Abstract
The antiinflammatory cytokine response during urosepsis was determined by measurement of concentrations of soluble tumor necrosis factor receptor (sTNFR) types I and II, interleukin 1 receptor antagonist (IL-1ra), soluble IL-1 receptor type II (sIL-1RII), and interleukin 10 in sera and urine of 30 patients with culture-proven urinary tract infections before and 4, 24, 48, and 72 h after initiation of antibiotic therapy and in 20 healthy individuals. In serum, the levels of sTNFR types I and II, IL-1ra, and IL-10 were higher in patients than in controls. In urine, only sTNFR type I and II levels were elevated in patients. The ratios of concentrations of both types of sTNFR in urine to concentrations in serum were higher in patients than in controls. These findings indicate that during urosepsis, the antiinflammatory cytokine response is generated predominantly at the systemic level.
The clinical spectrum of urinary tract infections ranges from asymptomatic bacteriuria to acute pyelonephritis. In the healthy urinary system, the dynamics of urine flow and a functional vesicoureteral junction protect against ascending urinary tract infections. In recent years, attention has been paid to the role of inflammation in resistance to urinary tract infections (29).
Cytokines are small proteins important for the orchestration of inflammatory processes. The most-potent proinflammatory cytokines are tumor necrosis factor alpha (TNF) and interleukin 1 (IL-1) (10, 32). Several endogenous mechanisms that can modulate the production and/or activity of TNF and/or IL-1 have been identified (31). TNF can bind to two distinct types of cellular receptors. Both TNF receptor species can be processed to soluble forms (sTNFR) that represent the extracellular domains of the respective transmembrane receptors. sTNFR retain their affinity for free TNF and can therefore act as competitive inhibitors of TNF activity when present in high concentrations (1, 34). Similarly, the extracellular part of the type II IL-1 receptor can be shed from the cell surface. Soluble IL-1 receptor type II (sIL-1R type II) is considered a negative regulator of IL-1 activity, since it binds free IL-1 without eliciting a cellular response (10, 28). Another endogenous IL-1 inhibitor is IL-1 receptor antagonist (IL-1ra), which preferentially binds to the signaling type I IL-1R without inducing any biological response (10). Furthermore, the production of proinflammatory cytokines can be inhibited by so-called antiinflammatory cytokines, of which IL-10 is the most potent (22).
Although animal studies have indicated that enhanced production of TNF and IL-1 plays an important role in the pathogenesis of bacterial sepsis, only a small subset of patients with sepsis have detectable TNF and IL-1 in their circulation (10, 32). However, a presumed increase in TNF and IL-1 activity in such patients is associated with elevated concentrations of inhibitors of these proinflammatory cytokines in plasma. Indeed, it is now well appreciated that the host response to sepsis involves both release of proinflammatory cytokines and release of soluble cytokine inhibitors and antiinflammatory cytokines. The latter response was recently given the name compensatory antiinflammatory response syndrome (CARS), as opposed to the designation systemic inflammatory response syndrome (SIRS) for the former response (6). At present, knowledge of the site of production of the antiinflammatory responses during human sepsis is highly limited. Therefore, in a first attempt to determine whether inhibitors of TNF and IL-1 are secreted locally at the site of the infection or predominantly at the systemic level, we sequentially measured the levels of TNF, sTNFR, IL-1β, IL-1ra, sIL-1R type II, and IL-10 in the urine and sera of patients with urosepsis during a 3-day follow-up period.
MATERIALS AND METHODS
Patients and design.
A total of 30 patients over 18 years of age with gram-negative urosepsis were studied. The diagnosis of urosepsis was based on the presence of a urine culture positive for a gram-negative micro-organism with pyuria (leukocytes, >100 cells/mm3, with few epithelial cells) and metabolic or hematologic signs of systemic infection, including two of the following six signs: tachycardia (>90/min); hypotension (systolic pressure, <90 mm Hg); hypoxemia (pO2 ≤ 75 mm Hg); leukocytosis (>10,000/mm3); abnormal prothrombin time, activated partial thromboplastin time, or thrombocytopenia (<100,000/mm3); and acute mental status change. Exclusion criteria included antibiotic use within the previous 72 h, a very poor clinical condition, severe renal insufficiency (estimated creatinine clearance, <30 ml/min), or pregnancy. Further details of the study have been published elsewhere (24). Patients were treated with 500 mg of intravenous imipenem every 8 h for the first 72 h or with 1,000 mg of intravenous ceftazidime every 8 h. Since the type of antibiotic regimen (imipenem versus ceftazidime) did not significantly influence the levels of TNF, sTNFR, IL-1β, IL-1ra, soluble IL-1R type II, or IL-10, data from the two groups were combined. Clinical data (APACHE II score) and blood and urine samples were collected immediately before the start of treatment (0 h) and at 4, 24, 48, and 72 h thereafter. Blood and urine samples were also collected from 20 healthy individuals for use as controls. Cultures of all control urines were sterile. Blood and urine samples were centrifuged at 1,500 × g for 20 min. Supernatants were collected and stored at −20°C until assays were performed.
Assays.
The amounts of TNF and IL-1β were measured by enzyme-linked immunosorbent assaying (ELISA) according to the instructions of the manufacturer (Medgenix, Fleurus, Belgium). Both the TNF and the IL-1β ELISAs detect total cytokine levels, i.e., irrespective of whether they are bound by soluble receptors (information supplied by the manufacturer [11]). sTNFR were measured by enzyme-linked immunological binding assaying (ELIBA) as described previously (7, 30). The reagents for sTNFR measurements were kindly donated by Hoffmann La Roche, Ltd. (Basel, Switzerland). The sTNFR assays make use of TNF-binding noninhibitory monoclonal antibodies against TNFR type I (clone htr-20) or TNFR type II (clone utr-4) as coating antibodies, peroxidase-conjugated recombinant human TNF as detecting reagent, and recombinant sTNFR type I or sTNFR type II as standards. The specificity of the sTNFR assays has been confirmed by experiments in which the binding of the detecting recombinant TNF to sTNFRs could be prevented either by addition of an excess (20 μg/ml) unlabeled TNF or by replacing the anti-TNFR monoclonal antibodies by nonspecific antibodies (7). In addition, the linearity of the assays has been verified with natural TNFRs from cell lysates of HL-60 cells as well as recombinant TNFR-p55 and TNFR-p75. The concentrations of both sTNFRs were calculated from the amount of bound labeled TNF by using a 1:1 binding stoichiometry between TNF and sTNFR. The concentrations calculated in this way were consistent with those obtained by using recombinant sTNFRs as the standard (7). IL-1ra was measured by ELISA with mouse anti-human IL-1ra monoclonal antibody (MAb) (4 μg/ml; Antibody Solutions SARL, Illkirch, France) as the coating antibody, biotinylated goat anti-human IL-1ra (0.1 μg/ml; R&D Systems, Abingdon, United Kingdom) as the detecting antibody, and human recombinant IL-1ra (R&D Systems) as the standard. sIL-1R type II was measured by ELISA essentially as described previously (15, 33). Mouse anti-human IL-1R type II MAb (5 μg/ml) was used as the coating antibody, polyclonal rabbit anti-IL-1R type II was used as the labeling antibody, horseradish peroxidase-labeled donkey anti-rabbit immunoglobulin G was used as the detecting antibody, and recombinant sIL-1R type II was used as the standard. The addition of exogenous recombinant IL-1β did not influence the performance of the ELISA. All reagents for the sIL-1R type II assay were kindly donated by John Sims (Immunex Corporation, Seattle, Wash.). IL-10 was measured by ELISA according to the instructions of the manufacturer (PharMingen, San Diego, Calif.). The detection limits of the assays were 7 pg/ml (TNF), 0.40 ng/ml (sTNFR types I and II), 25 pg/ml (IL-1β), 80 pg/ml (IL-1ra), 16 pg/ml (sIL-1RII), 25 pg/ml (IL-10 in serum), and 75 pg/ml (IL-10 in urine).
Statistical analysis.
Values are given as medians and ranges. Differences between healthy controls and patients were analyzed by the Mann-Whitney U test. In patients, changes in time were analyzed by one-way analysis of variance, followed by Dunnett’s t test for multiple comparisons. These two tests were performed after log transformation of the data. Correlations were investigated by calculating the Spearman correlation coefficient (rs). All tests were two tailed, and α was set at 0.05.
Urine concentrations are given in nanograms per milliliter. When levels of cytokines in urine were normalized for urinary creatinine concentrations, analyses of differences between patients and controls and the effect of antibiotic treatment yielded similar results (data not shown).
RESULTS
Patients.
Patient characteristics and cultured microorganisms have been reported previously (24). Escherichia coli was cultured from the urine of 28 patients (93%). A total of 10 patients had positive blood cultures for a microorganism that was also cultured from the urine (E. coli for nine patients and Pseudomonas aeruginosa for 1 patient). All patients recovered fully after treatment.
TNF and sTNFR.
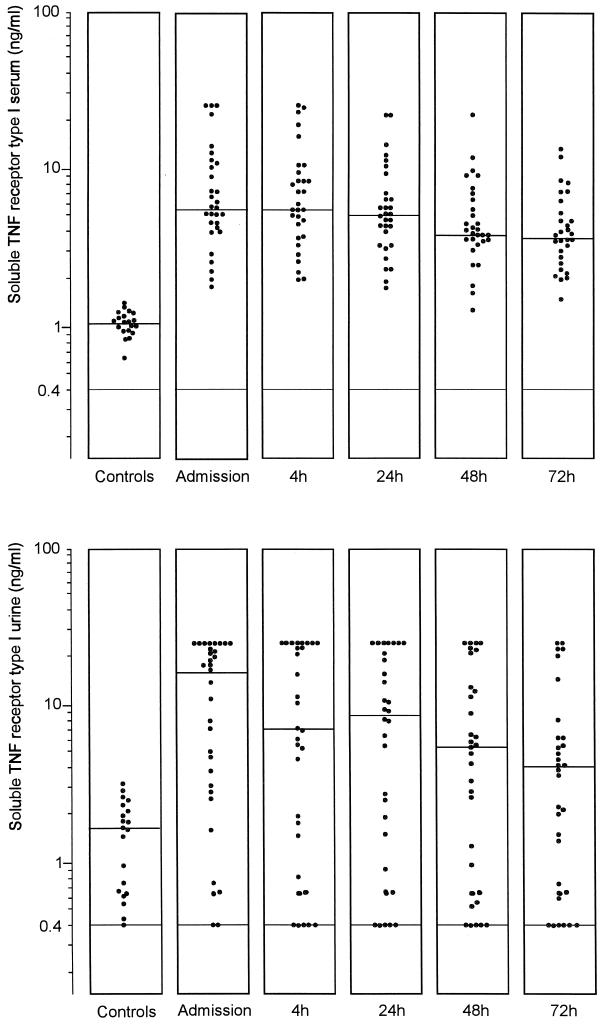
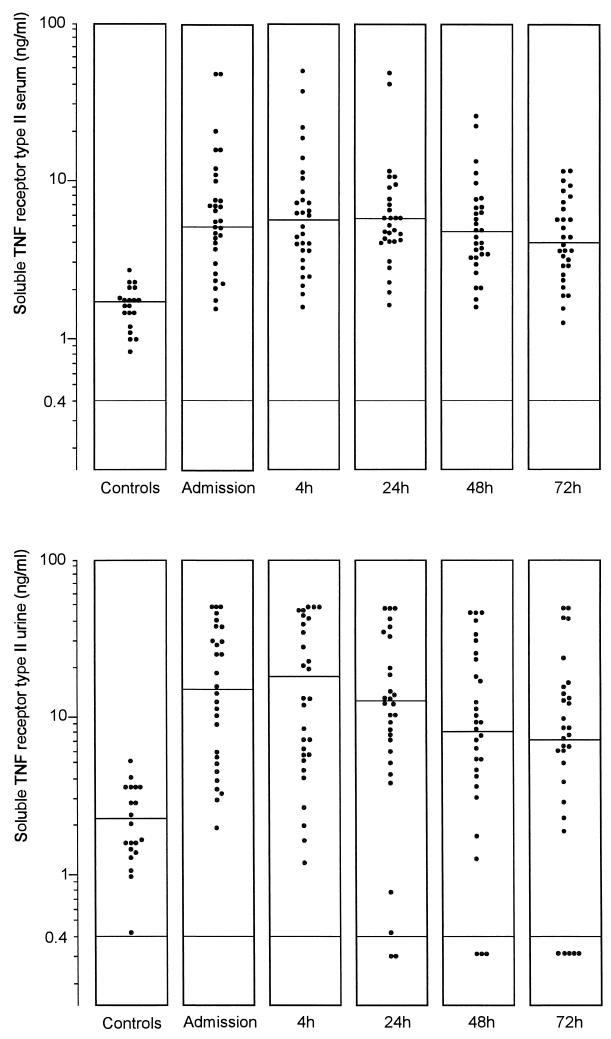
Concentrations of TNF in serum and urine were below the limit of detection in the vast majority of controls and patients, and no significant differences between groups were found (data not shown). In patients, levels of sTNFR types I and II were elevated in both serum and urine compared to controls (Fig. 1 and 2). Upon admission, the sTNFR type I concentrations in serum and urine were 5.79 (1.93 to 25.00) and 17.55 (<0.40 to 25.00) ng/ml, respectively, in patients compared to 1.10 (0.63 to 1.43) and 1.78 (0.40 to 3.32) ng/ml, respectively, in controls (P = 0.005 and P < 0.001). sTNFR type II levels in serum and urine were 5.27 (1.60 to 50.00) and 14.96 (<0.20 to 50.00) ng/ml, respectively, in patients upon admission and 1.77 (0.89 to 2.83) and 2.34 (0.50 to 5.34) ng/ml, respectively, in controls (P < 0.01 and 0.001). The ratio of the concentrations of soluble TNF receptors in urine to the concentrations in serum was higher in patients upon admission than that in controls, although the difference reached statistical significance only for sTNFR type II (Table 1). The level of sTNFR type I decreased significantly 72 h after initiation of the therapy in both serum and urine (for both, P < 0.05) while the decrease in the level of sTNFR type II was not significant. Levels of both types of sTNFR in serum but not in urine upon admission were higher in patients with a positive blood culture (P < 0.01 and <0.001) (Table 2). There was a positive correlation between levels of both types of sTNFR in serum and urine and the APACHE II score (sTNFR type I in serum, rs = 0.55 and P < 0.005; sTNFR type I in urine, rs = 0.61 and P < 0.001; sTNFR type II in serum, rs = 0.47 and P < 0.01; and sTNFR type II in urine, rs = 0.50 and P < 0.01).
FIG. 1.
Levels of sTNFR type I in the sera (upper panel) and urine (lower panel) of healthy subjects and patients with urosepsis upon admission and 4, 24, 48, and 72 h after initiation of antibiotic therapy. Horizontal lines represent the median. There was a significant difference between patients and controls in the values for serum (P = 0.005 [Mann-Whitney U test]) and urine (P <0.001) and a significant decrease in the levels of sTNFR type I in both serum (P <0.05 [Dunnett’s t test]) and urine (P <0.05) at 72 h.
FIG. 2.
Levels of sTNFR type II in the sera and urine of healthy subjects and patients with urosepsis upon admission and 4, 24, 48, and 72 h after initiation of antibiotic therapy. Horizontal lines represent the median. There was a significant difference between patients and controls in the values for serum (P <0.01 [Mann-Whitney U test]) and urine (P <0.001).
TABLE 1.
Median (range) ratios of concentrations of sTNFR types I and II and IL-1ra in urine to concentrations in sera of patients with acute urosepsis upon admission and in healthy controls
| Cytokine | Median ratio (range)
|
|
|---|---|---|
| Patients | Controls | |
| sTNFR type I | 1.84 (0.04–5.93) | 1.68 (0.28–3.41) |
| sTNFR type II | 2.45 (0.30–15.00) | 1.35 (0.20–3.20)a |
| IL-1ra | 0.10 (0.00–1.70) | 1.65 (0.10–12.30)b |
Significantly different (P <0.05) from the patient group (Mann-Whitney U test).
Significantly different (P <0.001) from the patient group (Mann-Whitney U test).
TABLE 2.
Median values and ranges of sTNFR types I and II, IL-1ra, and IL-10 in sera and urine of patients with urosepsis with positive and negative blood cultures
| Cytokine and Source | Median value (range) (ng/ml)
|
||
|---|---|---|---|
| Positive blood culture (n = 10) | Negative blood culture (n = 20) | Difference between the two groups | |
| sTNF type I | |||
| Serum | 12.44 (5.12–25.00) | 5.04 (1.93–25.00) | P <0.01 |
| Urine | 19.10 (0.76–25.00) | 12.40 (<0.4–25.00) | NSa |
| sTNF type II | |||
| Serum | 12.07 (6.63–>50.00) | 4.57 (1.60–>50.00) | P <0.001 |
| Urine | 12.07 (6.63–>50.00) | 4.57 (1.60–>50.00) | NS |
| IL-1ra | |||
| Serum | 17.22 (2.67–>20.00) | 1.91 (0.77–>20.00) | P <0.01 |
| Urine | 2.48 (<0.08–11.58) | 0.21 (<0.08–26.00) | NS |
| IL-10 | |||
| Serum | 0.69 (<0.05–27.32) | 0.09 (<0.05–5.86) | P <0.05 |
| Urine | <0.08 (<0.08–<0.08) | <0.08 (<0.08–0.08) | NS |
NS, not significant.
IL-1β, IL-1ra, and sIL-1R type II.
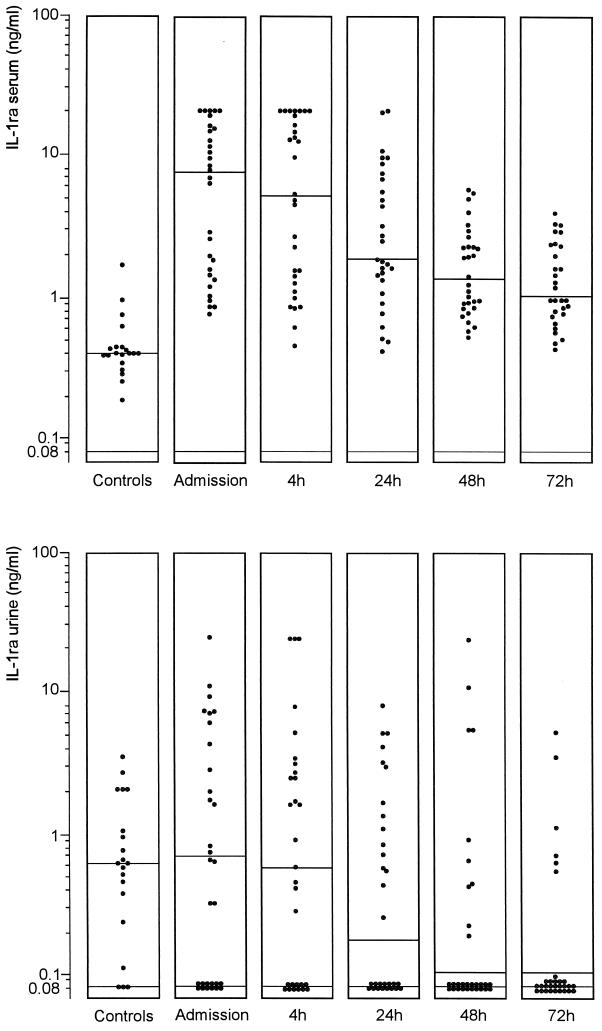
Concentrations of IL-1β in serum and urine were below the limit of detection in the vast majority of controls and patients, and no significant differences between groups were found (data not shown). Levels of IL-1ra in serum were significantly higher in patients (7.40 [0.77 to 20.00] ng/ml) than in controls (0.41 [0.19 to 0.94] ng/ml) (P < 0.001) (Fig. 3). Concentrations of IL-1ra in urine were similar in patients (0.67 [0.10 to 26.00] ng/ml) and controls (0.60 [<0.08 to 3.62] ng/ml). The ratio of the concentration of IL-1ra in urine to that in serum was significantly lower in patients than that in controls (Table 1). Levels of IL-1ra in serum significantly decreased (P <0.05) 24 h after initiation of the therapy. Levels of IL-1ra in serum were higher in patients with a positive blood culture (P <0.01) (Table 2). There was a positive correlation between levels of IL-1ra in serum and the APACHE II score (rs = 0.59; P = 0.001). Levels of sIL-1R type II in serum were 3.25 (1.33 to 10.92) ng/ml in patients and 3.60 (1.69 to 5.24) ng/ml in controls. Levels of sIL-1R type II in urine did not show a difference between the two groups either (0.05 [<0.02 to 3.71] ng/ml versus 0.07 [<0.02 to 1.12] ng/ml).
FIG. 3.
Levels of IL-1ra in the sera and urine of healthy subjects and patients with urosepsis upon admission and 4, 24, 48, and 72 h after initiation of antibiotic therapy. Horizontal lines represent the median. There was a significant difference between patients and controls in the values for serum (P <0.001 [Mann-Whitney U test]) and a significant decrease in the levels of IL-1ra in serum (P <0.05 [Dunnett’s t test]) at 24 h.
IL-10.
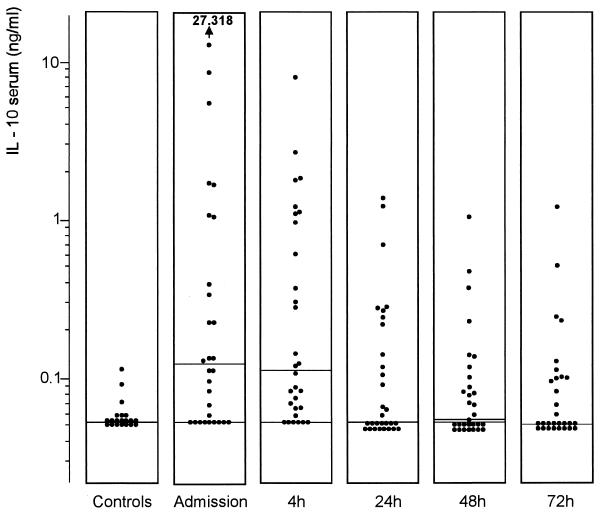
Levels of IL-10 in serum from patients were significantly higher (0.12 [<0.03 to 27.32] ng/ml) than those in controls (<0.03 [<0.03 to 0.11] ng/ml) (P = 0.001) (Fig. 4). IL-10 was undetectable in urine from all but one patient and all controls. Its levels in serum were significantly higher (P <0.05) in patients with positive blood cultures than in those whose cultures were negative (Table 2). Concentrations of IL-10 in serum decreased at 24 h after initiation of the therapy (P <0.05). Levels of IL-10 in serum correlated with levels of IL-1ra (rs = 0.70 and P <0.001), sTNFR type I (rs = 0.64 and P <0.001), and sTNFR type II (rs = 0.52 and P <0.005) in serum upon admission.
FIG. 4.
Levels of IL-10 in sera of healthy subjects and patients with urosepsis upon admission and 4, 24, 48, and 72 h after initiation of antibiotic therapy. Horizontal lines represent the median. There was a significant difference between patients and controls (P = 0.001 [Mann-Whitney U test]) and a significant decrease in the levels of IL-10 in serum (P <0.05 [Dunnett’s t test]) at 24 h.
DISCUSSION
In the present study, we sought to gain more insight into the systemic and localized pro- and antiinflammatory cytokine responses to a clinically well-defined bacterial infection by sequential measurements of concentrations of TNF, IL-1β, and their inhibitors in sera and urine of 30 patients with urosepsis and in 20 normal controls. The concentrations of sTNFR types I and II were elevated in both urine and serum during urinary tract infections, while TNF and IL-1β were undetectable in virtually all patients and controls. The concentrations of IL-1ra and the antiinflammatory cytokine IL-10 were elevated only in serum. The levels of all of these antagonistic members of the cytokine network decreased or tended to decrease during antibiotic therapy.
Proinflammatory cytokine production during urinary tract infection may predominantly occur locally, at the site of the infection. Indeed, deliberate colonization of the human urinary tract with E. coli resulted in detectable levels of IL-6 and IL-8 in urine, but not in serum (2, 16). Previous studies examining cytokine production during acute urinary tract infections reported increased concentrations of IL-6 and IL-8 in serum and urine, with higher levels in urine than in serum (4, 5, 17, 18, 24). Previous studies examining TNF and IL-1β concentrations in urine during urinary tract infections have yielded conflicting results. While one study found elevated TNF levels in urine in patients with bacterial cystitis (9), two other investigations could not reproduce this finding (5, 18). Similarly, levels of IL-1β in urine have been found to be elevated (9, 21) or not elevated (18) during urinary tract infections. In our study, neither TNF nor IL-1β could be detected in the urine of patients with urosepsis. These data suggest that the local production of these proinflammatory cytokines is not strongly enhanced or that these cytokines are not secreted from tissue to the urine in significant quantities. This possibility is supported by findings with a mouse model of pyelonephritis demonstrating an increase in TNF mRNA in the kidney without detectable TNF protein levels in urine or serum (26). Alternatively, TNF and IL-1β production occurs only for a brief period and/or intermittently, and elevated levels are missed due to their short half-lives.
sTNFR have been identified first in the urine of healthy individuals as naturally occurring inhibitors of TNF (12, 23, 27). It is clear now that they are derived from cell-associated TNFR by proteolytic cleavage. The role of sTNFR may be twofold; they can neutralize TNF activity, especially when present in a large molar excess over TNF (such as in the present study), or they may serve as carriers for TNF and even augment its effects by stabilizing its structure and prolonging its activity (1). During recovery from sepsis, a strong reduction of sTNFR levels in plasma toward normal values is usually seen (13). In accordance with this, in our study the levels of sTNFR were elevated both in sera and in urine of patients with urosepsis, with their concentrations positively correlating with the severity of disease as indicated by APACHE II score and decreasing during antibiotic therapy.
Our study does not elucidate whether sTNFR are produced within the urinary tract during urinary tract infection. However, it is of interest that median sTNFR concentrations were two to almost fourfold higher in urine than in concurrently collected serum and that the ratio of sTNFR concentrations in urine to those in serum was higher in patients than that in controls. Considering the dilution factor when levels of cytokines in urine, are measured, it is conceivable that at least part of the sTNFR present in urine is shed from cells in the urinary tract. It is well established that sTNFR are cleared from the circulation by the kidneys and that a decrease in renal function can result in elevated levels of sTNFR in serum (3, 7). Although positive correlations were found between levels of sTNFR and creatinine in serum (data not shown), impaired renal function is unlikely to contribute significantly to our findings since, due to the study inclusion criteria, the vast majority of patients had a normal renal function.
Endogenous IL-1 activity is regulated by IL-1ra and surface and sIL-1R type II. IL-1ra binds with high affinity to IL-1R but does not induce signal transduction (10). The type II IL-1 R serves as a decoy receptor and is not involved in cellular effects of IL-1. sIL-1R type II is generated by shedding of the extracellular domain of the surface receptor, a process that may result in levels at sites of inflammation much higher than those attainable on the cell surface (10). IL-1ra but not sIL-1R type II concentrations were increased in serum during urinary tract infections. This finding is remarkable, since earlier studies of patients with sepsis have documented similar increases in the levels of both IL-1 antagonists in serum (14, 15, 25, 33). It should be noted that low-dose endotoxemia in normal humans is associated only with an increase in levels of IL-1ra in serum while sIL-1R type II concentrations remain unchanged (14, 33). In our study population, we could detect endotoxin in sera from only 7 of our 30 patients (24). Together, these data suggest that shedding of the type II IL-1R to the circulation plays a significant role in the regulation of IL-1 activity only in severe systemic inflammation. We found similar levels of IL-1ra and sIL-1R type II in normal urine and in urine from patients with urinary tract infections. Hence, these data argue against local production of IL-1 inhibitors during urinary tract infections.
IL-10 is an antiinflammatory cytokine that potently inhibits the production of TNF and IL-1 (22). IL-10 concentrations are elevated in the sera of more than 80% of patients with sepsis (20, 33). In our study, 70% of patients with urosepsis had detectable IL-10 in serum, which decreased during therapy. IL-10 remained undetectable in urine, suggesting that local IL-10 production is not strongly stimulated during urinary tract infection and/or that IL-10 is not secreted in urine in large amounts. Apart from its inhibitory effect on proinflammatory cytokines, IL-10 can upregulate the expression of IL-1ra by polymorphonuclear leukocytes (8) and can induce shedding of sTNFR from mononuclear cells (19). Therefore, it is of interest that levels of IL-10 in serum were positively correlated with elevated levels of IL-1ra and sTNFR in serum.
Previously, we reported the concentrations of lipopolysaccharide, IL-6, and IL-8 in patients that are also reported in the present investigation (24). No correlations existed between these proinflammatory parameters and the antiinflammatory responses measured in the present study (data not shown).
In conclusion, we sequentially measured concentrations of inhibitors of two major proinflammatory cytokines in the sera and urine of a group of patients with gram-negative urinary tract infections. Our results demonstrate that in the absence of detectable TNF and IL-1β, levels of sTNFR, IL-1ra, and IL-10 in serum were elevated. In concurrently collected urine, only the levels of sTNFR were increased, with urine-to-serum ratios higher than those in healthy controls. Considering that in patients with acute febrile urinary tract infections, urine concentrations of the proinflammatory cytokines IL-6 and IL-8 exceed those measured in simultaneously obtained serum (4, 5, 17, 18, 24), these data suggest that in contrast to the response of the proinflammatory cytokines IL-6 and IL-8, the antiinflammatory response to acute urinary tract infection is generated for a large part at the systemic level and that cells within the urinary tract do not secrete significant quantities of antiinflammatory mediators into urine, with the possible exception of sTNFR.
ACKNOWLEDGMENTS
This work was financially supported by a grant from the Dutch Kidney Foundation to D. P. Olszyna and by a grant from the Royal Dutch Academy of Arts and Sciences to T. van der Poll.
We thank Anita de Boer for excellent technical assistance.
REFERENCES
- 1.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agace W, Hedges S, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bemelman F J, Jansen J, van der Poll T, van Deventer S J H, ten Berge R J M. Increase of sTNF receptor levels in acute renal allograft rejection after treatment with OKT3. Nephrol Dial Transplant. 1994;9:1786–1790. [PubMed] [Google Scholar]
- 4.Benson M, Jodal U, Agace W, et al. Interleukin (IL)-6 and IL-8 in children with febrile urinary tract infection and asympotmatic bacteriuria. J Infect Dis. 1996;174:1080–1084. doi: 10.1093/infdis/174.5.1080. [DOI] [PubMed] [Google Scholar]
- 5.Benson M, Jodal U, Andreasson A, Karlsson A, Rydberg J, Svanborg C. Interleukin 6 response to urinary tract infection in childhood. Pediatr Infect Dis J. 1994;13:612–616. doi: 10.1097/00006454-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Bone R C, Grodzin C J, Balk R A. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 7.Brockhaus M, Bar-Khayim Y, Gurwicz S, Frensdorff A, Hara N. Plasma tumor necrosis factor soluble receptors in chronic renal failure. Kidney Int. 1992;42:663–667. doi: 10.1038/ki.1992.332. [DOI] [PubMed] [Google Scholar]
- 8.Cassatella M A, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidoff R, Yamaguchi R, Leach G E, Park E, Lad P M. Multiple urinary cytokine levels of bacterial cystitis. J Urol. 1997;157:1980–1985. [PubMed] [Google Scholar]
- 10.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 11.Engelberts I, Stephens S, Francot G J M, van der Linden C J, Buurman W A. Evidence for different effects of soluble TNF-receptors on various TNF measurements in human biological fluids. Lancet. 1991;338:515–516. doi: 10.1016/0140-6736(91)90591-c. [DOI] [PubMed] [Google Scholar]
- 12.Engelmann H, Rubinstein M, Rotman D, Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989;264:11974–11980. [PubMed] [Google Scholar]
- 13.Ertel W, Scholl F A, Gallati H, Bonaccio M, Schildberg F W, Trentz O. Increased release of soluble tumor necrosis factor receptors into blood during clinical sepsis. Arch Surg. 1994;129:1330–1336. doi: 10.1001/archsurg.1994.01420360120017. [DOI] [PubMed] [Google Scholar]
- 14.Fischer E, Van Zee K J, Marano M, et al. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood. 1992;79:2196–2200. [PubMed] [Google Scholar]
- 15.Giri J G, Wells J, Dower S K, et al. Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. J Immunol. 1994;153:5802–5809. [PubMed] [Google Scholar]
- 16.Hedges S, Anderson P, Lidin-Janson G, de Man P, Svanborg C. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect Immun. 1991;59:421–427. doi: 10.1128/iai.59.1.421-427.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedges S, Stenqvist K, Lindin-Janson G, Martinell J, Sandberg T, Svanborg C. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J Infect Dis. 1992;166:653–656. doi: 10.1093/infdis/166.3.653. [DOI] [PubMed] [Google Scholar]
- 18.Ko Y, Mukaida N, Ishiyama S, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61:1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeuwenberg J F M, Jeunhomme T M A A, Buurman W A. Slow release of TNF receptors by monocytes in vitro. J Immunol. 1994;152:4036–4040. [PubMed] [Google Scholar]
- 20.Marchant A, Deviere J, Byl B, de Groote D, Vincent J L, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- 21.Martins S M, Darlin D J, Lad P M, Zimmern P E. Interleukin-1β: a clinically relevant urinary marker. J Urol. 1994;151:1198–1201. doi: 10.1016/s0022-5347(17)35212-6. [DOI] [PubMed] [Google Scholar]
- 22.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin 10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 23.Olsson I, Lantz M, Nilsson E, et al. Isolation and characterization of a tumor necrosis factor binding protein from urine. Eur J Haematol. 1989;42:270–275. doi: 10.1111/j.1600-0609.1989.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 24.Prins J M, Van Agtmael M A, Kuijper E J, Van Deventer S J H, Speelman P. Antibiotic induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis. 1995;172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 25.Pruitt J H, Welborn M B, Edwards P D, et al. Increased soluble interleukin 1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood. 1996;87:3282–3288. [PubMed] [Google Scholar]
- 26.Rugo H S, O’Hanley P, Bishop A G, et al. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J Clin Invest. 1992;89:1032–1039. doi: 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seckinger P, Isaaz S, Dayer J M. Purification and biologic characterization of a specific tumor necrosis factor α inhibitor. J Biol Chem. 1989;264:11966–11973. [PubMed] [Google Scholar]
- 28.Sims J E, Giri J G, Dower S K. The two interleukin-1 receptors play different roles in IL-1 actions. Clin Immunol Immunopathol. 1994;72:9–14. doi: 10.1006/clin.1994.1100. [DOI] [PubMed] [Google Scholar]
- 29.Svanborg C. Resistance to urinary tract infection. N Engl J Med. 1993;329:802–803. doi: 10.1056/NEJM199309093291111. [DOI] [PubMed] [Google Scholar]
- 30.Van der Poll T, Jansen J, van Leenen D, et al. Release of soluble receptors for tumor necrosis factor in clinical sepsis and experimental endotoxemia. J Infect Dis. 1993;168:955–960. doi: 10.1093/infdis/168.4.955. [DOI] [PubMed] [Google Scholar]
- 31.Van der Poll T, Lowry S F. Endogenous mechanisms regulating TNF and IL-1 during sepsis. In: Vincent J L, editor. Year-book of intensive care and emergency medicine. New York: Springer-Verlag; 1995. pp. 385–397. [Google Scholar]
- 32.Van der Poll T, Lowry S F. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995;3:1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Van der Poll T, de Waal Malefyt R, Coyle S M, Lowry S F. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10 and IL-13 concentrations. J Infect Dis. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- 34.Van Zee K J, Kohno T, Fischer E, Rock S C, Moldawer L L, Lowry S F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor α in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]