scholar]
- Bertoni L., Attolini, C., Faravelli, M., Simi, S. and Giulotto, E. (1996) Intrachromosomal telomere-like DNA sequences in Chinese hamster. Mamm. Genome, 7, 853–855. [DOI] [PubMed] [Google Scholar]
- Carine K., Solus, J., Waltzer, E., Manch-Citron, J., Hamkalo, B.A. and Scheffler, I.E. (1986) Chinese hamster cells with a minichromosome containing the centromeric region of human chromosome 1. Somat. Cell Mol. Genet., 12, 479–491. [DOI] [PubMed] [Google Scholar]
- Carine K., Jacquemin-Sablon, A., Waltzer, E., Mascarello, J.T. and Scheffler, I.E. (1989) Molecular characterization of human minichromosomes with centromere from chromosome 1 in human hamster hybrid cells. Cell. Mol. Genet., 14, 445–460. [DOI] [PubMed] [Google Scholar]
- Cooke H.J. and Hindley, J. (1979) Cloning of human satellite DNA: different components are on different chromosomes. Nucleic Acids Res., 6, 3177–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Sart D. et al. (1997) A functional neo-centromere formed through activation of a latent human centromere and consisting of non-alpha satellite DNA. Nature Genet., 16, 144–153. [DOI] [PubMed] [Google Scholar]
- Earnshaw W.C. and Rothfield, N. (1985) Identification of a family of human centromere proteins using autoimmune sera from patient with scleroderma. Chromosoma, 91, 313–321. [DOI] [PubMed] [Google Scholar]
- Ebersole T.A., Ross, A., Clark, E., McGill, N., Schindelhauer, D., Cooke, H. and Grimes, B. (2000) Mammalian artificial chromosome formation from circular alphoid input DNA does not require telomere repeats. Hum. Mol. Genet., 9, 1623–1631. [DOI] [PubMed] [Google Scholar]
- Farr C., Fantes, J., Goodfellow, P. and Cooke, H. (1991) Functional reintroduction of human telomeres into mammalian cells. Proc. Natl Acad. Sci. USA, 88, 7006–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner N.E., Vig, B., Echeverri, C.J., Wordeman, L. and Vallee, R.B. (1998) Localization of motor-related proteins and associated complexes to active, but not inactive centromeres. Hum. Mol. Genet., 7, 671–677. [DOI] [PubMed] [Google Scholar]
- Finelli P., Archidiacono, R., Marzella, R., Lanoce, A., Archidiacono, N. and Rocchi, M. (1996) Structural organization of multiple alphoid subsets coexisting on human chromosomes 1, 4, 5, 7, 15, 18 and 19. Genomics, 38, 325–330. [DOI] [PubMed] [Google Scholar]
- Guiducci C., Ascenzioni, F., Auriche, C., Piccolella, E., Guerrini, A.M. and Donini, P. (1999) Use of a human minichromosome as a cloning and expression vector for mammalian cells. Hum. Mol. Genet., 8, 1417–1424. [DOI] [PubMed] [Google Scholar]
- Haaf T. and Ward, D.C. (1994) Structural analysis of a-satellite DNA and centromere proteins using extended chromatin and chromosomes. Hum. Mol. Genet., 3, 697–709. [DOI] [PubMed] [Google Scholar]
- Harrington J.J., Van Bokkeln, G., Mays, R.W., Gustashaw, K. and Willard, H.F. (1997) Formation of de novo centromeres and construction of first-generation human artificial chromosomes. Nature Genet., 15, 345–355. [DOI] [PubMed] [Google Scholar]
- Karpen G.H. and Allshire, R.C.S. (1997) The case for epigenetic effects on centromere identity and function. Trends Genet., 13, 489–496. [DOI] [PubMed] [Google Scholar]
- Lee C., Wevrick, R., Fisher, R.B., Ferguson-Smith, M.A. and Lin, C.C. (1997) Human centromeric DNAs. Hum. Genet., 100, 291–304. [DOI] [PubMed] [Google Scholar]
- Mascarello J.T., Soderberg, K.L. and Scheffler, I.E. (1980) Assignment of a gene for succinate dehydrogenase to human chromosome 1 by somatic cell hybridization. Cytogenet. Cell Genet., 28, 121–135. [DOI] [PubMed] [Google Scholar]
- Mills W., Critcher, R., Lee, C. and Farr, C. (1999) Generation of an 2.4 Mb human centromere based minichromsome by targeted telomere-associated chromosome fragmentation in DT40. Hum. Mol. Genet., 8, 751–761. [DOI] [PubMed] [Google Scholar]
- Shen M.H., Mee, P.J., Nichols, J., Yang, J., Brook, F., Gardner, R.L., Smith, A.G. and Brown, W.R.A. (1999) A structurally defined minichromosome vector for the mouse germ line. Curr. Biol., 10, 31–34. [DOI] [PubMed] [Google Scholar]
- Yang J.W., Pendon, C., Yang, J., Haywood, N., Chand, A. and Brown, W.R.A. (2000) Human mini-chromosomes with minimal centromeres. Hum. Mol. Genet., 9, 1891–1902. [DOI] [PubMed] [Google Scholar]