Abstract
β-catenin/Armadillo are transcriptional co-activators that mediate Wnt signalling in normal development. Activated forms of β-catenin are oncogenic. We have constructed mutant forms of Drosophila Armadillo which correspond to common human oncogenic mutations, and find them to activate Armadillo constitutively. When expressed in the Drosophila eye, these eventually induce apoptosis in all cell types. Intriguingly, cells in the eye are resistant to the effects of activated Armadillo for a long period prior to the onset of cell death at the mid-pupal stage. This latency is conferred by EGF receptor (EGFR)/MAP kinase signalling, which prevents activated Armadillo from inducing apoptosis; when EGFR signalling naturally ceases, the cells rapidly die. Nemo, the Drosophila homologue of NLK in mice and LIT-1 in Caenorhabditis elegans, does not antagonize activated Armadillo, suggesting that the Nemo-like MAP kinases may not generally interact with Armadillo/β-catenin. Thus, our results show that activated Armadillo is subject to a specific negative control by EGFR/Rolled MAP kinase signalling.
INTRODUCTION
β-catenin and its Drosophila homologue Armadillo have two distinct functions during development. Membrane-associated β-catenin/Armadillo is a constituent of E-cadherin junctions, which mediate cellular adhesion, while free β-catenin/Armadillo effects the nuclear response to Wnt signalling. In normal cells, free cytoplasmic β-catenin/Armadillo is destabilized by the Axin complex, which also contains glycogen synthase kinase 3β (GSK3) and the tumour suppressor Adenomatous polyposis coli (APC). Wnt stimulation inhibits this complex, thus allowing the stabilization of β-catenin/Armadillo and its accumulation in the nucleus where it serves as a transcriptional co-activator of TCF (T-cell factor). Mutations of the putative GSK3 phosphorylation sites within the N-terminus of β-catenin which stabilize this protein have been found in many different human cancers (Polakis, 2000).
Stabilization of β-catenin/Armadillo also results from mutational loss of APC during normal development (Ahmed et al., 1998; McCartney et al., 1999; Yu et al., 1999) or in human cancers (Morin et al., 1997). Loss of APC is found in ∼85% of all colorectal cancers, and is known to be an early event in tumorigenesis (Kinzler and Vogelstein, 1996). Direct experimental evidence shows that stabilized forms of β-catenin cause tumours in the mouse intestine and skin (Gat et al., 1998; Harada et al., 1999). The earliest manifestation of these tumours in the intestine is an abnormal tissue architecture (i.e. an outpocketing of the epithelium); however, at these first stages of tumorigenesis, cell proliferation is normal (Oshima et al., 1997; Harada et al., 1999).
Drosophila provides a powerful genetic system for analysing the function of proteins and for identifying interacting proteins. We have, therefore, constructed mutant forms of Armadillo that are equivalent to common oncogenic mutations in human β-catenin. We examined whether these changes are able to activate Armadillo by examining their effects on Drosophila eye development. Ectopic expression of this activated Armadillo leads ultimately to the apoptosis of all cell types in the eye. This is consistent with the previous discovery that dAPC mutation affects neuronal cell differentiation, causing photoreceptor death (Ahmed et al., 1998). Intriguingly, we find that cells only become vulnerable to activated Armadillo in the pupal retina, at a time when EGFR signalling ceases. Our results show that EGFR and MAP kinase signalling protect epithelial cells from responding to activated Armadillo.
RESULTS
Activated Armadillo causes late onset of apoptosis in the pupal eye disc
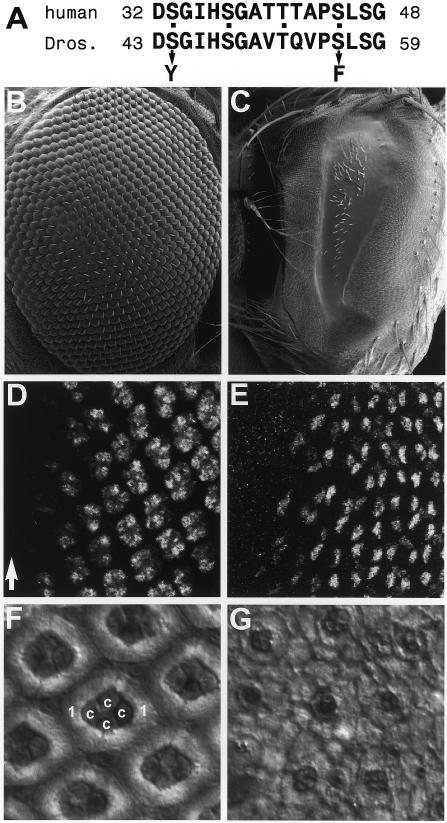
We introduced two of the most common oncogenic point mutations of human β-catenin (Polakis, 2000) into Armadillo (S56F and S44Y; Figure 1A) and expressed these mutant Armadillo genes in Drosophila directly from an eye-specific enhancer. These transformants had severely disrupted eyes which are rough and glazed, and often substantially smaller than wild-type eyes (Figure 1B and C). There was no discernible quantitative or qualitative difference between the eyes from Y or F transformants. We shall thus refer to both types of mutated Armadillo collectively as Arm*.
Fig. 1. The Arm* rough eye phenotype. (A) Mutations in S44Y and S56F Armadillo. The putative GSK3 phosphorylation sites (dots) in the N-termini of human β-catenin and Drosophila Armadillo are shown. The mutations indicated by arrows correspond to some of the most common oncogenic mutations found in human tumours. (B) Wild-type eye. (C) Eye from a homozygous Y55 fly; this eye is very narrow, rough and glazed as a consequence of Arm* expression in the eye disc. (D and E) Eye discs from third instar homozygous Y55 larvae, stained with α-Elav (D) or α-Cut (E); photoreceptor (D) and cone cell recruitment (E) are completely normal, despite expression of Arm* in all cells behind the morphogenetic furrow (arrow) [note, in (E) the furrow is beyond the left edge of the panel]. (F and G) Pupal eye discs (44 h after pupariation), stained with cobalt sulfide to visualize cone cells. (F) wild type; at this focal plane four cone cells (c) and two primary pigment cells (1) are seen in each ommatidium. (G) Y55 homozygote; the Arm* retina is beginning to be disorganized, and both cone and pigment cells are missing. Examination at different focal planes (not shown) indicates that there are also missing photoreceptors. At later stages the tissue becomes completely disrupted. Posterior is to the right in this and subsequent figures.
To examine the basis for the rough eye phenotype, we stained larval eye discs from moderate Y and F lines with antibodies against Elav, a neuronal marker, and against Cut, a marker for the non-neuronal cone cells (Blochlinger et al., 1990). To our surprise, we found that the larval discs of these Arm* lines are completely normal in every respect (Figures 1D and E, and 2B), despite expressing increased levels of Armadillo (not shown). Thus, we examined pupal eye discs with cobalt sulfide staining. This revealed that the early pupal discs were also completely normal. Prior to ∼30 h post-pupariation, they are indistinguishable from wild type. However, ∼30–36 h into pupal development, we begin to see a loss of cells and general breakdown of the retinal morphology (Figure 1F and G). All cell types (neuronal, cone and pigment cells) are affected. Later in pupal development, the retina largely disintegrates.
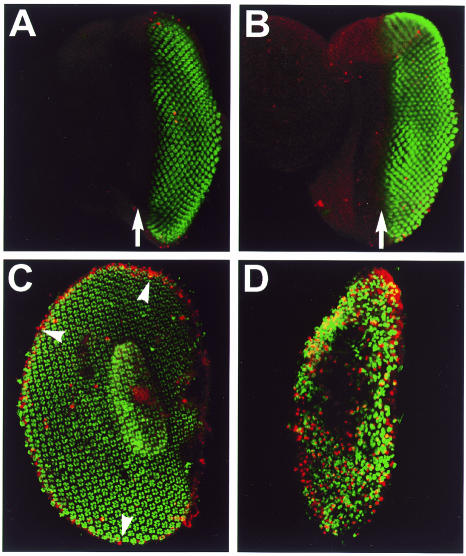
Fig. 2. Apoptosis in wild-type and Arm* eye discs. Third instar larval (A and B) and 46 h pupal eye discs (C and D), TUNEL labelled (red) and stained with α-Elav (green). (A and C) wild type; (B and D) Y55 homozygote. There is little TUNEL staining in wild-type larval discs (A), or in Y55 discs (B) which express Arm* in all cells behind the morphogenetic furrow (arrow). Wild-type pupal discs also have very few dying cells, except at the margin of the retina (C). In contrast, there is massive cell death throughout the retina of 46-h-old pupal Y55 discs (D). Note that the Y55 retinas are by this stage smaller than the wild type.
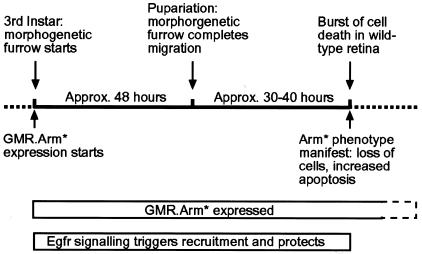
This phenotype had the hallmarks of cell death. We performed TUNEL staining of pupal discs and found large numbers of apoptotic cells throughout the retina of 40–48 h-old pupal discs from Arm* transformants (Figure 2D) compared with low numbers of TUNEL-positive cells in control discs at this stage (Figure 2C; note that most of the TUNEL-positive cells in wild-type retinas are at the margin). Earlier pupal and larval discs from the same lines have very few TUNEL-positive cells (Figure 2B, compare with A; and not shown). Activated Armadillo, expressed throughout ommatidial development, therefore induces onset of apoptosis in the pupal eye disc, ∼3–4 days after its expression is initiated (Figure 3).
Fig. 3. Timeline of Arm*-induced cell death.
We also expressed with GMR.Gal4 a constitutively active form of Armadillo called S10 in which the putative GSK3 phosphorylation sites and flanking residues are deleted (Pai et al., 1997). The mutant phenotypes caused by this misexpression were indistinguishable from those due to Arm*, both in pupal and adult eyes. This confirms that the human oncogenic β-catenin mutations are sufficient to constitutively activate Drosophila Armadillo. Finally, we similarly expressed dTCF, the transcription factor activated by Armadillo, and found this to cause essentially the same eye defects, although they were less severe. In other words, the larval and early pupal discs were completely normal, but the 40–48-h-old pupal discs showed extensive disruptions. This suggests that the disruptive effects of activated Armadillo are mediated by dTCF.
Activated Armadillo is antagonized by EGFR signalling
We crossed representatives of weak, moderate and severe Arm* lines with dTCF mutants to halve the dTCF gene dosage. The eyes of these flies were much more normal, exhibiting only a mild roughening (Figure 4A and B). This suppression strongly supports the notion that the disruptive activity of Arm* is mediated by dTCF. We also examined the same lines in a genetic background heterozygous for armadillo (Table I). The eyes of these flies were indistinguishable from their controls, indicating that the S56F and S44Y mutations result in a fully activated form of Armadillo whose disruptive activity does not depend on endogenous Armadillo.
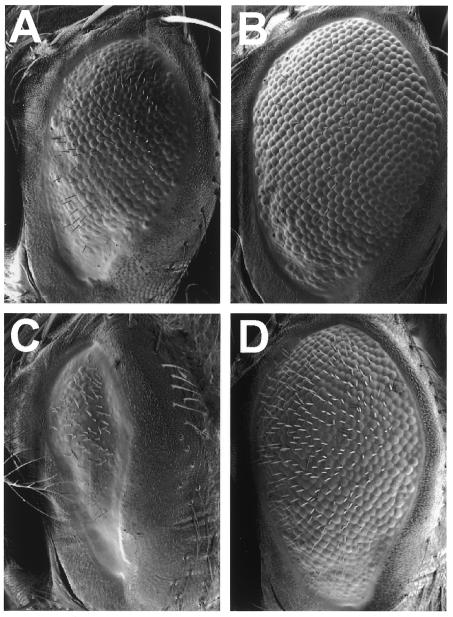
Fig. 4. Modification of the Arm* phenotype by altered EGFR signalling. Eyes from F76/+ flies (A), also heterozygous for dTCF2 (B), EGFRflb1K35 (C) or argosΔ7 (D). Halving the dose of dTCF suppresses the Arm* phenotype (B), whereas halving the dose of EGFR enhances it (C). The Arm* phenotype is also suppressed by halving the dose of argos (D) and other inhibitors of EGFR signalling (see Table I).
Table I. Genetic interactions with Arm*.
| Strong enhancement | EGFRflb1K35, rase1B |
| Weak enhancement | rho7M43, spiA14, drke0A, sose2H, rl5, pntΔ88 |
| Strong suppression | dTCF2, dTCF3, argoslΔ7 |
| Weak suppression | sproutyΔ5 |
| No interaction | arm4, armXM19, Df(3L)H99, svb1, aop2, N55e11, wgcx4, dshv26, zw3M11–1, h14H, nemoP1, nemoi147–1, dppS4, Mad12, hh13C, ptcG12 |
Several representative Arm* transformant lines were crossed to the mutants indicated. The adult eye phenotypes of progeny which carried one copy of Arm*, and were heterozygous for the mutations indicated, were phenotypically scored (see text).
We asked whether the prolonged resistance of cells in the eye to excess Arm* was due to antagonism by another signal. To identify this putative protective signal, we tested a number of genes with known roles in eye development or developmental signalling for their ability to interact genetically with Arm*. In most cases, heterozygosity for these genes did not modify the Arm* mutant phenotype (Table I), suggesting that their corresponding pathways do not affect the activity of Arm* in the developing eye. However, there was one exception: we found that the rough eyes due to Arm* were consistently modified by mutations in the EGFR signalling pathway. For example, halving the dose of EGFR itself (Figure 4C), or of its effector Dras1, strongly enhanced the Arm* phenotype. Conversely, halving Argos, an extracellular inhibitor of EGFR (Schweitzer et al., 1995), suppressed this phenotype (Figure 4D). Generally, mutation of positively acting EGFR pathway components enhances the rough eye phenotype due to Arm*, while mutation of negatively acting components suppresses it (Table I). We, therefore, conclude that the EGFR signalling specifically antagonizes the activity of the activated Armadillo in the eye.
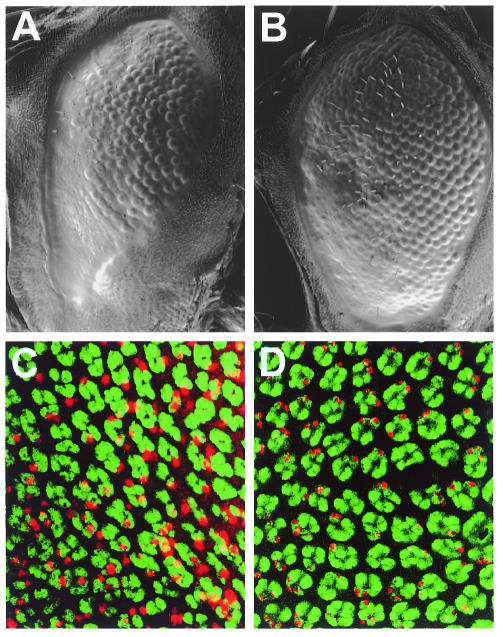
Larval and early pupal eye discs experience multiple waves of EGFR signalling which trigger the recruitment of cells to various fates (Freeman, 1996, 1997). These waves abate in the pupal disc, during a period that coincides roughly with the period in which the disruptive effects of Arm* are observed. We surmised that the cessation of EGFR signalling may cause the sharp onset of the disruptive effects of Arm*. If so, prolonged EGFR signalling might delay this onset, thereby suppressing the rough eye phenotype caused by Arm*. This was tested by co-expressing Arm* with either an activated form of the receptor itself (TorDEGFR; Reichman-Fried et al., 1994) or with Rhomboid, a protein responsible for EGFR activation. TorDEGFR overexpression suppressed the Arm* phenotype in the adult eye considerably (Figure 5A and B). Likewise, in TUNEL-stained pupal discs (Figure 5C and D), we found that TorDEGFR suppressed the rate of apoptosis due to Arm* on average by a factor of three (ranging from 1.5× to 5.3× in four different experiments). Rhomboid overexpression also suppressed the Arm* rough eye phenotype, but to a lesser extent than the activated receptor (not shown). Evidently, sustained EGFR stimulation extends the protection against the disruptive effects of Arm*. Furthermore, our evidence suggests that wild-type levels of EGFR signalling prevent cells from responding to activated Armadillo throughout larval and early pupal stages of eye development.
Fig. 5. Sustained EGFR signalling antagonizes Arm*. (A and B) Eyes from F76/+ flies (A) also expressing TorDEGFR (B) in the eye disc throughout larval and pupal development (see Methods). This treatment leads to a significant suppression of the Arm* rough eye phenotype (B) compared with untreated controls (A). (C and D) Forty-six hour pupal discs from F76/+ transformants (C) also expressing TorDEGFR (D), TUNEL labelled (red) and stained with α-Elav (green). The suppression of the Arm* rough eye phenotype by sustained EGFR signalling reflects a suppression of the rate of cell death in the pupal disc (D, compare with C).
It has recently been reported that the Nemo-like MAP kinase LIT-1 in Caenorhabditis elegans downregulates the TCF homologue POP-1 during cell polarization in the early embryo (Meneghini et al., 1999). Furthermore, mouse Nemo-like kinase (NLK) antagonizes TCF/β-catenin by apparently obliterating their binding to DNA (Ishitani et al., 1999). In Drosophila, the only Nemo-like MAP kinase is encoded by nemo, a non-essential gene whose only reported function is in the control of ommatidial rotation in the eye (Choi and Benzer, 1994; Zheng et al., 1995). However, despite the fact that Nemo is expressed and functions in the larval eye disc, nemo heterozygosity does not modify the rough eye phenotype caused by Arm* (Table I). Furthermore, there is no increase in larval cell death when Arm* is expressed in the complete absence of Nemo (also see Supplementary data available at EMBO reports Online). Thus, our results imply that Nemo has no protective or antagonistic effect on Arm* signalling in the Drosophila eye disc.
DISCUSSION
We have shown that the Drosophila Armadillo protein is made constitutively active by introducing point mutations known to activate its human counterpart, β-catenin. Expression of these activated forms of Armadillo causes a late onset of apoptosis in the developing Drosophila eye. These effects are similar to those of overexpressing dTCF, and genetic interactions (Table I) indicate that they are mediated by endogenous dTCF. The disruptive effects of these conditions closely mimic those of dAPC mutations except that, since dAPC expression is restricted to neuronal cells, cell death in that case is confined to photoreceptors (Ahmed et al., 1998). Therefore, in flies as in humans, the consequences of activated Armadillo/β-catenin are essentially equivalent to those caused by APC loss. Importantly, these new forms of activated Armadillo provide us with powerful tools for genetic screens which might identify ancillary signalling proteins conserved between humans and Drosophila.
A precedent for an antagonistic effect between Wingless and EGFR signalling is found in the embryonic epidermis (O’Keefe et al., 1997; Szüts et al., 1997). In this case, signalling by these pathways appears to be integrated at the level of a common target gene, svb (Payre et al., 1999). Given that, in the eye disc, overexpression of dTCF causes the same delayed cell death as Arm*, and that the Arm* rough eye phenotype is suppressed by dTCF heterozygosity, this indicates that the antagonism between Arm* and EGFR signalling also occurs at the level of target gene transcription. As in the embryonic epidermis, antagonistic inputs from the two pathways could be integrated at a transcriptional enhancer of a common target gene. However, svb is not a good candidate in this case since it is not expressed in the eye (see Flybase, http://flybase.bio.indiana.edu/) and we have not found any genetic interaction between svb and Arm* (Table I).
Although we have argued that the delayed apoptotic effect of Arm* is based primarily on dTCF-mediated transcription, there may also be a post-transcriptional contribution. This is suggested by our observation that Armadillo levels were only moderately elevated in larval eye discs overexpressing Arm*, but strongly increased in 40–48-h-old pupal discs that showed severely disrupted ommatidia (not shown). As was observed for Armadillo S10 (Pai et al., 1997), both cytoplasmic and nuclear levels were increased simultaneously, indicating that both types of activating mutations cause stabilization of free protein rather than altering its relative distribution between cytoplasm and nucleus. However, since this increase in Armadillo level correlates in time with the onset of apoptosis, we cannot tell whether the accumulation is a cause or an effect of the putative transcriptional changes induced by Arm*.
Our most interesting observation was that, in contrast to other tissues where activated Armadillo has immediate effects (e.g. Pai et al., 1997; Payre et al., 1999; Yu et al., 1999), Arm* was expressed for 2–4 days in eye disc cells before causing a detectable phenotype; onset of apoptosis occurred only at the mid-pupal stage (Figure 3). This delayed manifestation of the effects of Arm* could not have been inferred from the phenotype of dAPC mutants. Our results imply that this latency is a consequence of protection conferred by EGFR signalling. The EGFR has multiple functions in the developing eye, including a protective effect against cell death in normal development (Domínguez et al., 1998; Miller and Cagan, 1998). Furthermore, a strong genetic interaction has been observed between the cell death-inducing factor Hid and Ras1 signalling in the eye, and the latter apparently promotes cell survival by downregulating Hid directly at the transcriptional and post-transcriptional level (Bergmann et al., 1998; Kurada and White, 1998).
In contrast, we see no suppression of the Arm* rough eye phenotype by heterozygosity for the H99 deficiency, which uncovers Hid and two further cell-death-inducing factors (Table I; see also Kurada and White, 1998). This argues that the antagonism we observe between Arm* and EGFR signalling is specific, and distinct from the more general survival-promoting function of the receptor, which is mediated directly by Hid. In support of this, a large number of genes including dE2F, p53 and presenilins have been found to induce cell death when expressed in the larval eye disc (see Supplementary references); in all cases, the apoptosis occurs in the third instar disc, despite the fact that normal EGFR signalling is very active at this stage. In the case of dE2F, this early-onset apoptosis has been shown to be antagonized by EGFR signalling (Staehling-Hampton et al., 1999), reflecting the ongoing survival-promoting role of the EGFR throughout disc development. The distinction between this and the phenomenon we report here is demonstrated by the fact that the rough eye phenotype caused by simultaneous overexpression of dE2F, dDP and p35 is not suppressed by dTCF or arm heterozygosity (see Methods). Also, there is no known requirement for arm in the larval disc (see Methods; and Boutros et al., 1998). Taken together, these observations suggest that the dramatic EGFR-mediated delay of the Arm*-induced apoptotic phenotype is unique, and that the mechanism on which it is based is distinct from that employed by the receptor in generally promoting cell survival in the disc.
Our results are reminiscent of those of Gat et al. (1998) who found that activated β-catenin was tumorigenic only in certain specific regions of the mouse epidermis, despite being expressed throughout this cell layer. Similarly, stabilized β-catenin produced adenomas in a particular section of the crypt-villus axis of the mouse intestinal epithelium, again despite being expressed throughout this epithelium (Harada et al., 1999). Therefore, a theme emerges of additional regulation of strongly oncogenic signalling by activated Armadillo/β-catenin. Bearing in mind possible differences between flies and mammals, the effects we see in the Drosophila eye may nevertheless provide a useful model for studying this regulation.
In conclusion, we have established a powerful genetic system for analysing the mechanism by which oncogenic forms of Armadillo/β-catenin disrupt normal cellular controls. Furthermore, we have provided evidence that EGFR/MAP kinase signalling effectively protects epithelial cells in the eye imaginal disc against activated Armadillo. More generally, our results imply that even strongly activated forms of Armadillo/β-catenin remain susceptible to restraint by other signalling pathways.
METHODS
Constructs and transformants
The following base substitutions were introduced into a plasmid encoding full-length Armadillo with an artificial BamHI site upstream of the ATG codon (Riese et al., 1997): codon 44 TCC→TAC (for S44Y; for Y lines) and codon 56 TCG→TTC (for S56F; for F lines). Mutant cDNAs were subcloned as 2.7 kb BamHI fragments into pGMR1 (Hay et al., 1994) cut with BglII. Nine transformant Y and F lines were isolated (host y w). These were categorized as strong (slit eyes; e.g. F36), moderate (oval eyes; e.g. lines F76, Y55) or weak (nearly round eyes; e.g. F32), based on the severity of their rough eye phenotype; the eyes of the latter two, but not of the former, were substantially narrowed in flies homozygous for the transposon.
Somatic clones mutant for armXM19 and arm25B were induced at 48–72 h of development, using standard FRT chromosomes. These clones, though narrow and preferentially found around the disc equator, were normal at the late third larval instar as well as in the adult eye. The mutant alleles and stocks used are described in FlyBase.
GMR.dE2FdDPp35 or GMR.p21 flies (Staehling-Hampton et al., 1999) were crossed to dTCF2, arm4, dAxinP and dAPCQ8 mutants (as described in Table I), but no modification of the rough eye phenotype was found in any of these trans-heterozygotes.
Histology
All techniques have been previously described. Scanning electron microscopy is described in Kimmel et al. (1990). Antibodies, immunostaining and TUNEL labelling are described in Domínguez et al. (1998), cobalt sulfide staining in Wolff and Ready (1991).
Expression of TorDEGFR (Reichman-Fried et al., 1994), under the control of the sevenless enhancer and the hsp70 promoter, was achieved by 30 min 37°C heat shocks every 6 h throughout pupal life.
Supplementary data
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank R. Smith and S. Eresh for technical assistance, M. Wehrli, F. Payre and N. Dyson for fly strains, and E. Saller and T. Brabletz for comments on the manuscript.
REFERENCES
- Ahmed Y., Hayashi, S., Levine, A. and Wieschaus, E. (1998) Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell, 93, 1171–1182. [DOI] [PubMed] [Google Scholar]
- Bergmann A., Agapite, J., McCall, K. and Steller, H. (1998) The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell, 95, 331–341. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer, R., Jan, L.Y. and Jan, Y.N. (1990) Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev., 4, 1322–1331. [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio, N., Strutt, D.I. and Mlodzik, M. (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell, 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Choi K.W. and Benzer, S. (1994) Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell, 78, 125–136. [DOI] [PubMed] [Google Scholar]
- Domínguez M., Wasserman, J.D. and Freeman, M. (1998) Multiple functions of the EGF receptor in Drosophila eye development. Curr. Biol., 8, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell, 87, 651–660. [DOI] [PubMed] [Google Scholar]
- Freeman M. (1997) Cell determination strategies in the Drosophila eye. Development, 124, 261–270. [DOI] [PubMed] [Google Scholar]
- Gat U., DasGupta, R., Degenstein, L. and Fuchs, E. (1998) De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell, 95, 605–614. [DOI] [PubMed] [Google Scholar]
- Harada N., Tamai, Y., Ishikawa, T., Sauer, B., Takaku, K., Oshima, M. and Taketo, M.M. (1999) Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J., 18, 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B.A., Wolff, T. and Rubin, G.M. (1994) Expression of baculovirus P35 prevents cell death in Drosophila. Development, 120, 2121–2129. [DOI] [PubMed] [Google Scholar]
- Ishitani T. et al. (1999) The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature, 399, 798–802. [DOI] [PubMed] [Google Scholar]
- Kimmel B.E., Heberlein, U. and Rubin, G.M. (1990) The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev., 4, 712–727. [DOI] [PubMed] [Google Scholar]
- Kinzler K.W. and Vogelstein, B. (1996) Lessons from hereditary colorectal cancer. Cell, 87, 159–170. [DOI] [PubMed] [Google Scholar]
- Kurada P. and White, K. (1998) Ras promotes cell survival in Drosophila by downregulating hid expression. Cell, 95, 319–329. [DOI] [PubMed] [Google Scholar]
- McCartney B.M., Dierick, H.A., Kirkpatrick, C., Moline, M.M., Baas, A., Peifer, M. and Bejsovec, A. (1999) Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol., 146, 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini M.D., Ishitani, T., Carter, J.C., Hisamoto, N., Ninomiya-Tsuji, J., Thorpe, C.J., Hamill, D.R., Matsumoto, K. and Bowerman, B. (1999) MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature, 399, 793–797. [DOI] [PubMed] [Google Scholar]
- Miller D.T. and Cagan, R.L. (1998) Local induction of patterning and cell death in the developing Drosophila retina. Development, 125, 2327–2335. [DOI] [PubMed] [Google Scholar]
- Morin P.J., Sparks, A.B., Korinek, V., Barker, N., Clevers, H., Vogelstein, B. and Kinzler, K.W. (1997) Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science, 275, 1787–1790. [DOI] [PubMed] [Google Scholar]
- O’Keefe L., Dougan, S.T., Gabay, L., Raz, E., Shilo, B.Z. and DiNardo, S. (1997) Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development, 124, 4837–4845. [DOI] [PubMed] [Google Scholar]
- Oshima H., Oshima, M., Kobayashi, M., Tsutsumi, M. and Taketo, M.M. (1997) Morphological and molecular processes of polyp formation in ApcD716 knockout mice. Cancer Res., 57, 1644–1649. [PubMed] [Google Scholar]
- Pai L.M., Orsulic, S., Bejsovec, A. and Peifer, M. (1997) Negative regulation of Armadillo, a Wingless effector in Drosophila. Development, 124, 2255–2266. [DOI] [PubMed] [Google Scholar]
- Payre F., Vincent, A. and Carreno, S. (1999) ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature, 400, 271–275. [DOI] [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Reichman-Fried M., Dickson, B., Hafen, E. and Shilo, B.-Z. (1994) Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev., 8, 428–439. [DOI] [PubMed] [Google Scholar]
- Riese J., Yu, X., Munnerlyn, A., Eresh, S., Hsu, S.C., Grosschedl, R. and Bienz, M. (1997) LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell, 88, 777–787. [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Howes, R., Smith, R., Shilo, B.Z. and Freeman, M. (1995) Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature, 376, 699–702. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K., Ciampa, P.J., Brook, A. and Dyson, N. (1999) A genetic screen for modifiers of E2F in Drosophila melanogaster. Genetics, 153, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szüts D., Freeman, M. and Bienz, M. (1997) Antagonism between EGFR and Wingless signalling in the larval cuticle of Drosophila. Development, 124, 3209–3219. [DOI] [PubMed] [Google Scholar]
- Wolff T. and Ready, D.F. (1991) Cell death in normal and rough eye mutants of Drosophila. Development, 113, 825–839. [DOI] [PubMed] [Google Scholar]
- Yu X., Waltzer, L. and Bienz, M. (1999) A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nature Cell Biol., 1, 144–151. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang, J. and Carthew, R.W. (1995) frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development, 121, 3045–3055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.